Abstract
In this study, the density functional theory (DFT) and CCSD(T) method have been performed to gain insight into the possible products and detailed reaction mechanism of the Criegee intermediate (CI) of anti-PhCHOO with SO2 for the first time. The potential energy surfaces (PESs) have been depicted at the UCCSD(T)/6-311++G(d,p)//UB3LYP/6-311++G(d,p) levels of theory with ZPE correction. Two different five-membered ring adducts, viz., endo PhCHOOS(O)O (IM1) and exo PhCHOOS(O)O (IM2) have been found in the entrance of reaction channels. Both direct and indirect reaction pathways from IM1 and IM2 have been considered for the title reaction. Our calculations show that the formation of PhCHO+SO3 (P1) via indirect reaction pathways from IM1 is predominant in all the pathways, and the production of P1 via direct dissociation pathway of IM1 and indirect reaction pathways of IM2 cannot be neglected. Moreover, PhCOOH+SO2 (P2) initiated from IM2 is identified as the minor product. According to the kinetic calculation, the total rate constant for the anti-PhCHOO+SO2 reaction is estimated to be 6.98 × 10−10 cm3·molecule−1·s−1 at 298 K.
1. Introduction
Criegee intermediate (CI) is an important species formed in the ozonolysis of alkenes, and it is a carbonyl oxide with diradical electronic structure [1]. Since the first direct observation [2] and direct kinetic measurements [3] for the simplest CI, CH2OO, recently, much attention has been given to the reactions of CIs in atmospheric chemistry. Due to the significance in ozonolysis mechanism and possessing high reactivity, it has been suggested that CIs can be added as a new type of atmospheric active species in atmospheric oxidation chemistry, besides OH, NO3, and O3 et al. [4].
When released into the atmosphere, the CIs can react with some atmospheric species, such as H2O [3,5,6,7,8,9,10,11,12,13,14], HO2 [15,16], NO2 [3,10,17,18,19], SO2 [3,9,10,11,14,19,20,21,22,23,24,25,26,27,28,29], OH [30] and CH2=C(CH3)CHO [31] et al., to form corresponding products. The reactions of CIs with SO2 in particular play an important role in atmospheric chemistry, due to the formation of SO3 and subsequent H2SO4, which is the main component of aerosols and acid rain [32,33,34,35], and this provides another feasible channel for the formation of H2SO4 in the atmosphere.
For the reactions of CIs with SO2, many experimental and theoretical studies have been carried out to investigate the kinetics and reaction mechanism for the simplest CH2OO [3,5,9,10,11,20,21,22,23,24,25,31], anti- or syn-CH3CHOO and (CH3)2COO [11,19,21,22,24,26,27,28,30]. Moreover, the reactions of SO2 with those CIs produced from czonolysis of limonene, α- pinene, β-pinene [20,29], and styrene [14] etc., have also been studied to investigate the influence of these reactions of CIs+SO2 to the formation of sulfuric acid or the secondary aerosols.
In 2017, Díaz-de-Mera et al. [14] studied the ozonolysis of styrene in the presence of SO2 at atmospheric pressure and room temperature. The formation of SO3 is expected to be major in reaction of Criegee intermediates with SO2. They found that lower concentrations of reactants were required in the ozonolysis of styrene with low concentrations of SO2 than those required in experiments without SO2. Furthermore, under high H2O concentrations, the formation of SO3 and subsequent H2SO4 in the smog chamber is inhibited, due to the competitive reactions of CIs with water. However, the rate constants ratio of (2.8 ± 0.7) × 10−5 (errors are 2σ ± 20%) for k(H2O)/k(SO2) illustrates that the reaction of CIs with SO2 will be fast under atmospheric conditions.
In the ozonolysis of styrene, both CH2OO and C6H5CHOO (denoted as PhCHOO) will be formed. For the case of CH2OO, the reactions with SO2 have been studied extensively [3,5,9,10,11,20,21,22,23,24,25,31], both in experiment and theory, while for the reaction of PhCHOO+SO2, as far as we know, no corresponding theoretical study has been done. Thus, the main goal of this work is to explore the reaction mechanism and kinetics of anti-PhCHOO+SO2, give the possible product channels, and compare with the available experimental results. The proposed reaction pathways for the reaction of anti-PhCHOO+SO2 are presented in Scheme 1.
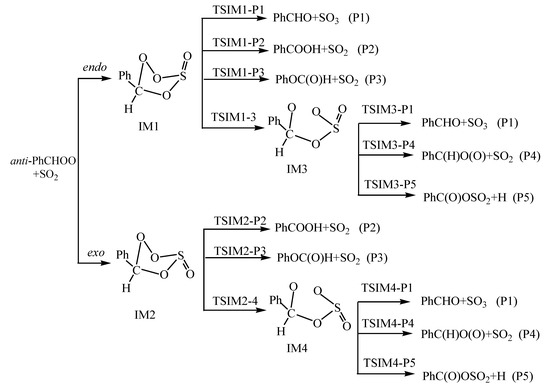
Scheme 1.
Proposed mechanisms for the anti-PhCHOO+SO2 reactions.
2. Computational Method
The Gaussian 09 package [36] was used to all ab initio calculations. Due to the ability of accurate describing the geometries of transition states and providing some properties that can be comparable in accuracy to higher levels of theory, the hybrid B3LYP [37,38] method has been employed extensively in the CI reaction systems [5,7,13,20,21,24], thus, the geometries of all species involved in the title reaction have been optimized at the B3LYP/6-311++G(d,p) level of theory. Harmonic vibrational frequency calculations were performed at the same level of theory to confirm the character of each stationary point, i.e., all real frequencies for a minimum and only one imaginary frequency for a transition state structure. Moreover, zero-point vibrational energy (ZPE) correction was also obtained from such calculations. Intrinsic reaction coordinate (IRC) [39,40] calculations were also carried out, to verify the connectivity of the transition state structures. To obtain more reliable relative energies, single-point energies of all stationary points at the B3LYP/6-311++G(d,p) geometries were calculated using coupled-cluster theory including single, double, and noniterative triple excitations [CCSD(T)] [41] using the 6-311++G(d,p) basis set. The ZPE corrections with a scale factor of 0.9688 [42] were included in the determination of energy barriers and reaction energies. We noted that the B3LYP/6-311++G(d,p) calculations were unable to locate the reactant complexes RC1, RC2, and transition states TSRC1-1, TSRC2-2 in the entrance channels. However, they could be obtained using the BH&HLYP method [38,43]. Therefore, the stationary points of RC1, RC2, TSRC1-1, and TSRC2-2 were reoptimized at the BH&HLYP/6-311++G(d,p) level. The single-point energy of each optimized geometry at the BH&LYP/6-311++G(d,p) level was recalculated, using the CCSD(T)/6-311++G(d,p) level of theory, with the ZPE correction scaled by 0.9335 [42]. Here, we would like to explain that how the ZPE correction obtained by the DFT method is transformed into the one obtained by the CCSD(T) method. The PESs obtained at 0 K are useful for kinetic analysis, especially for the rate constant calculation at different temperatures. The reliable energies at 0 K of all stationary points can be determined directly by geometry optimization and frequency calculation, using the CCSD(T) method. However, the geometry optimization and frequency calculations at CCSD(T) method are very expensive indeed. In general, the CCSD(T) total energy at 0 K is obtained by adding the DFT-determined ZPE correction to the CCSD(T) single point energy on the basis of DFT geometry optimization. This method is widely used in the PESs construction of reaction systems [44,45]. Moreover, due to the neglection of anharmonicity effects in the theoretical treatment, incomplete incorporation of electron correlation, and the use of finite basis sets, the computed quantum chemical harmonic vibrational frequencies are typically larger than the fundamentals observed experimentally. Therefore, the frequency scale factors are applied to obtain fundamentals and ZPEs [42].
It should be pointed out that, due to the special singlet diradical structure, the closed-shell paradigm is not suitable to CIs. Thus, similar to the reaction of CH2OO+SO2 [23], the unrestricted broken symmetry approach—mixing the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) option proposed by Noodleman [46]—was used for these species in the geometry optimizations and single-point energies calculations. Moreover, the %TAE[(T)] diagnostic method was used to estimate the magnitude of post-CCSD(T) contributions. It has been shown that %TAE[(T)] diagnostic can provide a useful a priori indicator of the magnitude of the post-CCSD(T) contributions to the total atomization energies (TAEs) [47,48]. According to the %TAE[(T)] values, (%TAE[(T)] = 100 × (TAE[CCSD(T)]−TAE[CCSD])/TAE[CCSD(T)], we can see from Table S1 of Supplementary Materials that all the %TAE[(T)] diagnostic values are small (below 0.02%), which suggests that the post-CCSD(T) contributions should not exceed 0.5 kcal/mol [48], and the application of CCSD(T)/6-311++G(d,p) for this system is suitable.
3. Results and Discussion
3.1. Reaction Mechanism
In the ozonolysis of styrene, both syn- and anti- PhCHOO will be formed in the initial steps. The anti-PhCHOO species has a smaller steric hindrance than syn-PhCHOO in the formation of the corresponding complexes and (or) adducts when drawing near SO2, as illustrated in the similar reactions of anti-CH3CHOO with H2O [8] and SO2 [26] etc., thus, only the anti-PhCHOO conformer is selected as the initial reactant.
The optimized geometries and critical structural parameters for various species, including reactants, reactant complexes (RC), intermediates (IM), transition states (TS), and products associated with the reaction of anti-PhCHOO with SO2 are shown in Figure S1, Figure S2, and Figure S3 of the Supplementary Materials. In Table S2 of the Supplementary Materials, the ZPE, total energies, and relative energies (relative to the isolated species) without and with ZPE corrections of all stationary points calculated at the UCCSD(T)/6-311++G(d,p)//UB3LYP/6-311++G(d,p) levels of theory with ZPE corrections (denoted as UCCSD(T)//UB3LYP) are listed.
The potential energy surfaces (PESs) for the reaction of anti-PhCHOO+SO2 plotted by the relative energies are shown in Figure 1 and Figure 2, together with the labeling of atoms in the reactant complexes.
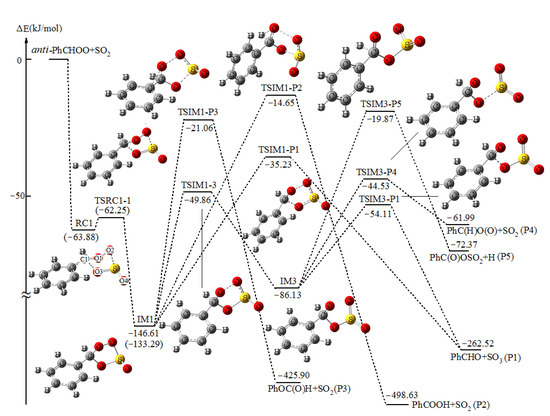
Figure 1.
Potential energy profile for the formation and subsequent reaction pathways of endo PhCHOOS(O)O (IM1) in the reaction of anti-PhCHOO+SO2 at the UCCSD(T)//UB3LYP level with zero-point vibrational energy (ZPE) corrections. The values in parentheses are derived from UCCSD(T)//UBH&HLYP level with ZPE corrections.
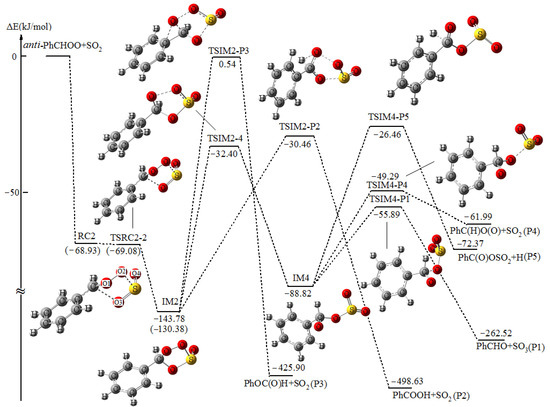
Figure 2.
Potential energy profile for the formation and subsequent reaction pathways of exo PhCHOOS(O)O (IM2) in the reaction of anti-PhCHOO+SO2 at the UCCSD(T)//UB3LYP level with ZPE corrections. The values in parentheses are derived from UCCSD(T)//UBH&HLYP level with ZPE corrections.
Similar to other CIs, the anti-PhCHOO produced in the ozonolysis of styrene is highly reactive, and it can be stabilized by collision, followed by the reaction with SO2, to form corresponding products.
As shown in Figure 1 and Figure 2, with the approach of SO2 from different directions, the reaction can proceed through the formation of two reactant complexes RC1 and RC2 in the entrance channels. Subsequently, with the S atom of SO2 bonding to the CI terminal O2 atom and the O3 atom of SO2 bonding to the C atom of CI simultaneously, two cyclic adducts endo PhCHOOS(O)O (IM1) and exo PhCHOOS(O)O (IM2) will be formed via TSRC1-1 and TSRC2-2, respectively. It has been mentioned in the Computational Method section that, at the B3LYP level, no corresponding reactant complexes and transition states have been found in the formation of IM1 and IM2, despite numerous attempts. The structures and energies of RC1, RC2, TSRC1-1, and TSRC2-2 shown in Figure 1 and Figure 2 are obtained at the UBH&HLYP/6-311++G(d,p) and UCCSD(T)/6-311++G(d,p)//UBH&HLYP/6-311++G(d,p), with ZPE corrections (denoted as UCCSD(T)//UBH&HLYP), levels of theory, respectively. The relative energies of RC1 and RC2 are −63.88 and −68.93 kJ/mol, and the corresponding transition states TSRC1-1 and TSRC2-2 lie slightly higher (1.63 kJ/mol for TSRC1-1), or even lower (−0.15 kJ/mol for TSRC2-2) than that of RC1 and RC2, respectively. Obviously, the formation of reactant complexes RC1 and RC2 and the subsequent adducts IM1 and IM2 is (or almost) barrierless. These results can find support from the frontier orbital analysis. As shown in Figure 3, at the UBH&HLYP/6-311++G(d,p) level, the electron density distribution localized on the fragment of –CHOO in the HOMO of anti-PhCHOO and the LUMO of SO2 is symmetry matching. Furthermore, the energy difference of 0.18539 a.u. between the HOMO of anti-PhCHOO and the LUMO of SO2 is smaller than 0.34151 a.u. between the HOMO of SO2 and the LUMO of anti-PhCHOO, thus, the reaction can proceed without barriers via interaction between the HOMO of anti-PhCHOO and the LUMO of SO2, to form RC1 and RC2.
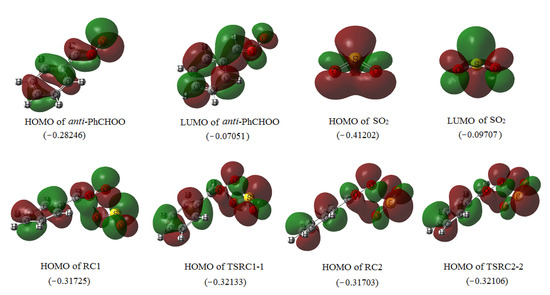
Figure 3.
Highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) orbital pictures of anti-PhCHOO and SO2, together with the HOMO of reactant complexes (RC)1, TSRC1-1, RC2 and TSRC2-2. The values in parentheses are the orbital energies (a.u.) calculated at the UBH&HLYP/6-311++G(d,p) level.
Comparing the HOMO of RC1 with that of TSRC1-1 shown in Figure 3, we can see that they have similar electronic density distribution and approximate orbital energies, that is to say, from RC1 to TSRC1-1, there is no obvious change of electron density distribution, which results in the formation of IM1 with almost no barrier. The similar instance can also be derived in the formation of IM2, according to the electronic density distribution in the HOMO of RC2 and TSRC2-2.
As depicted in Figure 1 and Figure 2, IM1 and IM2 are a pair of conformational isomers with the S=O double bond pointing to the outside and inside the plane, respectively. IM1 and IM2 lie 146.61 and 143.78 kJ/mol below the initial reactants, and this suggests that they have enough internal energy for the subsequent reactions.
Both isomerization and dissociation pathways of IM1 and IM2 have been considered, and they are discussed as follows.
Firstly, with the simultaneous fracture of O1-O2 and C-O3 bonds initiated from IM1, the products of benzaldehyde (PhCHO)+SO3 (P1) will be formed via the transition state TSIM1-P1, by overcoming a barrier of 111.38 kJ/mol.
Secondly, when the H atom shifting from the C1 atom to the adjacent O1 atom, the SO2 fragment (O2-S-O4) is also departed from the parent molecule via transition state TSIM1-P2, to produce benzoic acid (PhCOOH). The relative energy of TSIM1-P2 is -14.65 kJ/mol and it is 20.58 kJ/mol higher than that TSIM1-P1. Obviously, the production of P1 is more favorable than that of PhCOOH+SO2 (P2), due to its lower energy barrier.
The formation of singlet bisoxy diradical PhC(H)O(O) has also been considered, starting from IM1. However, an attempt to locate the corresponding TS at the B3LYP level was not successful. With the departure of SO2 via breaking the O1-O2 and S-O3 bonds, the product of phenyl formate (PhOC(O)H) will be formed via TSIM1-P3 after climbing the barrier height of 125.55 kJ/mol. The IRC analysis of TSIM1-P3 shown in Figure S4 verifies that it really connects IM1 and products of PhOC(O)H+SO2 (P3). The formation of PhOC(O)H is a new product channel that has not been discussed in other similar CIs+SO2 reactions.
In addition to three direct reaction pathways, IM1 can also isomerize to another intermediate IM3 via ring opening transition state TSIM1-3, with a lower barrier of 96.75 kJ/mol. In TSIM1-3, the breaking O1-O2 bond is 1.976 Å. Subsequently, three reaction pathways of IM3 will be open. As shown in Figure 1, the most feasible reaction pathways of IM3 is the formation of P1 via TSIM3-P1. TSIM3-P1 is 54.11 kJ/mol lower in energy than the initial reactants and the barrier height of it is only 32.02 kJ/mol.
As discussed above, the singlet bisoxy diradical PhC(H)O(O) cannot be produced directly from IM1, whereas, via TSIM3-P4, the products of PhC(H)O(O)+SO2 (P4) will be formed starting from IM3 by surmounting a barrier of 41.60 kJ/mol. It is worth noting that TSIM3-P4 lies above TSIM3-P1 by about 10 kJ/mol. Moreover, the reaction from IM3 to P4 is endergonic, and the process is reversible, so we can conclude that P4 would never be formed in the reaction.
The third reaction pathway of IM3 is the formation of PhC(O)OSO2+H (P5) via C1-H bond rupture transition state TSIM3-P5. The barrier height of TSIM3-P5 is 66.26 kJ/mol, and apparently the formation of P5 is not competitive with that of P1 initiated from IM3, due to it being the highest barrier.
As can be seen from Figure 1, because of the level of the lowest barrier, the formation of P1 through indirect reaction processes, viz. IM1→TSIM1-3→IM3→TS3-P1→P1 is more favorable than that through direct reaction pathway, and is the major product channel, and our calculational result is consistent with the conclusion that the reaction of CI+SO2 is surprisingly contributive to the formation of atmospheric H2SO4 [3]. As for the formation of PhCOOH+SO2 (P2), although it is the most favored product channel thermodynamically, the highest barrier height makes this pathway infeasible from IM1.
Similar to IM1, both the direct and indirect reaction pathways of IM2 have been considered, and they are depicted in Figure 2.
The product pathway of IM2 for the formation of P1 has been studied firstly, whereas no right transition state has been located at the B3LYP level. The search for such structure results in the transition state connecting the intermediate formed between syn-PhCHOO with SO2 and P1.
Starting from IM2, the PhCOOH+SO2 (P2) will be formed via TSIM2-P2 with a barrier of 113.32 kJ/mol. As shown in Figure 2, TSIM2-P2 is 15.81 kJ/mol lower in energy than that TSIM1-P2, and this indicates that the formation of P2 via TSIM2-P2 is more feasible. As for the production of P3 from IM2, the transition state TSIM2-P3 involves the highest barrier of 144.32 kJ/mol, thus, the contribution from this product channel can be negligible.
With the fracture of O1-O2 bond, the intermediate IM4 will be formed via TSIM2-4 with a barrier height of 111.38 kJ/mol. IM4 has similar reaction pathways to IM3, viz. the formation of P1, P4, and P5 via TSIM4-P1, TSIM4-P4, and TSIM4-P5, respectively. The relative energies of TSIM4-P1, TSIM4-P4, and TSIM4-P5 are −55.89, −49.29 and −26.46 kJ/mol, respectively. Similar to the reaction from IM3 to P4, the formation of P4 and P5 starting from IM4 can be almost ruled out, since these two pathways are not favorable thermodynamically, and are dynamically compared with the formation of P1 from IM4. As a result, P1 and P2 formed through TSIM4-P1 and TSIM2-P2 are the major and minor products of IM2, respectively.
Comparing Figure 1 with Figure 2, we can see that from both IM1 and IM2, the formation of P1 via indirect reaction pathways is the main product channels. IM1 and IM2 are a pair of isomers with similar structures and closer energies, however, due to their different spatial conformation, the energy of TSIM1-3 is 17.46 kJ/mol lower than that of TSIM2-4, which results in the formation of P1 starting from IM1 to be more feasible, that is to say, IM1 and IM2 have the conformation-dependent reactivity. Moreover, the formation of P2 from IM2 may also have slight contribution to the final product distribution, based on our calculations. In the experiment, besides the dominant SO3, no other possible products have been given [14]. Fortunately, our theoretical prediction for the possible product distribution of the title reaction is consistent with the similar reaction of CH2OO+SO2 [24]. In addition, we can see that during the formation of P1 via indirect reaction pathways of IM1, all the transition states lie below the initial reactants by about 50 kJ/mol, which suggests that the reaction of anti-PhCHOO+SO2 will be fast. This is attributed to the fact that the effective activation energy ΔEeff≠ (ΔEeff≠ = ETS − EReactants) [49,50] for the rate-determining step transition state TSIM1-3 is negative. A negative ΔEeff≠ is crucial to the reaction activity and product distribution [51,52]. Nevertheless, this behavior will be testified by further kinetic investigation (see next section).
3.2. Kinetic Calculation
The rate constant for the reaction of SO2 with the Criegee intermediate can be estimated in terms of the steady state approximation [21] for the IM1 adduct:
Then, the reaction rate starting from IM1 can be represented as:
Therefore, the rate constant k1 can be expressed as:
where kcap represents the capture rate constant between SO2 and anti-PhCHOO that form the IM1 adduct, kdiss,1 stands for the rate constant for the IM1 adduct dissociates back to the reactants, and kTSIM1-3, kTSIM1-P1, kTSIM1-P2 and kTSIM1-P3 are the unimolecular rate constants of the dissociation or isomerization channels of the IM1 adduct, and they can be calculated using transition state theory (TST) [53,54,55]:
where Q represents the partition functions of the respective subscripted species, while E are the zero-point corrected total energies of the respective subscripted species. Γ denotes the Wigner’s tunneling correction [56]. kB, h, T, and R represent Boltzmann’s constant (1.38 × 10−23 J K−1), Planck’s constant (6.63 × 10−34 J·s), the absolute temperature, and the ideal gas constant (8.314 J mol−1 K−1), respectively.
As stated above, the formation of IM1 and IM2 is barrierless. The capture rate constant kcap can be calculated using the long-range variational TST expression derived by Georgievskii and Klippenstein [57], which can be written as:
where d1 and d2 are the dipole moments of the SO2 and anti-PhCHOO, μ is the reduced mass of the anti-PhCHOO−SO2 collision, T is the absolute temperature, and C is a constant of proportionality whose value is 5.87 [57] for the dipole-dipole interaction between two nonlinear molecules.
The value of kcap for the IM1 adduct at 298 K was found to be 6.02 × 10−10 cm3 molecule−1 s−1 by using the dipole of anti-PhCHOO and SO2 (6.5619 D and 2.0269 D at the B3LYP/6-311++G(d,p) level of theory). This value is similar to that obtained in barrierless CH2OO-SO2 association study [24]. From elementary TST, and using the B3LYP-determined moments of inertia, and the B3LYP-determined vibrational frequencies and the zero-point-corrected CCSD(T) electronic energies, we obtain the following unimolecular rate constants: kdiss,1 = 4.87 × 10−7 s−1, kTSIM1-3 = 1.08 × 10−4 s−1, kTSIM1-P1 = 4.12×10−7 s−1, kTSIM1-P2 = 1.50×10−10 s−1, kTSIM1-P3 = 5.25×10−10 s−1 at 298 K. Thus, the rate constant k1 is computed to be 5.99 × 10−10 cm3 molecule−1 s−1.
By following a similar procedure, the rate constant k2 for the IM2 adduct can also be obtained as follows:
The unimolecular rate constants beginning with IM2 at 298 K were computed to be kdiss,2 = 1.65 × 10−6 s−1, kTSIM2-4 = 3.58 × 10−7 s−1, kTSIM2-P2 = 1.73 × 10−7 s−1, kTSIM2-P3 =3.29 × 10−13 s−1, respectively. As a result, the computed value for the rate constant k2 at 298 K is 9.94 × 10−11 cm3 molecule−1 s−1.
Finally, the overall bimolecular rate constant for the anti-PhCHOO+SO2 reaction was determined to be 6.98 × 10−10 cm3 molecule−1 s−1 by adding up the individual bimolecular rate constant k1 and k2.
There are no experimental data about the bimolecular rate constant for the anti-PhCHOO + SO2 reaction. Information on the bimolecular rate constant in the reactions of SO2 with Criegee intermediates is available in the case of CH2OO and (CH3)2COO [3,21]. Our calculated value matches within 1 order of magnitude compared with the experimental value of (3.9 ± 0.7) × 10−11 cm3 molecule−1 s−1 for the CH2OO+SO2 reaction recommended by Welz et al. [3]. Moreover, the obtained rate constant presented here agrees well with the computed value of 4 × 10−10 cm3 molecule−1 s−1 for both the CH2OO+SO2 and the (CH3)2COO+SO2 oxidation reactions reported by Kurtén et al. [21].
4. Conclusions
Quantum chemical calculations have been performed to characterize the potential energy surface of the anti-PhCHOO+SO2 reaction at the UCCSD(T)/6-311++G(d,p) level of energy calculations based on UB3LYP/6-311++G(d,p) optimized geometries together with ZPE corrections. Various possible reaction pathways have been probed. The calculated results show that the reaction begins with the formation of two reactant complexes RC1 and RC2 and produces two energy-rich adducts IM1 and IM2 barrierlessly in the process of SO2 association with anti-PhCHOO from different directions. IM1 and IM2 are a pair of isomers with similar structures and closer energies, however, IM1 shows higher reactivity than that IM2 in the formation of the most favorable product of P1 via indirect reaction pathways, which suggests the conformation-dependent reactivity of anti-PhCHOO with SO2. P2 is found to be the less competitive product, followed by the almost negligible products of P3, P4 and P5. Based on the quantum chemical calculations and transition state theory, the overall reaction rate constant is predicted to be 6.98 × 10−10 cm3 molecule−1 s−1 at 298 K, which is in agreement with the similar CH2OO+SO2 and (CH3)2COO+SO2 reactions.
Supplementary Materials
Figure S1: Optimized geometries of species including reactants, reactant complexes, transition states and intermediates involved in the reaction of anti-PhCHOO+SO2 at the UB3LYP/6-311++G(d,p) level. Figure S2: Optimized geometries of various species involved in the subsequent reaction pathways of IM1 at the UB3LYP/6-311++G(d,p) level. Figure S3: Optimized geometries of various species involved in the subsequent reaction pathways of IM2 at the UB3LYP/6-311++G(d,p) level. Figure S4: The intrinsic reaction coordinate of TSIM1-P3. Table S1: The total energies (hartree) obtained at the UCCSD(T)//UB3LYP and UCCSD//UB3LYP levels of theory together with the %TAE(T) of various species in the reaction of anti-PhCHOO+SO2. Table S2: The ZPE (hartree), total energies (E, hartree) and relative energies (ΔE, Δ(E+ZPE), kJ/mol) without and with ZPE corrections of various species calculated at the UCCSD(T)//UB3LYP levels of theory in the reaction of anti-PhCHOO+SO2. Cartesian coordinates for all optimized structures at the UB3LYP/6-311++G(d,p) and UBH&HLYP/6-311++G(d,p) levels of theory.
Author Contributions
Conceptualization, B.D.; Methodology, B.D. and W.Z.; Software, W.Z.; Validation, B.D. and W.Z.; Formal Analysis, W.Z.; Investigation, B.D. and W.Z.; Resources, B.D.; Data Curation, B.D. and W.Z.; Writing-Original Draft Preparation, B.D. and W.Z.; Writing-Review & Editing, W.Z.; Visualization, W.Z.; Supervision, W.Z.; Project Administration, W.Z.; Funding Acquisition, B.D. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Doctoral Scientific Research Foundation of Jiangsu Normal University (Grant No. 13XLR003).
Acknowledgments
The authors would like to acknowledge the support of the Top-notch Academic Programs Project of Jiangsu Higher Education Institutions (TAPP).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Su, Y.T.; Huang, Y.H.; Witek, H.A.; Lee, Y.P. Infrared Absorption Spectrum of the Simplest Criegee Intermediate CH2OO. Science 2013, 340, 174–176. [Google Scholar] [CrossRef]
- Taatjes, C.A.; Meloni, G.; Selby, T.M.; Trevitt, A.J.; Osborn, D.L.; Percival, C.J.; Shallcross, D.E. Direct Observation of the Gas-Phase Criegee Intermediate (CH2OO). J. Am. Chem. Soc. 2008, 130, 11883–11885. [Google Scholar] [CrossRef] [PubMed]
- Welz, O.; Savee, J.D.; Osborn, D.L.; Vasu, S.S.; Percival, C.J.; Shallcross, D.E.; Taatjes, C.A. Direct Kinetic Measurements of Criegee Intermediate (CH2OO) Formed by Reaction of CH2I with O2. Science 2012, 335, 204–207. [Google Scholar] [CrossRef]
- Kjaergaard, H.G.; Kurtén, T.; Nielsen, L.B.; Jørgensen, S.; Wennberg, P.O. Criegee Intermediates React with Ozone. J. Phys. Chem. Lett. 2013, 4, 2525–2529. [Google Scholar] [CrossRef]
- Aplincourt, P.; Ruiz-López, M.F. Theoretical Investigation of Reaction Mechanisms for Carboxylic Acid Formation in the Atmosphere. J. Am. Chem. Soc. 2000, 122, 8990–8997. [Google Scholar] [CrossRef]
- Fenske, J.D.; Hasson, A.S.; Ho, A.W.; Paulson, S.E. Measurement of Absolute Unimolecular and Bimolecular Rate Constants for CH3CHOO Generated by the trans-2-Butene Reaction with Ozone in the Gas Phase. J. Phys. Chem. A 2000, 104, 9921–9932. [Google Scholar] [CrossRef]
- Ryzhkov, A.B.; Ariya, P.A. The Importance of Water Clusters (H2O)n (n = 2,...,4) in the Reaction of Criegee Intermediate with Water in the Atmosphere. Chem. Phys. Lett. 2006, 419, 479–485. [Google Scholar] [CrossRef]
- Anglada, J.M.; González, J.; Torrent-Sucarrat, M. Effects of the Substituents on the Reactivity of Carbonyl Oxides. A Theoretical Study on the Reaction of Substituted Carbonyl Oxides with Water. Phys. Chem. Chem. Phys. 2011, 13, 13034–13035. [Google Scholar] [CrossRef]
- Berndt, T.; Voigtländer, J.; Stratmann, F.; Junninen, H.; Mauldin, R.L., III; Sipilä, M.; Kulmala, M.; Herrmann, H. Competing Atmospheric Reactions of CH2OO with SO2 and Water Vapour. Phys. Chem. Chem. Phys. 2014, 16, 19130–19136. [Google Scholar] [CrossRef]
- Stone, D.; Blitz, M.; Daubney, L.; Howes, N.U.M.; Seakins, P. Kinetics of CH2OO Reactions with SO2, NO2, NO, H2O and CH3CHO as a Function of Pressure. Phys. Chem. Chem. Phys. 2014, 16, 1139–1149. [Google Scholar] [CrossRef]
- Newland, M.J.; Rickard, A.R.; Alam, M.S.; Vereecken, L.; Munoz, A.; Ródenas, M.; Bloss, W.J. Kinetics of Stabilized Criegee Intermediates Derived from Alkene Ozonolysis: Reactions with SO2, H2O and Decomposition under Boundary Layer Conditions. Phys. Chem. Chem. Phys. 2015, 17, 4076–4088. [Google Scholar] [CrossRef] [PubMed]
- Chao, W.; Hsieh, J.-T.; Chang, C.-H.; Lin, J.J.-M. Direct Kinetic Measurement of the Reaction of the Simplest Criegee Intermediate with Water Vapor. Science 2015, 347, 751–754. [Google Scholar] [CrossRef] [PubMed]
- Long, B.; Bao, J.L.; Truhlar, D.G. Atmospheric Chemistry of Criegee Intermediates. Unimolecular Reactions and Reactions with Water. J. Am. Chem. Soc. 2016, 138, 14409–14422. [Google Scholar] [CrossRef]
- Díaz-de-Mera, Y.; Aranda, A.; Martínez, E.; Rodríguez, A.A.; Rodríguez, D.; Rodríguez, A. Formation of Secondary Aerosols from the Ozonolysis of Styrene: Effect of SO2 and H2O. Atmos. Environ. 2017, 171, 25–31. [Google Scholar] [CrossRef]
- Long, B.; Tan, X.F.; Long, Z.W.; Wang, Y.B.; Ren, D.S.; Zhang, W.J. Theoretical Studies on Reactions of the Stabilized H2COO with HO2 and the HO2···H2O Complex. J. Phys. Chem. A 2011, 115, 6559–6567. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Huang, Y.; Xue, Y.G.; Cao, J.J.; Wang, W.L. Competition between HO2 and H2O2 Reactions with CH2OO/anti-CH3CHOO in the Oligomer Formation: A Theoretical Perspective. J. Phys. Chem. A 2017, 121, 6981–6991. [Google Scholar] [CrossRef]
- Ouyang, B.; McLeod, M.W.; Jones, R.L.; Bloss, W.J. NO3 Radical Production from the Reaction between the Criegee Intermediate CH2OO and NO2. Phys. Chem. Chem. Phys. 2013, 15, 17070–17075. [Google Scholar] [CrossRef]
- Vereecken, L.; Nguyen, H.M.T. Theoretical Study of the Reaction of Carbonyl Oxide with Nitrogen Dioxide: CH2OO + NO2. Int. J. Chem. Kinet. 2017, 49, 752–760. [Google Scholar] [CrossRef]
- Chhantyal-Pun, R.; Welz, O.; Savee, J.D.; Eskola, A.J.; Lee, E.P.F.; Blacker, L.; Hill, H.R.; Ashcroft, M.; Khan, M.A.H.; Lloyd-Jones, G.C.; et al. Direct Measurements of Unimolecular and Bimolecular Reaction Kinetics of the Criegee Intermediate (CH3)2COO. J. Phys. Chem. A 2017, 121, 4–15. [Google Scholar] [CrossRef]
- Jiang, L.; Xu, Y.S.; Ding, A.Z. Reaction of Stabilized Criegee Intermediates from Ozonolysis of Limonene with Sulfur Dioxide: Ab Initio and DFT Study. J. Phys. Chem. A 2010, 114, 12452–12461. [Google Scholar] [CrossRef]
- Kurtén, T.; Lane, J.R.; Jørgensen, S.; Kjaergaard, H.G. A Computational Study of the Oxidation of SO2 to SO3 by Gas-Phase Organic Oxidants. J. Phys. Chem. A 2011, 115, 8669–8681. [Google Scholar] [CrossRef] [PubMed]
- Berndt, T.; Jokinen, T.; Mauldin, R.L., III; Petäjä, T.; Herrmann, H.; Junninen, H.; Paasonen, P.; Worsnop, D.R.; Sipilä, M. Gas-Phase Ozonolysis of Selected Olefins: The Yield of Stabilized Criegee Intermediate and the Reactivity toward SO2. J. Phys. Chem. Lett. 2012, 3, 2892–2896. [Google Scholar] [CrossRef]
- Vereecken, L.; Harder, H.; Novelli, A. The Reaction of Criegee Intermediates with NO, RO2, and SO2, and Their Fate in the Atmosphere. Phys. Chem. Chem. Phys. 2012, 14, 14682–14695. [Google Scholar] [CrossRef] [PubMed]
- Kuwata, K.T.; Guinn, E.J.; Hermes, M.R.; Fernandez, J.A.; Mathison, J.M.; Huang, K. A Computational Re-examination of the Criegee Intermediate−Sulfur Dioxide Reaction. J. Phys. Chem. A 2015, 119, 10316–10335. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Q.; Liu, F.H.; Liu, S.Y.; Dai, D.X.; Dong, W.R.; Yang, X.M. A Kinetic Study of the CH2OO Criegee Intermediate Reaction with SO2, (H2O)2, CH2I2 and I Atoms using OH Laser Induced Fluorescence. Phys. Chem. Chem. Phys. 2017, 19, 20786–20794. [Google Scholar] [CrossRef]
- Taatjes, C.A.; Welz, O.; Eskola, A.J.; Savee, J.D.; Scheer, A.M.; Shallcross, D.E.; Rotavera, B.; Lee, E.P.F.; Dyke, J.M.; Mok, D.K.W.; et al. Direct Measurements of Conformer-Dependent Reactivity of the Criegee Intermediate CH3CHOO. Science 2013, 340, 177–180. [Google Scholar] [CrossRef]
- Berndt, T.; Jokinen, T.; Sipilä, M.; Mauldin, R.L., III; Herrmann, H.; Stratmann, F.; Junninen, H.; Kulmala, M. H2SO4 Formation from the Gas-Phase Reaction of Stabilized Criegee Intermediates with SO2: Influence of Water Vapour Content and Temperature. Atmos. Environ. 2014, 89, 603–612. [Google Scholar] [CrossRef]
- Lin, H.-Y.; Huang, Y.-H.; Wang, X.; Bowman, J.M.; Nishimura, Y.; Witek, H.A.; Lee, Y.-P. Infrared Identification of the Criegee Intermediates syn- and anti-CH3CHOO, and Their Distinct Conformation-Dependent Reactivity. Nat. Commun. 2015, 6, 7012–7018. [Google Scholar] [CrossRef]
- Boy, M.; Mogensen, D.; Smolander, S.; Zhou, L.; Nieminen, T.; Paasonen, P.; Plass-Dülmer, C.; Sipilä, M.; Petäjä, T.; Mauldin, L.; et al. Oxidation of SO2 by Stabilized Criegee Intermediate (sCI) Radicals as a Crucial Source for Atmospheric Sulfuric Acid Concentrations. Atmos. Chem. Phys. 2013, 13, 3865–3879. [Google Scholar] [CrossRef]
- Saheb, V.; Nazari, A. The Reaction of OH Radical with the Criegee Intermediate Propanone Oxide: Theoretical Investigations. Comput. Theor. Chem. 2020, 1175, 112726. [Google Scholar] [CrossRef]
- Cai, J.; Lu, Y.S.; Wang, W.N.; Chen, L.; Liu, F.Y.; Wang, W.L. Reaction Mechanism and Kinetics of Criegee Intermediate CH2OO with CH2=C(CH3)CHO. Comput. Theor. Chem. 2019, 1170, 112644. [Google Scholar] [CrossRef]
- Mauldin, R.L., III; Berndt, T.; Sipilä, M.; Paasonen, P.; Petäjä, T.; Kim, S.; Kurten, T.; Stratmann, F.; Kerminen, V.M.; Kulmala, M. A New Atmospherically Relevant Oxidant of Sulphur Dioxide. Nature 2012, 488, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Ehn, M.; Thornton, J.A.; Kleist, E.; Sipilä, M.; Junninen, J.; Pullinen, I.; Springer, M.; Rubach, F.; Tillmann, R.; Lee, B.; et al. A Large Source of Low-Volatility Secondary Organic Aerosol. Nature 2014, 506, 476–479. [Google Scholar] [CrossRef] [PubMed]
- Berresheim, H.; Adam, M.; Monahan, C.; O’Dowd, C.; Plane, J.M.C.; Bohn, B.; Rohrer, F. Missing SO2 Oxidant in the Coastal Atmosphere?–Observations from High-Resolution Measurements of OH and Atmospheric Sulfur Compounds. Atmos. Chem. Phys. 2014, 14, 12209–12223. [Google Scholar] [CrossRef]
- Sarwar, G.; Simon, H.; Fahey, K.; Mathur, R.; Goliff, W.; Stockwell, W. Impact of Sulfur Dioxide Oxidation by Stabilized Criegee Intermediate on Sulfate. Atmos. Environ. 2014, 85, 204–214. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision, D.01; Gaussian Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Becke, A.D. Density-Functional Exchange-Energy Approximation with Correct Asymptotic Behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron Density. Phys. Rev. B: Condens. Matter Mater. Phys. 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, C.; Schlegel, H.B. An Improved Algorithm for Reaction Path Following. J. Chem. Phys. 1989, 90, 2154–2161. [Google Scholar] [CrossRef]
- Gonzalez, C.; Schlegel, H.B. Reaction Path Following in Mass-Weighted Internal Coordinates. J. Phys. Chem. 1990, 94, 5523–5527. [Google Scholar] [CrossRef]
- Pople, J.A.; Head-Gordon, M.; Raghavachari, K. Quadratic Configuration Interaction. A General Technique for Determining Electron Correlation Energies. J. Chem. Phys. 1987, 87, 5968–5975. [Google Scholar] [CrossRef]
- Merrick, J.P.; Moran, D.; Radom, L. An Evaluation of Harmonic Vibrational Frequency Scale Factors. J. Phys. Chem. A 2007, 111, 11683–11700. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. A New Mixing of Hartree–Fock and Local Density-Functional Theories. J. Chem. Phys. 1993, 98, 1372–1377. [Google Scholar] [CrossRef]
- Raghunath, P.; Lee, Y.-P.; Lin, M.C. Computational Chemical Kinetics for the Reaction of Criegee Intermediate CH2OO with HNO3 and Its Catalytic Conversion to OH and HCO. J. Phys. Chem. A 2017, 121, 3871–3878. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.M.; Zheng, R.H.; Pan, Y.L.; Wu, Y.K.; Yang, F.; Hong, S. Ozone Dissociation to Oxygen Affected by Criegee Intermediate. J. Phys. Chem. A 2014, 118, 1644–1650. [Google Scholar] [CrossRef]
- Noodleman, L. Valence Bond Description of Antiferromagnetic Coupling in Transition Metal Dimers. J. Chem. Phys. 1981, 74, 5737–5743. [Google Scholar] [CrossRef]
- Karton, A.; Rabinovich, E.; Martin, J.M.L.; Ruscic, B. W4 Theory for Computational Thermochemistry: In Pursuit of Confident Sub-kJ/mol Predictions. J. Chem. Phys. 2006, 125, 144108–144125. [Google Scholar] [CrossRef]
- Karton, A. A Computational Chemist’s Guide to Accurate Thermochemistry for Organic Molecules. WIREs Comput. Mol. Sci. 2016, 6, 292–310. [Google Scholar] [CrossRef]
- Iuga, C.; Alvarez-Idaboy, J.R.; Reyes, L.; Vivier-Bunge, A. Can a Single Water Molecule Really Catalyze the Acetaldehyde + OH Reaction in Tropospheric Conditions? J. Phys. Chem. Lett. 2010, 1, 3112–3115. [Google Scholar] [CrossRef]
- Iuga, C.; Alvarez-Idaboy, J.R.; Vivier-Bunge, A. On the Possible Catalytic Role of a Single Water Molecule in the Acetone + OH Gas Phase Reaction: A Theoretical Pseudo-second-order Kinetics Study. Theor. Chem. Acc. 2011, 129, 209–217. [Google Scholar] [CrossRef]
- Asatryan, R.; da Silva, G.; Bozzelli, J.W. Quantum Chemical Study of the Acrolein (CH2CHCHO) + OH + O2 Reactions. J. Phys. Chem. A 2010, 114, 8302–8311. [Google Scholar] [CrossRef]
- da Silva, G. Reaction of Methacrolein with the Hydroxyl Radical in Air: Incorporation of Secondary O2 Addition into the MACR + OH Master Equation. J. Phys. Chem. A 2012, 116, 5317–5324. [Google Scholar] [CrossRef]
- Pilling, M.J.; Seakins, P.W. Reaction Kinetics; Oxford University Press Inc.: New York, NY, USA, 1999. [Google Scholar]
- Li, J.Y.; Tsona, N.T.; Du, L. The Role of (H2O)1-2 in the CH2O + ClO Gas-Phase Reaction. Molecules 2018, 23, 2240. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.S.; Gan, Q.; Yu, Q.; Zhang, X.M.; Li, R.; Qian, S.C.; Feng, C.G. Initial Mechanisms for the Unimolecular Thermal Decomposition of 2,6-Diamino-3,5-dinitropyrazine-1-oxide. Molecules 2019, 24, 125. [Google Scholar] [CrossRef] [PubMed]
- Wigner, E. Calculation of the Rate of Elementary Association Reactions. J. Chem. Phys. 1937, 5, 720–725. [Google Scholar] [CrossRef]
- Georgievskii, Y.; Klippenstein, S.J. Long-Range Transition State Theory. J. Chem. Phys. 2005, 122, 194103–194117. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).