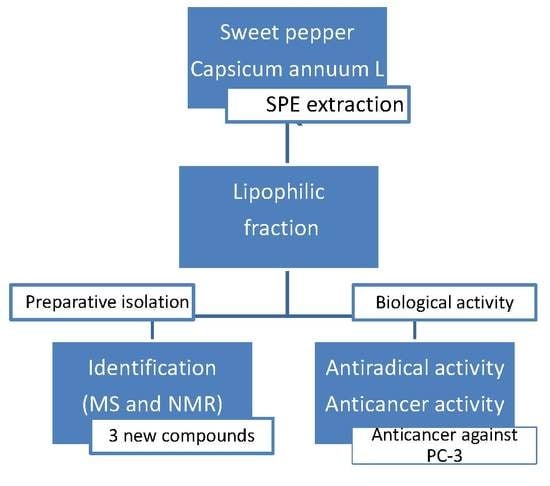
Anticancer Potential and Capsianosides Identification in Lipophilic Fraction of Sweet Pepper (Capsicum annuum L.)
Abstract
1. Introduction
2. Results and Discussion
2.1. Phenolic Content
2.2. Antiradical Activity
2.3. Biological Activity
2.4. Isolation and Identification
3. Materials and Methods
3.1. Plant Material
3.2. Extract Preparation
3.3. Total Phenolic Compounds (TP)
3.4. Total Flavonoids (TF)
3.5. Total Dihydroxycinnamic Acids (TDHCA)
3.6. Antiradical Activity
3.7. Biological Activity
3.8. HPLC Analysis
3.9. Isolation and Identification of Lipophilic Fraction Components
3.10. Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Grochowicz, J.; Fabisiak, A. Żywność funkcjonalna – aspekty prawne i znaczenie wybranych składników bioaktywnych. ZNUV 2018, 60, 143–153. [Google Scholar]
- Tomas –Barberan, F.A.; Andres-Lacueva, C. Polyphenols and health: Current state and progress. J. Agric. Food Chem. 2012, 60, 8773–8775. [Google Scholar] [CrossRef] [PubMed]
- Szajdek, A.; Borowska, J. Właściwości utleniające żywności pochodzenia roślinnego. Żywność Nauka Technologia Jakość 2004, 4, 5–28. [Google Scholar]
- Si, W.; Liang, Y.; Ma, K.Y.; Chung, H.Y.; Chen, Z.-Y. Antioxidant activity of capsaicinoid in canola oil. J. Agric. Food Chem. 2012, 60, 6230–6234. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, Y.; Zu, Y.; Yang, L.; Lu, Q.; Wang, W. Antioxidant effects of rosemary extracts on sunflower oil compared with synthetic antioxidants. Inter. J. Food Sci. Tech. 2014, 49, 385–391. [Google Scholar] [CrossRef]
- Kukula-Koch, W.; Aligiannis, N.; Halabalaki, M.; Skaltsounis, A.-L.; Glowniak, K.; Kalpoutzakis, E. Influence of extraction procedures on phenolic content and antioxidant activity of Cretan barberry herb. Food Chem. 2013, 138, 406–413. [Google Scholar] [CrossRef]
- Si, W.; Chen, Y.P.; Zhang, J.; Chen, Z.Y.; Chumg, H.Y. Antioxidant activities of ginger extract and its constituents toward lipids. Food Chem. 2018, 239, 1117–1125. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, M.; Dong, L.; Jia, X.; Liu, L.; Ma, Y.; Huang, F.; Zhang, R. Phytochemical profile, bioactivity, and prebiotic potential of bound phenolics released from rice bran dietary fiber during in vitro gastrointestinal digestion and colonic fermentation. J. Agric. Food Chem. 2019, 67, 12796–12805. [Google Scholar] [CrossRef]
- Materska, M. The scavenging effect and flavonoid glycosides content in fractions from fruits of hot pepper Capsicum annuum L. Acta Sci. Pol. Technol. Aliment. 2012, 11, 363–371. [Google Scholar]
- Naidu, K.A. Vitamin C in human health and disease is still a mystery an overview. J. Nutr. 2003, 2, 7–16. [Google Scholar] [CrossRef]
- Wang, Z.; Li, S.; Ge, S.; Lin, S. Review of distribution, extraction methods, and health benefits of bound phenolics in food plants. J. Agric. Food Chem. 2020, 68, 3330–3343. [Google Scholar] [CrossRef] [PubMed]
- Parus, A. Antioxidant and pharmacological properties of phenolic acids. Postępy Fitoterapii 2013, 1, 48–53. [Google Scholar]
- Slavin, J.; Marquart, L.; Jakoby, D.I. Comsumption of whole-grain food and decreased risk of cancer: Proposed mechanisms. Cereal Foods World 2000, 2, 54–58. [Google Scholar]
- Kujawska, M.; Jodynis-Liebert, J. Polyphenols in Parkinson’s disease: A systematic review of in vivo studies. Nutrients 2018, 10, 642. [Google Scholar] [CrossRef] [PubMed]
- Chao, X.; He, X.; Yang, Y.; Zhou, X.; Jin, M.; Liu, S.; Cheng, Z.; Liu, P.; Wang, Y.; Yu, J.; et al. Design, synthesis and pharmacological evaluation of novel tacrine−caffeic acid hybrids as multi-targeted compounds against Alzheimer’s disease. Bioorg. Med. Chem. Lett. 2012, 22, 6498–6502. [Google Scholar] [CrossRef] [PubMed]
- Sgarbossa, A.; Giacomazza, D.; Di Carlo, M. Ferulic acid: A hope for Alzheimer’s disease therapy from plants. Nutrients 2015, 7, 5764–5782. [Google Scholar] [CrossRef]
- Materska, M. Bioactive phenolics of fresh and freeze-dried sweet and semi-spicy pepper fruits (Capsicum annuum L.). J. Funct Foods. 2014, 7, 269–277. [Google Scholar] [CrossRef]
- Wahyuni, Y.; Ballester, A.-R.; Sudarmonowati, E.; Bino, R.J.; Bovy, A.G. Secondary metabolites of Capsicum species and their importance in the human diet. J. Nat. Prod. 2013, 76, 783–793. [Google Scholar] [CrossRef]
- Luo, X.J.; Peng, J.; Li, Y.J. Recent advances in the study on capsaicinoids and capsinoids. Eur. J. Pharmacol. 2011, 650, 1–7. [Google Scholar] [CrossRef]
- Joo, J.I.; Kim, D.H.; Choi, J.W.; Yun, J.W. Proteomic analysis for antiobesity potential of capsaicin on white adipose tissue in rats fed with a high fat diet. J. Proteome Res. 2010, 9, 2977–2987. [Google Scholar] [CrossRef]
- Kwon, D.Y.; Kim, Y.S.; Ryn, S.Y.; Ryu, S.Y.; Cha, M.R.; Yon, G.H.; Kim, M.J.; Kang, S.; Park, S. Capsiate improves glucose matabolism by improving insulin sensitivity better than capsaicin in diabetic rats. J. Nutr. Bioch. 2013, 24, 1078–1085. [Google Scholar] [CrossRef] [PubMed]
- Bode, A.M.; Dong, Z. The two faces of capsaicin. Cancer Res. 2011, 71, 2809–2814. [Google Scholar] [CrossRef] [PubMed]
- Ohyama, K.; Nogusa, Y.; Suzuki, K.; Shinoda, K.; Kajimura, S.; Bannai, M. A combination of exercise and capsinoid supplementation of. additively suppreses diet-induced obesity by increasing energy expenditure in mice. Am. J. Physiol Endocrinol. Metab. 2016, 308, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Kobata, K.; Sugawara, M.; Mimura, M.; Yazawa, S.; Watanabe, T. Potent production of capsaicinoids and capsinoids by Capsicum peppers. J. Agric. Food Chem. 2013, 61, 11127–11132. [Google Scholar] [CrossRef] [PubMed]
- Knatkova, H.; Pappagallo, M.; Szallasi, A. Capsaicin (TRPV1 Agronist) therapy for pain, relief: Forewell or revival? Clin. J. Pain. 2008, 24, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.Y.; Gavva, N.R. Therapeutic potential of vanilloid receptor TRPV1 agonists and antagonists as analgesics: Recent advances and setbacks. Brain Res. Rev. 2009, 60, 267–277. [Google Scholar] [CrossRef]
- Yang, C.Y.; Mandal, P.K.; Han, K.H.; Fukushima, M.; Choi, K.; Kim, C.I.; Lee, C.H. Capsaicin and tocopherol in red pepper seed oil enhances the thermal oxidative stability during frying. J. Food Sci. Technol. 2010, 47, 162–165. [Google Scholar] [CrossRef]
- Macho, A.L.; Lucena, C.; Sancho, R.; Daddario, N.; Minassi, A.; Muñoz, E.; Appendino, G. Non-pungent capsaicinoids from sweet pepper synthesis and evaluation of the chemopreventive and anticancer potential. Eur. J. Nutr. 2003, 42, 2–9. [Google Scholar] [CrossRef]
- Wanabata, T.; Ohnuki, K.; Kabata, K. Studies on the metabolism and toxicology of emerging capsinoids. Expert Opin. Drug Metab. Toxical. 2011, 7, 533–542. [Google Scholar]
- Fan, L.; Xu, H.; Yang, R.; Zang, Y.; Chen, J.; Qin, H. Combination of capsaicin and capsiate induces browning in 3T3-L1 white adipocytes via activation of the peroxisome proliferator-activated receptor γ/β3–adrenergic receptor signaling pathways. J. Agric. Food Chem. 2019, 67, 6232–6240. [Google Scholar] [CrossRef]
- Alvarez-Parrilla, E.; Rosa, L.; Amarowicz, R.; Shahidi, F. Antioxidant activity of fresh and processed Jalapeno and Serrano peppers. J. Agric. Food Chem. 2011, 59, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Kevers, C.; Falkowski, M.; Tabart, J.; Defraigne, J.O.; Dommes, J.; Pincemail, J. Evolution of antioxidant capacity during storage of selected fruits and vegetable. J. Agric. Food Chem. 2007, 55, 8596–8603. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B. Some methodological problems in the determination of antioxidant activity using chromogen radicals: A practical case. Food Scien. Tech. 2000, 11, 419–421. [Google Scholar] [CrossRef]
- Nenadis, N.; Tsimidou, M. Observations on the estimation of scavenging activity of phenolic compounds using rapid 1,1-diphenyl-2-picrylhydrazyl (DPPH•) tests. J. Am. Chem. Soc. 2002, 79, 1191. [Google Scholar] [CrossRef]
- Siriamornpun, S.; Kaisoon, O.; Meeso, N. Changes in colour, antioxidant activities and carotenoids (lycopene, ß-carotene, lutein) of marigold flower (Tageteserecta L.) resulting from different drying processes. J. Function. Food 2012, 4, 757–766. [Google Scholar] [CrossRef]
- Yao, L.H.; Jiang, Y.M.; Shi, J.; Thomas-Barberan, F.A.; Datta, N.; Singanusong, R.; Chen, S.S. Flavonoids in food and their health benefits. Plant Foods for Hum. Nutr. 2004, 59, 113–122. [Google Scholar] [CrossRef]
- Marino, D.S.; Borbane, N.; Gala, F.; Zollo, F.; Fico, G.; Pagiotti, R.; Iorizzi, M. New constituents of sweet Capsicum annuum L. fruits and evaluation of their biological activity. J. Agric Food Chem. 2006, 54, 7508–7516. [Google Scholar] [CrossRef]
- Zhang, D.; Yan, Y.; Tian, H.; Jiang, G.; Li, X.; Liu, W. Resveratrol supplementation improves lipid and glucose metabolism in high-fat diet-fed blunt snout bream. Fish Physiol. Biochem. 2018, 44, 163–173. [Google Scholar] [CrossRef]
- Pyun, B.J.; Choi, S.; Lee, Y.; Kim, T.W.; Min, J.K.; Kim, Y.; Kim, B.D.; Kim, J.H.; Kim, T.Y.; Kim, Y.M.; et al. Capsiate, a nonpungent capsaicin-like compound, inhibits angiogenesis and vascular permeability via a direct inhibition of Src kinase activity. Cancer Res. 2008, 68, 227–235. [Google Scholar] [CrossRef]
- Jassbi, A.R.; Zamanizadehnajari, S.; Kessler, D.; Baldwin, I.T. A new acyclic diterpene glycoside from nicotiana attenuate with a mild deterrent effect on feeding manduca sexta Larvae. Z. Naturforschung B 2006, 61, 1138–1142. [Google Scholar] [CrossRef]
- Izumitani, Y.; Sahara, S.; Nohara, T. Novel acyclic diterpene glycosides, capsianosides A-F and I-V from Capsicum plants (solanceus Studies. XVI). Chem. Pharm. Bull. 1999, 38, 1299–1307. [Google Scholar] [CrossRef][Green Version]
- Kowalska, I.; Jedrejek, D.; Ciesla, Ł.; Pecio, Ł.; Masullo, M.; Piacente, S.; Oleszek, W.; Stochmal, A. Isolation, chemical and free radical scavenging characterization of phenolics from Trifolium scabrum L. aerial parts. J. Agric. Food Chem. 2013, 61, 4417–4423. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, S. Antioxidant activity and phenolic compounds in selected herbs. J. Agric. Food Chem. 2001, 49, 5165–5170. [Google Scholar] [CrossRef] [PubMed]
- Zhisten, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Nicolle, C.; Carnat, A.; Fraisse, D.; Lamaison, J.L.; Rock, E.; Michel, H.; Amouroux, P.; Remesy, C. Characterisation and variation of antioxidant micronutrients in lettuce (Lactuca sativa folium). J. Sci. Food Agric. 2004, 84, 2061–2069. [Google Scholar] [CrossRef]
- Conforti, F.; Statti, G.A.; Menichini, F. Chemical and biological variability of hot pepper fruits (Capsicum annuum var. acuminatum L.) in relation to maturity stage. Food Chem. 2007, 102, 1096–1104. [Google Scholar] [CrossRef]
- Yang, S.A.; Jeon, S.K.; Lee, E.J.; Shim, C.H.; Lee, I.S. Comparative study of the chemical composition and antioxidant activity of six essential oils and their components. J. Nat. Prod. Res. 2010, 24, 140–151. [Google Scholar] [CrossRef]
- Abe, K.; Matsuki, N. Measurement of cellular 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyltetrazolium bromide (MTT) reduction activity and lactate dehydrogenase release using MTT. Neurosci. Res. 2000, 38, 325–329. [Google Scholar] [CrossRef]
- Perez, J.; Pecio, Ł.; Kowalczyk, M.; Kontek, R.; Gajek, G.; Stopinesk, L.; Mirt, I.; Stichmal, A.; Oleszek, W. Cytotoxic triterpenoids isolated from sweet chestnut heartwood (Castanea sativa) and their health benefits implication. Food Chem. Toxicol. 2017, 109, 863–870. [Google Scholar] [CrossRef]
- Łudzik, K.; Kustrzepa, K.; Kowalewicz-Kulbat, M.; Kontek, R.; Kontek, B.; ·Wróblewska, A.; Jóźwiak, M.; Lulo, D. Antimicrobial and cytotoxic properties of bis quaternary ammonium bromides of different spacer length. J. Surfac. Deterg. 2018, 21, 91–99. [Google Scholar]
Sample Availability: Samples of the compounds 2, 3 and 4 are available from the authors. |
| Yield 1 | Phenolics Content | Antiradical Activity 5 | Biological Activity 6 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| TP 2 | TF 3 | TDHCA 4 | DPPH • | ABTS +• | HCT116 | PC-3 | L929 | ||
| Extract | 6.98 a ± 0.05 | 83.25 ± 1.23 | 7.56 b ± 0.3 | 27.05 c ± 0.08 | 278 b ± 4.00 | 70.4 b ± 1.10 | 134 b ± 4.33 | 78b ± 3.83 | 90 b ± 2.65 |
| F1 | 5.82 b ± 0.07 | 155.64 c ± 3.70 | 7.32 b ± 0.01 | 26.08 c ± 0.13 | 284 b ± 3.75 | 44.6 c ± 1.01 | 158 a ± 3.82 | 60 c ± 3.42 | 64 c ± 2.70 |
| F2 | 0.06 c ± 0.01 | 386.47 a ± 1.25 | 41.79 a ± 0.5 | 44.92 a ± 1.19 | 73 c ± 1.3 | 17.0 d ± 0.91 | 154 a ± 5.0 | 101 a ± 2.82 | 118 a ± 3.14 |
| F3 | 0.11 c ± 0.03 | 172.09b ± 0.41 | 6.49 c ± 0.3 | 33.56 b ± 0.04 | 570 a ± 8.1 | 111.7 a ± 1.20 | 160 a ± 3.86 | 51 d ± 4.42 | 94 b ± 3.20 |
| Trolox | n.a.8 | n.a. | n.a. | n.a. | 5.5 d ± 0.08 | 3.4 e ± 0.03 | n.a. | n.a. | n.a. |
| Ascorbic acid 5-fluorouracil 7 | n.a. n.a. | n.a. n.a. | n.a. n.a. | n.a. n.a. | 3.2 e ± 0.01 n.a. | 0.2 f ± 0.03 n.a. | n.a. 32.87 ± 3.21 | n.a. 23.3 ± 2.58 | n.a. 7.5 ± 3.97 |
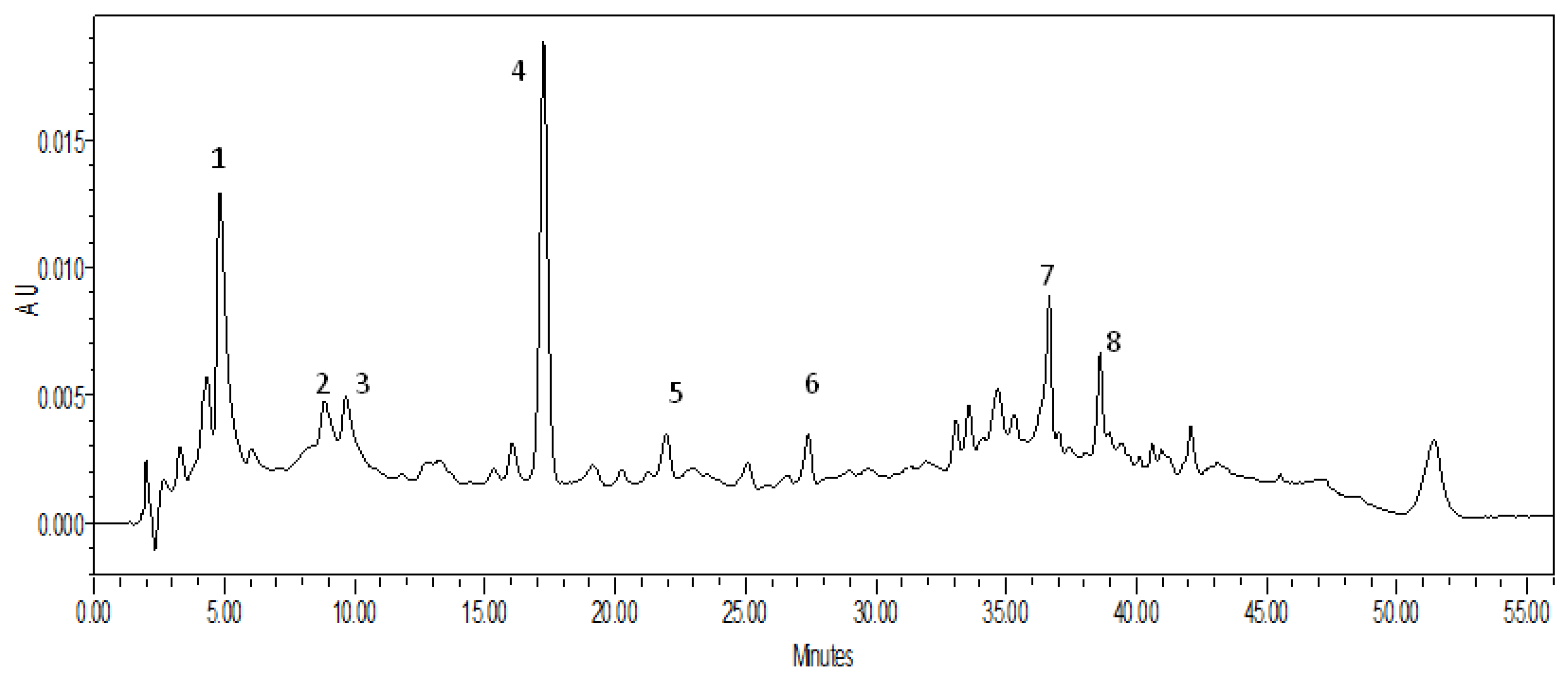
| No. | Chemical Name | Tr | [M-H]- m/z | Amount (mg) | Chemical Formula |
|---|---|---|---|---|---|
| 1 | Capsianoside IX | 5.088 | 938 | 5.96 | C44H74O21 |
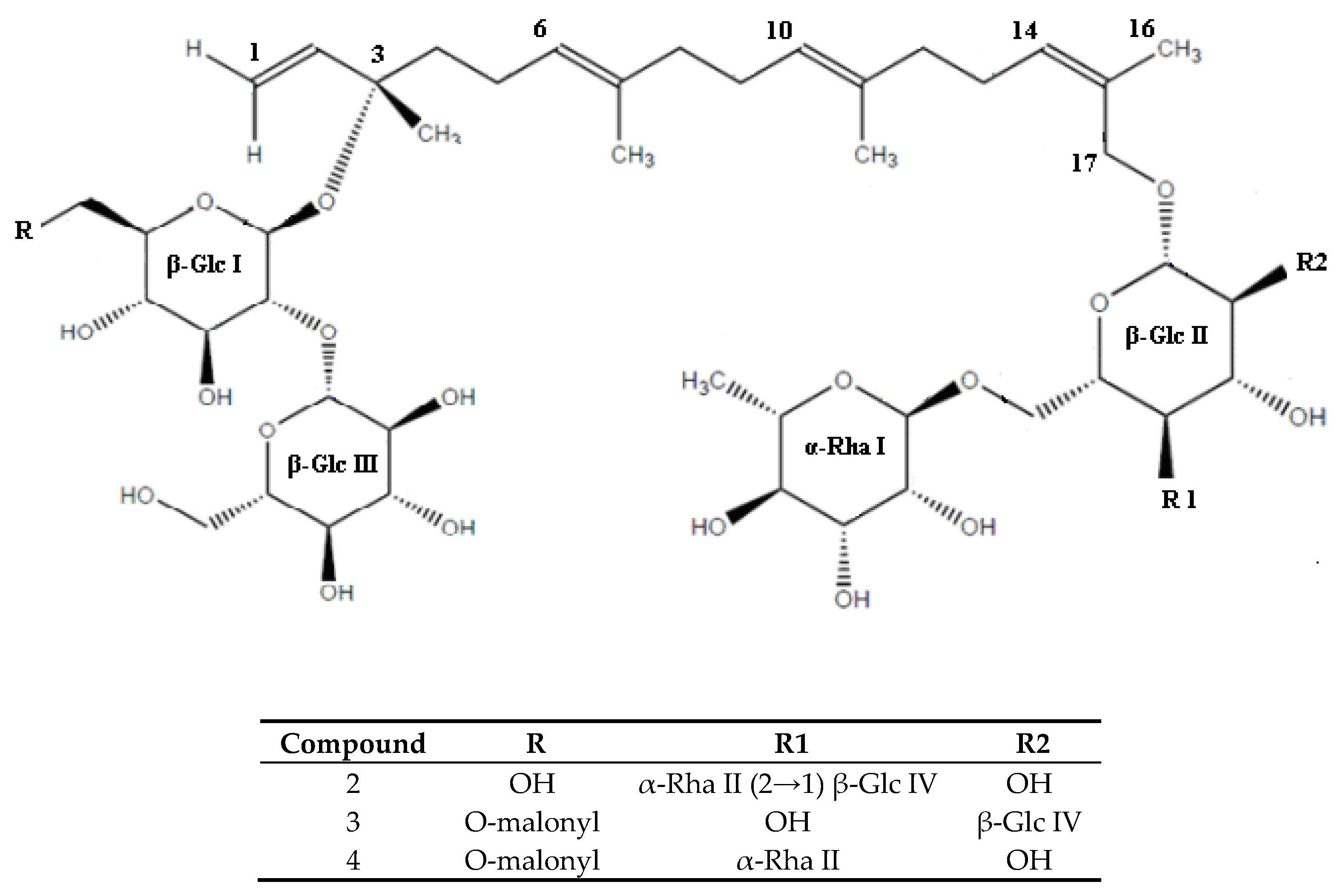
| 2 | new | 9.664 | 1246 | 2.07 | C56H94O30 |
| 3 | new | 10.296 | 1186 | 34.21 | C53H86O29 |
| 4 | new | 17.398 | 1170 | 100 | C53H86O28 |
| 5 | Capsianoside VIII | 21.832 | 1083 | 14.9 | C50H84O25 |
| 6 | Capsianoside I | 27.124 | 659 | 1.47 | C32H52O14 |
| 7 | Capsianoside IV | 36.531 | 805 | 1.6 | C38H62O18 |
| 8 | Capsianoside III | 38.743 | 1099 | 13.91 | C50H84O26 |
| 2 | 3 | 4 | ||||
|---|---|---|---|---|---|---|
| Position | δHa | δC CD3OD (25 °C) | δHa | δC CD3OD (25 °C) | δHa | δC CD3OD (25 °C) |
| 1 | 5.22 dd(10.5. 1.4) 5.23 dd(17.8. 1.4) | 116.1 | 5.23 dd(11.0; 1.3) 5.25 dd(17.8. 1.4) | 116.3 | 5.23 dd(11.0; 1.4) 5.25 dd(17.8. 1.4) | 116.2 |
| 2 | 6.13 dd(18.1. 10.7) | 144.4 | 6.08 dd(17.7; 11.0) | 144.1 | 6.8 dd(17.7; 11.0) | 144.1 |
| 3 | - | 82.1 | - | 82.2 | - | 82.2 |
| 4 | 1.60 m | 43.1 | 1.59 | 43.0 | 1.60 m | 43.0 |
| 5 | 2.06 m | 23.6 | 2.05 o | 23.5 | 2.05 o | 23.5 |
| 6 | 5.13 m | 125.8 | 5.13 o | 125.7 | 5.13 o | 125.7 |
| 7 | 136 | 136 | 136 | |||
| 8 | 1.99 m | 40.8 | 2.00 o | 40.8 | 1.99 o | 40.8 |
| 9 | 2.09 m | 27.7 | 2.09 o | 27.7 | 2.09 o | 27.7 |
| 10 | 5.13 m | 125.9 | 5.13 o | 126.0 | 5.12 o | 125.9 |
| 11 | - | 135.5 | - | 135.4 | - | 135.5 |
| 12 | 2.01 t(4.5) | 40.9 | 2.02 t(7.1) | 40.9 | 2.01 t(7.4) | 40.9 |
| 13 | 2.17 m(7.30) | 27.3 | 2.17 m(7.4) | 27.2 | 2.17 m(6.9) | 27.3 |
| 14 | 5.40 dd(8.0 6.4) | 131.3 | 5.39 dd(8.0; 6.2) | 131.3 | 5.40 td(7.3; 1.7) | 131.3 |
| 15 | - | 132.4 | - | 132.4 | - | 132.4 |
| 16 | 1.77 d(1.4) | 21.9 | 1.79 d(1.5) | 22.0 | 1.77 d(1.5) | 21.9 |
| 17 | 4.33 d(11.5) 4.13 d(11.6) | 67.7 | 4.30 d(11.4) 4.21 d(11.6) | 68.2 | 4.33 d(11.5) 4.13 d(11.5) | 67.7 |
| 18 | 1.61 s | 16.3 | 1.61 s | 16.3 | 1.61 s | 16.3 |
| 19 | 1.61 s | 16.2 | 1.61 s | 16.3 | 1.61 s | 16.3 |
| 20 | 1.39 s | 23.4 | 1.36 s | 23.5 | 1.39 s | 23.5 |
| 1′ | - | 168.7 | - | 168.7 | ||
| 2′ | 3.37 o | 41.9 | 3.37 o | 41.9 | ||
| 3′ | - | 170.1 | - | 170.1 | ||
| ß Glc (1) 1JCH = 158 | ||||||
| 1 | 4.47 d(7.7) | 98.4 | 4.48 d(7.6) | 98.2 | 4.48 d(7.7) | 98.2 |
| 2 | 3.44 dd(9.3. 7.7) | 83.2 | 3.46 dd(9.9; 7.8) | 82.9 | 3.46 dd(9.4; 7.8) | 82.9 |
| 3 | 3.51 t(9.0) | 78.1 | 3.52 t(8.4) | 77.9 | 3.52 t(8.9) | 77.9 |
| 4 | 3.31 t(9.3) | 71.6 | 3.30 t(9.1) | 71.7 | 3.29 dd(9.8; 8.8) | 71.7 |
| 5 | 3.16 ddd(9.0. 5.5. 2.4.) | 77.5 | 3.41ddd(9.5; 5.5; 2.0) | 74.8 | 3.41 ddd(9.2; 6.8; 2.2) | 74.8 |
| 6 | 3.81 dd(12.0 2.2) 3.64 dd(11.9. 5.6) | 62.7 | 4.42 dd(11.8; 2.0) 4.22 dd(12.0; 5.5) | 65.6 | 4.43 dd(11.8; 2.1) 4.22 dd(12.5; 5.8) | 65.6 |
| ß Glc (2) 1JCH = 160 | ||||||
| 1 | 4.2 d(7.9) | 102.1 | 4.36 d(7.7) | 101.0 | 4.21 d(7.9) | 102.1 |
| 2 | 3.21 dd(9.1. 7.8) | 75.3 | 3.48 dd(9.3; 7.5) | 81.9 | 3.22 dd(9.1; 7.9) | 75.2 |
| 3 | 3.42 t(9.0) | 76.7 | 3.56 t(9.0) | 78.1 | 3.44 t(9.0) | 76.7 |
| 4 | 3.58 t(9.4) | 78.9 | 3.36 o | 71.3 | 3.58 t(9.3) | 79.2 |
| 5 | 3.37 ddd(9.7; 3.8; 2.0) | 75.4 | 3.36 o | 76.6 | 3.36 ddd(9.7; 3.9; 1.7) | 75.4 |
| 6 | 3.93 dd(11.0; 1.8) 3.61 dd(11.0; 3.9) | 66.8 | 3.97 d(11.0) 3.63 dd(11.0; 4.0) | 67.6 | 3.93 dd(11.4; 1.4) 3.62 dd(11.1; 3.8) | 66.8 |
| ß Glc (3) 1JCH = 158 | ||||||
| 1 | 4.56 dd(7.7) | 105.9 | 4.56 d(7.7) | 105.8 | 4.56 d(7.7) | 105.8 |
| 2 | 3.25 t(8.4) | 76.6 | 3.24 t(8.9) | 76.6 | 3.24 dd(9.1; 7.08) | 76.6 |
| 3 | 3.39 t (9.1) | 77.7 | 3.37 t (8.7) | 77.7 | 3.38 t(9.0) | 77.7 |
| 4 | 3.35 t(8.6) | 71.4 | 3.34 t(8.5) | 71.4 | 3.34 t(8.9) | 71.4 |
| 5 | 3.26 ddd(9.5;4.9.; 2.3) | 78.3 | 3.26 o | 78.3 | 3.26 ddd (9.3; 5.0; 2.4) | 78.3 |
| 6 | 3.83 dd(11.6; 2.4) 3.71 dd(11.9; 5.0) | 62.7 | 3.82 d(11.4) 3.70 dd(11.9; 4.8) | 62.7 | 3.82 dd(11.7; 2.4) 3.70 dd(11.9; 5.0) | 62.7 |
| ß Glc (4) 1JCH = 160 | ||||||
| 1 | 4.58 d(7.8) | 105.6 | 4.63 d(7.8) | 104.8 | ||
| 2 | 3.21 dd(9.1; 7.8) | 76.0 | 3.24 dd(9.2; 7.8) | 75.9 | ||
| 3 | 3.37 t(8.8) | 78.2 | 3.37 t(8.8) | 77.6 | ||
| 4 | 3.31 dd(9.5; 8.2) | 71.5 | 3.30 t(9.7)(9.5; 8.2) | 71.5 | ||
| 5 | 3.28 ddd(9.3; 5.2; 2.1) | 78 | 3.26 o | 78.2 | ||
| 6 | 3.85 dd(11.9; 2.3) 3.69 dd(11.8; 5.0) | 62.7 | 3.83 dd(12.0; 2.3) 3.67 dd(11.9; 5.0) | 62.8 | ||
| α Rha (1) 1JCH = 168 | ||||||
| 1 | 4.71 d(1.7) | 101.6 | 4.75 d(1.7) | 102.1 | 4.71 d(1.7) | 101.6 |
| 2 | 3.85 dd(3.6; 1.70 | 72.2 | 3.85 dd(3.5; 1.7) | 72.2 | 3.85 dd(3.5; 1.5) | 72.2 |
| 3 | 3.69 dd(9.5; 3.3) | 72.3 | 3.66 dd(9.5; 3.3) | 72.4 | 3.69 dd(9.5; 3.3) | 72.3 |
| 4 | 3.37 t(9.5) | 74.0 | 3.37 t(9.5) | 74.0 | 3.37 t(9.5) | 74.0 |
| 5 | 3.72 dq(9.5; 6.2) | 69.8 | 3.67 dq(9.4; 6.2) | 69.7 | 3.72 dq(9.7; 6.2) | 69.8 |
| 6 | 1.27 d (6.2) | 18.2 | 1.27 d(6.2) | 18.1 | 1.27 d(6.2) | 18.2 |
| α Rha (2) 1JCH = 168 | ||||||
| 1 | 4.82 d(1.7) | 102.3 | 4.82 d(1.8) | 102.6 | ||
| 2 | 3.85 dd(3.3; 1.8) | 72.3 | 3.83 dd(3.4; 1.8) | 72.5 | ||
| 3 | 3.88 dd(9.1; 3.7) | 72.3 | 3.64 dd(9.4; 3.3) | 72.2 | ||
| 4 | 3.63 t(9.3) | 83.3 | 3.40 t(9.5) | 73.8 | ||
| 5 | 4.09 dq(9.6; 6.1) | 69.2 | 3.99 dq(9.6; 6.2) | 70.6 | ||
| 6 | 1.34 d(6.2) | 18 | 1.26 d (6.2) | 17.8 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chilczuk, B.; Marciniak, B.; Stochmal, A.; Pecio, Ł.; Kontek, R.; Jackowska, I.; Materska, M. Anticancer Potential and Capsianosides Identification in Lipophilic Fraction of Sweet Pepper (Capsicum annuum L.). Molecules 2020, 25, 3097. https://doi.org/10.3390/molecules25133097
Chilczuk B, Marciniak B, Stochmal A, Pecio Ł, Kontek R, Jackowska I, Materska M. Anticancer Potential and Capsianosides Identification in Lipophilic Fraction of Sweet Pepper (Capsicum annuum L.). Molecules. 2020; 25(13):3097. https://doi.org/10.3390/molecules25133097
Chicago/Turabian StyleChilczuk, Barbara, Beata Marciniak, Anna Stochmal, Łukasz Pecio, Renata Kontek, Izabella Jackowska, and Małgorzata Materska. 2020. "Anticancer Potential and Capsianosides Identification in Lipophilic Fraction of Sweet Pepper (Capsicum annuum L.)" Molecules 25, no. 13: 3097. https://doi.org/10.3390/molecules25133097
APA StyleChilczuk, B., Marciniak, B., Stochmal, A., Pecio, Ł., Kontek, R., Jackowska, I., & Materska, M. (2020). Anticancer Potential and Capsianosides Identification in Lipophilic Fraction of Sweet Pepper (Capsicum annuum L.). Molecules, 25(13), 3097. https://doi.org/10.3390/molecules25133097