Curcumin Containing PEGylated Solid Lipid Nanoparticles for Systemic Administration: A Preliminary Study
Abstract
1. Introduction
2. Results and Discussion
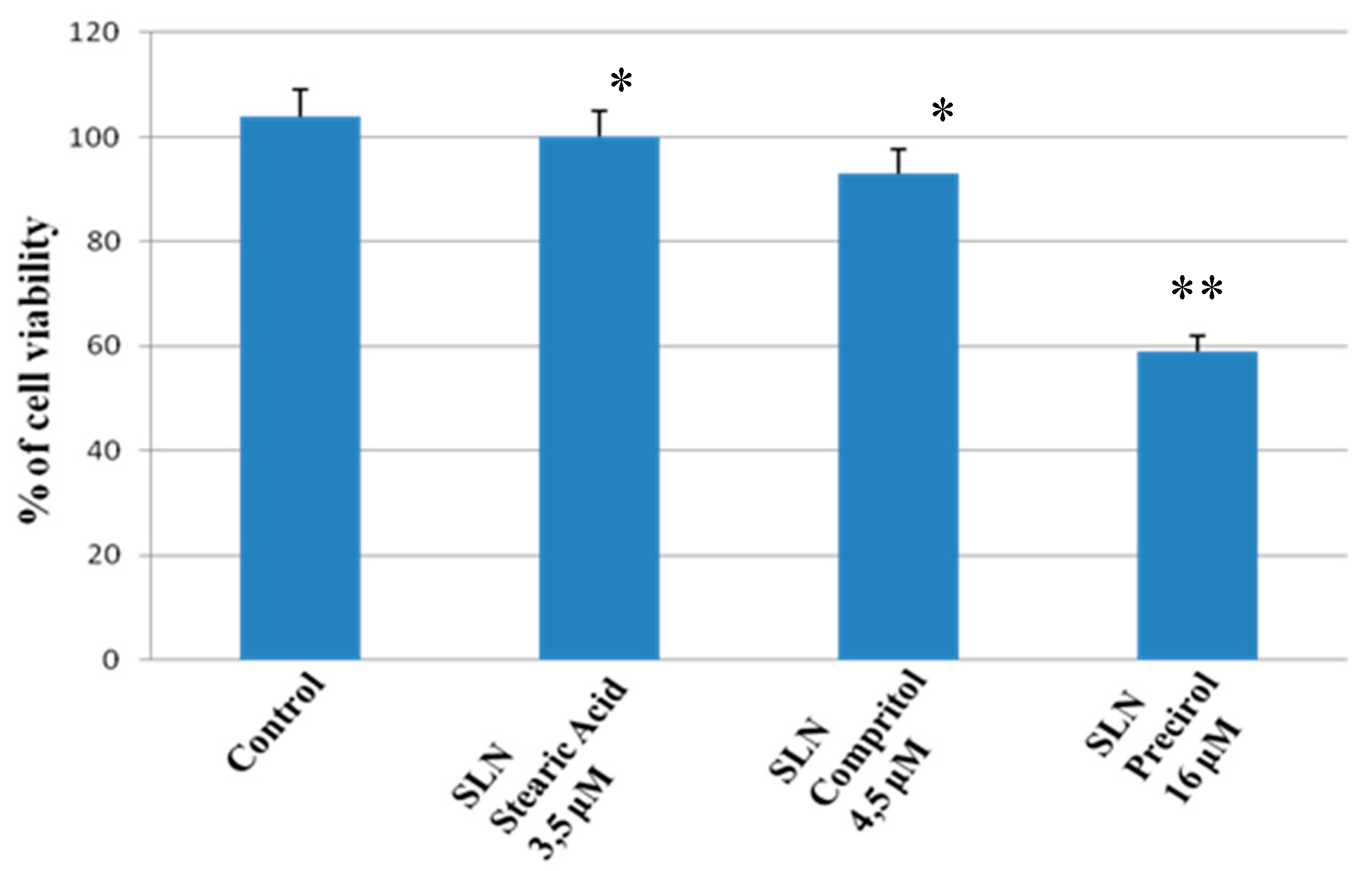
2.1. In Vitro Study
2.2. SLNs Characterization
2.3. Stability Studies
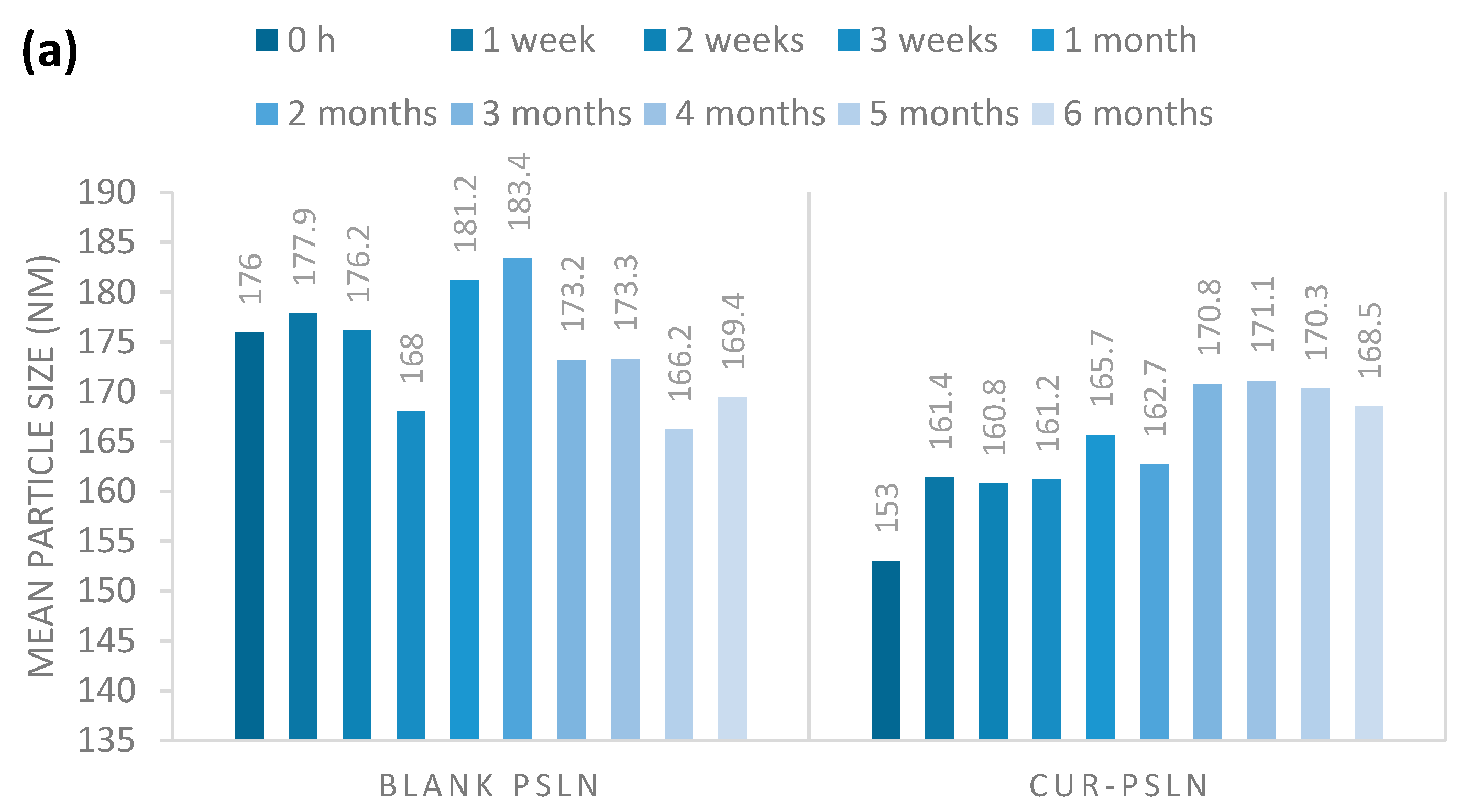
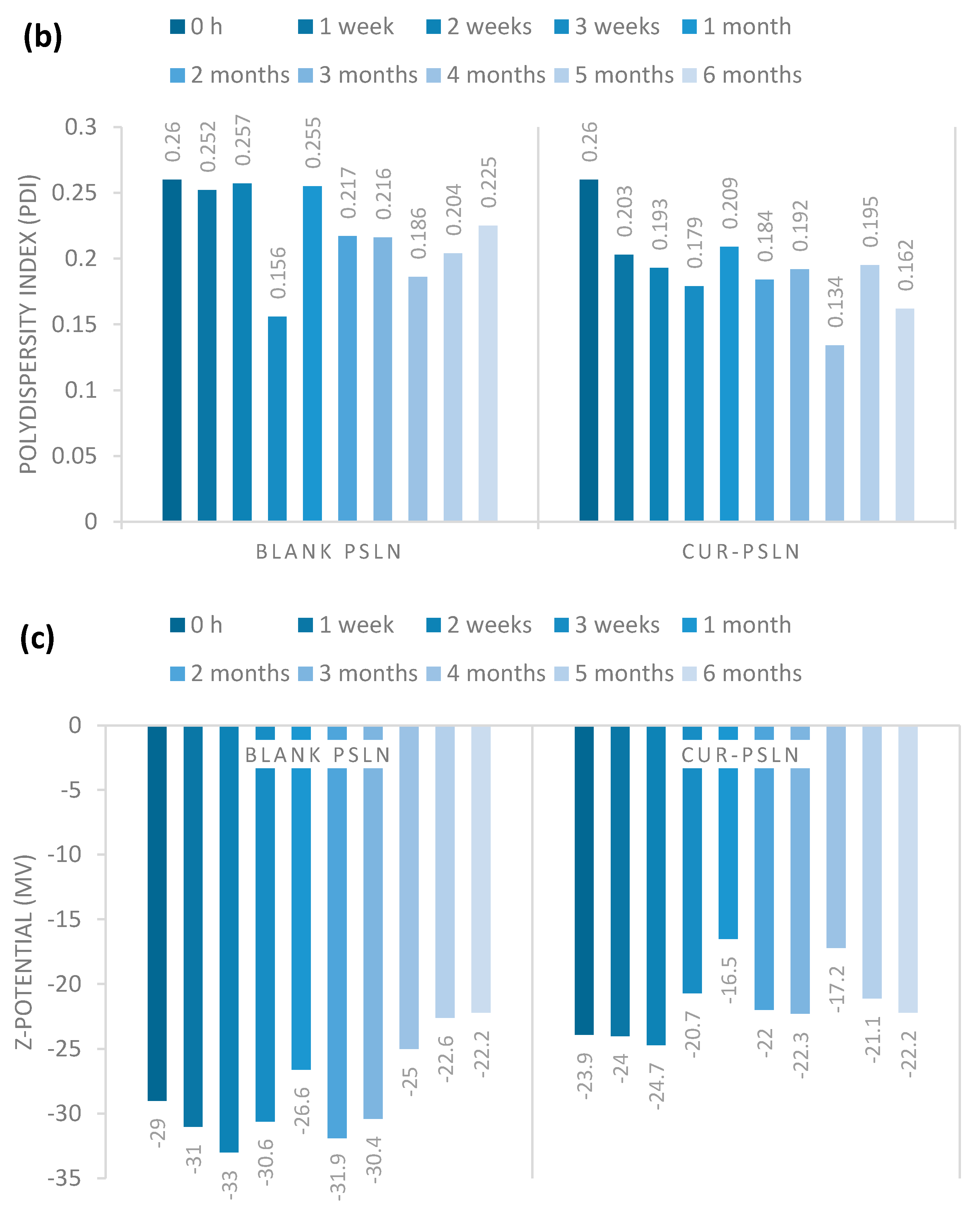
2.3.1. Long-Term Stability
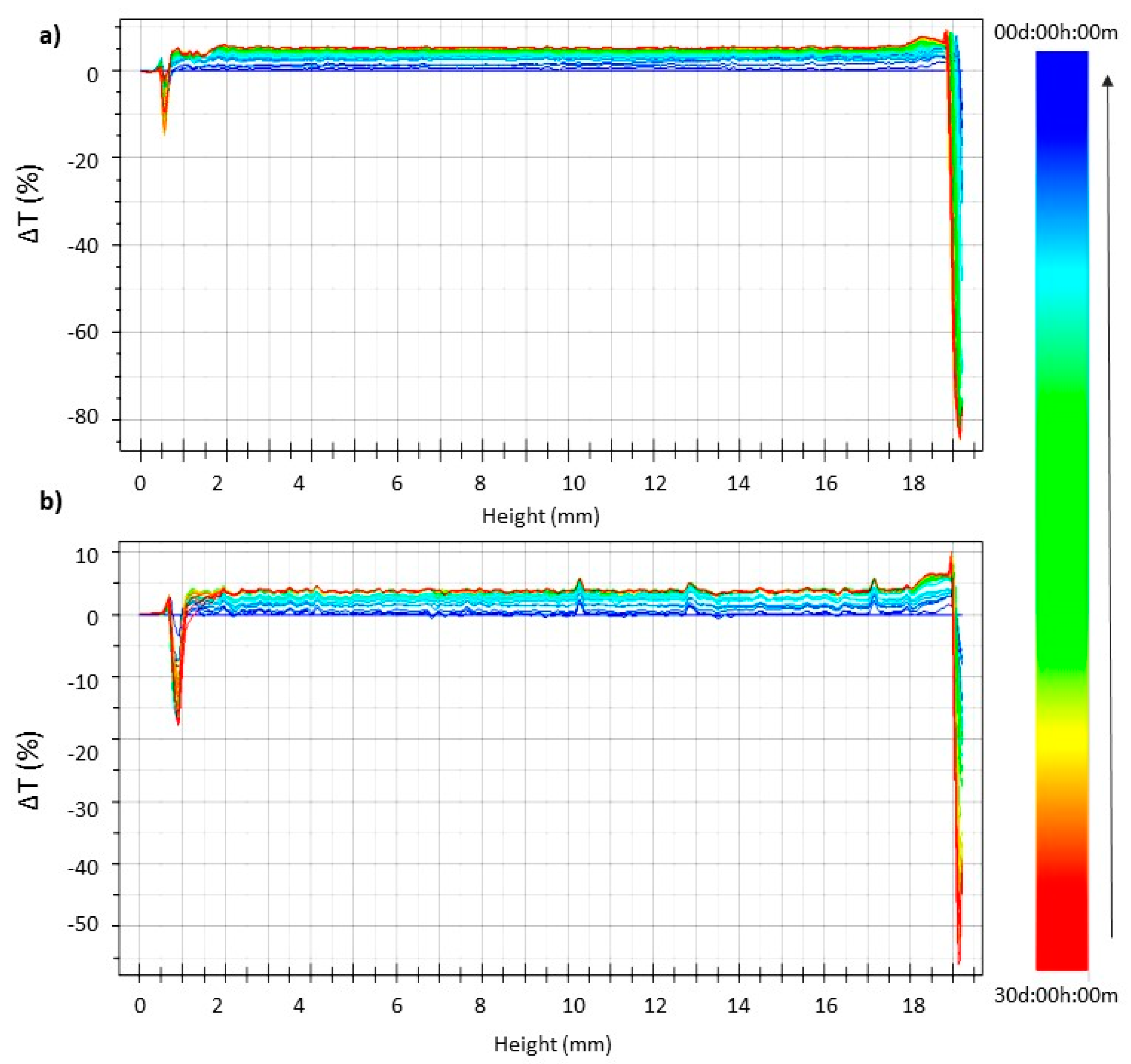
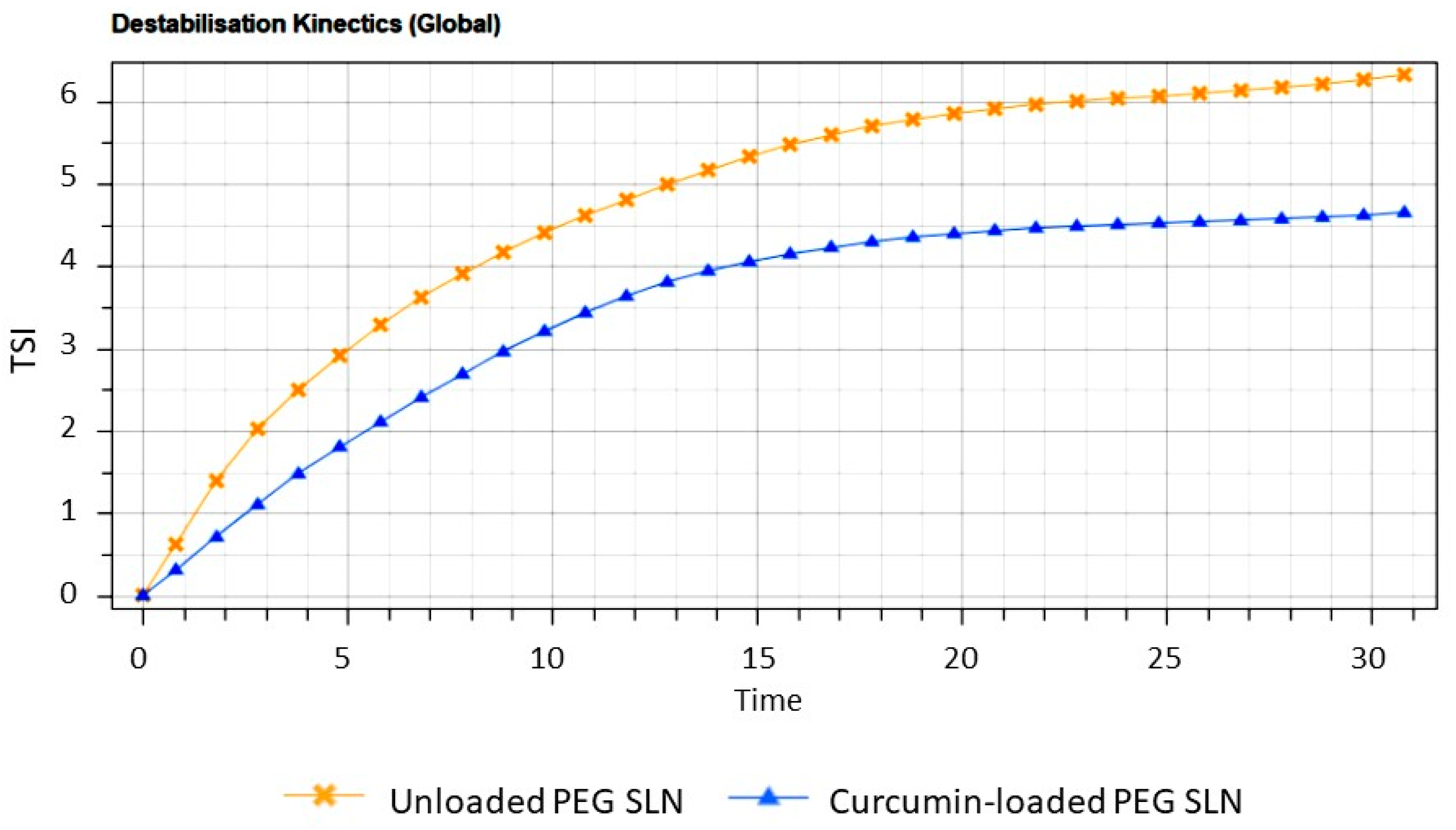
2.3.2. Turbiscan Stability Studies
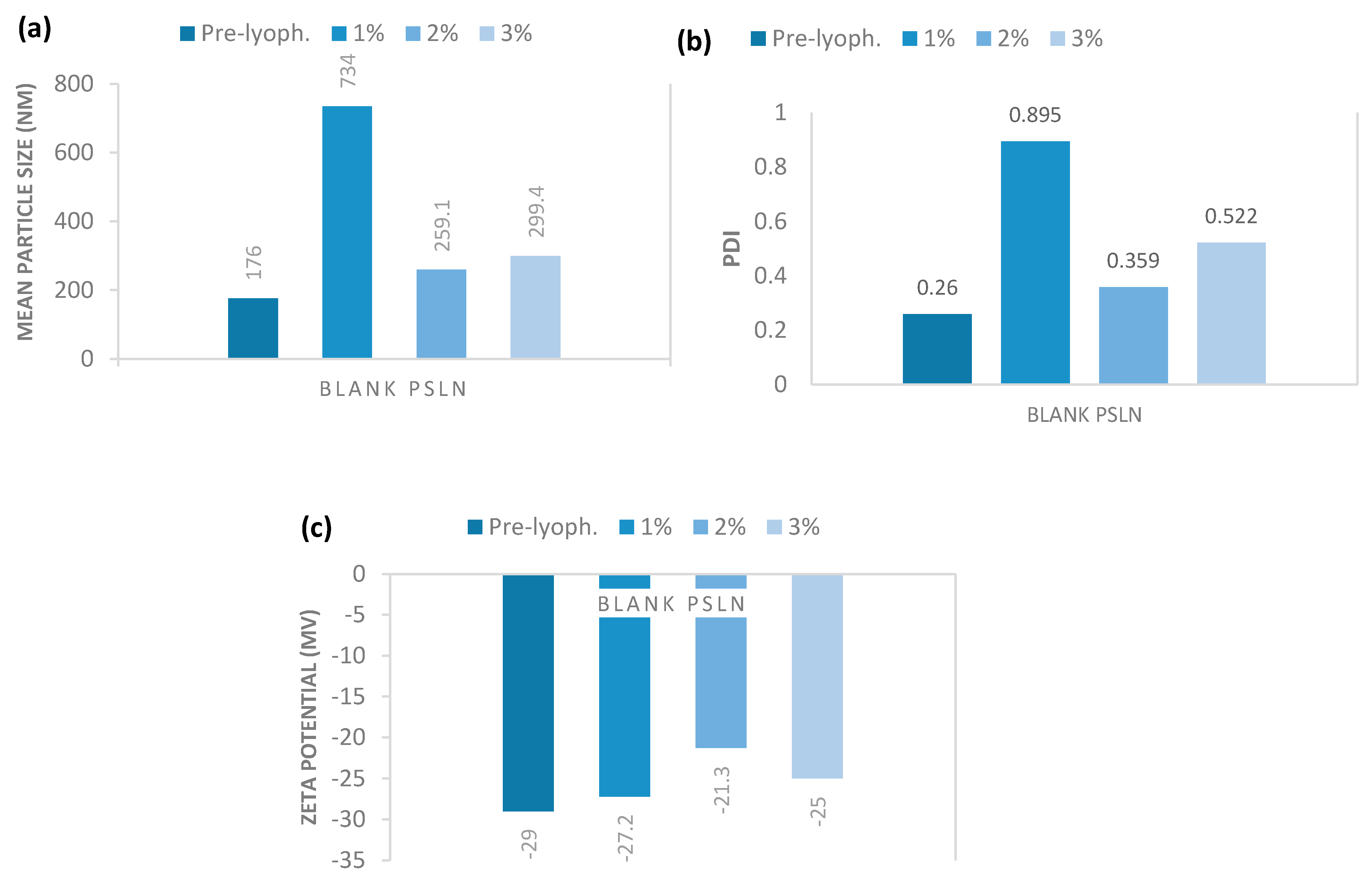
2.3.3. Lyophilization Effect
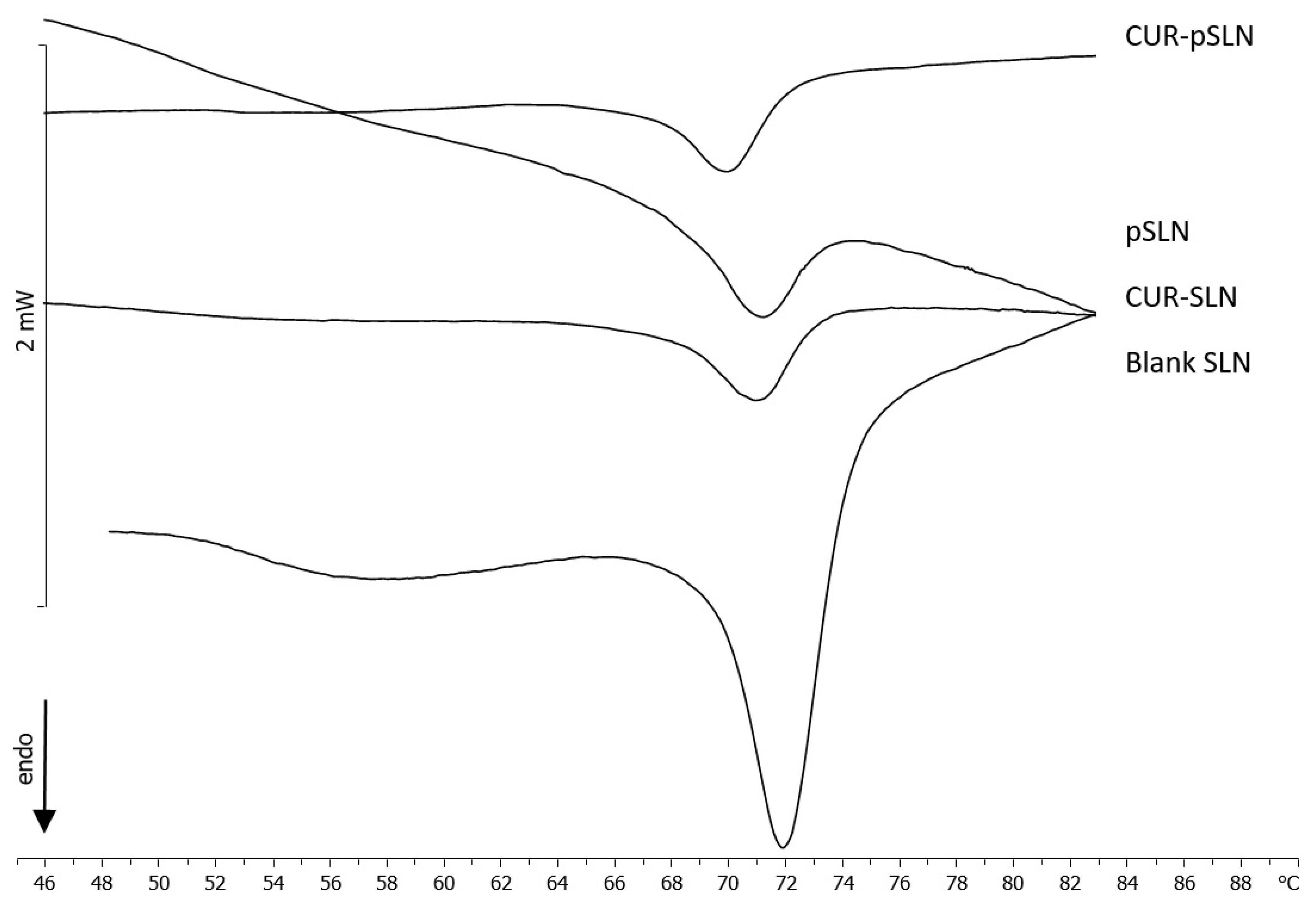
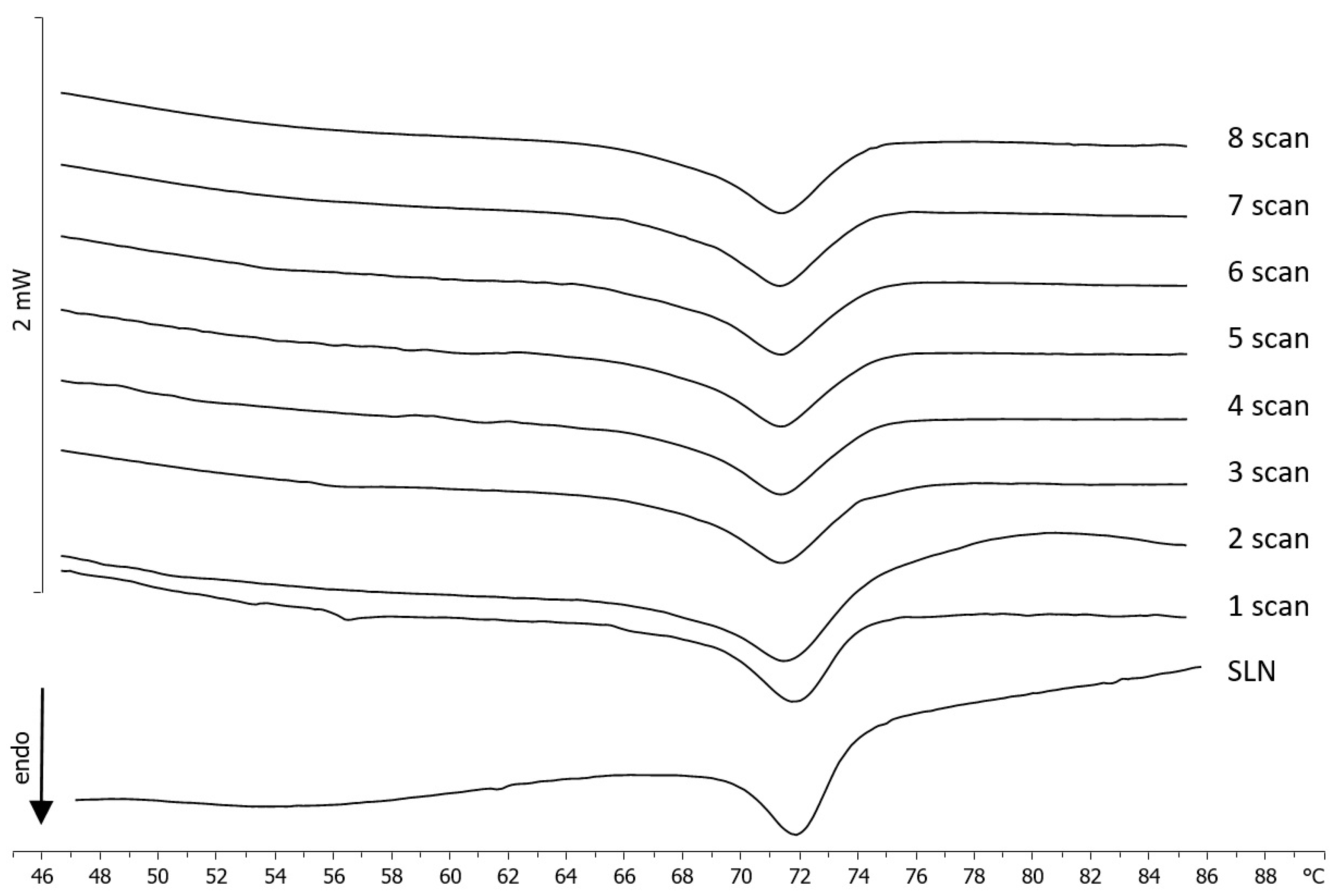
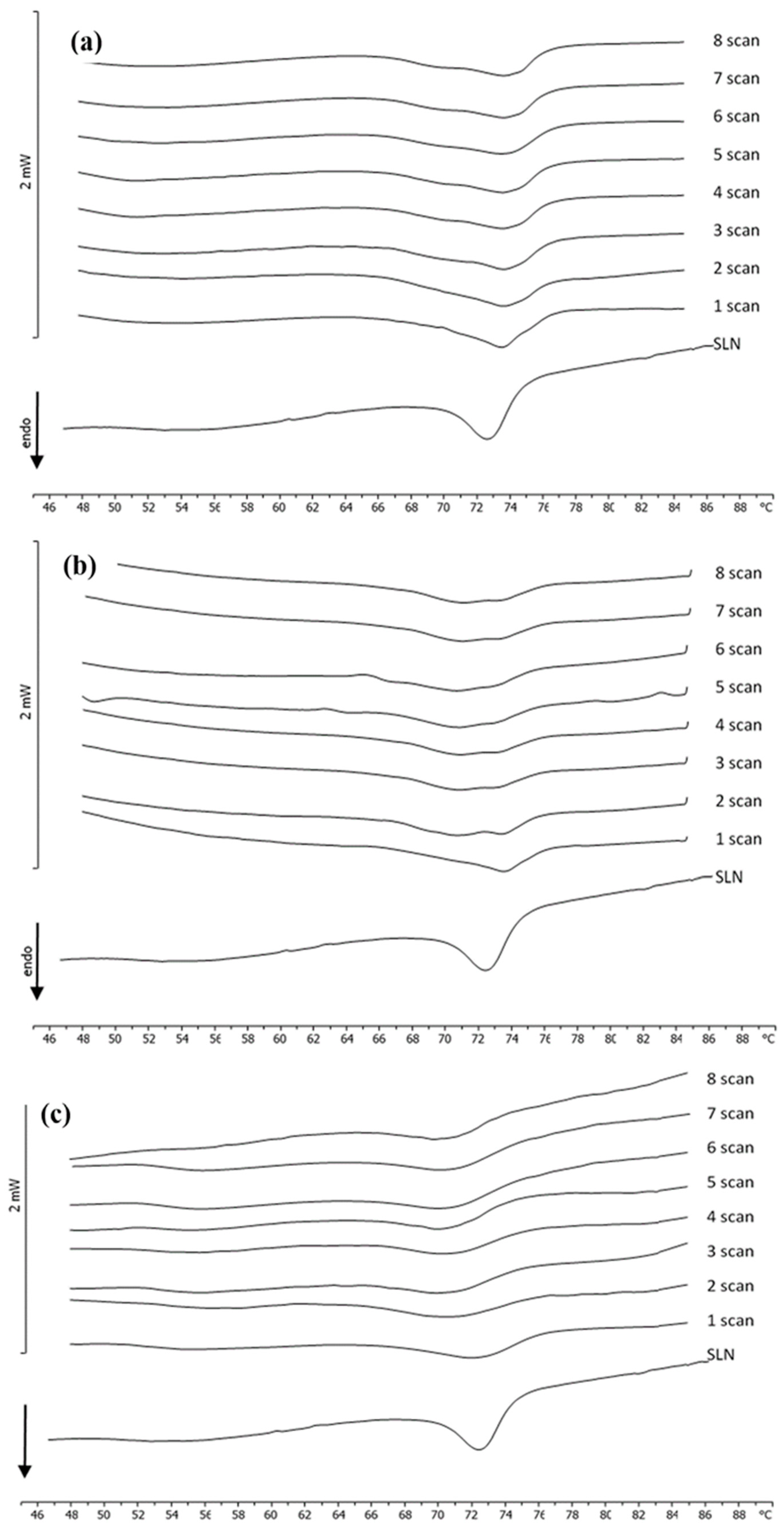
2.4. Differential Scanning Calorimetry (DSC)
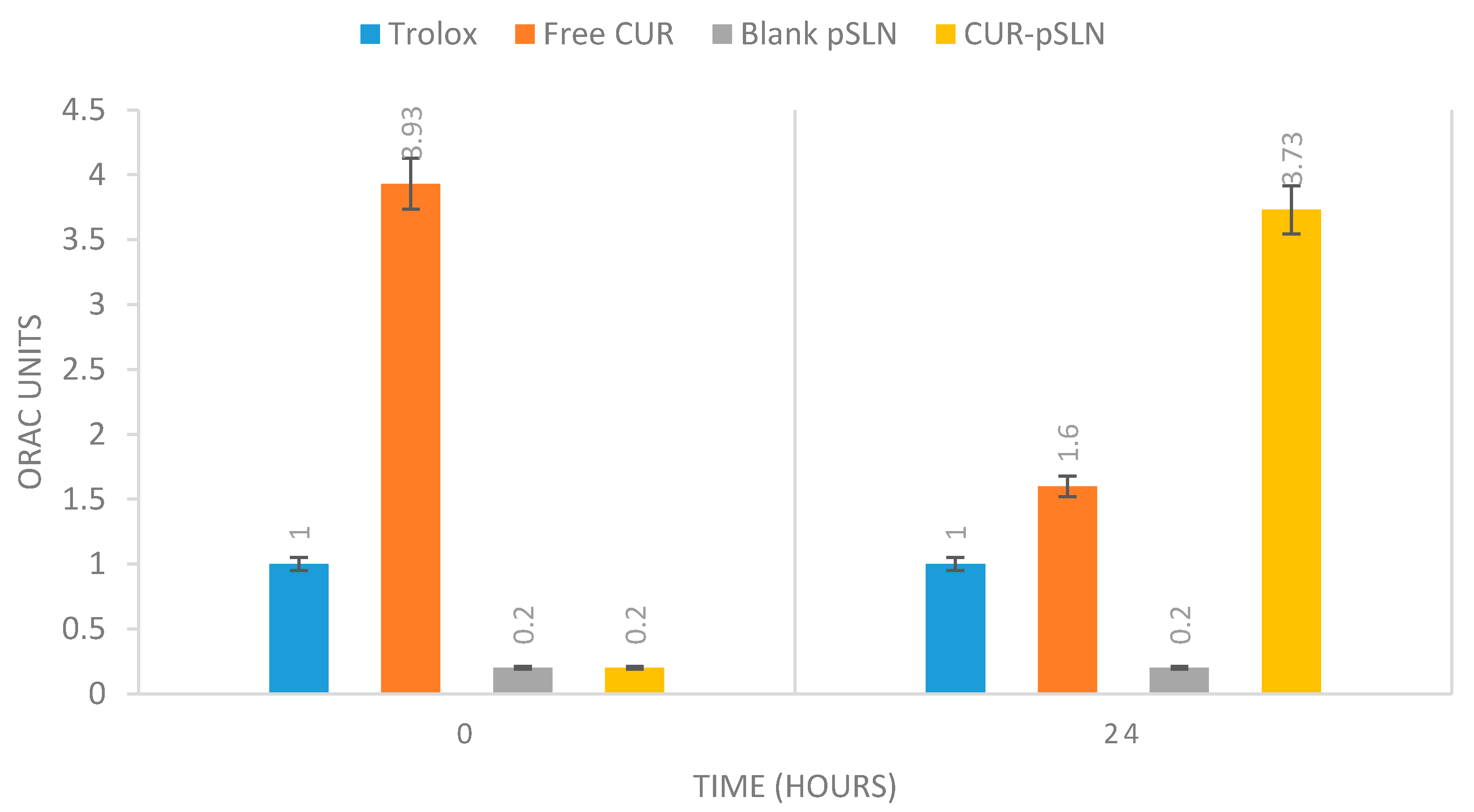
2.5. In Vitro Antioxidant Activity
3. Materials and Methods
3.1. Materials
3.2. Cell Culture
3.2.1. Primary DPSC Cell Culture
3.2.2. Treatment of Cell Cultures
3.2.3. MTT Assay
3.2.4. Statistical Analysis
3.3. SLNs Preparation
3.4. SLNs Characterization
3.5. Determination of Drug Loading
3.6. Stability Tests
3.6.1. Long-Term Stability
3.6.2. Samples Stability by Turbiscan® Aging Station (TAGS)
3.6.3. Freeze Drying of SLNs
3.7. PEG Micelles Preparation
3.8. Differential Scanning Calorimetry (DSC)
3.9. Oxygen Radical Absorbance Capacity (ORAC) Assay
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Aggarwal, B.B.; Harikumar, K.B. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int. J. Biochem. Cell Biol. 2009, 41, 40–59. [Google Scholar] [CrossRef] [PubMed]
- Rakotoarisoa, M.; Angelov, B.; Garamus, V.M.; Angelova, A. Curcumin- and Fish Oil-Loaded Spongosome and Cubosome Nanoparticles with Neuroprotective Potential against H2O2-Induced Oxidative Stress in Differentiated Human SH-SY5Y Cells. ACS Omega 2019, 4, 3061–3073. [Google Scholar] [CrossRef]
- Rakotoarisoa, M.; Angelov, B.; Espinoza, S.; Khakurel, K.; Bizien, T.; Angelova, A. Cubic Liquid Crystalline Nanostructures Involving Catalase and Curcumin: BioSAXS Study and Catalase Peroxidatic Function after Cubosomal Nanoparticle Treatment of Differentiated SH-SY5Y Cells. Molecules 2019, 24, 3058. [Google Scholar] [CrossRef] [PubMed]
- Rakotoarisoa, M.; Angelova, A. Amphiphilic Nanocarrier Systems for Curcumin Delivery in Neurodegenerative Disorders. Medicines 2018, 5, 126. [Google Scholar] [CrossRef]
- Guerzoni, L.P.B.; Nicolas, V.; Angelova, A. In Vitro Modulation of TrkB Receptor Signaling upon Sequential Delivery of Curcumin-DHA Loaded Carriers Towards Promoting Neuronal Survival. Pharm. Res. 2017, 34, 492–505. [Google Scholar] [CrossRef]
- Chattopadhyay, I.; Biswas, K.; Bandyopadhyay, U.; Banerjee, R.K. Turmeric and curcumin. Biological actions and medicinal applications. Curr. Sci. 2004, 87, 44–50. [Google Scholar]
- Panico, A.; Maccari, R.; Cardile, V.; Avondo, S.; Crascì, L.; Ottanà, R. Evaluation of the anti-inflammatory/chondroprotective activity of aldose reductase inhibitors in human chondrocyte cultures. Med. Chem. Commun. 2015, 6, 823–830. [Google Scholar] [CrossRef]
- Crascì, L.; Cardile, V.; Longhitano, G.; Nanfitò, F.; Panico, A. Anti-degenerative effect of Apigenin, Luteolin and Quercetin on human keratinocyte and chondrocyte cultures: SAR evaluation. Drug Res. 2018, 68, 132–138. [Google Scholar] [CrossRef]
- Ninfali, P.; Mea, G.; Giorgini, S.; Rocchi, M.; Bacchiocca, M. 2005. Antioxidant capacity of vegetables, spices and dressings relevant to nutrition. Br. J. Nutr. 2005, 93, 257–266. [Google Scholar] [CrossRef]
- Maheshwari, R.K.; Singh, A.K.; Gaddipati, J.; Srimal, R.C. Multiple biological activities of curcumin: A short review. Life Sci. 2006, 78, 2081–2087. [Google Scholar] [CrossRef]
- Lee, H.Y.; Kim, S.W.; Lee, G.H.; Choi, M.K.; Chung, H.W.; Lee, Y.C.; Kim, H.R.; Kwon, H.J.; Chae, H.J. Curcumin and Curcuma longa L. extract ameliorate lipid accumulation through the regulation of the endoplasmic reticulum redox and ER stress. Sci. Rep. 2017, 7, 6513. [Google Scholar] [CrossRef] [PubMed]
- Panaro, M.A.; Corrado, A.; Benameur, T.; Paolo, C.F.; Cici, D.; Porro, C. The Emerging Role of Curcumin in the Modulation of TLR-4 Signaling Pathway: Focus on Neuroprotective and Anti-Rheumatic Properties. Int. J. Mol. Sci. 2020, 21, 2299. [Google Scholar] [CrossRef] [PubMed]
- Esposito, E.; Ravani, L.; Mariani, P.; Contado, C.; Drechsler, M.; Puglia, C.; Cortesi, R. Curcumin containing monoolein aqueous dispersions: A preformulative study. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 4923–4934. [Google Scholar] [CrossRef] [PubMed]
- Mandal, M.; Jaiswal, P.; Mishra, A. Role of curcumin and its nanoformulations in neurotherapeutics: A comprehensive review. J. Biochem. Mol. Toxicol. 2020, 34, e22478. [Google Scholar] [CrossRef]
- Prasad, S.; Tyagi, A.K. Curcumin and its analogues: A potential natural compound against HIV infection and AIDS. Food Funct. 2015, 6, 3412–3419. [Google Scholar] [CrossRef]
- Hatcher, H.; Planalp, R.; Cho, J.; Torti, F.M.; Torti, S.V. Curcumin: From ancient medicine to current clinical trials. Cell Mol. Life Sci. 2008, 65, 1631–1652. [Google Scholar] [CrossRef]
- Jiang, T.; Liao, W.; Charcosset, C. Recent advances in encapsulation of curcumin in nanoemulsions: A review of encapsulation technologies, bioaccessibility and applications. Food Res. Int. 2020, 132, 109035. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Farokhzad, O.C.; Langer, R. Impact of nanotechnology on drug delivery. ACS Nano 2009, 3, 16–20. [Google Scholar] [CrossRef]
- Muller, R.H.; Mehnert, W.; Lucks, J.S.; Schwarz, C.; Zur, M.A. Solid Lipid nanoparticles (SLNs)—An alternative collidal carrier system for controlled drug delivery. Eur. J. Pharm. Biopharm. 1995, 41, 62–69. [Google Scholar]
- Wissing, S.A.; Kayser, O.; Muller, R.H. Solid Lipid Nanoparticles for Parenteral Drug Delivery. Adv. Drug Deliv. Rev. 2004, 56, 1257–1272. [Google Scholar] [CrossRef] [PubMed]
- Puglia, C.; Offerta, A.; Tirendi, G.G.; Tarico, M.S.; Curreri, S.; Bonina, F.; Perrotta, R.E. Design of solid lipid nanoparticles for caffeine topical administration. Drug Deliv. 2016, 23, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Frank, M.M.; Fries, L.F. The role of complement in inflammation and phagocytosis. Frank MM1, Fries LF. Immunol. Today 1991, 12, 322–326. [Google Scholar] [CrossRef]
- Johnson, R.J. The complement system. In Biomaterials Science: An Introduction to Materials in Medicine, 2nd ed.; Ratner, B.D., Hoffman, A.S., Schoen, F.J., Lemons, J.E., Eds.; Elsevier Academic Press: Amsterdam, The Netherlands, 2004; pp. 318–328. [Google Scholar]
- Illum, L.; Hunneyball, I.M.; Davis, S.S. The effect of hydrophilic coating on the uptake of the colloidal particles by the liver and by peritoneal-macrophages. Int. J. Pharm. 1986, 29, 53–65. [Google Scholar] [CrossRef]
- Gref, R.; Domb, A.; Quellec, P.; Blunk, T.; Muller, R.H.; Verbavatz, J.M.; Langer, R. The controller intravenous delivery of drugs using PEG-coated sterically stabilized nanospheres. Adv. Drug Deliv. Rev. 1995, 16, 215–233. [Google Scholar] [CrossRef]
- Panagi, Z.; Beletsi, A.; Evangelatos, G.; Livaniou, E.; Ithakissios, D.S.; Avgoustakis, K. Effect of dose on the biodistribution and pharmacokinetics of PLGA and PLGA-mPEG nanoparticles. Int. J. Pharm. 2001, 221, 143–152. [Google Scholar] [CrossRef]
- Arranja, A.; Gouveia, L.F.; Gener, P.; Rafael, D.F.; Pereira, C.; Schwartz, S.J.; Videira, M.A. Self-assembly PEGylation assists SLNs-paclitaxel delivery inducing cancer cell apoptosis upon internalization. Int. J. Pharm. 2016, 501, 180–189. [Google Scholar] [CrossRef]
- Betancourt, T.; Byrne, J.D.; Sunaryo, N.; Crowder, S.W.; Kadapakkam, M.; Patel, S.; Casciato, S.; Brannon-Peppas, L. PEGylation strategies for active targeting of PLA/PLGA nanoparticles. J. Biomed. Mater. Res. A 2009, 91, 263–276. [Google Scholar] [CrossRef]
- Kommareddy, S.; Amiji, M. Biodistribution and pharmacokinetic analysis of long-circulating thiolated gelatin nanoparticles following systemic administration in breast cancer-bearing mice. J. Pharm. Sci. 2007, 96, 397–407. [Google Scholar] [CrossRef]
- Gref, R.; Minamitake, Y.; Peracchia, M.T.; Trubetskoy, V.; Torchilin, V.; Langer, R. Biodegradable long circulating polymeric nanospheres. Science 1994, 263, 1600–1603. [Google Scholar] [CrossRef]
- Hu, X.; Kang, X.; Ying, X.; Wang, L.; Du, Y. Enhanced oral absorption of saquinavir mediated by PEGylated solid lipid nanoparticles. RSC Adv. 2015, 5, 40341. [Google Scholar] [CrossRef]
- Kouchakzadeh, H.; Shojaosadati, S.; Maghsoudi, A.; Vasheghani Farahani, E. Optimization of PEGylation Conditions for BSA Nanoparticles Using Response Surface Methodology. AAPS PharmSciTech 2010, 11, 1206–1211. [Google Scholar] [CrossRef] [PubMed]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef]
- Bonaventura, G.; Incontro, S.; Iemmolo, R.; La Cognata, V.; Barbagallo, I.; Costanzo, E.; Barcellona, M.L.; Pellitteri, R.; Cavallaro, S. Dental mesenchymal stem cells and neuro-regeneration: A focus on spinal cord injury. Cell Tissue Res. 2019, 67, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Ueda, T.; Inden, M.; Ito, T.; Kurita, H.; Hozumi, I. Characteristics and Therapeutic Potential of Dental Pulp Stem Cells on Neurodegenerative Diseases. Front. Neurosci. 2020, 14, 407. [Google Scholar] [CrossRef] [PubMed]
- Freitas, C.; Lucks, J.S.; Muller, R.H. Effect of storage condition on long-term stability of solid lipid nanoparticles (SLNs) in aqueous dispersion. In Proceedings of the 1st World Meeting on Pharmaceutics, Biopharmaceutics, Pharmaceutical Technology, APGI/APV, Budapest, Hungary, 9–11 May 1995; pp. 493–494. [Google Scholar]
- Freitas, C.; Muller, R.H. Correlation between long-term stability of solid lipid nanoparticles (SLNs) and crystallinity of lipid phase. Eur. J. Pharm. Biopharm. 1999, 47, 125–132. [Google Scholar] [CrossRef]
- Coffin, M.D.; McGinity, J.W. Biodegradable pseudolatexes: The chemical stability of poly (D, L-lactide) and poly(epsilon-caprolactone) nanoparticles in aqueous media. Pharm. Res. 1992, 9, 200–205. [Google Scholar] [CrossRef]
- Abdelwahed, W.; Degobert, G.; Stainmesse, S.; Fessi, H. Freeze-drying of nanoparticles: Formulation, process and storage consideration. Adv. Drug Deliv. Rev. 2006, 58, 1688–1713. [Google Scholar] [CrossRef]
- Schwarz, C.; Mehnert, W. Freeze-drying of drug-free and drug-loaded solid lipid nanoparticles (SLNs). Int. J. Pharm. 1997, 157, 171–179. [Google Scholar] [CrossRef]
- Mehnert, W.; Mader, K. Solid lipid nanoparticles: Production, characterization and applications. Adv. Drug Deliv. Rev. 2001, 47, 165–196. [Google Scholar] [CrossRef]
- Abdelwahed, W. Lyophilization of solid lipid nanoparticles for brain targeting. Int. J. Pharm. Pharm. Sci. 2015, 7, 381–385. [Google Scholar]
- Awika, J.M.; Rooney, L.W.; Wu, X.; Prior, R.L.; Cisneros-Zevallos, L. Screening methods to measure antioxidant activity of sorghum (sorghum bicolor) and sorghum products. J. Agric. Food Chem. 2003, 51, 6657–6662. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Puglia, C.; Santonocito, D.; Musumeci, T.; Cardile, V.; Graziano, A.C.E.; Salerno, L.; Raciti, G.; Crascì, L.; Panico, A.M.; Puglisi, G. Nanotechnological Approach to Increase the Antioxidant and Cytotoxic Efficacy of Crocin and Crocetin. Planta Med. 2019, 85, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.F.; Hou, N.; Tian, J.; Yuan, M.; Liu, S.M.; Xiong, L.G.; Luo, J.D.; Chen, M.S. Metabolic profile of puerarin in rats after intragastric administration of puerarin solid lipid nanoparticles. Int. J. Nanomed. 2013, 8, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Shuhendler, A.J.; Prasad, P.; Leung, M.; Rauth, A.M.; Dacosta, R.S.; Wu, X.Y. A novel solid lipid nanoparticle formulation for active targeting to tumor α(v) β(3) integrin receptors reveals cyclic RGD as a double-edged sword. Adv. Healthc. Mater. 2012, 1, 600–608. [Google Scholar] [CrossRef]
- Carbone, C.; Campisi, A.; Manno, D.; Serra, A.; Spatuzza, M.; Musumeci, T.; Bonfanti, R.; Puglisi, G. The critical role of didodecyldimethylammonium bromide on physico-chemical, technological and biological properties of NLC. Colloids Surf. B Biointerfaces 2014, 121, 1–10. [Google Scholar] [CrossRef]
- Carbone, C.; Musumeci, T.; Lauro, M.R.; Puglisi, G. Eco-friendly aqueous core surface-modified nanocapsules. Colloids Surf. B Biointerfaces 2015, 125, 190–196. [Google Scholar] [CrossRef]
- Caddeo, C.; Pons, R.; Carbone, C.; Fernandez-Busquets, X.; Cardia, M.C.; Maccioni, A.M.; Fadda, A.M.; Manconi, M. Physico-chemical characterization of succinyl chitosan-stabilized liposomes for the oral co-delivery of quercetin and resveratrol. Carbohydr. Polym. 2017, 157, 1853–1861. [Google Scholar] [CrossRef]
- Puglia, C.; Santonocito, D.; Ostacolo, C.; Maria Sommella, E.; Campiglia, P.; Carbone, C.; Drago, F.; Pignatello, R.; Bucolo, C. Ocular formulation based on palmitoylethanolamide-loaded nanostructured lipid carriers: Technological and pharmacological profile. Nanomaterials 2020, 10, 287. [Google Scholar] [CrossRef]
- Carbone, C.; Fuochi, V.; Zielińska, A.; Musumeci, T.; Souto, E.B.; Bonaccorso, A.; Puglia, C.; Petronio Petronio, G.; Furneri, P.M. Dual-drugs delivery in solid lipid nanoparticles for the treatment of Candida albicans mycosis. Colloids Surf. B Biointerfaces 2020, 186, 110705. [Google Scholar] [CrossRef] [PubMed]
- Carbone, C.; Caddeo, C.; Grimaudo, M.A.; Manno, D.E.; Serra, A.; Musumeci, T. Ferulic Acid-NLC with Lavandula Essential Oil: A Possible Strategy for Wound-Healing? Nanomaterials 2020, 10, 898. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kho, K.; Cheow, W.S.; Hadinoto, K. A comparison between spray drying and spray freeze drying for dry powder inhaler formulation of drug-loaded lipid-polymer hybrid nanoparticles. Int. J. Pharm. 2012, 424, 98–106. [Google Scholar] [CrossRef]
- Kastantin, M.; Ananthanarayanan, B.; Karmali, P.; Ruoslahti, E.; Tirrell, M. Effect of the Lipid Chain Melting Transition on the Stability of DSPE-PEG (2000) Micelles. Langmuir 2009, 25, 7279–7286. [Google Scholar] [CrossRef]
- Paolino, D.; Accolla, M.L.; Cilurzo, F.; Cristiano, M.C.; Cosco, D.; Castelli, F.; Sarpietro, M.G.; Fresta, M.; Celia, C. Interaction between PEG lipid and DSPE/DSPC phospholipids: An insight of PEGylation degree and kinetics of de-PEGylation. Colloids Surf. B Biointerfaces 2017, 155, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Sarpietro, M.G.; Accolla, M.L.; Celia, C.; Grattoni, A.; Castelli, F.; Fresta, M.; Ferrari, M.; Paolino, D. Differential scanning calorimetry as a tool to investigate the transfer of anticancer drugs to biomembrane model. Curr. Drug Targets 2013, 14, 1053–1060. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Alessio, H.M.; Cutler, R.G. Oxygen-radical absorbance capacity assay for antioxidants. Free Radic. Biol. Med. 1993, 14, 303–311. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef]
Sample Availability: Samples blank and CUR-pSLNs are available from the authors. |

| Formulation | Z-Ave [nm ± SD] | PDI [–] ± SD | ZP [mV ± SD] |
|---|---|---|---|
| Blank SLNs | 161.8 ± 0.1 | 0.24 ± 0.1 | −31.4 ± 0.3 |
| Blank pSLNs | 176.0 ± 0.2 | 0.26 ± 0.1 | −29.0 ± 0.1 |
| CUR-SLNs | 132.0 ± 0.1 | 0.25 ± 0.0 | −24.3 ± 0.2 |
| CUR-pSLNs | 153.3 ± 0.2 | 0.26 ± 0.2 | −23.9 ± 0.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santonocito, D.; Sarpietro, M.G.; Carbone, C.; Panico, A.; Campisi, A.; Siciliano, E.A.; Sposito, G.; Castelli, F.; Puglia, C. Curcumin Containing PEGylated Solid Lipid Nanoparticles for Systemic Administration: A Preliminary Study. Molecules 2020, 25, 2991. https://doi.org/10.3390/molecules25132991
Santonocito D, Sarpietro MG, Carbone C, Panico A, Campisi A, Siciliano EA, Sposito G, Castelli F, Puglia C. Curcumin Containing PEGylated Solid Lipid Nanoparticles for Systemic Administration: A Preliminary Study. Molecules. 2020; 25(13):2991. https://doi.org/10.3390/molecules25132991
Chicago/Turabian StyleSantonocito, Debora, Maria Grazia Sarpietro, Claudia Carbone, Annamaria Panico, Agata Campisi, Edy Angela Siciliano, Giovanni Sposito, Francesco Castelli, and Carmelo Puglia. 2020. "Curcumin Containing PEGylated Solid Lipid Nanoparticles for Systemic Administration: A Preliminary Study" Molecules 25, no. 13: 2991. https://doi.org/10.3390/molecules25132991
APA StyleSantonocito, D., Sarpietro, M. G., Carbone, C., Panico, A., Campisi, A., Siciliano, E. A., Sposito, G., Castelli, F., & Puglia, C. (2020). Curcumin Containing PEGylated Solid Lipid Nanoparticles for Systemic Administration: A Preliminary Study. Molecules, 25(13), 2991. https://doi.org/10.3390/molecules25132991