The Lipoxin Receptor/FPR2 Agonist BML-111 Protects Mouse Skin Against Ultraviolet B Radiation
Abstract
1. Introduction
2. Results
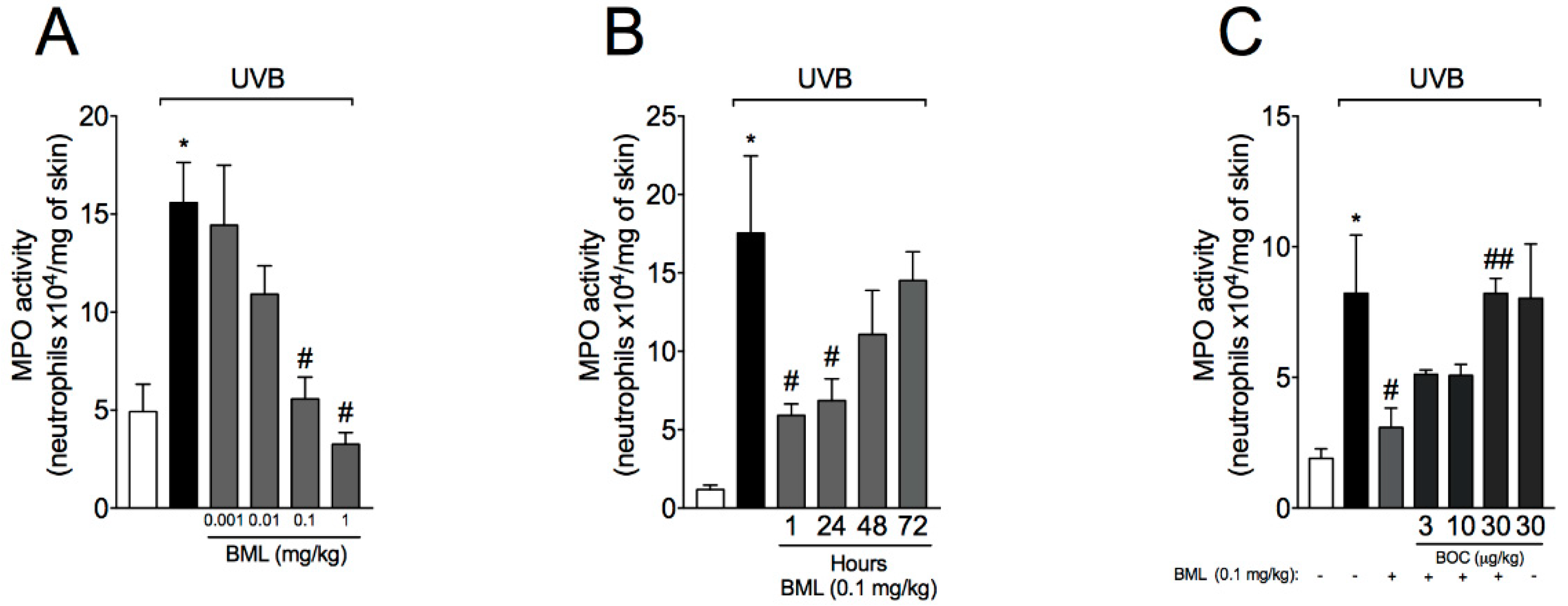
2.1. BML-111 Reduces Neutrophil Recruitment in a Dose-Dependent Manner and an ALX/FPR2-Sensitive Manner
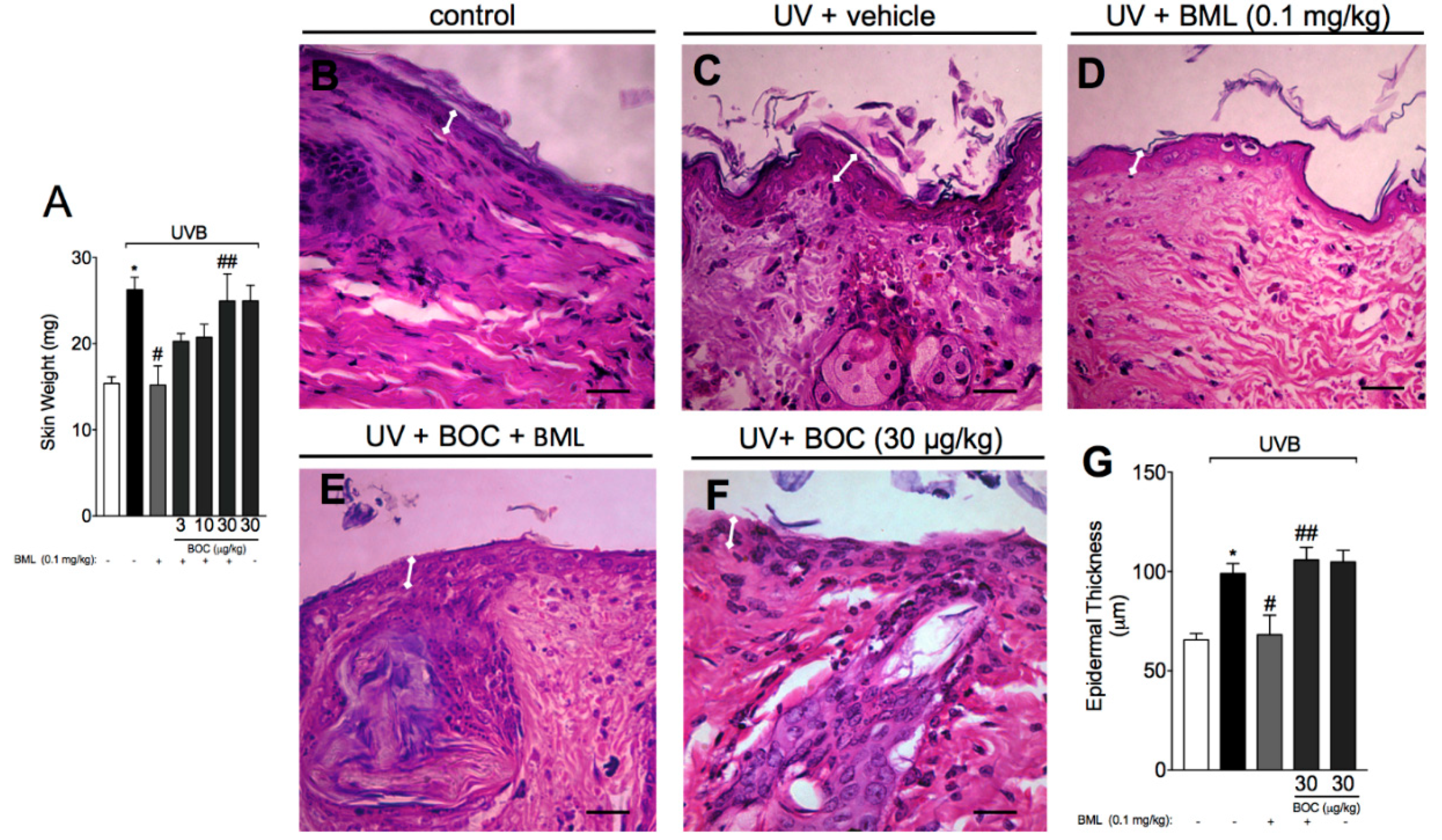
2.2. BML-111 Reduces Skin Edema and the Increase in Epidermal Thickness Induced by UVB Radiation
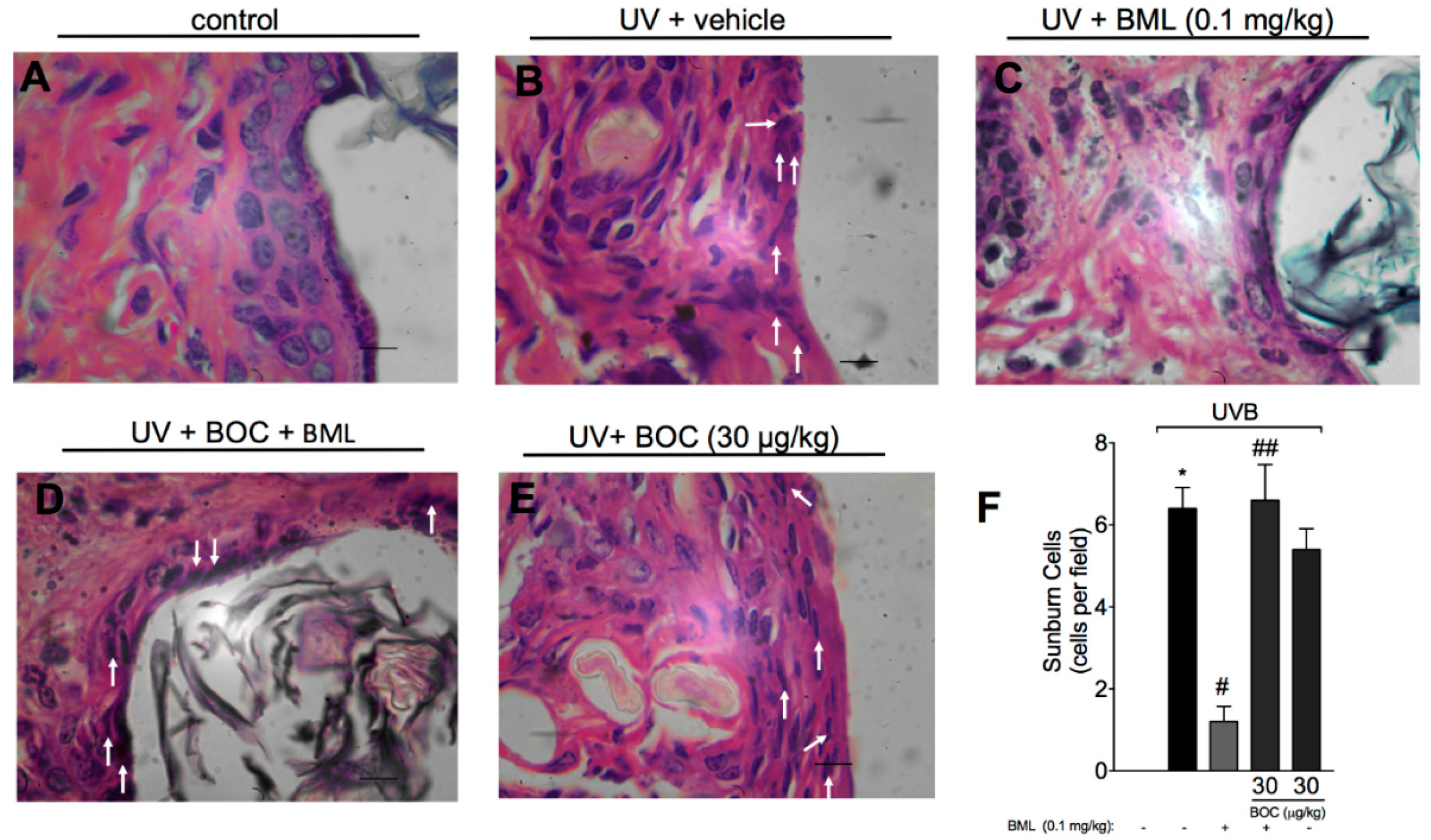
2.3. BML-111 Reduces UVB-Induced Sunburn Cells
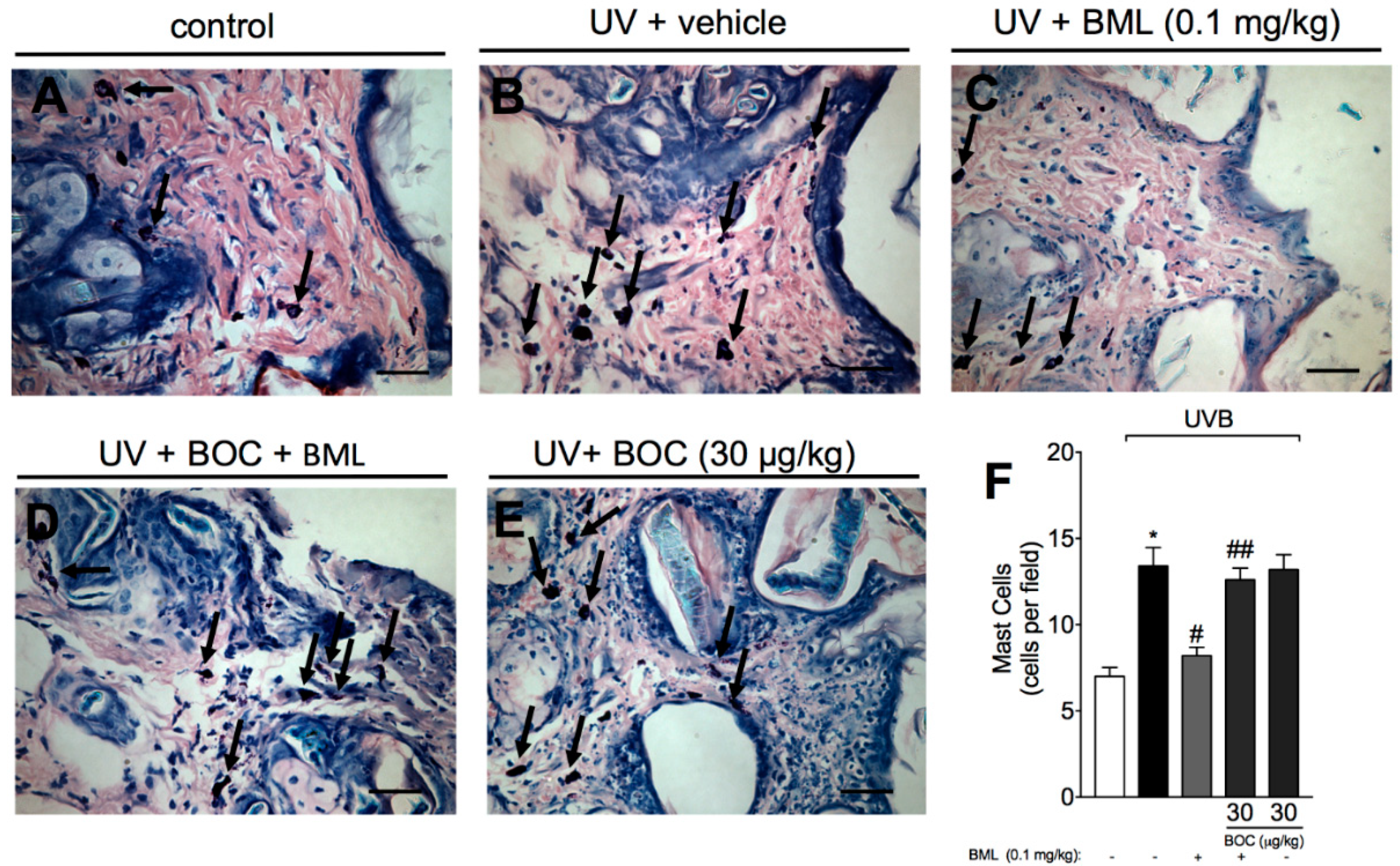
2.4. BML-111 Reduces UVB Irradiation-Induced Increase of Mast Cell Count
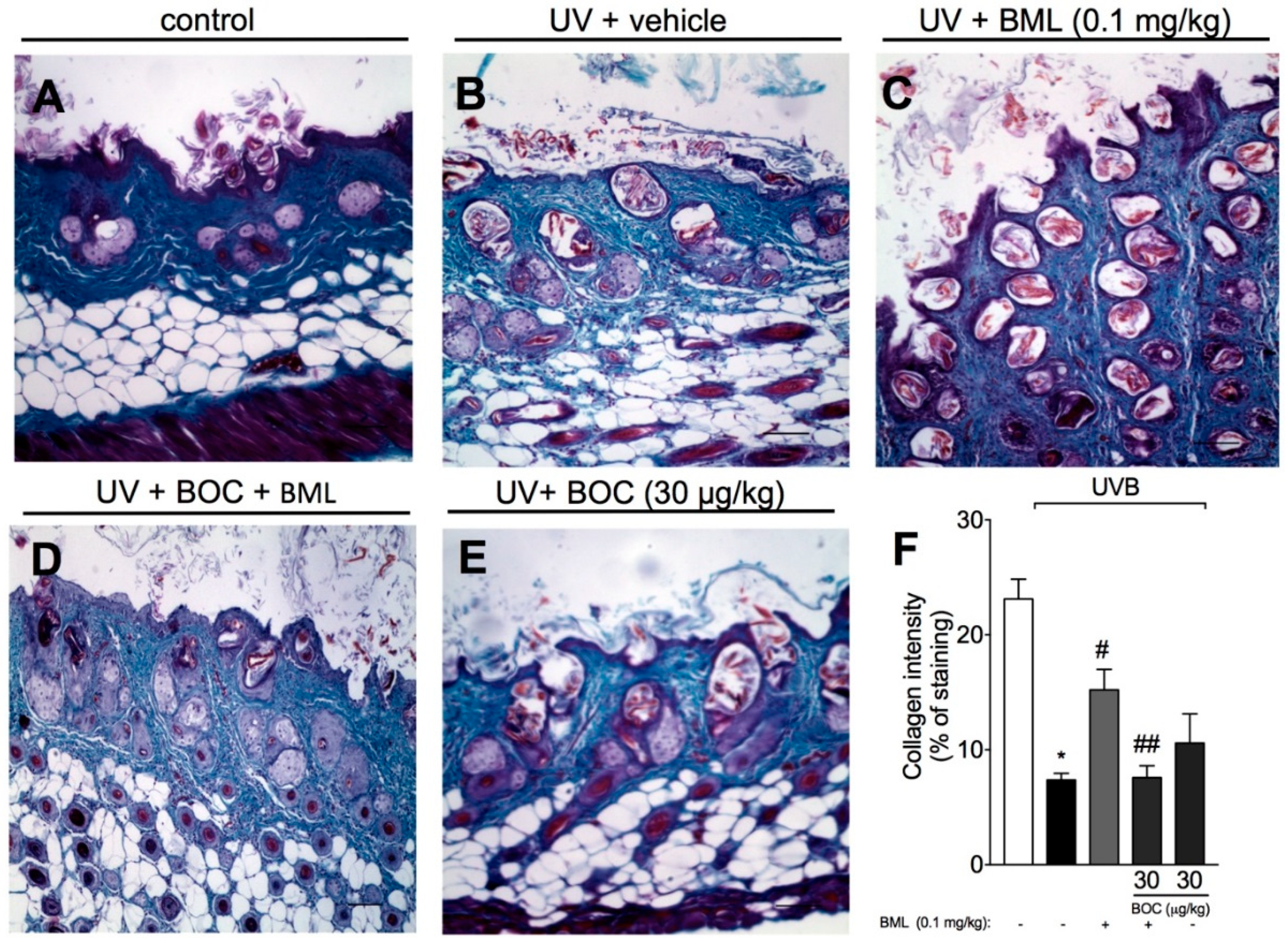
2.5. BML-111 Prevents UVB Irradiation-Induced Collagen Degradation
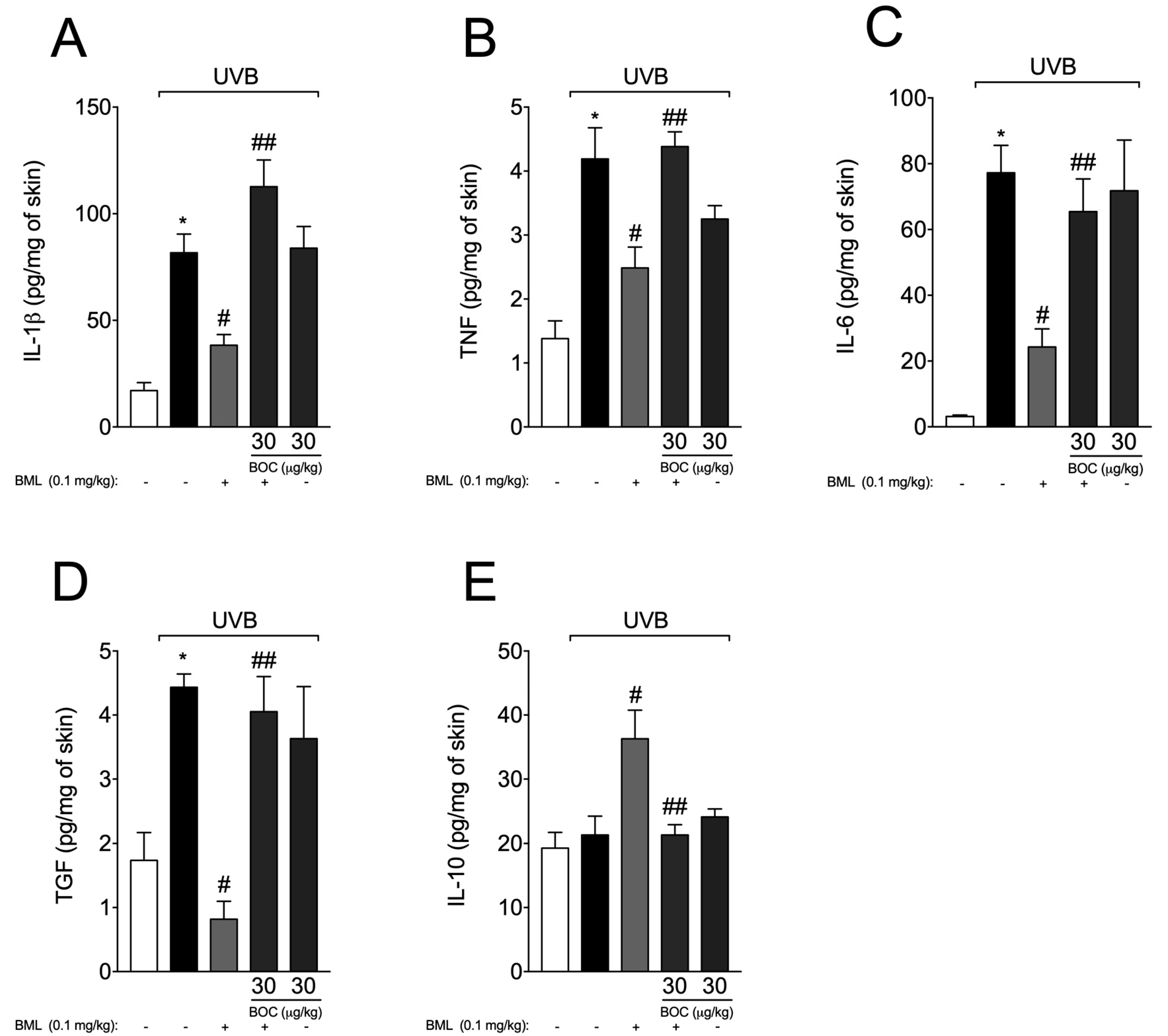
2.6. BML-111 Reduces Cytokine Production During UVB-Induced Skin Inflammation
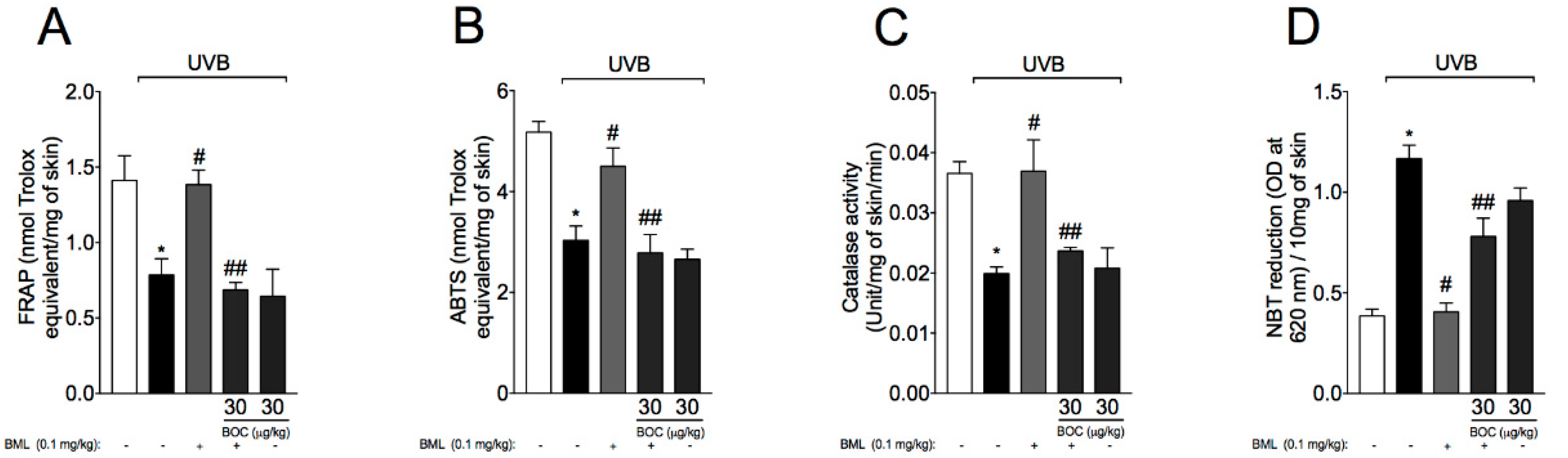
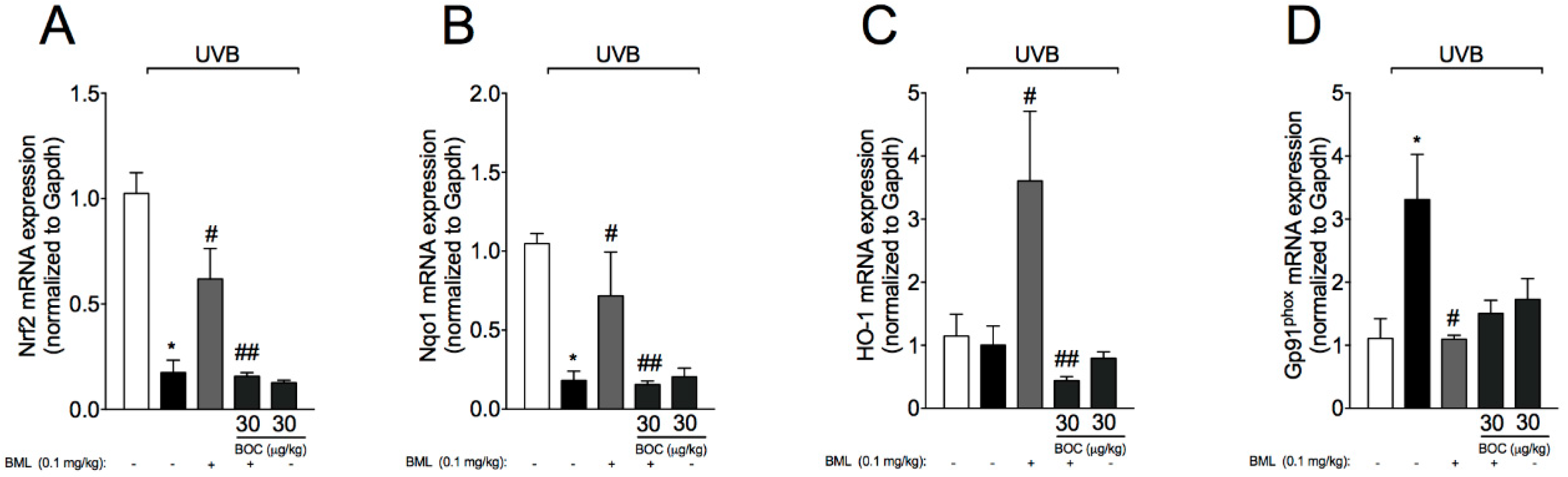
2.7. BML-111 Reduces UVB-Induced Oxidative Stress
3. Discussion
4. Material and Methods
4.1. Animals
4.2. Drugs and Treatment Regimen
4.3. Irradiation Protocol
4.4. MPO Activity Assay
4.5. Skin Edema
4.6. Histopathological Analysis
4.7. Cytokine Measurement
4.8. Total Antioxidant Capacity: ABTS and FRAP Assays
4.9. Catalase Assay
4.10. Superoxide Anion Production
4.11. Real Time and Quantitative Polymerase Chain Reaction (RT-qPCR)
4.12. Data Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV radiation and the skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef]
- Afaq, F.; Adhami, V.M.; Mukhtar, H. Photochemoprevention of ultraviolet B signaling and photocarcinogenesis. Mutat. Res. 2005, 571, 153–173. [Google Scholar] [CrossRef] [PubMed]
- Podda, M.; Traber, M.G.; Weber, C.; Yan, L.J.; Packer, L. UV-irradiation depletes antioxidants and causes oxidative damage in a model of human skin. Free Radic. Biol. Med. 1998, 24, 55–65. [Google Scholar] [CrossRef]
- Vitale, N.; Kisslinger, A.; Paladino, S.; Procaccini, C.; Matarese, G.; Pierantoni, G.M.; Mancini, F.P.; Tramontano, D. Resveratrol couples apoptosis with autophagy in UVB-irradiated HaCaT cells. PLoS ONE 2013, 8, e80728. [Google Scholar] [CrossRef] [PubMed]
- Saija, A.; Tomaino, A.; Trombetta, D.; De Pasquale, A.; Uccella, N.; Barbuzzi, T.; Paolino, D.; Bonina, F. In vitro and in vivo evaluation of caffeic and ferulic acids as topical photoprotective agents. Int. J. Pharm. 2000, 199, 39–47. [Google Scholar] [CrossRef]
- Touitou, E.; Godin, B. Skin nonpenetrating sunscreens for cosmetic and pharmaceutical formulations. Clin. Dermatol. 2008, 26, 375–379. [Google Scholar] [CrossRef]
- Printz, C. Skin cancer prevention advocates target indoor tanning: As tanning beds increasingly turn up outside of traditional salons, experts are trying innovative approaches to curb their use. Cancer 2019, 125, 493–494. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer Working Group on Artificial Ultraviolet (UV) Light and Skin Cancer. The association of use of sunbeds with cutaneous malignant melanoma and other skin cancers: A systematic review. Int. J. Cancer 2007, 120, 1116–1122. [Google Scholar] [CrossRef]
- Levy, B.D.; Clish, C.B.; Schmidt, B.; Gronert, K.; Serhan, C.N. Lipid mediator class switching during acute inflammation: Signals in resolution. Nat. Immunol. 2001, 2, 612–619. [Google Scholar] [CrossRef]
- Bannenberg, G.L.; Chiang, N.; Ariel, A.; Arita, M.; Tjonahen, E.; Gotlinger, K.H.; Hong, S.; Serhan, C.N. Molecular circuits of resolution: Formation and actions of resolvins and protectins. J. Immunol. 2005, 174, 4345–4355. [Google Scholar] [CrossRef]
- Chiang, N.; Serhan, C.N. Structural elucidation and physiologic functions of specialized pro-resolving mediators and their receptors. Mol. Asp. Med. 2017, 58, 114–129. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N. Treating inflammation and infection in the 21st century: New hints from decoding resolution mediators and mechanisms. FASEB J. 2017, 31, 1273–1288. [Google Scholar] [CrossRef] [PubMed]
- Romano, M.; Cianci, E.; Simiele, F.; Recchiuti, A. Lipoxins and aspirin-triggered lipoxins in resolution of inflammation. Eur. J. Pharmacol. 2015, 760, 49–63. [Google Scholar] [CrossRef]
- Fattori, V.; Zaninelli, T.H.; Rasquel-Oliveira, F.S.; Casagrande, R.; Verri, W.A., Jr. Specialized pro-resolving lipid mediators: A new class of non-immunosuppressive and non-opioid analgesic drugs. Pharm. Res. 2020, 151, 104549. [Google Scholar] [CrossRef] [PubMed]
- Dalli, J.; Serhan, C.N. Identification and structure elucidation of the pro-resolving mediators provides novel leads for resolution pharmacology. Br. J. Pharmacol. 2019, 176, 1024–1037. [Google Scholar] [CrossRef] [PubMed]
- Martinez, R.M.; Fattori, V.; Saito, P.; Melo, C.B.P.; Borghi, S.M.; Pinto, I.C.; Bussmann, A.J.C.; Baracat, M.M.; Georgetti, S.R.; Verri, W.A., Jr.; et al. Lipoxin A4 inhibits UV radiation-induced skin inflammation and oxidative stress in mice. J. Dermatol. Sci. 2018. [Google Scholar] [CrossRef] [PubMed]
- Cezar, T.L.C.; Martinez, R.M.; Rocha, C.D.; Melo, C.P.B.; Vale, D.L.; Borghi, S.M.; Fattori, V.; Vignoli, J.A.; Camilios-Neto, D.; Baracat, M.M.; et al. Treatment with maresin 1, a docosahexaenoic acid-derived pro-resolution lipid, protects skin from inflammation and oxidative stress caused by UVB irradiation. Sci. Rep. 2019, 9, 3062. [Google Scholar] [CrossRef]
- Saito, P.; Melo, C.P.B.; Martinez, R.M.; Fattori, V.; Cezar, T.L.C.; Pinto, I.C.; Bussmann, A.J.C.; Vignoli, J.A.; Georgetti, S.R.; Baracat, M.M.; et al. The Lipid Mediator Resolvin D1 Reduces the Skin Inflammation and Oxidative Stress Induced by UV Irradiation in Hairless Mice. Front. Pharmacol. 2018, 9, 1242. [Google Scholar] [CrossRef]
- Serhan, C.N.; Petasis, N.A. Resolvins and Protectins in Inflammation Resolution. Chem. Rev. 2011, 111, 5922–5943. [Google Scholar] [CrossRef]
- Conte, F.P.; Menezes-de-Lima, O., Jr.; Verri, W.A., Jr.; Cunha, F.Q.; Penido, C.; Henriques, M.G. Lipoxin A(4) attenuates zymosan-induced arthritis by modulating endothelin-1 and its effects. Br. J. Pharmacol. 2010, 161, 911–924. [Google Scholar] [CrossRef]
- Xu, J.; Li, H.B.; Chen, L.; Wang, Y.X.; Lu, S.; Li, S.N.; Cui, S.N.; Xiao, H.R.; Qin, L.; Hu, H.; et al. BML-111 accelerates the resolution of inflammation by modulating the Nrf2/HO-1 and NF-kappaB pathways in rats with ventilator-induced lung injury. Int. Immunopharmacol. 2019, 69, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, X.; Duan, X.; Poorun, D.; Xu, J.; Zhang, S.; Gan, L.; He, M.; Zhu, K.; Ming, Z.; et al. Lipoxin A4 and its analog suppress inflammation by modulating HMGB1 translocation and expression in psoriasis. Sci. Rep. 2017, 7, 7100. [Google Scholar] [CrossRef]
- Recchiuti, A.; Serhan, C.N. Pro-Resolving Lipid Mediators (SPMs) and Their Actions in Regulating miRNA in Novel Resolution Circuits in Inflammation. Front. Immunol. 2012, 3, 298. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Wang, C.F.; Serhan, C.N.; Strichartz, G. Enduring prevention and transient reduction of postoperative pain by intrathecal resolvin D1. Pain 2011, 152, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Fattori, V.; Pinho-Ribeiro, F.A.; Staurengo-Ferrari, L.; Borghi, S.M.; Rossaneis, A.C.; Casagrande, R.; Verri, W.A., Jr. The specialized pro-resolving lipid mediator Maresin-1 reduces inflammatory pain with a long-lasting analgesic effect. Br. J. Pharmacol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Quan, T.; Qin, Z.; Xia, W.; Shao, Y.; Voorhees, J.J.; Fisher, G.J. Matrix-degrading metalloproteinases in photoaging. J. Investig. Dermatol. Symp. Proc. 2009, 14, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, A.; Bhardwaj, R.; Aragane, Y.; Mahnke, K.; Riemann, H.; Metze, D.; Luger, T.A.; Schwarz, T. Ultraviolet-B-induced apoptosis of keratinocytes: Evidence for partial involvement of tumor necrosis factor-alpha in the formation of sunburn cells. J. Investig. Dermatol. 1995, 104, 922–927. [Google Scholar] [CrossRef]
- Martinez, R.M.; Pinho-Ribeiro, F.A.; Steffen, V.S.; Caviglione, C.V.; Vignoli, J.A.; Baracat, M.M.; Georgetti, S.R.; Verri, W.A., Jr.; Casagrande, R. Hesperidin methyl chalcone inhibits oxidative stress and inflammation in a mouse model of ultraviolet B irradiation-induced skin damage. J. Photochem. Photobiol. B 2015, 148, 145–153. [Google Scholar] [CrossRef]
- Yoshizumi, M.; Nakamura, T.; Kato, M.; Ishioka, T.; Kozawa, K.; Wakamatsu, K.; Kimura, H. Release of cytokines/chemokines and cell death in UVB-irradiated human keratinocytes, HaCaT. Cell Biol. Int. 2008, 32, 1405–1411. [Google Scholar] [CrossRef]
- Ivan, A.L.; Campanini, M.Z.; Martinez, R.M.; Ferreira, V.S.; Steffen, V.S.; Vicentini, F.T.; Vilela, F.M.; Martins, F.S.; Zarpelon, A.C.; Cunha, T.M.; et al. Pyrrolidine dithiocarbamate inhibits UVB-induced skin inflammation and oxidative stress in hairless mice and exhibits antioxidant activity in vitro. J. Photochem. Photobiol. B 2014, 138, 124–133. [Google Scholar] [CrossRef]
- Natarajan, V.T.; Ganju, P.; Ramkumar, A.; Grover, R.; Gokhale, R.S. Multifaceted pathways protect human skin from UV radiation. Nat. Chem. Biol. 2014, 10, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Casagrande, R.; Georgetti, S.R.; Verri, W.A., Jr.; Dorta, D.J.; dos Santos, A.C.; Fonseca, M.J. Protective effect of topical formulations containing quercetin against UVB-induced oxidative stress in hairless mice. J. Photochem. Photobiol. B 2006, 84, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Madigan, L.M.; Lim, H.W. Tanning beds: Impact on health, and recent regulations. Clin. Dermatol. 2016, 34, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Walsh, L.J. Ultraviolet B irradiation of skin induces mast cell degranulation and release of tumour necrosis factor-alpha. Immunol. Cell Biol. 1995, 73, 226–233. [Google Scholar] [CrossRef]
- Pinho-Ribeiro, F.A.; Verri, W.A., Jr.; Chiu, I.M. Nociceptor Sensory Neuron-Immune Interactions in Pain and Inflammation. Trends Immunol. 2017, 38, 5–19. [Google Scholar] [CrossRef]
- Mocsai, A. Diverse novel functions of neutrophils in immunity, inflammation, and beyond. J. Exp. Med. 2013, 210, 1283–1299. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, H.; Gomi, T.; Shishido, M.; Watanabe, H.; Suenobu, N. Neutrophil elastase contributes to extracellular matrix damage induced by chronic low-dose UV irradiation in a hairless mouse photoaging model. J. Dermatol. Sci. 2010, 60, 151–158. [Google Scholar] [CrossRef]
- Faustin, B.; Reed, J.C. Sunburned skin activates inflammasomes. Trends Cell Biol. 2008, 18, 4–8. [Google Scholar] [CrossRef]
- Enk, C.D.; Sredni, D.; Blauvelt, A.; Katz, S.I. Induction of IL-10 gene expression in human keratinocytes by UVB exposure in vivo and in vitro. J. Immunol. 1995, 154, 4851–4856. [Google Scholar]
- Lee, H.S.; Kooshesh, F.; Sauder, D.N.; Kondo, S. Modulation of TGF-beta 1 production from human keratinocytes by UVB. Exp. Dermatol. 1997, 6, 105–110. [Google Scholar] [CrossRef]
- Verri, W.A., Jr.; Cunha, T.M.; Parada, C.A.; Poole, S.; Cunha, F.Q.; Ferreira, S.H. Hypernociceptive role of cytokines and chemokines: Targets for analgesic drug development? Pharmacol. Ther. 2006, 112, 116–138. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Wu, S.H.; Zhang, L.; Chen, X.Q. Roles of lipoxin A4 receptor activation and anti-interleukin-1beta antibody on the toll-like receptor 2/mycloid differentiation factor 88/nuclear factor-kappaB pathway in airway inflammation induced by ovalbumin. Mol. Med. Rep. 2015, 12, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Hughes, E.L.; Becker, F.; Flower, R.J.; Buckingham, J.C.; Gavins, F.N.E. Mast cells mediate early neutrophil recruitment and exhibit anti-inflammatory properties via the formyl peptide receptor 2/lipoxin A4 receptor. Br. J. Pharmacol. 2017, 174, 2393–2408. [Google Scholar] [CrossRef] [PubMed]
- Vicentini, F.T.; He, T.; Shao, Y.; Fonseca, M.J.; Verri, W.A., Jr.; Fisher, G.J.; Xu, Y. Quercetin inhibits UV irradiation-induced inflammatory cytokine production in primary human keratinocytes by suppressing NF-kappaB pathway. J. Dermatol. Sci. 2011, 61, 162–168. [Google Scholar] [CrossRef]
- Oliveira, M.M.; Ratti, B.A.; Dare, R.G.; Silva, S.O.; Truiti, M.; Ueda-Nakamura, T.; Auzely-Velty, R.; Nakamura, C.V. Dihydrocaffeic Acid Prevents UVB-Induced Oxidative Stress Leading to the Inhibition of Apoptosis and MMP-1 Expression via p38 Signaling Pathway. Oxidative Med. Cell. Longev. 2019. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-kappaB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2. [Google Scholar] [CrossRef]
- Fattori, V.; Amaral, F.A.; Verri, W.A., Jr. Neutrophils and arthritis: Role in disease and pharmacological perspectives. Pharm. Res. 2016, 112, 84–98. [Google Scholar] [CrossRef]
- Proebstl, D.; Voisin, M.B.; Woodfin, A.; Whiteford, J.; D’Acquisto, F.; Jones, G.E.; Rowe, D.; Nourshargh, S. Pericytes support neutrophil subendothelial cell crawling and breaching of venular walls in vivo. J. Exp. Med. 2012, 209, 1219–1234. [Google Scholar] [CrossRef]
- Hattori, H.; Subramanian, K.K.; Sakai, J.; Jia, Y.; Li, Y.; Porter, T.F.; Loison, F.; Sarraj, B.; Kasorn, A.; Jo, H.; et al. Small-molecule screen identifies reactive oxygen species as key regulators of neutrophil chemotaxis. Proc. Natl. Acad. Sci. USA 2010, 107, 3546–3551. [Google Scholar] [CrossRef]
- Anrather, J.; Racchumi, G.; Iadecola, C. NF-kappaB regulates phagocytic NADPH oxidase by inducing the expression of gp91phox. J. Biol. Chem. 2006, 281, 5657–5667. [Google Scholar] [CrossRef]
- Martinez, R.M.; Ivan, A.L.M.; Vale, D.L.; Campanini, M.Z.; Ferreira, V.S.; Steffen, V.S.; Vicentini, F.; Vilela, F.M.P.; Fonseca, M.J.V.; Baracat, M.M.; et al. Topical emulsion containing pyrrolidine dithiocarbamate: Effectiveness against ultraviolet B irradiation-induced injury of hairless mouse skin. J. Pharm. Pharmacol. 2018, 70, 1461–1473. [Google Scholar] [CrossRef] [PubMed]
- Martinez, R.M.; Pinho-Ribeiro, F.A.; Steffen, V.S.; Caviglione, C.V.; Vignoli, J.A.; Barbosa, D.S.; Baracat, M.M.; Georgetti, S.R.; Verri, W.A., Jr.; Casagrande, R. Naringenin Inhibits UVB Irradiation-Induced Inflammation and Oxidative Stress in the Skin of Hairless Mice. J. Nat. Prod. 2015, 78, 1647–1655. [Google Scholar] [CrossRef] [PubMed]
- Gunaseelan, S.; Balupillai, A.; Govindasamy, K.; Ramasamy, K.; Muthusamy, G.; Shanmugam, M.; Thangaiyan, R.; Robert, B.M.; Prasad Nagarajan, R.; Ponniresan, V.K.; et al. Linalool prevents oxidative stress activated protein kinases in single UVB-exposed human skin cells. PLoS ONE 2017, 12, e0176699. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.H.; Qu, J.; Shen, X. NF-kappaB/p65 antagonizes Nrf2-ARE pathway by depriving CBP from Nrf2 and facilitating recruitment of HDAC3 to MafK. Biochim. Biophys. Acta 2008, 1783, 713–727. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Li, H.; Liu, Q.; Liu, F.; Tang, L.; Li, C.; Yuan, Y.; Zhan, Y.; Xu, W.; Li, W.; et al. Nuclear factor p65 interacts with Keap1 to repress the Nrf2-ARE pathway. Cell Signal. 2011, 23, 883–892. [Google Scholar] [CrossRef]
- Verri, W.A., Jr.; Vicentini, F.T.M.C.; Baracat, M.M.; Georgetti, S.R.; Cardoso, R.D.; Cunha, T.M.; Ferreira, S.H.; Cunha, F.Q.; Fonseca, M.J.; Casagrande, R. Flavonoids as Anti-Inflammatory and Analgesic Drugs: Mechanisms of Action and Perspectives in the Development of Pharmaceutical Forms. In Studies in Natural Products Chemistry, 1st ed.; Rahman, A.U., Ed.; Elsevier: Amsterdam, The Netherlands, 2012; Volume 36, pp. 297–330. [Google Scholar]
- Wakabayashi, N.; Slocum, S.L.; Skoko, J.J.; Shin, S.; Kensler, T.W. When NRF2 talks, who’s listening? Antioxid. Redox Signal. 2010, 13, 1649–1663. [Google Scholar] [CrossRef]
- Chiang, N.; Fredman, G.; Backhed, F.; Oh, S.F.; Vickery, T.; Schmidt, B.A.; Serhan, C.N. Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature 2012, 484, 524–528. [Google Scholar] [CrossRef]
- Aberg, K.M.; Radek, K.A.; Choi, E.H.; Kim, D.K.; Demerjian, M.; Hupe, M.; Kerbleski, J.; Gallo, R.L.; Ganz, T.; Mauro, T.; et al. Psychological stress downregulates epidermal antimicrobial peptide expression and increases severity of cutaneous infections in mice. J. Clin. Investig. 2007, 117, 3339–3349. [Google Scholar] [CrossRef]
- Tristao, F.S.; Rocha, F.A.; Moreira, A.P.; Cunha, F.Q.; Rossi, M.A.; Silva, J.S. 5-Lipoxygenase activity increases susceptibility to experimental Paracoccidioides brasiliensis infection. Infect. Immun. 2013, 81, 1256–1266. [Google Scholar] [CrossRef]
- El-Agamy, D.S.; Makled, M.N.; Gamil, N.M. Protective effects of BML-111 against acetaminophen-induced acute liver injury in mice. J. Physiol. Biochem. 2014, 70, 141–149. [Google Scholar] [CrossRef]
- Martinez, R.M.; Pinho-Ribeiro, F.A.; Steffen, V.S.; Caviglione, C.V.; Fattori, V.; Bussmann, A.J.C.; Bottura, C.; Fonseca, M.J.V.; Vignoli, J.A.; Baracat, M.M.; et al. trans-Chalcone, a flavonoid precursor, inhibits UV-induced skin inflammation and oxidative stress in mice by targeting NADPH oxidase and cytokine production. Photochem. Photobiol. Sci. 2017, 16, 1162–1173. [Google Scholar] [CrossRef] [PubMed]
- Katalinic, V.; Modun, D.; Music, I.; Boban, M. Gender differences in antioxidant capacity of rat tissues determined by 2,2’-azinobis (3-ethylbenzothiazoline 6-sulfonate; ABTS) and ferric reducing antioxidant power (FRAP) assays. Comp. Biochem. Physiol. C Toxicol. Pharmcol. 2005, 140, 47–52. [Google Scholar] [CrossRef] [PubMed]
- John, A.; Tuszynski, G. The role of matrix metalloproteinases in tumor angiogenesis and tumor metastasis. Pathol. Oncol. Res. 2001, 7, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.F.; Schafer, F.Q.; Buettner, G.R.; Rodgers, V.G. The rate of cellular hydrogen peroxide removal shows dependency on GSH: Mathematical insight into in vivo H2O2 and GPx concentrations. Free Radic. Res. 2007, 41, 1201–1211. [Google Scholar] [CrossRef]
- Bray, T.M.; Taylor, C.G. Tissue glutathione, nutrition, and oxidative stress. Can. J. Physiol. Pharmacol. 1993, 71, 746–751. [Google Scholar] [CrossRef]
- Harper, J.I.; Godwin, H.; Green, A.; Wilkes, L.E.; Holden, N.J.; Moffatt, M.; Cookson, W.O.; Layton, G.; Chandler, S. A study of matrix metalloproteinase expression and activity in atopic dermatitis using a novel skin wash sampling assay for functional biomarker analysis. Br. J. Dermatol. 2010, 162, 397–403. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds can be acquired from the cited Companies. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez, R.M.; Fattori, V.; Saito, P.; Pinto, I.C.; Rodrigues, C.C.A.; Melo, C.P.B.; Bussmann, A.J.C.; Staurengo-Ferrari, L.; Bezerra, J.R.; Vignoli, J.A.; et al. The Lipoxin Receptor/FPR2 Agonist BML-111 Protects Mouse Skin Against Ultraviolet B Radiation. Molecules 2020, 25, 2953. https://doi.org/10.3390/molecules25122953
Martinez RM, Fattori V, Saito P, Pinto IC, Rodrigues CCA, Melo CPB, Bussmann AJC, Staurengo-Ferrari L, Bezerra JR, Vignoli JA, et al. The Lipoxin Receptor/FPR2 Agonist BML-111 Protects Mouse Skin Against Ultraviolet B Radiation. Molecules. 2020; 25(12):2953. https://doi.org/10.3390/molecules25122953
Chicago/Turabian StyleMartinez, Renata M., Victor Fattori, Priscila Saito, Ingrid C. Pinto, Camilla C. A. Rodrigues, Cristina P. B. Melo, Allan J. C. Bussmann, Larissa Staurengo-Ferrari, Julia Rojo Bezerra, Josiane A. Vignoli, and et al. 2020. "The Lipoxin Receptor/FPR2 Agonist BML-111 Protects Mouse Skin Against Ultraviolet B Radiation" Molecules 25, no. 12: 2953. https://doi.org/10.3390/molecules25122953
APA StyleMartinez, R. M., Fattori, V., Saito, P., Pinto, I. C., Rodrigues, C. C. A., Melo, C. P. B., Bussmann, A. J. C., Staurengo-Ferrari, L., Bezerra, J. R., Vignoli, J. A., Baracat, M. M., Georgetti, S. R., Verri Jr., W. A., & Casagrande, R. (2020). The Lipoxin Receptor/FPR2 Agonist BML-111 Protects Mouse Skin Against Ultraviolet B Radiation. Molecules, 25(12), 2953. https://doi.org/10.3390/molecules25122953






