Quasi-Isostructural Co(II) and Ni(II) Complexes with Mefenamato Ligand: Synthesis, Characterization, and Biological Activity
Abstract
1. Introduction
2. Results and Discussion
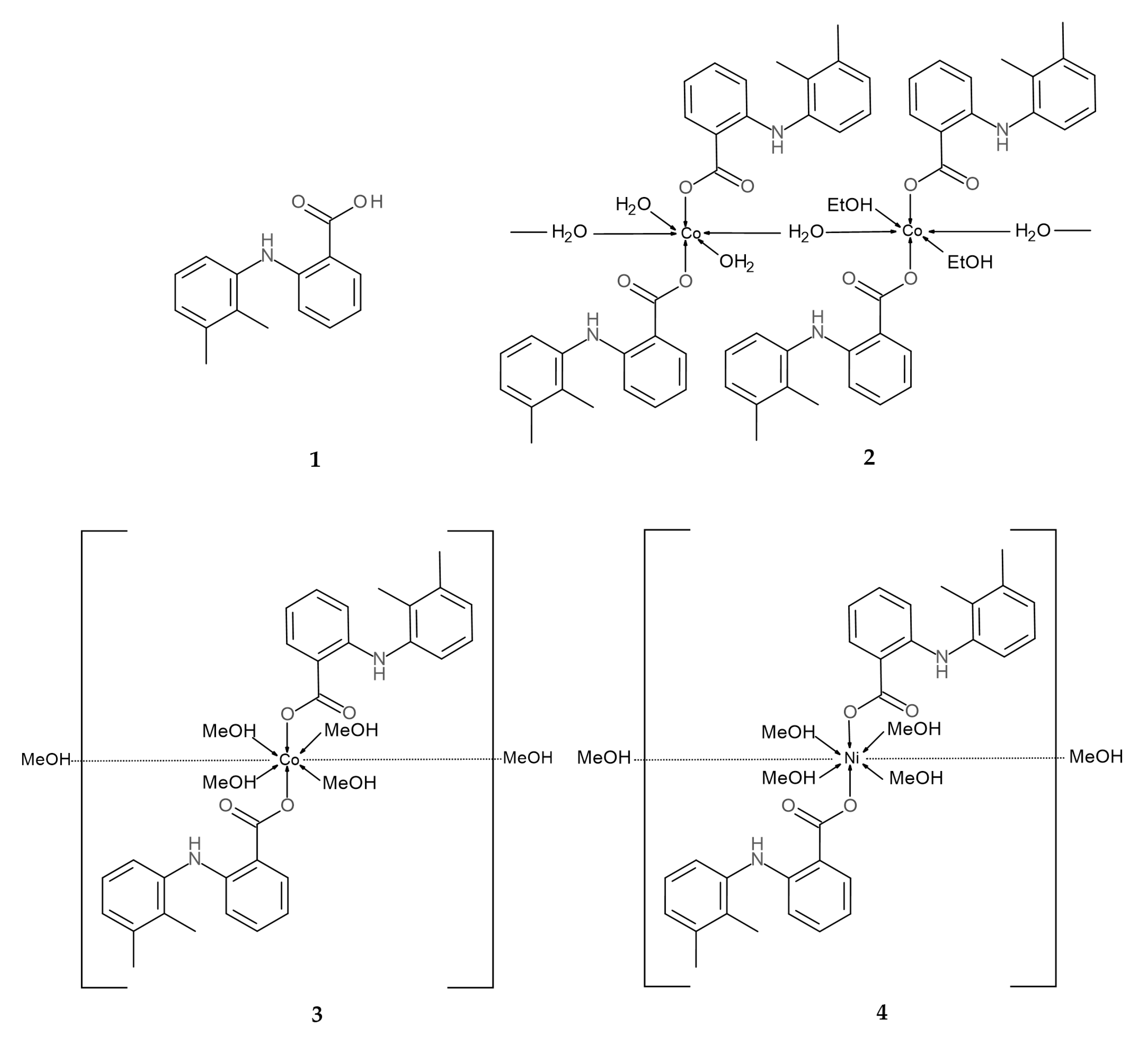
2.1. Description of Crystal Structures
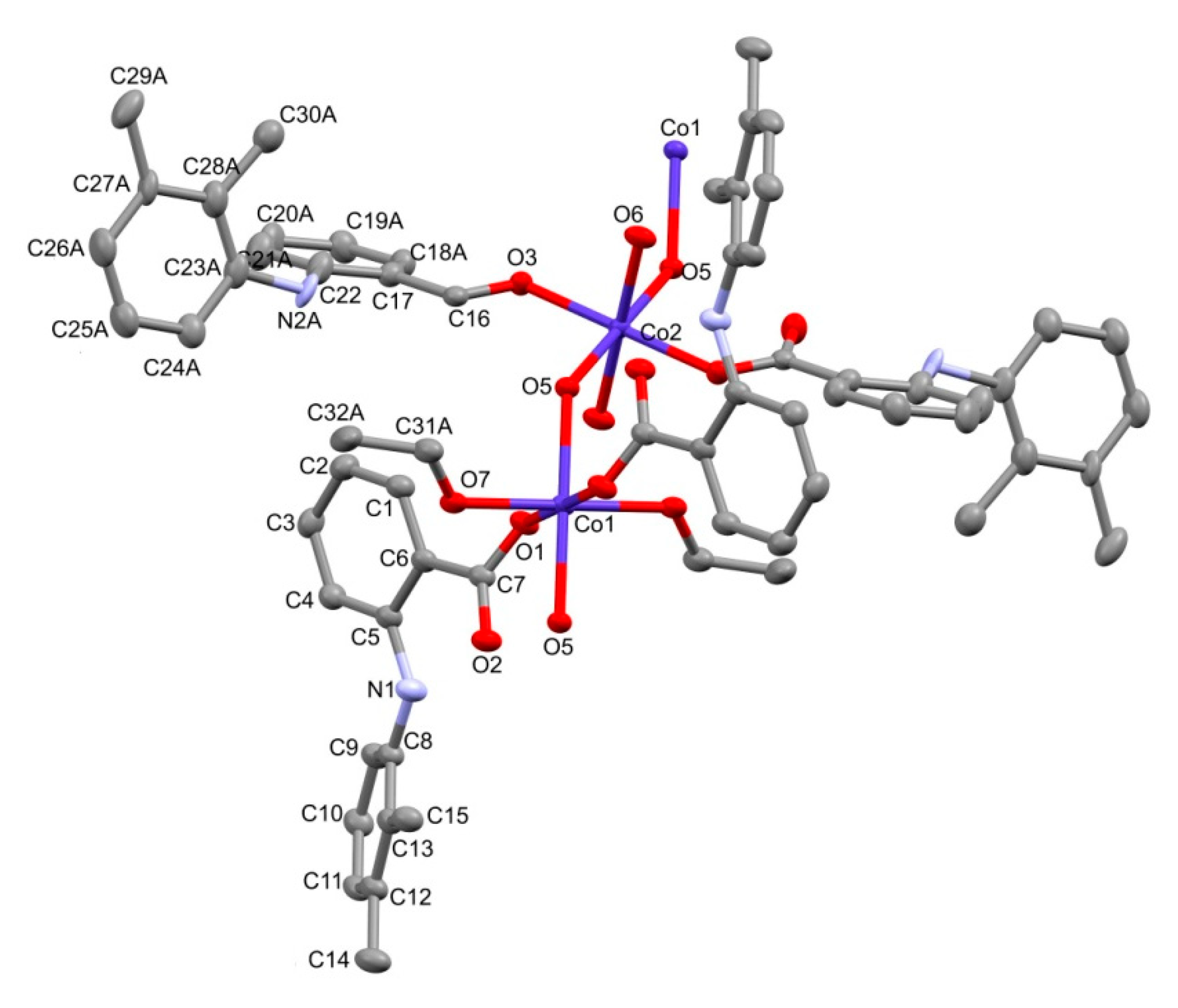
2.1.1. Crystal Structure of 2
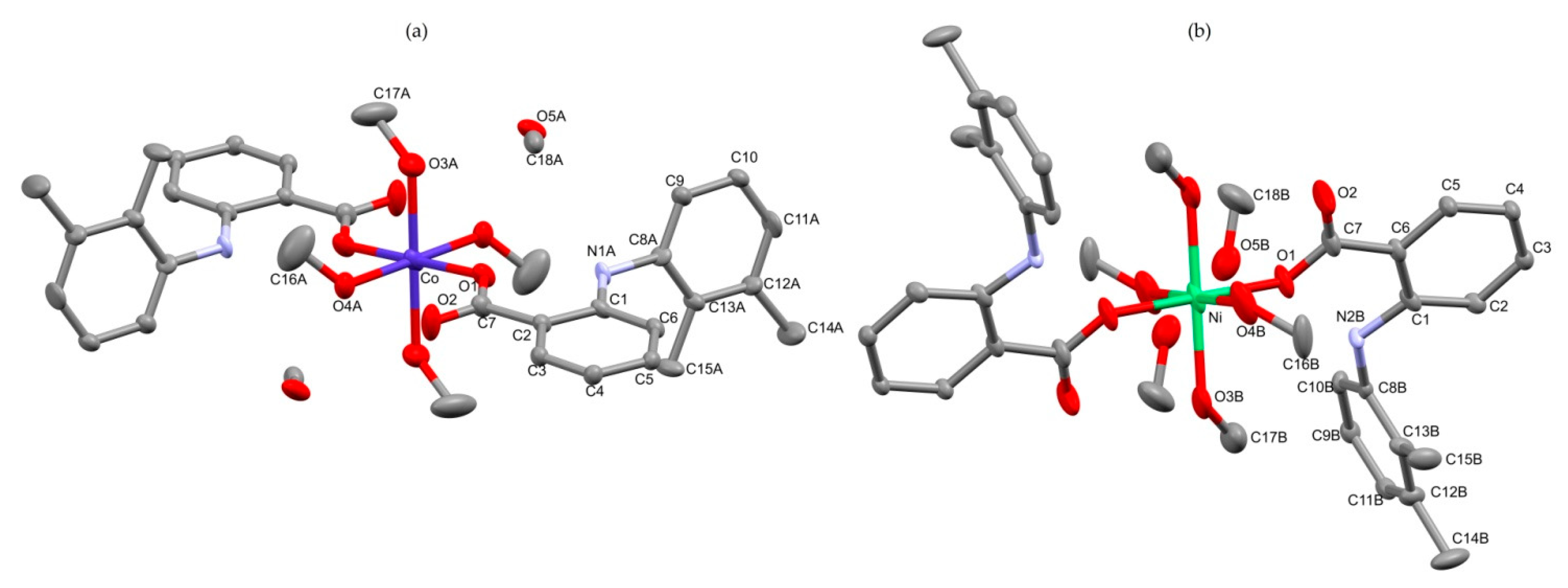
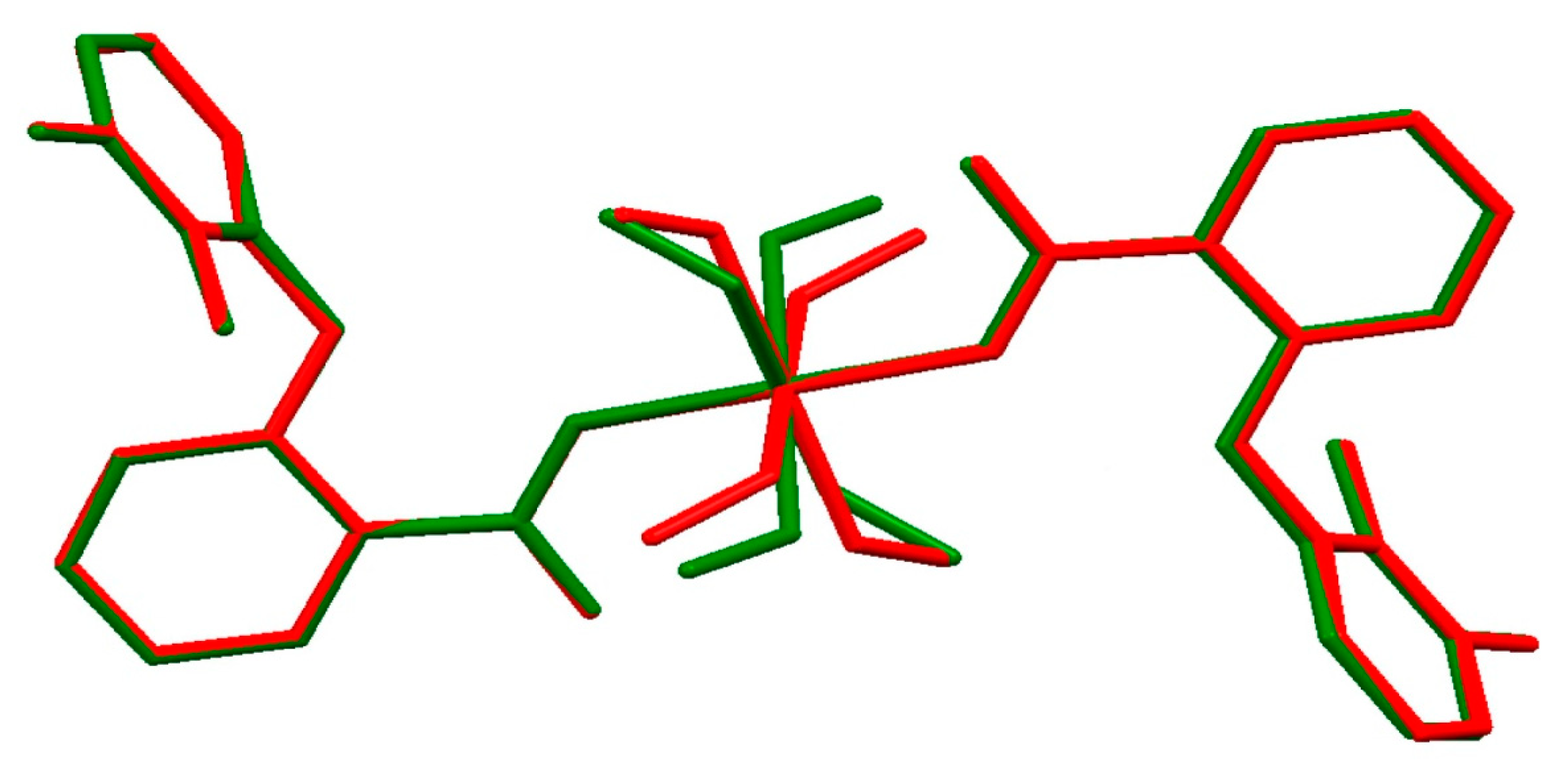
2.1.2. Crystal Structure of 3 and 4
2.1.3. Crystal Packing Analysis of 2–4
2.2. Infrared Spectra of 2–4
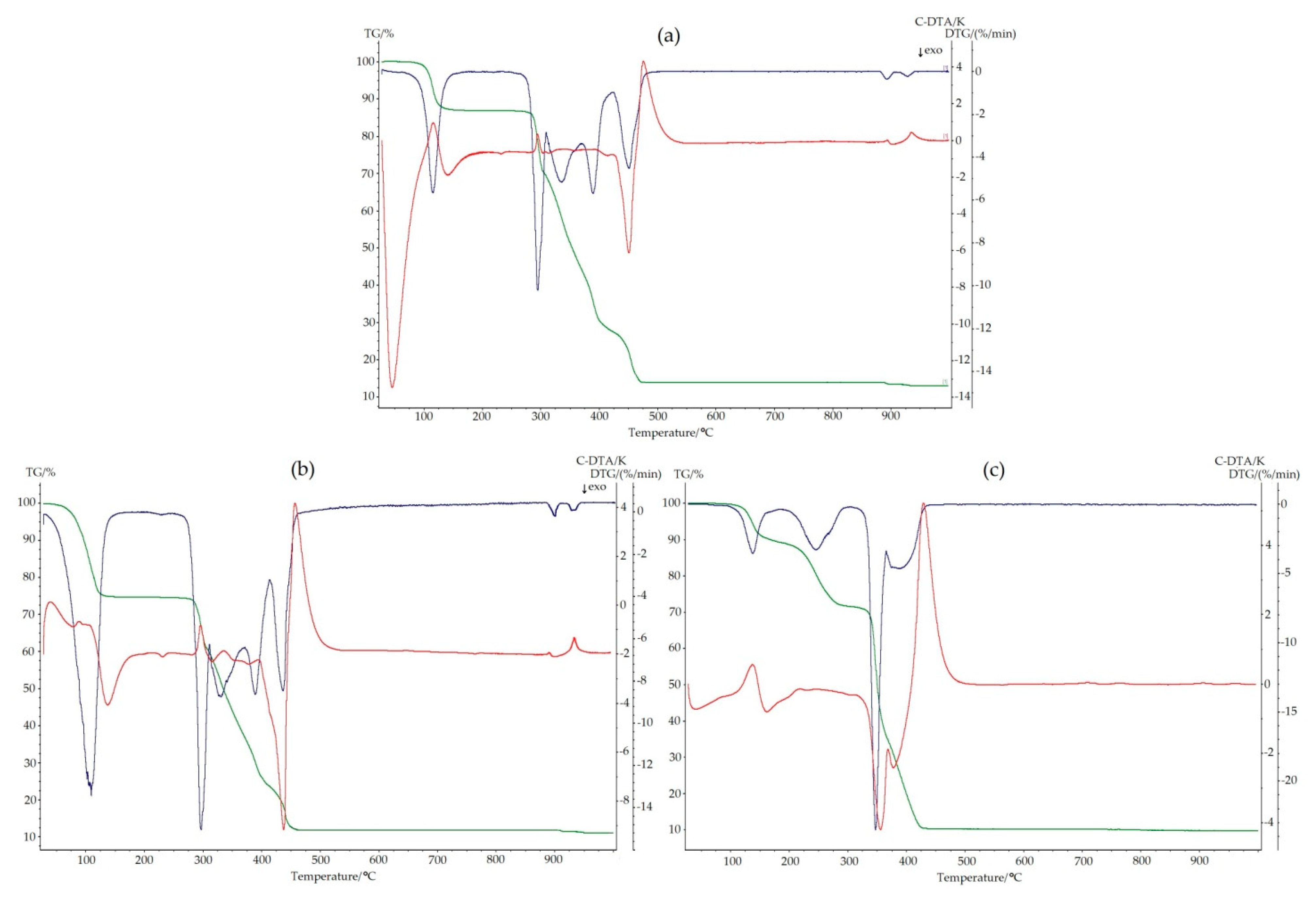
2.3. Thermal Analysis of 2–4
2.4. Antioxidant and Antimicrobial Activities
3. Experimental
3.1. Preparation of Complexes and Crystallization
3.2. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Krawczyk, M.S.; Majerz, I. The Na-O Bond In sodium fenamate. Acta Cryst. 2019, B75, 766–774. [Google Scholar] [CrossRef]
- Gacki, M.; Kafarska, K.; Wolf, W.M. A supramolecular polymeric chain in the cobalt(II) complex with diclofenac: Synthesis, crystal structure, spectroscopic, thermal and antioxidant activity. J. Coord. Chem. 2019, 72, 3481–3494. [Google Scholar] [CrossRef]
- Tarushi, A.; Geromuchalos, G.D.; Lafazanis, K.; Raptopoulou, C.P.; Psycharis, V.; Lalioti, L.; Pantazaki, A.A.; Kessissoglou, D.P.; Tangoulis, V.; Psomas, G. A step-ladder manganese(III) metallacrown hosting mefenamic acid and a manganese(II)–mefanamato complex: Synthesis, characterization and cytotoxicactivity. N. J. Chem. 2018, 42, 6955–6976. [Google Scholar] [CrossRef]
- De Groot, D.J.A.; de Vries, E.G.E.; Groen, H.J.M.; de Jong, S. Non-steroidal anti-inflammatory drugs to potentiate chemotherapy effects: From lab to clinic. Crit. Rev. Oncol. Hematol. 2007, 61, 52–59. [Google Scholar] [CrossRef]
- Orido, T.; Fujino, H.; Hasegawa, Y.; Toyomura, K.; Kawashima, T.; Murayama, T. Indomethacin Decreases Arachidonic Acid Uptake in HCA-7 Human Colon Cancer Cells. J. Pharmacol. Sci. 2008, 108, 389–392. [Google Scholar] [CrossRef]
- Altay, A.; Caglar, S.; Ceglar, B.; Sahin, O. Synthesis, structural, thermal elucidation and in vitro anticancer activity of novel silver(I) complex with non-steroidal anti-inflammatory drugs diclofenac and mefenamic acid including picoline derivatives. Polyhedron 2018, 151, 160–170. [Google Scholar] [CrossRef]
- Herendeen, J.M.; Lindley, C. Use of NSAIDs for the chemoprevention of colorectal cancer. Ann. Pharmacother. 2003, 37, 1664–1674. [Google Scholar] [CrossRef]
- Rao, C.V.; Reddy, B.S. NSAIDs and chemoprevention. Curr. Cancer Drug Targets 2004, 4, 29–42. [Google Scholar] [CrossRef]
- Bernstein, J. Polymorphism in Molecular Crystals; Oxford University Press: Oxford, UK, 2002. [Google Scholar]
- Salbego, P.R.S.; Bender, C.R.; Orlando, T.; Moraes, G.A.; Copetti, J.P.P.; Weimer, G.H.; Bonacorso, H.G.; Zanatta, N.; Hoerner, M.; Martins, M.A.P. Supramolecular similarity in polymorphs: Use of similarity indices (Ix). ACS Omega 2019, 4, 9697–9709. [Google Scholar] [CrossRef]
- Resnati, G.; Boldyreva, E.; Bombicz, P.; Kawano, M. Supramolecular interactions in the solid state. IUCrJ 2015, 2, 675–690. [Google Scholar] [CrossRef]
- Ranjan, S.; Devarapalli, R.; Kundu, S.; Saha, S.; Deolka, S.; Vangala, V.R.; Reddy, C.M. Isomorphism: ‘molecular similarity of crystal structure similarity’ in multicompponent forms of analgesic drugs tolfenamic and mefenamic acid. IUCrJ 2020, 7, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Bombicz, P. The way from isostructurality to polymorphism. Where are the borders? The role of supramolecular interactions and crystal symmetries. Crystallogr. Rev. 2017, 23, 118–151. [Google Scholar] [CrossRef]
- Scherrer, R.A.; Arbor, A. Anthranilic Acid Derivatives. U.S. Patent 3138636, 23 June 1960. [Google Scholar]
- Whitehouse, M.W. Drugs to treat inflammation: A historical introduction. Curr. Med. Chem. 2005, 12, 2931–2942. [Google Scholar] [CrossRef] [PubMed]
- Smolková, R.; Smolko, L.; Zeleňák, V.; Kuchárc, J.; Gyepes, R.; Talian, I.; Sabo, J.; Biščáková, Z.; Rabajdová, M. Impact of the central atom on human genomic DNA and human serum albumin binding properties in analogous Zn(II) and Cd(II) complexes with mefenamic acid. J. Mol. Struct. 2019, 1188, 42–50. [Google Scholar] [CrossRef]
- Adam, A.; Schrimpl, L.; Schmidt, P.C. Some physicochemical properties of mefenamic acid. Drug Dev. Ind. Pharm. 2000, 26, 477–487. [Google Scholar] [CrossRef]
- Batool, S.S.; Gilani, S.R.; Zainab, S.S.; Tahir, M.N.; Harrison, W.T.A.; Syed, Q.; Mazhar, S. Synthesis and structural characterization of a monomeric mixed ligand copper(II) complex involving N, N,N’,N’-tetramethylethylenediamine and mefenamate. J. Struct. Chem. 2019, 60, 1156–1165. [Google Scholar] [CrossRef]
- Joo, Y.; Kim, H.-S.; Woo, R.-S.; Hyoung Park, H.; Shin, K.-Y.; Lee, J.-P.; Chang, K.-A.; Kim, S.; Suh, Y.-H. Mefenamic acid shows neuroprotective effects and improves cognitive impairment in in vitro and in vivo alzheimer’s disease models. Mol. Pharmacol. 2006, 69, 76–84. [Google Scholar] [CrossRef]
- SeethaLekshmi, S.; Guru Row, T.N. Conformational polymorphism in a non-steroidal anti-inflammatory drug, mefenamic acid. Cryst. Growth Des. 2012, 12, 4283–4289. [Google Scholar] [CrossRef]
- Abbas, N.; Oswald, I.D.H.; Pulham, C.R. Accessong mefenamic acid form II through high-pressure recrystallisation. Pharmaceutics 2017, 9, 16. [Google Scholar] [CrossRef]
- Aguiar, A.J.; Zelmer, J.E. Dissolution behavior of polymorphs of chloramphenicol palmitate and mefenamic acid. J. Pharm. Sci. 1969, 58, 983–987. [Google Scholar] [CrossRef]
- Romero, S.; Escalera, B.; Bustamante, P. Solubility behavior of polymorphs I and II of mefenamic acid in solvent mixtures. Int. J. Pharm. 1999, 178, 193–202. [Google Scholar] [CrossRef]
- McConnell, J.; Company, F.Z. N-(2,3-xylyl) anthranilic acid, C15H15NO2. Mefenamic acid. Cryst. Struct. Commun. 1976, 5, 861–864. [Google Scholar]
- Lee, E.H.; Byrn, S.R.; Carvajal, M.T. Additive-induced metastable single crystal of mefenamic acid. Pharm. Res. 2006, 23, 2375–2380. [Google Scholar] [CrossRef] [PubMed]
- Amidon, G.L.; Lennernas, H.; Shah, V.P.; Crison, J.R.A. A theoretical basis for a bio-pharmaceutical drug classification: The correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm. Res. 1995, 12, 413–420. [Google Scholar] [CrossRef]
- Reiss, A.; Cioatera, N.; Chifiriuc, M.C.; Munteanu, G.; Ganescu, A.; Dabuleanu, I.; Avram, G.; Spinu, I.C.; Rotaru, P.J. New biologically active mixed-ligand Co(II) and Ni(II) complexes of enrofloxacin. Therm. Anal. Calorim. 2018, 134, 527–541. [Google Scholar] [CrossRef]
- Mewis, R.; Archibald, S.J. Biomedical applications of macrocyclic ligand complexes. Coord. Chem. Rev. 2010, 254, 1686–1712. [Google Scholar] [CrossRef]
- Badea, M.; Pătraşcu, F.; Cerc Korošec, R.; Bukovec, P.; Raita, M.; Chifiriuc, M.C.; Măruţescu, L.; Bleotu, C.; Velescu, B.; Marinescu, D.; et al. Thermal, spectral, magnetic and biologic characterization of new Ni(II), Cu(II) and Zn(II) complexes with a hexaazamacrocyclic ligand bearing ketopyridine moieties. J. Therm. Anal. Calorim. 2014, 118, 1183–1193. [Google Scholar] [CrossRef]
- Kovala-Demertzi, D.; Staninska, M.; Garcia-Santos, I.; Castineiras, A.; Demertzis, M.A. Synthesis, crystal structures and spectroscopy of meclofenamic acid and its metal complexes with manganese(II), copper(II), zinc(II) and cadmium(II). Antiproliferative and superoxide dismutase activity. J. Inorg. Biochem. 2011, 105, 1187–1195. [Google Scholar] [CrossRef]
- Kovala-Demertzi, D.; Dokorou, V.; Primikiri, A.; Vargas, R.; Silvestru, C.; Russo, U.; Demertzisa, M.A. Organotin meclofenamic complexes: Synthesis, crystal structures and antiproliferative activity of the first complexes of meclofenamic acid—Novel anti-tuberculosis agents. J. Inorg. Biochem. 2009, 103, 738–744. [Google Scholar] [CrossRef]
- Feng, J.; Du, X.; Liu, H.; Sui, X.; Zhang, C.; Tang, Y.; Zhang, J. Manganese-mefenamic acid complexes exhibit high lipoxygenase inhibitory activity. Dalton Trans. 2014, 43, 10930–10939. [Google Scholar] [CrossRef]
- Dimiza, F.; Papadopoulos, A.N.; Tangoulis, V.; Psycharis, V.; Raptopoulou, C.P.; Kessissoglou, D.P.; Psomas, G. Biological evaluation of non-steroidal anti-inflammatory drugs-cobalt(ii) complexes. Dalton Trans. 2010, 39, 4517–4528. [Google Scholar] [CrossRef] [PubMed]
- Tottaa, X.; Papadopoulou, A.A.; Hatzidimitriou, A.G.; Papadopoulos, A.; Psomas, G. Synthesis, structure and biological activity of nickel(II) complexes with mefenamato and nitrogen-donor ligands. J. Inorg. Biochem. 2015, 145, 79–93. [Google Scholar] [CrossRef]
- Facchin, G.; Torre, M.H.; Kremer, E.; Piro, O.E.; Baran, E.J. Crystal structure and spectroscopic behaviour of a binuclear copper(II) complex of mefenamic acid and dimethylsulfoxide. Z. Nat. 1998, B53, 871–874. [Google Scholar] [CrossRef]
- Sharma, R.P.; Kumar, S.; Venugopalan, P.; Ferretti, V.; Tarushi, A.; Psomas, G.; Witwicki, M. New copper(ii) complexes of the anti-inflammatory drug mefenamic acid: A concerted study including synthesis, physicochemical characterization and their biological evaluation. RSC Adv. 2016, 6, 88546–88558. [Google Scholar] [CrossRef]
- Dimiza, F.; Fountoulaki, S.; Papadopoulos, A.N.; Kontogiorgis, C.A.; Tangoulis, V.; Raptopoulou, C.P.; Psycharis, V.; Terzis, A.; Kessissoglou, A.P.; Psomas, G. Non-steroidal antiinflammatory drug–copper(ii) complexes: Structure and biological perspectives. Dalton Trans. 2011, 40, 8555–8568. [Google Scholar] [CrossRef] [PubMed]
- Tarushi, A.; Karaflou, Z.; Kljun, J.; Turel, I.; Psomas, G.; Papadopoulos, A.N.; Kessissoglou, D.P. Antioxidant capacity and DNA-interaction studies of zinc complexes with a non-steroidal anti-inflammatory drug, mefenamic acid. J. Inorg. Biochem. 2013, 128, 85–96. [Google Scholar] [CrossRef]
- Dokorou, V.; Ciunik, Z.; Russo, U.; Kovala-Demertzi, D. Synthesis, crystal structures and spectroscopic studies of diorganotin derivatives with mefenamic acid. Crystal and molecular structures of 1,2:3,4-di-μ2-2-[(2,3-dimethylphenyl)amino]-benzoato-O,O-1,3-bis-2-[(-[(2,3-dimethylphenyl)amino]benzoato-O-1,2,4:2,3,4-di-μ3-oxo-tetrakis[di-methyltin(IV)] and 1,2:3,4-di-μ2-2-[(-[(-[(2,3-dimethylphenyl)amino]-benzoato-O,O-1,3-bis-2-[(-[(-[(2,3-dimethylphenyl)amino]benzoato-O-1,2,4:2,3,4-di-μ3-oxo-tetrakis[di-n-butyltin(IV)]. J. Organomet. Chem. 2001, 630, 205–214. [Google Scholar]
- Kafarska, K.; Gacki, M.; Wolf, W.M. Synthesis, spectroscopic, and thermal investigations of metal complexes with mefenamic acid. J. Chem. 2017, 2017, 1–7. [Google Scholar] [CrossRef]
- Bernstein, J.; Davis, R.; Shimoni, L.; Chang, N.-L. Patterns in hydrogen bonding: Functionality and graph set analysis in crystals. Angew. Chem. Int. Ed. 1995, 34, 1555–1573. [Google Scholar] [CrossRef]
- Etter, M.C. Encoding and decoding hydrogen-bond patterns of organic compounds. Acc. Chem. Res. 1990, 23, 120–126. [Google Scholar] [CrossRef]
- Dey, D.; Thomas, S.P.; Spackman, M.A.; Chopra, D. ‘Quasi-isostructural polymorphism’ in molecular crystals: Inputs from interaction hierarchy and energy frameworks. Chem. Commun. 2016, 52, 2141–2144. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds; John Wiley & Sons, Inc.: New York, NY, USA, 2009. [Google Scholar]
- Altun, O.; Suözer, M. Synthesis, spectral analysis, stability contants, antioxidant and biological activities of Co(II), Ni(II) and Cu(II) mixed ligand complexes of nicotinamide, theophylline and thiocyanate. J. Mol. Struct. 2017, 1149, 307–314. [Google Scholar] [CrossRef]
- O’Donnell, F.; Smyth, T.J.P.; Ramachandran, V.N.; Smyth, W.F. A study of the antimicrobial activity of selected synthetic and naturally occurring quinolines. Int. J. Antimicrob. Agents 2009, 35, 30–38. [Google Scholar] [CrossRef] [PubMed]
- CrysAlis Pro; 1.171.39.33c; Rigaku Oxford Diffraction: Tokyo, Japan, 2017.
- Sheldrick, G.M. SHELXT-Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- HüBschle, C.B.; Sheldrick, G.M.; Dittrich, B. ShelXle: A Qt graphical user interface for SHELXL. J. Appl. Cryst. 2011, 44, 1281–1284. [Google Scholar] [CrossRef] [PubMed]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; Mccabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; Van De Streek, J.; Wood, P.A. Mercury: Visualization and analysis of crystal structures. J. Appl. Cryst. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Spek, A.L. Structure validation in chemical crystallography. Acta Crystallogr. Sect. D Biol. Crystallogr. 2009, 65, 148–155. [Google Scholar] [CrossRef]
- McKinnon, J.J.; Jayatilaka, D.; Spackman, M.A. Towards quantitative analysis of intermolecular interactions with Hirshfeld surfaces. Chem. Commun. 2007, 37, 3814–3816. [Google Scholar] [CrossRef]
- Żesławska, E.; Korona-Głowniak, I.; Szczesio, M.; Olczak, A.; Żylewska, A.; Tejchman, W.; Malm, A. Structural analysis and antimicrobial activity of 2[1H]-pyrimidinethione/selenone derivatives. J. Mol. Struct. 2017, 1142, 261–266. [Google Scholar] [CrossRef]
Sample Availability: Not available. |


| No. | Compounds | Empirical Formula | Color | Formula Weight (g/mol) | Elemental Analysis, Found (calc.) (%) | |||
|---|---|---|---|---|---|---|---|---|
| C | H | N | M * | |||||
| 2 | [Co2(mef)4(EtOH)2(H2O)4] | C64H74Co2N4O14 | pale pink | 1241.16 | 62.21 (61.93) | 5.87 (6.01) | 4.87 (4.51) | 9.96 (9.50) |
| 3 | [Co(mef)2(MeOH)4]∙2MeOH | C36H52CoN2O10 | pink | 731.72 | 59.88 (59.09) | 7.02 (7.16) | 3.27 (3.83) | 8.78 (8.05) |
| 4 | [Ni(mef)2(MeOH)4]∙2MeOH | C36H52NiN2O10 | pale green | 731.50 | 59.58 (59.11) | 7.61 (7.17) | 3.97 (3.83) | 8.48 (8.02) |
| 2 | 3 | 4 | |
|---|---|---|---|
| T (K) | 100.0 | 100 | 100 |
| Crystal system | triclinic | triclinic | triclinic |
| Space group | P | P | P |
| a (Å) | 7.80610(10) | 7.6291(2) | 7.6176(2) |
| b (Å) | 13.0847(4) | 7.8052(2) | 7.8118(4) |
| c (Å) | 15.7219(5) | 15.7376(3) | 15.7014(3) |
| α (°) | 105.091(3) | 89.971(2) | 89.966(3) |
| β (°) | 97.760(2) | 80.7510(10) | 80.276(2) |
| γ (°) | 97.208(3) | 87.376(3) | 87.349(3) |
| V (Å3) | 1514.45(7) | 923.95(4) | 919.91(6) |
| Z | 2 | 1 | 1 |
| ρcalc (g/cm3) | 1.363 | 1.315 | 1.320 |
| F(000) | 654 | 389 | 390 |
| Radiation | CuKα (λ = 1.54184) | MoKα (λ = 0.71073) | MoKα (λ = 0.71073) |
| 2θ range (°) | 5.914–148.962 | 5.224–61.012 | 5.432–63.01 |
| Reflections collected | 6987 | 64201 | 45789 |
| Independent reflections | 6134 | 5622 | 6094 |
| Goodness-of-Fit (GOF) | 1.050 | 1.089 | 1.044 |
| R [I ≥ 2σ (I)] | 0.0388 | 0.0347 | 0.0368 |
| wR2 [I ≥ 2σ (I)] | 0.1027 | 0.0941 | 0.0953 |
| D-H…A | D-H | H…A | D…A | D-H…A |
|---|---|---|---|---|
| O6-H6A…O1 a | 0.83(2) | 1.85(2) | 2.672(2) | 170(3) |
| O7-H6…O3 | 0.83(3) | 1.84(3) | 2.670(2) | 174(3) |
| O5-H5B…O2 | 0.85(2) | 1.74(2) | 2.560(2) | 161(2) |
| O5-H5A…O4 b | 0.835(18) | 1.723(19) | 2.541(2) | 166(2) |
| N1-H1…O2 | 0.90(3) | 1.87(3) | 2.661(3) | 145(3) |
| N2A-H2A…O4 | 0.84(6) | 1.94(5) | 2.58(2) | 132(6) |
| D-H…A | D-H | H…A | D…A | D-H…A |
|---|---|---|---|---|
| N1A-H1A…O1 | 0.813(18) | 1.95(2) | 2.552(5) | 130(2) |
| O3A-H3A…O5A | 0.72(3) | 2.13(3) | 2.835(5) | 168(3) |
| O4A-H4A…O2 | 0.92(3) | 1.72(3) | 2.6045(16) | 162(3) |
| O5B-H5B…O2 a | 0.82(6) | 2.04(6) | 2.676(11) | 134(5) |
| D-H…A | D-H | H…A | D…A | D-H…A |
|---|---|---|---|---|
| N2B-H2B…O1 | 0.83(2) | 1.83(3) | 2.521(6) | 133(4) |
| O3A-H3A…O2 a | 0.84(2) | 1.772(19) | 2.5989(17) | 166(3) |
| O4A-H4A…O5A | 0.84(3) | 1.86(3) | 2.663(5) | 161(4) |
| O5A-H5A…O2 b | 0.83(7) | 2.12(7) | 2.732(6) | 131(8) |
| Bacteria | MIC (mg/L) | ||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | Van | |
| S. aureus ATCC 25923 | 1000 | 125 | 125 | 125 | 250 | 125 | 125 | 250 | 125 | 62.5 | 0.98 |
| S. epidermidis ATCC 12228 | >1000 | 250 | 250 | 250 | 500 | 250 | 250 | 250 | 125 | 62.5 | 0.98 |
| M. luteus ATCC 10240 | 62.5 | 125 | 62.5 | 125 | 250 | 62.5 | 125 | 62.5 | 125 | 3.9 | 0.12 |
| E. faecalis ATCC 29212 | >1000 | 1000 | >1000 | 500 | 1000 | 500 | 500 | 1000 | 500 | 15.6 | 1.95 |
| B. subtilis ATCC 6633 | 62.5 | 125 | 62.5 | 125 | 250 | 125 | 125 | 125 | 125 | 7.8 | 0.24 |
| B. cereus ATCC 10876 | 62.5 | 62.5 | 62.5 | 125 | 125 | 62.5 | 125 | 62.5 | 62.5 | 62.5 | 0.98 |
| S, pyogenes ATCC 19615 | 125 | 125 | 62.5 | 62.5 | 250 | 31.3 | 125 | 125 | 250 | 31.3 | 0.24 |
| S. pneumoniae ATCC 49619 | 125 | 62.5 | 62.5 | 62.5 | 250 | 31.3 | 125 | 125 | 125 | 7.8 | 0.24 |
| S. mutans ATCC 25175 | 500 | 125 | 125 | 250 | >1000 | 125 | 50 | 250 | 250 | 15.6 | 0.98 |
| Yeasts | MIC (mg/L) | ||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | Nys | |
| C. albicans ATCC 102231 | 1000 | 1000 | 1000 | 1000 | 500 | 1000 | 250 | 1000 | 500 | 250 | 0.48 |
| C. parapsilosis ATCC 22019 | 1000 | 1000 | 1000 | 1000 | 500 | 500 | 250 | 500 | 500 | 15.6 | 0.24 |
| C. glabrata ATCC 90030 | 1000 | 500 | 500 | 1000 | 250 | 500 | 500 | 1000 | 1000 | 125 | 0.24 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gacki, M.; Kafarska, K.; Pietrzak, A.; Korona-Głowniak, I.; Wolf, W.M. Quasi-Isostructural Co(II) and Ni(II) Complexes with Mefenamato Ligand: Synthesis, Characterization, and Biological Activity. Molecules 2020, 25, 3099. https://doi.org/10.3390/molecules25133099
Gacki M, Kafarska K, Pietrzak A, Korona-Głowniak I, Wolf WM. Quasi-Isostructural Co(II) and Ni(II) Complexes with Mefenamato Ligand: Synthesis, Characterization, and Biological Activity. Molecules. 2020; 25(13):3099. https://doi.org/10.3390/molecules25133099
Chicago/Turabian StyleGacki, Michał, Karolina Kafarska, Anna Pietrzak, Izabela Korona-Głowniak, and Wojciech M. Wolf. 2020. "Quasi-Isostructural Co(II) and Ni(II) Complexes with Mefenamato Ligand: Synthesis, Characterization, and Biological Activity" Molecules 25, no. 13: 3099. https://doi.org/10.3390/molecules25133099
APA StyleGacki, M., Kafarska, K., Pietrzak, A., Korona-Głowniak, I., & Wolf, W. M. (2020). Quasi-Isostructural Co(II) and Ni(II) Complexes with Mefenamato Ligand: Synthesis, Characterization, and Biological Activity. Molecules, 25(13), 3099. https://doi.org/10.3390/molecules25133099







