Complete Microbial Fuel Cell Fabrication Using Additive Layer Manufacturing
Abstract
1. Introduction
2. Results and Discussion
2.1. Anode Materials
2.2. Cathode Materials
2.3. Membrane-less 3D Printed MFCs
2.4. Practical Application of the AM Built MFCs
3. Materials and Methods
3.1. Initial MFC Design
3.2. Fused Deposition Modelling (FDM)
3.3. 3D Printed Electrode Surface Modification
3.4. Final MFC Design for Practical Demonstration
3.5. MFC Operation and Data Recording
3.6. Electrochemical Analysis
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wong, K.V.; Hernandez, A. A Review of Additive Manufacturing. ISRN Mech. Eng. 2012, 2012, 208760. [Google Scholar] [CrossRef]
- Yuan, L.; Ding, S.; Wen, C. Additive manufacturing technology for porous metal implant applications and triple minimal surface structures: A review. Bioact. Mater. 2019, 4, 56–70. [Google Scholar] [CrossRef] [PubMed]
- Tai, X.Y.; Zhakeyev, A.; Wang, H.; Jiao, K.; Zhang, H.; Xuan, J. Accelerating Fuel Cell Development with Additive Manufacturing Technologies: State of the Art, Opportunities and Challenges. Fuel Cells 2019, 19, 636–650. [Google Scholar] [CrossRef]
- Griffiths, L. 3D printing for Aerospace: “Additive manufacturing will change the game forever”. TCT Magazine, July 2015. Available online: https://www.tctmagazine.com/additive-manufacturing-3d-printing-news/1000-3d-printed-parts-on-an-aeroplane/ (accessed on 16 March 2020).
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Rasperini, G.; Pilipchuk, S.P.; Flanagan, C.L.; Park, C.H.; Pagni, G.; Hollister, S.J.; Giannobile, W.V. 3D-printed bioresorbable scaffold for periodontal repair. J. Dent. Res. 2015, 94, 153S–157S. [Google Scholar] [CrossRef]
- Walters, P.; Davies, K. 3D printing for artists: Research and creative practice. Rapp. J. Nor. Print Assoc. 2010, 1, 12–15. [Google Scholar]
- Zhakeyev, A.; Wang, P.; Zhang, L.; Shu, W.; Wang, H.; Xuan, J. Additive Manufacturing: Unlocking the Evolution of Energy Materials. Adv. Sci. 2017, 4, 1700187. [Google Scholar] [CrossRef]
- Walters, P.; Ieropoulos, I.; McGoran, D. Digital fabrication of a novel bio-actuator for bio-robotic art and design. In Proceedings of the International Conference on Digital Printing Technologies, Minneapolis, MN, USA, 2−6 October 2011; pp. 496–499. [Google Scholar]
- Papaharalabos, G.; Greenman, J.; Melhuish, C.; Ieropoulos, I. A novel small scale Microbial Fuel Cell design for increased electricity generation and waste water treatment. Int. J. Hydro. Energy 2015, 40, 4263–4268. [Google Scholar] [CrossRef]
- Massaglia, G.; Gerosa, M.; Agostino, V.; Cingolani, A.; Sacco, A.; Saracco, G.; Margaria, V.; Quaglio, M. Fluid Dynamic Modeling for Microbial Fuel Cell Based Biosensor Optimization. Fuel Cells 2017, 17, 627–634. [Google Scholar] [CrossRef]
- Agostino, V.; Massaglia, G.; Gerosa, M.; Sacco, A.; Saracco, G.; Margaria, V.; Quaglio, M. Environmental electroactive consortia as reusable biosensing element for freshwater toxicity monitoring. New Biotechnol. 2020, 55, 36–45. [Google Scholar] [CrossRef]
- Philamore, H.; Rossiter, J.; Walters, P.; Winfield, J.; Ieropoulos, I. Cast and 3D printed ion exchange membranes for monolithic microbial fuel cell fabrication. J. Power Sources 2015, 289, 91–99. [Google Scholar] [CrossRef]
- You, J.; Preen, R.J.; Bull, L.; Greenman, J.; Ieropoulos, I. 3D printed components of microbial fuel cells: Towards monolithic microbial fuel cell fabrication using additive layer manufacturing. Sustain Energy Techn. 2017, 19, 94–101. [Google Scholar] [CrossRef]
- Theodosiou, P.; Greenman, J.; Ieropoulos, I. Towards monolithically printed Mfcs: Development of a 3d-printable membrane electrode assembly (mea). Int. J. Hydro. Energy 2019, 44, 4450–4462. [Google Scholar] [CrossRef]
- Calignano, F.; Tommasi, T.; Manfredi, D.; Chiolerio, A. Additive Manufacturing of a Microbial Fuel Cell—A detailed study. Sci. Rep. 2015, 5, 17373. [Google Scholar] [CrossRef]
- Bian, B.; Wang, C.; Hu, M.; Yang, Z.; Cai, X.; Shi, D.; Yang, J. Application of 3D Printed Porous Copper Anode in Microbial Fuel Cells. Front. Energy Res. 2018, 6, 50. [Google Scholar] [CrossRef]
- Bian, B.; Shi, D.; Cai, X.; Hu, M.; Guo, Q.; Zhang, C.; Wang, Q.; Sun, A.X.; Yang, J. 3D printed porous carbon anode for enhanced power generation in microbial fuel cell. Nano Energy 2018, 44, 174–180. [Google Scholar] [CrossRef]
- Shive, M.S.; Anderson, J.M. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv. Drug Deliv. Rev. 1997, 28, 5–24. [Google Scholar] [CrossRef] [PubMed]
- Santoro, C.; Arbizzani, C.; Erable, B.; Ieropoulos, I. Microbial fuel cells: From fundamentals to applications. A review. J. Power Sources 2017, 356, 225–244. [Google Scholar] [CrossRef]
- Rismani-Yazdi, H.; Carver, S.M.; Christy, A.D.; Tuovinen, O.H. Cathodic limitations in microbial fuel cells: An overview. J. Power Sources 2008, 180, 683–694. [Google Scholar] [CrossRef]
- Du, Z.; Li, Q.; Tong, M.; Li, S.; Li, H. Electricity Generation Using Membrane-less Microbial Fuel Cell during Wastewater Treatment. Chin. J. Chem. Eng. 2008, 16, 772–777. [Google Scholar] [CrossRef]
- Leong, J.X.; Daud, W.R.W.; Ghasemi, M.; Ben Liew, K.; Ismail, M. Ion exchange membranes as separators in microbial fuel cells for bioenergy conversion: A comprehensive review. Renew. Sustain. Energy Rev. 2013, 28, 575–587. [Google Scholar] [CrossRef]
- He, Z. Microbial Fuel Cells: Now Let us Talk about Energy. Environ. Sci. Technol. 2012, 47, 332–333. [Google Scholar] [CrossRef] [PubMed]
- He, Z. Development of Microbial Fuel Cells Needs To Go beyond “Power Density”. ACS Energy Lett. 2017, 2, 700–702. [Google Scholar] [CrossRef]
- Preen, R.J.; You, J.; Bull, L.; Ieropoulos, I. Design mining microbial fuel cell cascades. Soft Comput. 2018, 23, 4673–4683. [Google Scholar] [CrossRef]
- Gajda, I.; Greenman, J.; Santoro, C.; Serov, A.; Atanassov, P.; Melhuish, C.; Ieropoulos, I. Multi-functional microbial fuel cells for power, treatment and electro-osmotic purification of urine. J. Chem. Technol. Biotechnol. 2018, 94, 2098–2106. [Google Scholar] [CrossRef]
- Walter, X.A.; Greenman, J.; Ieropoulos, I. Binder materials for the cathodes applied to self-stratifying membraneless microbial fuel cell. Bioelectrochemistry 2018, 123, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Winfield, J.; Chambers, L.D.; Stinchcombe, A.; Rossiter, J.; Ieropoulos, I. The power of glove: Soft microbial fuel cell for low-power electronics. J. Power Sources 2014, 249, 327–332. [Google Scholar] [CrossRef]
- Schwarz, H.A. Gesammelte Mathematische Abhandlungen; Springer: Berlin/Heidelberg, Germany, 1890. [Google Scholar]
- Shin, J.; Kim, S.; Jeong, D.; Lee, H.G.; Lee, D.; Lim, J.Y.; Kim, J. Finite Element Analysis of Schwarz P Surface Pore Geometries for Tissue-Engineered Scaffolds. Math. Probl. Eng. 2012, 2012, 1–13. [Google Scholar] [CrossRef]
- Song, Y.E.; Boghani, H.; Lee, T.; Premier, G.; Kim, H.S.; Kim, B.G.; Jeon, B.-H.; Kim, J.R. Maximum Power Point Tracking to Increase the Power Production and Treatment Efficiency of a Continuously Operated Flat-Plate Microbial Fuel Cell. Energy Technol. 2016, 4, 1427–1434. [Google Scholar] [CrossRef]
- Nahir, T.M.; Clark, R.A.; Bowden, E.F. Linear-Sweep Voltammetry of Irreversible Electron Transfer in Surface-Confined Species Using the Marcus Theory. Anal. Chem. 1994, 66, 2595–2598. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

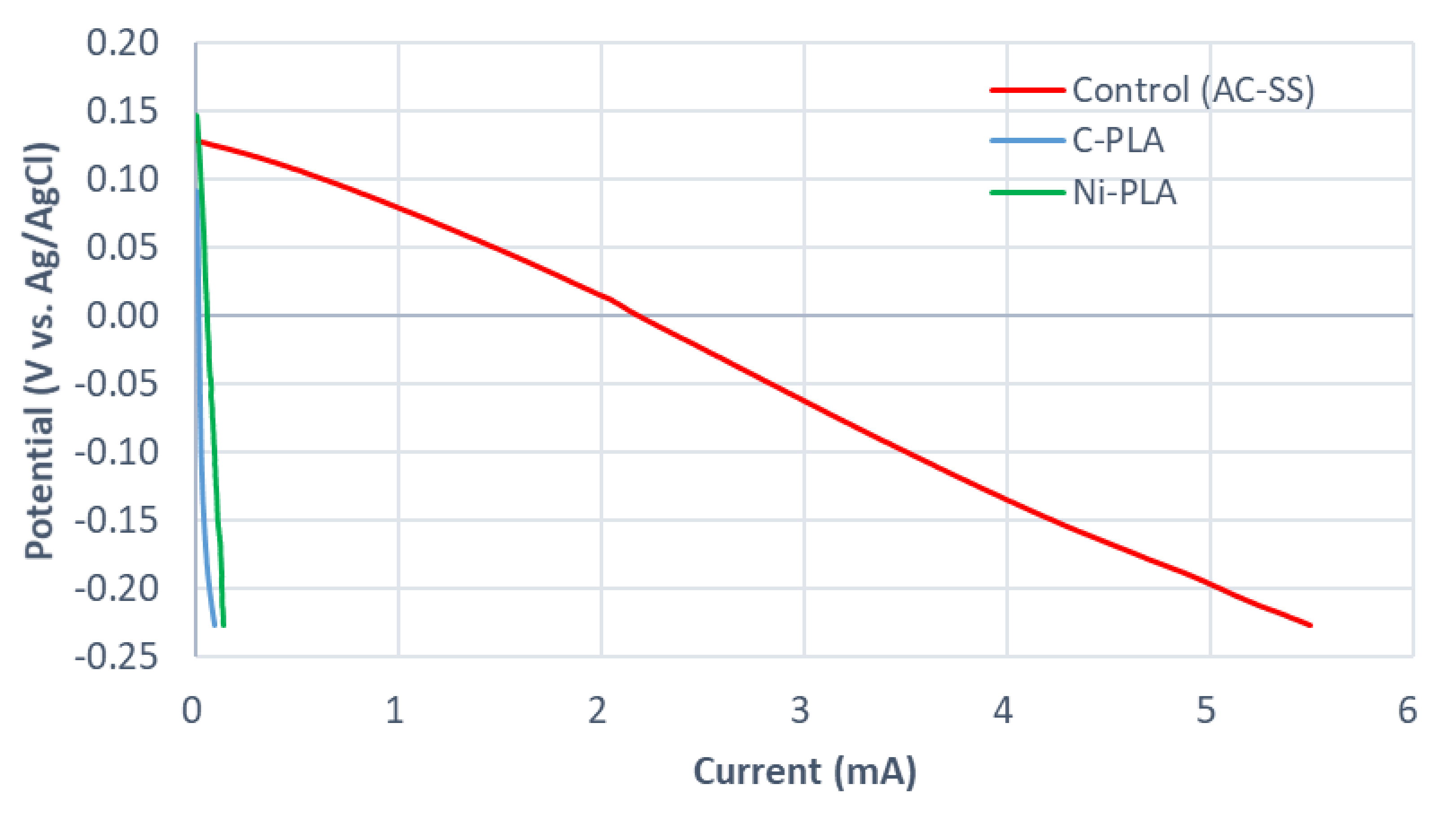

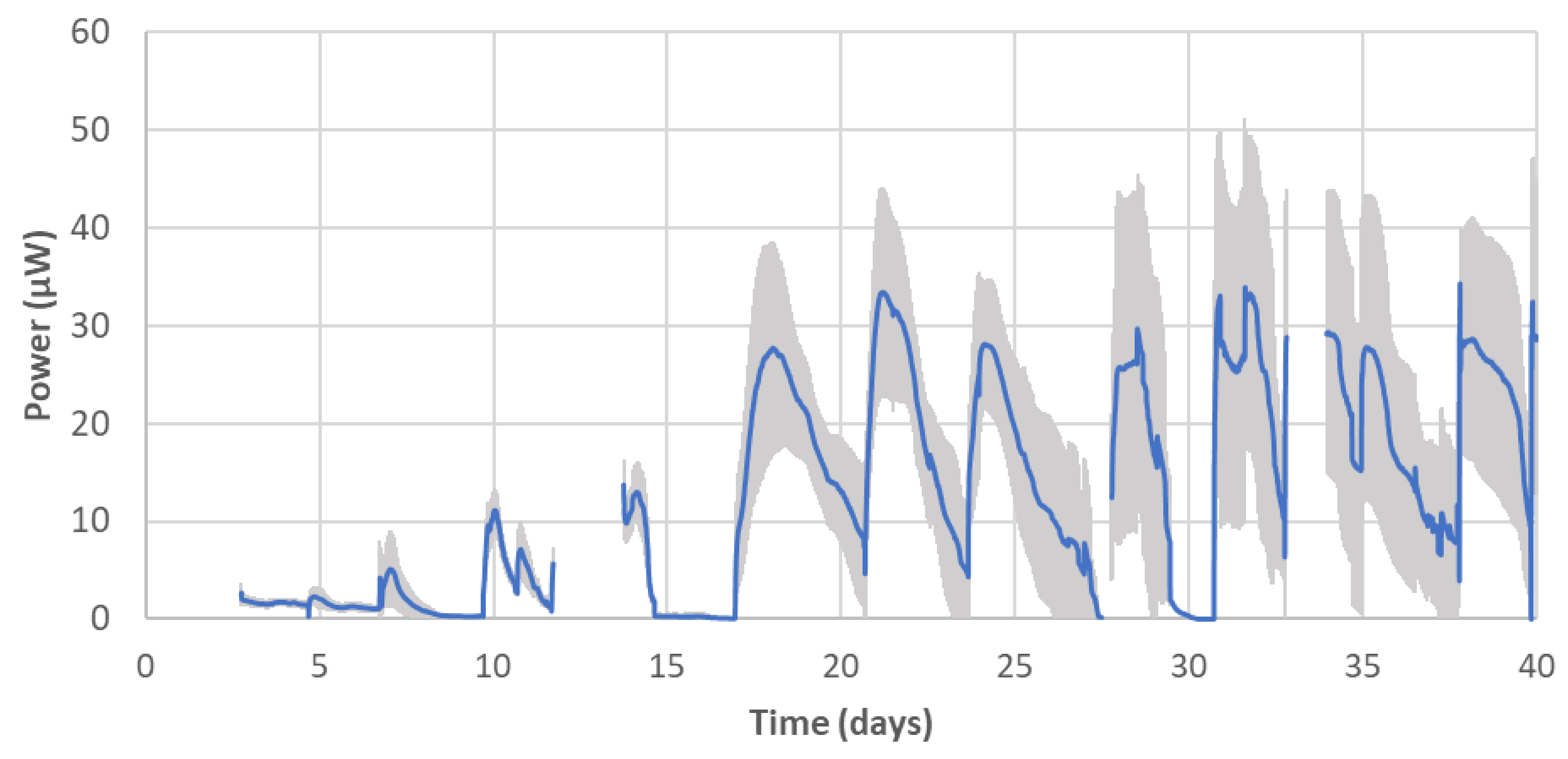
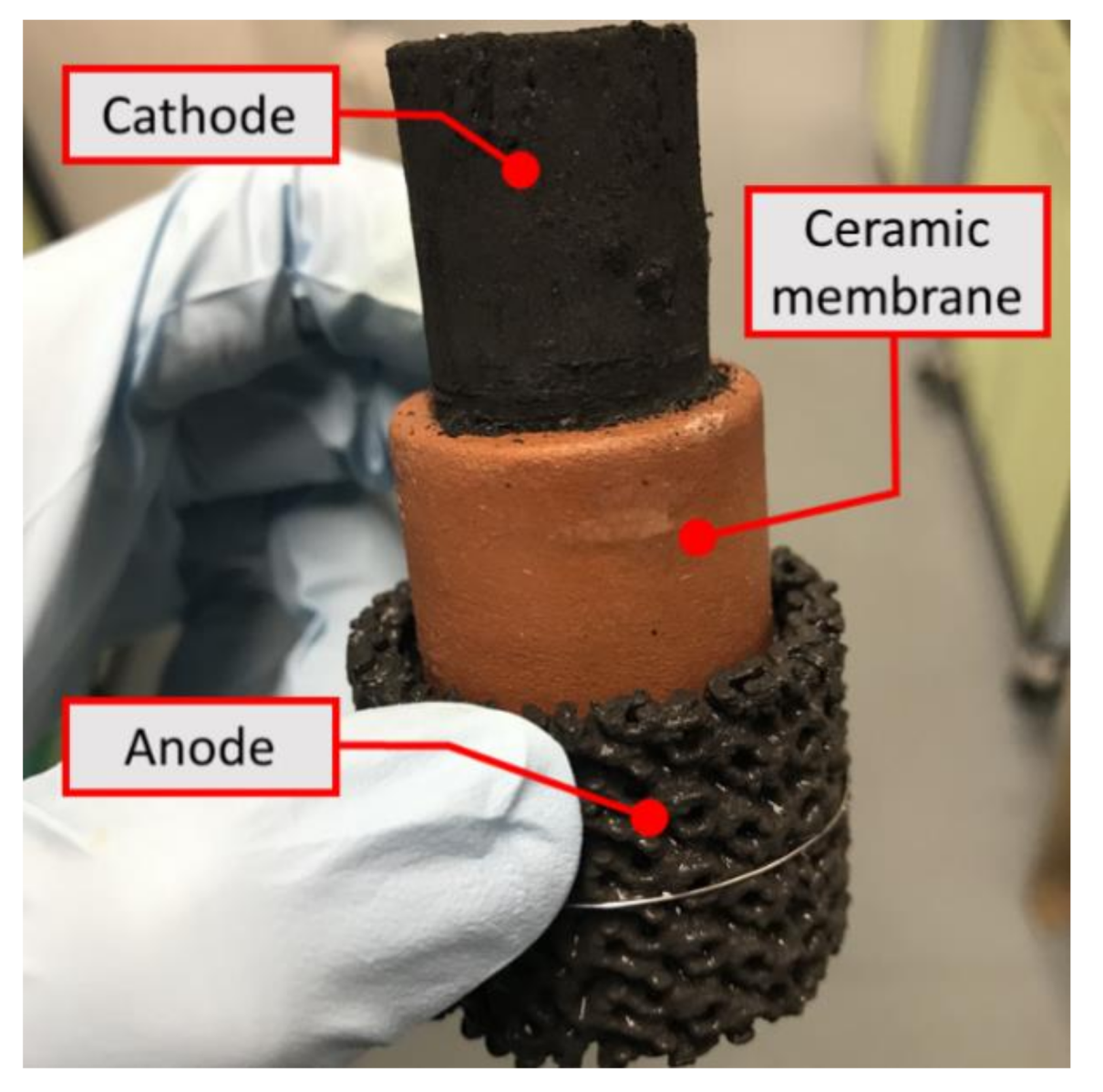


| Samples | Resistance-Parallel to the Direction of Layers 1) (Ω) | Resistance-Perpendicular to the Direction of Layers 1) (Ω) | Carbon Increase (mg cm−2) |
|---|---|---|---|
| PLA | n/m 2) | n/m | n/a 3) |
| Single coated C-PLA 4) | 1100 | 2025 | 3.0 |
| Double coated C-PLA | 240 | 370 | 12.5 |
| cPLA 5) | 1235 | 1955 | n/a |
| Single coated C-cPLA | 385 | 540 | 2.6 |
| Double coated C-cPLA | 175 | 305 | 12.4 |
| Membrane Type | Peak Power of Each Feed | Energy Generation from Each Feed | ||
|---|---|---|---|---|
| Absolute power (µW) | Power to volume 1) (W m−3) | Energy (mWh) | Energy to volume 2) (kWh m−3) | |
| Membrane-less | 520–570 | 7.4–8.1 | 11.38 ± 0.20 | 0.57 ± 0.01 |
| Ceramic membrane | 320–400 | 6.4–8.0 | 13.83 ± 0.41 | 0.69 ± 0.20 |
| Sample Name | PLA | C-PLA | Ni-PLA | cPLA | C-cPLA |
|---|---|---|---|---|---|
| Base filament material | Non-conductive PLA | Conductive PLA | |||
| Surface modification | n/a | Carbon coating | Nickel coating | n/a | Carbon coating |
| Tested as | Anode | Anode, cathode | Anode, cathode | Anode | Anode |
| Dimension | (Anode) external diameter: 41 mm, height: 30 mm, thickness: 3.5 mm) (Cathode) external diameter: 20 mm, height: 70 mm, thickness: 4.0 mm) | ||||
| Surface area 1) | (Anode) 158.5 cm2 (Cathode) 96.2 cm2 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
You, J.; Fan, H.; Winfield, J.; Ieropoulos, I.A. Complete Microbial Fuel Cell Fabrication Using Additive Layer Manufacturing. Molecules 2020, 25, 3051. https://doi.org/10.3390/molecules25133051
You J, Fan H, Winfield J, Ieropoulos IA. Complete Microbial Fuel Cell Fabrication Using Additive Layer Manufacturing. Molecules. 2020; 25(13):3051. https://doi.org/10.3390/molecules25133051
Chicago/Turabian StyleYou, Jiseon, Hangbing Fan, Jonathan Winfield, and Ioannis A. Ieropoulos. 2020. "Complete Microbial Fuel Cell Fabrication Using Additive Layer Manufacturing" Molecules 25, no. 13: 3051. https://doi.org/10.3390/molecules25133051
APA StyleYou, J., Fan, H., Winfield, J., & Ieropoulos, I. A. (2020). Complete Microbial Fuel Cell Fabrication Using Additive Layer Manufacturing. Molecules, 25(13), 3051. https://doi.org/10.3390/molecules25133051






