Biological Activity of Porcine Gastric Mucin on Stress Resistance and Immunomodulation
Abstract
1. Introduction
2. Results
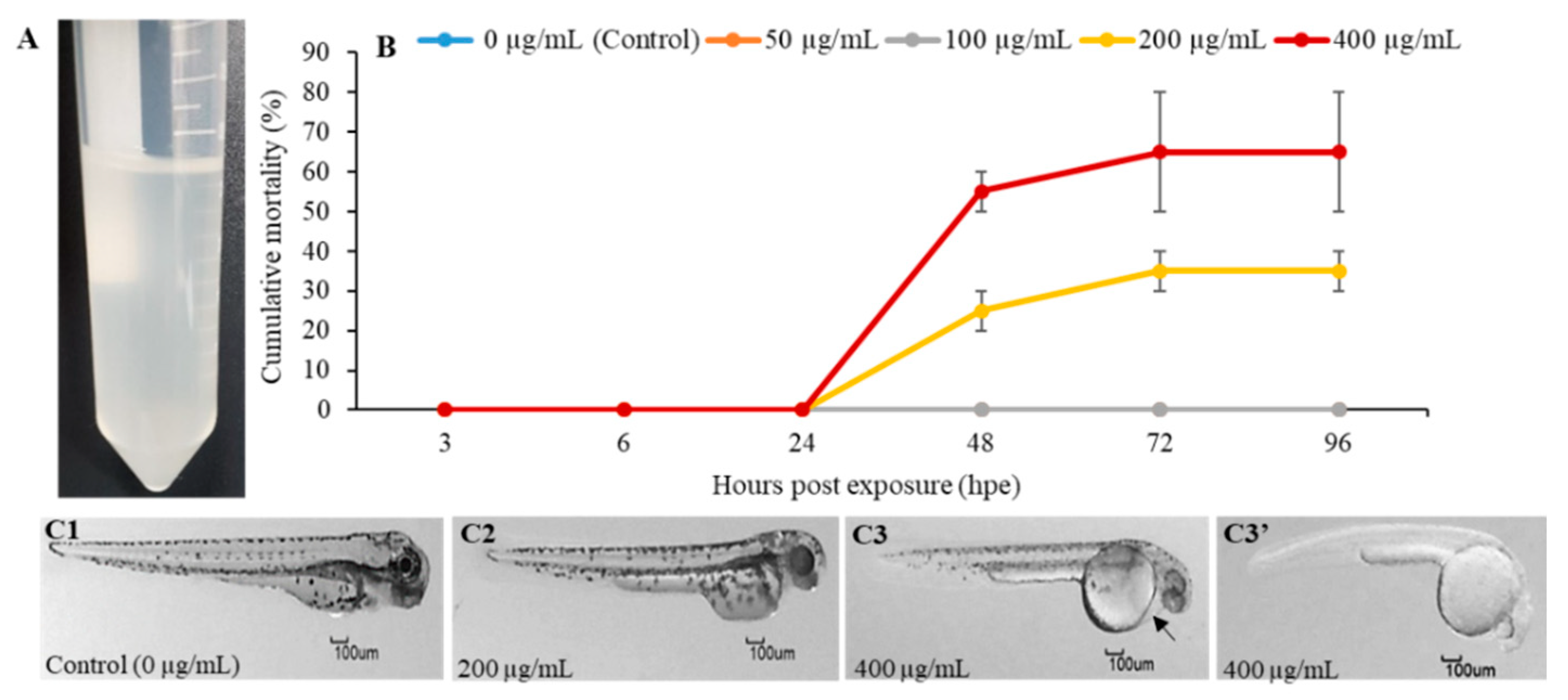
2.1. The In Vivo and In Vitro Toxic Effects of PGM
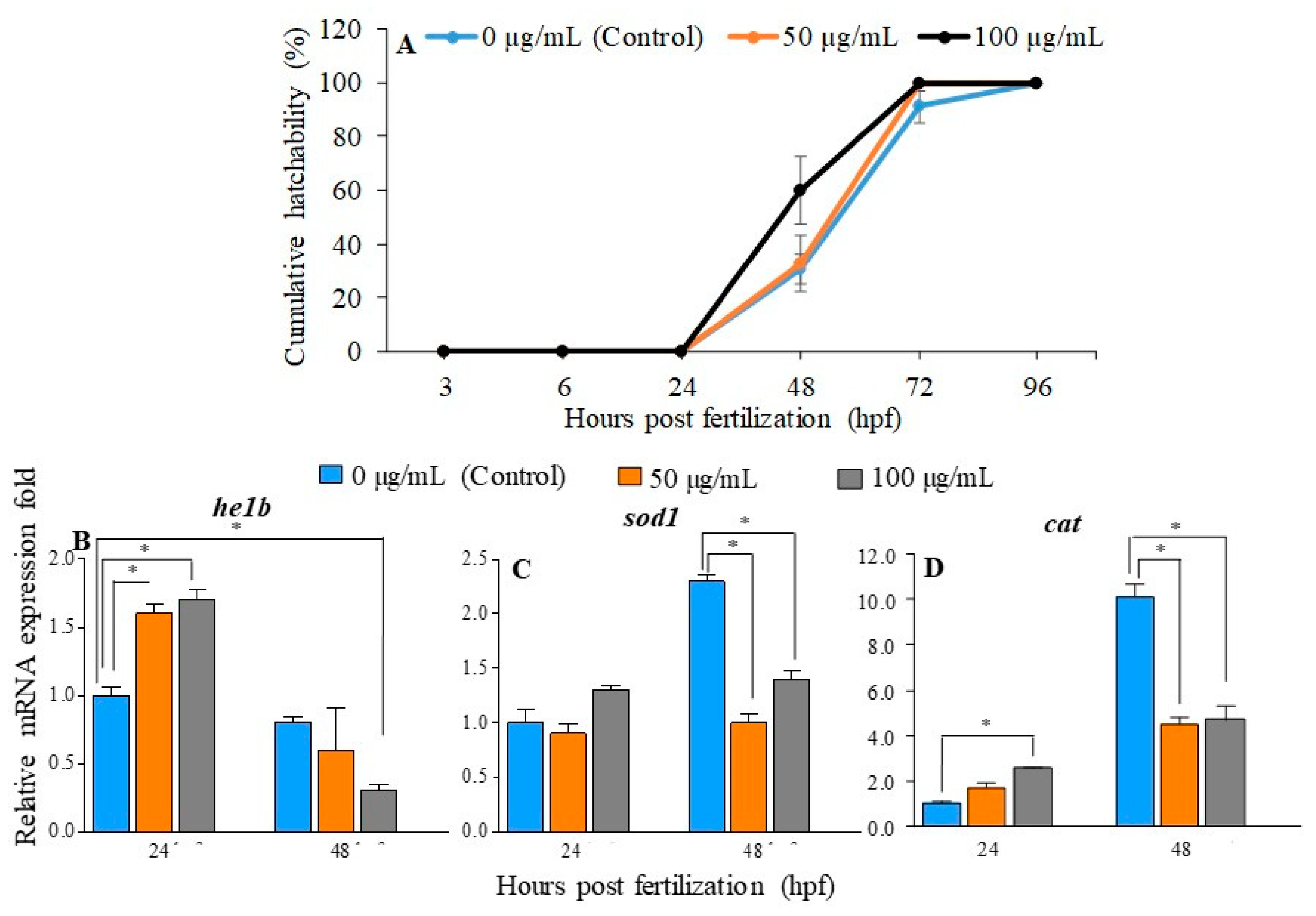
2.2. Effect of PGM Exposure on Embryo Hatching in Zebrafish
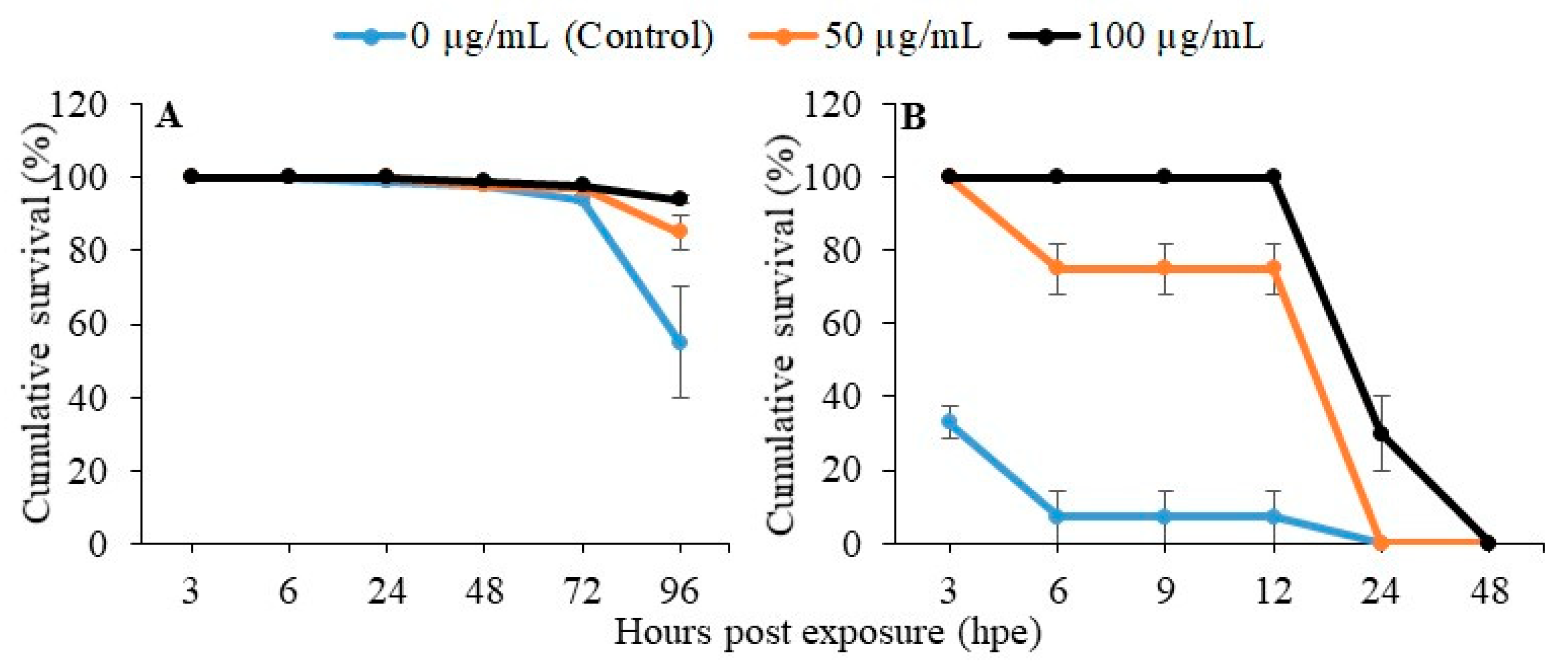
2.3. Disease Resistance and Heat Tolerance of Zebrafish Larvae upon PGM Exposure
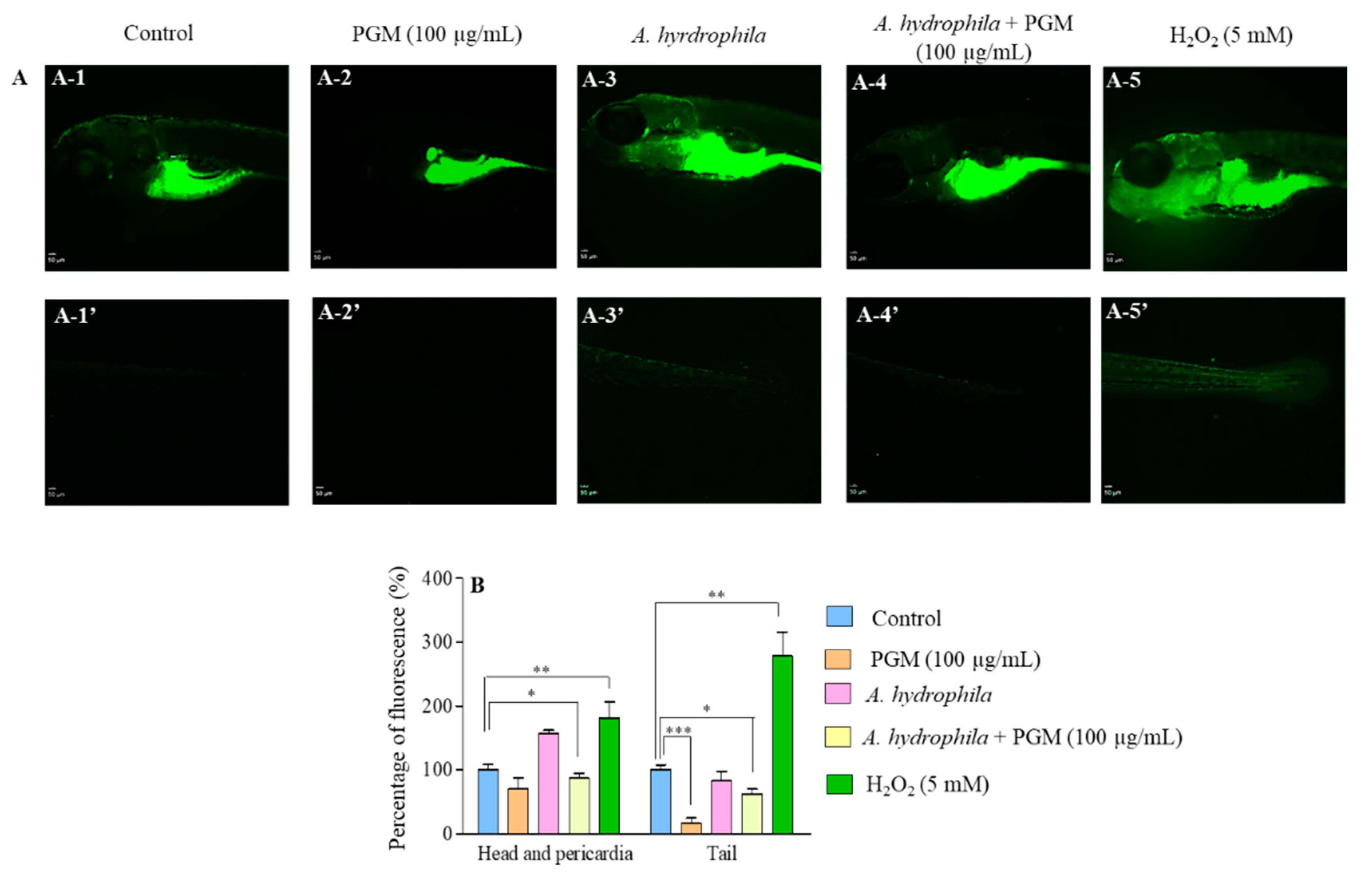
2.4. Detoxification Effect of PGM on Bacteria-Induced Oxidative Stress
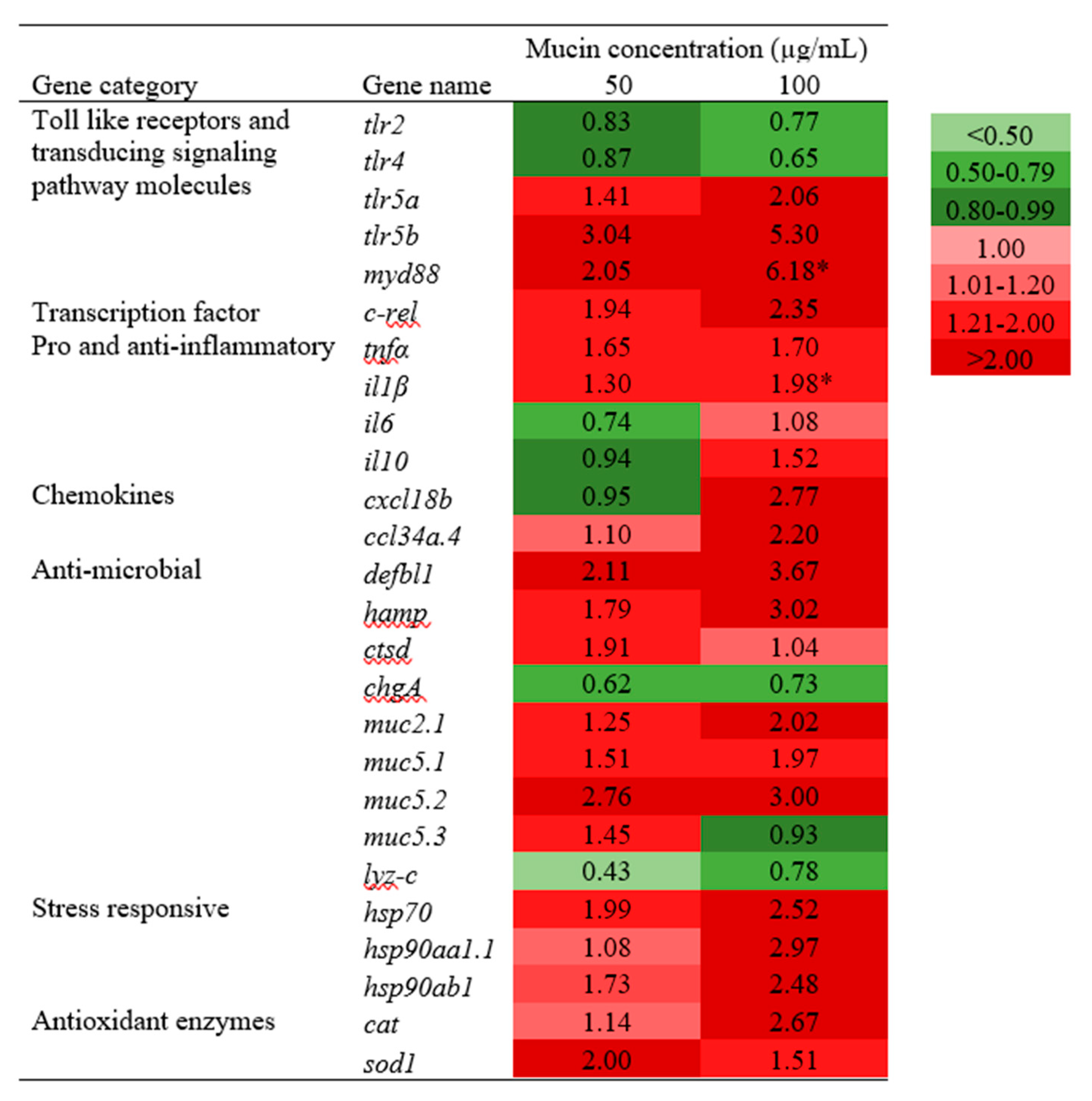
2.5. Transcriptional Analysis of Immune-Related Genes in PGM-Exposed Zebrafish Larvae
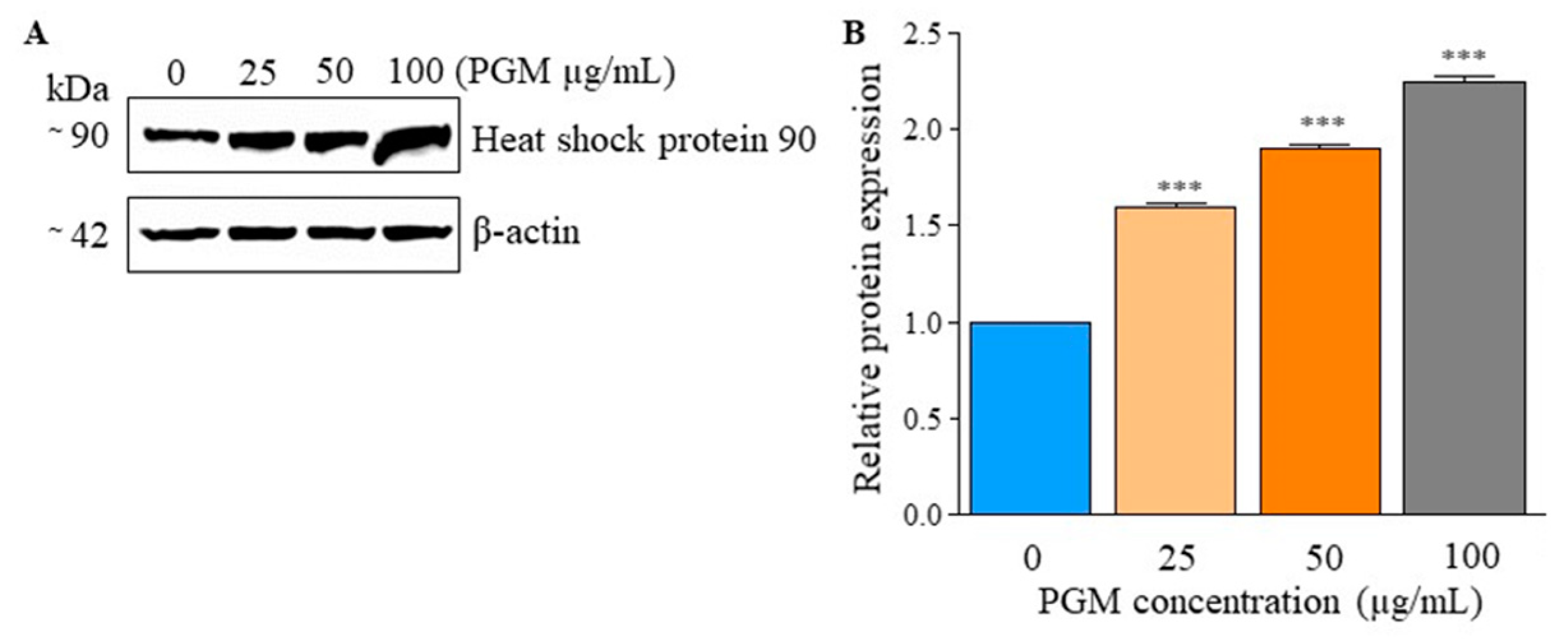
2.6. PGM Effect on Hsp90 Protein Expression
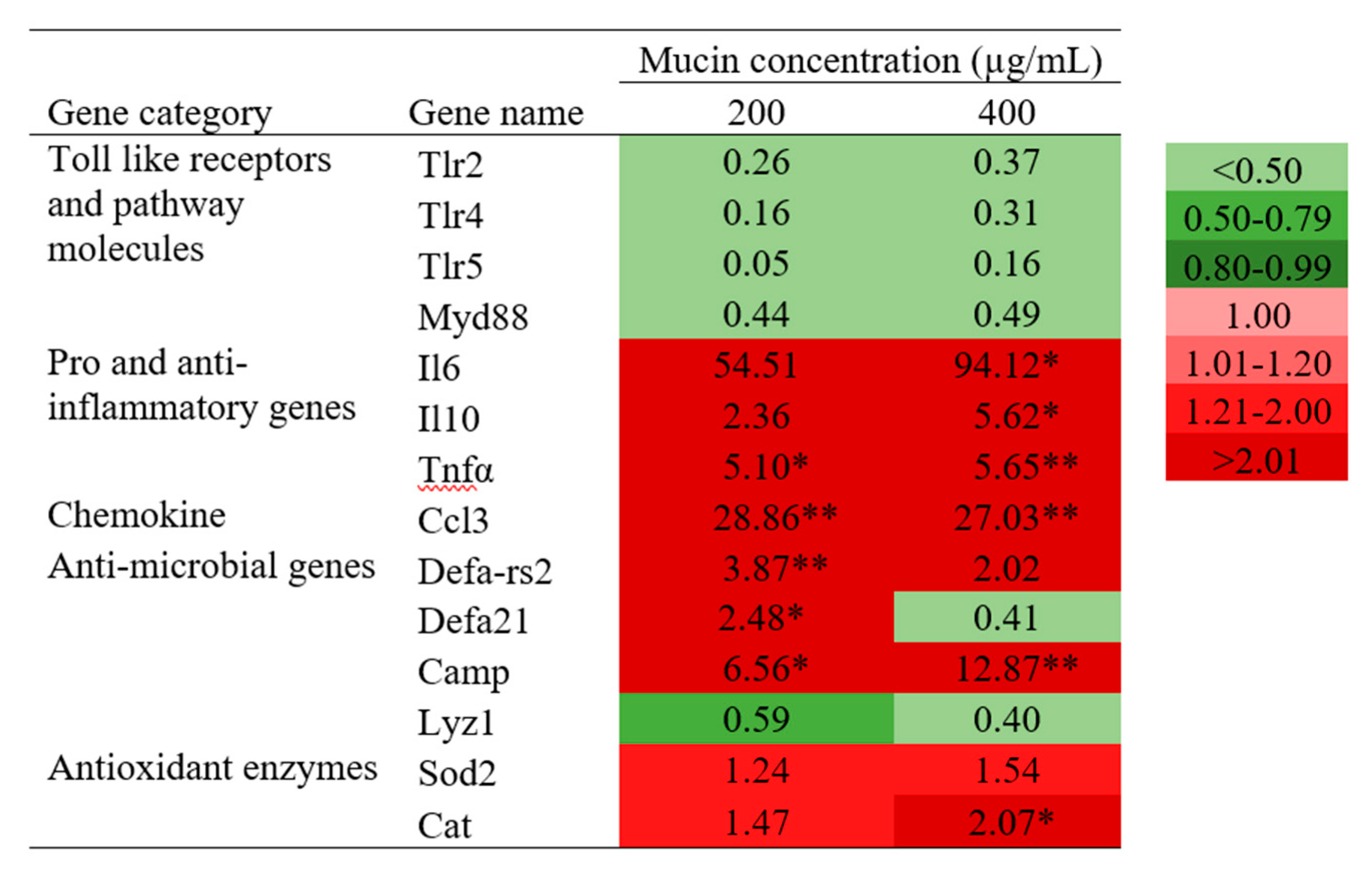
2.7. Transcriptional Analysis of Immune-Related Genes in PGM-Exposed RAW 264.7 Cells
3. Discussion
4. Materials and Methods
4.1. Preparation of PGM Solution
4.2. Cell Culture and Cytotoxicity of PGM
4.3. Determination of In Vivo Toxicity of PGM on Zebrafish Embryos
4.4. Effect of PGM on Zebrafish Hatching
4.5. PGM Effect on A. hydrophila Challenge and Heat Resistance in Zebrafish Larvae
4.6. Analysis of ROS Production in PGM Exposed Larvae Upon A. hydrophila Challenge
4.7. Isolation of Total RNA and Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
4.8. Immunoblot Analysis for Hsp90 Expression in Zebrafish Larvae
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Ethics Approval
References
- John, G.; Mesalhy, S.; Rezk, M.; El-Naggar, G.; Fathi, M. Effect of some immunostimulants as feed additives on the survival and growth performance of Nile tilapia, Oreochromis niloticus and their response to artificial infection. Egypt. J. Aquat. Biol. Fish. 2007, 11, 1299–1308. [Google Scholar]
- Wang, W.; Sun, J.; Liu, C.; Xue, Z. Application of immunostimulants in aquaculture: Current knowledge and future perspectives. Aquac. Res. 2017, 48, 1–23. [Google Scholar] [CrossRef]
- Devine, P.L.; McKenzie, I.F.C. Mucins: Structure, function, and associations with malignancy. BioEssays 1992, 14, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Lesuffleur, T.; Zweibaum, A.; Real, F.X. Mucins in normal and neoplastic human gastrointestinal tissues. Crit. Rev. Oncol. Hematol. 1994, 17, 153–180. [Google Scholar] [CrossRef]
- Gendler, S.J.; Spicer, A.P. Epithelial mucin genes. Annu. Rev. Physiol. 1995, 57, 607–634. [Google Scholar] [CrossRef]
- Seregni, E.; Botti, C.; Massaron, S.; Lombardo, C.; Capobianco, A.; Bogni, A.; Bombardieri, E. Structure, function and gene expression of epithelial mucins. Tumori J. 1997, 83, 625–632. [Google Scholar] [CrossRef]
- Van Klinken, B.J.W.; Einerhand, A.W.C.; Büller, H.A.; Dekker, J. Strategic biochemical analysis of mucins. Anal. Biochem. 1998, 265, 103–116. [Google Scholar] [CrossRef]
- Strous, G.J.; Dekker, J. Mucin-type glycoproteins. Crit. Rev. Biochem. Mol. Biol. 1992, 27, 57–92. [Google Scholar] [CrossRef]
- Johansson, M.E.V.; Sjövall, H.; Hansson, G.C. The gastrointestinal mucus system in health and disease. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 352–361. [Google Scholar] [CrossRef]
- Leal, J.; Smyth, H.D.C.; Ghosh, D. Physicochemical properties of mucus and their impact on transmucosal drug delivery. Int. J. Pharm. 2017, 532, 555–572. [Google Scholar] [CrossRef]
- Dhanisha, S.S.; Guruvayoorappan, C.; Drishya, S.; Abeesh, P. Mucins: Structural diversity, biosynthesis, its role in pathogenesis and as possible therapeutic targets. Crit. Rev. Oncol. Hematol. 2018, 122, 98–122. [Google Scholar] [CrossRef] [PubMed]
- Çelebioğlu, H.Y.; Lee, S.; Chronakis, I.S. Interactions of salivary mucins and saliva with food proteins: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 64–83. [Google Scholar] [CrossRef]
- Pelaseyed, T.; Bergström, J.H.; Gustafsson, J.K.; Ermund, A.; Birchenough, G.M.H.; Schütte, A.; van der Post, S.; Svensson, F.; Rodríguez-Piñeiro, A.M.; Nyström, E.E.L.; et al. The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunol. Rev. 2014, 260, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Birchenough, G.M.H.; Johansson, M.E.V.; Gustafsson, J.K.; Bergström, J.H.; Hansson, G.C. New developments in goblet cell mucus secretion and function. Mucosal Immunol. 2015, 8, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Sáez, N.; Peregrina, J.M.; Corzana, F. Principles of mucin structure: Implications for the rational design of cancer vaccines derived from MUC1-glycopeptides. Chem. Soc. Rev. 2017, 46, 7154–7175. [Google Scholar] [CrossRef] [PubMed]
- Schömig, V.J.; Käsdorf, B.T.; Scholz, C.; Bidmon, K.; Lieleg, O.; Berensmeier, S. An optimized purification process for porcine gastric mucin with preservation of its native functional properties. RSC Adv. 2016, 6, 44932–44943. [Google Scholar] [CrossRef]
- Neuhaus, H.; Van der Marel, M.; Caspari, N.; Meyer, W.; Enss, M.L.; Steinhagen, D. Biochemical and histochemical study on the intestinal mucosa of the common carp Cyprinus carpio L., with special consideration of mucin glycoproteins. J. Fish Biol. 2007, 70, 1523–1534. [Google Scholar] [CrossRef]
- Schroers, V.; van der Marel, M.; Neuhaus, H.; Steinhagen, D. Changes of intestinal mucus glycoproteins after peroral application of Aeromonas hydrophila to common carp (Cyprinus carpio). Aquaculture 2009, 288, 184–189. [Google Scholar] [CrossRef]
- Padra, J.T.; Pagneux, Q.; Bouckaert, J.; Jijie, R.; Sundh, H.; Boukherroub, R.; Lindén, S.K. Mucin modified SPR interfaces for studying the effect of flow on pathogen binding to Atlantic salmon mucins. Biosens. Bioelectron. 2019, 146, 111736. [Google Scholar] [CrossRef]
- Jessberger, N.; Dietrich, R.; Mohr, A.K.; Da Riol, C.; Märtlbauer, E. Porcine gastric mucin triggers toxin production of enteropathogenic Bacillus cereus. Infect. Immun. 2019, 87. [Google Scholar] [CrossRef]
- Lieleg, O.; Lieleg, C.; Bloom, J.; Buck, C.B.; Ribbeck, K. Mucin biopolymers as broad-spectrum antiviral agents. Biomacromolecules 2012, 13, 1724–1732. [Google Scholar] [CrossRef] [PubMed]
- Korwin-Kossakowski, M. Fish hatching strategies: A review. Rev. Fish Biol. Fish. 2012, 22, 225–240. [Google Scholar] [CrossRef]
- Armant, O.; Gombeau, K.; El Houdigui, S.M.; Floriani, M.; Camilleri, V.; Cavalie, I.; Adam-Guillermin, C. Zebrafish exposure to environmentally relevant concentration of depleted uranium impairs progeny development at the molecular and histological levels. PLoS ONE 2017, 12, e0177932. [Google Scholar] [CrossRef] [PubMed]
- Valavanidis, A.; Vlahogianni, T.; Dassenakis, M.; Scoullos, M. Molecular biomarkers of oxidative stress in aquatic organisms in relation to toxic environmental pollutants. Ecotoxicol. Environ. Saf. 2006, 64, 178–189. [Google Scholar] [CrossRef]
- Vinagre, C.; Madeira, D.; Narciso, L.; Cabral, H.N.; Diniz, M. Effect of temperature on oxidative stress in fish: Lipid peroxidation and catalase activity in the muscle of juvenile seabass, Dicentrarchus labrax. Ecol. Indic. 2012, 23, 274–279. [Google Scholar] [CrossRef]
- Brownlee, I.A.; Knight, J.; Dettmar, P.W.; Pearson, J.P. Action of reactive oxygen species on colonic mucus secretions. Free Radic. Biol. Med. 2007, 43, 800–808. [Google Scholar] [CrossRef]
- Bricknell, I.; Dalmo, R.A. The use of immunostimulants in fish larval aquaculture. Fish Shellfish Immunol. 2005, 19, 457–472. [Google Scholar] [CrossRef]
- Oyarbide, U.; Rainieri, S.; Pardo, M.A. Zebrafish (Danio rerio) larvae as a system to test the efficacy of polysaccharides as immunostimulants. Zebrafish 2012, 9, 74–84. [Google Scholar] [CrossRef]
- Brun, N.R.; Lenz, M.; Wehrli, B.; Fent, K. Comparative effects of zinc oxide nanoparticles and dissolved zinc on zebrafish embryos and eleuthero-embryos: Importance of zinc ions. Sci. Total Environ. 2014, 476–477, 657–666. [Google Scholar] [CrossRef]
- Udayangani, R.M.C.; Dananjaya, S.H.S.; Fronte, B.; Kim, C.H.; Lee, J.; De Zoysa, M. Feeding of nano scale oats β-glucan enhances the host resistance against Edwardsiella tarda and protective immune modulation in zebrafish larvae. Fish Shellfish Immunol. 2017, 60, 72–77. [Google Scholar] [CrossRef]
- Nikapitiya, C.; Dananjaya, S.H.S.; De Silva, B.C.J.; Heo, G.J.; Oh, C.; De Zoysa, M.; Lee, J. Chitosan nanoparticles: A positive immune response modulator as display in zebrafish larvae against Aeromonas hydrophila infection. Fish Shellfish Immunol. 2018, 76, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Agata, N.; Chen, D.; Li, Y.; Yu, W.; Huang, L.; Raina, D.; Chen, W.; Kharbanda, S.; Kufe, D. Human MUC1 carcinoma-associated protein confers resistance to genotoxic anticancer agents. Cancer Cell 2004, 5, 163–175. [Google Scholar] [CrossRef]
- Martínez-Álvarez, R.M.; Morales, A.E.; Sanz, A. Antioxidant defenses in fish: Biotic and abiotic factors. Rev. Fish Biol. Fish. 2005, 15, 75–88. [Google Scholar] [CrossRef]
- Hong, Y.; Jiang, W.; Kuang, S.; Hu, K.; Tang, L.; Liu, Y.; Jiang, J.; Zhang, Y.; Zhou, X.; Feng, L. Growth, digestive and absorptive capacity and antioxidant status in intestine and hepatopancreas of sub-adult grass carp Ctenopharyngodon idella fed graded levels of dietary threonine. J. Anim. Sci. Biotechnol. 2015, 6, 34. [Google Scholar] [CrossRef][Green Version]
- Grisham, M.B.; Von Ritter, C.; Smith, B.F.; Lamont, J.T.; Granger, D.N. Interaction between oxygen radicals and gastric mucin. Am. J. Physiol. 1987, 253, G93–G96. [Google Scholar] [CrossRef]
- Zapata, A.G.; Torroba, M.; Alvarez, F.; Anderson, D.P.; Dixon, O.W.; Wisniewski, M. Electron microscopic examination of antigen uptake by salmonid gill cells after bath immunization with a bacterin. J. Fish Biol. 1987, 31, 209–217. [Google Scholar] [CrossRef]
- Dalmo, R.A.; Bøgwald, J. β-glucans as conductors of immune symphonies. Fish Shellfish Immunol. 2008, 25, 384–396. [Google Scholar] [CrossRef]
- Raida, M.K.; Buchmann, K. Bath vaccination of rainbow trout (Oncorhynchus mykiss Walbaum) against Yersinia ruckeri: Effects of temperature on protection and gene expression. Vaccine 2008, 26, 1050–1062. [Google Scholar] [CrossRef]
- Takeda, K.; Kaisho, T.; Akira, S. Toll-Like receptors. Annu. Rev. Immunol. 2003, 2, 335–376. [Google Scholar] [CrossRef]
- Hayashi, F.; Smith, K.D.; Ozinsky, A.; Hawn, T.R.; Yi, E.C.; Goodlett, D.R.; Eng, J.K.; Akira, S.; Underhill, D.M.; Aderem, A. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 2001, 410, 1099–1103. [Google Scholar] [CrossRef]
- Iqbal, M.; Philbin, V.J.; Withanage, G.S.K.; Wigley, P.; Beal, R.K.; Goodchild, M.J.; Barrow, P.; McConnell, I.; Maskell, D.J.; Young, J.; et al. Identification and functional characterization of chicken toll-like receptor 5 reveals a fundamental role in the biology of infection with Salmonella enterica serovar typhimurium. Infect. Immun. 2005, 73, 2344–2350. [Google Scholar] [CrossRef] [PubMed]
- Stockhammer, O.W.; Zakrzewska, A.; Hegedûs, Z.; Spaink, H.P.; Meijer, A.H. Transcriptome profiling and functional analyses of the zebrafish embryonic innate immune response to Salmonella infection. J. Immunol. 2009, 182, 5641–5653. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.I.; Kurnasov, O.; Natarajan, V.; Hong, M.; Gudkov, A.V.; Osterman, A.L.; Wilson, I.A. Structural basis of TLR5-flagellin recognition and signaling. Science 2012, 335, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Takeda, K. Functions of toll-like receptors: Lessons from KO mice. C. R. Biol. 2004, 327, 581–589. [Google Scholar] [CrossRef]
- Van der Vaart, M.; van Soest, J.J.; Spaink, H.P.; Meijer, A.H. Functional analysis of a zebrafish myd88 mutant identifies key transcriptional components of the innate immune system. Dis. Model. Mech. 2013, 6, 841–854. [Google Scholar] [CrossRef]
- Fullard, N.; Wilson, C.L.; Oakley, F. Roles of c-Rel signalling in inflammation and disease. Int. J. Biochem. Cell. Biol. 2012, 44, 851–860. [Google Scholar] [CrossRef]
- Zheng, X.; Dai, W.; Chen, X.; Wang, K.; Zhang, W.; Liu, L.; Hou, J. Caffeine reduces hepatic lipid accumulation through regulation of lipogenesis and ER stress in zebrafish larvae. J. Biomed. Sci. 2015, 22, 1–12. [Google Scholar] [CrossRef]
- Rojo, I.; de Ilárduya, Ó.M.; Estonba, A.; Pardo, M.Á. Innate immune gene expression in individual zebrafish after Listonella anguillarum inoculation. Fish Shellfish Immunol. 2007, 23, 1285–1293. [Google Scholar] [CrossRef]
- Bhatt, P.; Kumaresan, V.; Palanisamy, R.; Ravichandran, G.; Mala, K.; Amin, S.M.N.; Arshad, A.; Yusoff, F.M.; Arockiaraj, J. A mini review on immune role of chemokines and its receptors in snakehead murrel Channa striatus. Fish Shellfish Immunol. 2018, 72, 670–678. [Google Scholar] [CrossRef]
- Cobo, E.R.; Kissoon-Singh, V.; Moreau, F.; Chadee, K. Colonic MUC2 mucin regulates the expression and antimicrobial activity of β-defensin 2. Mucosal Immunol. 2015, 8, 1360–1372. [Google Scholar] [CrossRef]
- Milton, J.S. Heat Shock Proteins. J. Biol. Chem. 1990, 25, 1211–1214. [Google Scholar] [CrossRef]
- Gunderson, L.L.; Tepper, J.E. Clinical Radiation Oncology, 4th ed.; Elsevier Health Sciences: Philadelphia, PA, USA, 2015. [Google Scholar]
- Kono, T.; Sakai, M. The analysis of expressed genes in the kidney of Japanese flounder, Paralichthys olivaceus, injected with the immunostimulant peptidoglycan. Fish Shellfish Immunol. 2001, 11, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.J.; Yoon, W.J.; Kim, K.N.; Ahn, G.N.; Kang, S.M.; Kang, D.H.; Affan, A.; Oh, C.; Jung, W.K.; Jeon, Y.J. Evaluation of anti-inflammatory effect of fucoxanthin isolated from brown algae in lipopolysaccharide-stimulated RAW 264.7 macrophages. Food Chem. Toxicol. 2010, 48, 2045–2051. [Google Scholar] [CrossRef] [PubMed]
- Grayfer, L.; Hodgkinson, J.W.; Hitchen, S.J.; Belosevic, M. Characterization and functional analysis of goldfish (Carassius auratus L.) interleukin-10. Mol. Immunol. 2011, 48, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Chandrarathna, H.P.S.U.; Nikapitiya, C.; Dananjaya, S.H.S.; Wijerathne, C.U.B.; Wimalasena, S.H.M.P.; Kwun, H.J.; Heo, G.J.; Lee, J.; De Zoysa, M. Outcome of co-infection with opportunistic and multidrug resistant Aeromonas hydrophila and A. veronii in zebrafish: Identification, characterization, pathogenicity and immune responses. Fish Shellfish Immunol. 2018, 80, 573–581. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liyanage, T.D.; Dahanayake, P.S.; Edirisinghe, S.L.; Nikapitiya, C.; Heo, G.-J.; De Zoysa, M.; Whang, I. Biological Activity of Porcine Gastric Mucin on Stress Resistance and Immunomodulation. Molecules 2020, 25, 2981. https://doi.org/10.3390/molecules25132981
Liyanage TD, Dahanayake PS, Edirisinghe SL, Nikapitiya C, Heo G-J, De Zoysa M, Whang I. Biological Activity of Porcine Gastric Mucin on Stress Resistance and Immunomodulation. Molecules. 2020; 25(13):2981. https://doi.org/10.3390/molecules25132981
Chicago/Turabian StyleLiyanage, Thiloma D., Pasan S. Dahanayake, Shan L. Edirisinghe, Chamilani Nikapitiya, Gang-Joon Heo, Mahanama De Zoysa, and Ilson Whang. 2020. "Biological Activity of Porcine Gastric Mucin on Stress Resistance and Immunomodulation" Molecules 25, no. 13: 2981. https://doi.org/10.3390/molecules25132981
APA StyleLiyanage, T. D., Dahanayake, P. S., Edirisinghe, S. L., Nikapitiya, C., Heo, G.-J., De Zoysa, M., & Whang, I. (2020). Biological Activity of Porcine Gastric Mucin on Stress Resistance and Immunomodulation. Molecules, 25(13), 2981. https://doi.org/10.3390/molecules25132981




