Thermodynamic Hydricity of Small Borane Clusters and Polyhedral closo-Boranes †
Abstract
1. Introduction
2. Results and Discussion
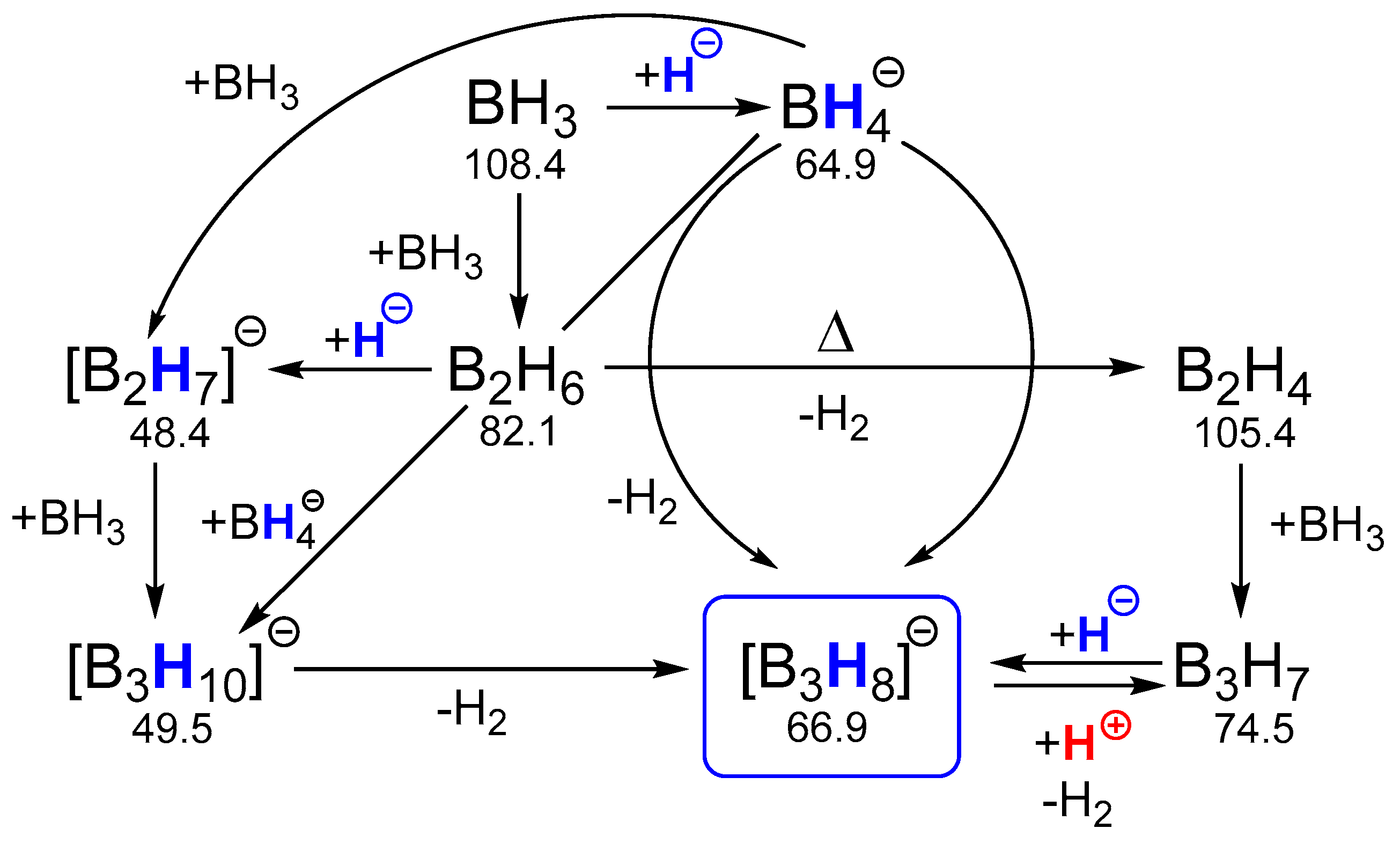
2.1. Thermodynamic Hydricity of Small Borane Clusters
2.1.1. General Pattern in Thermodynamic Hydricity of Borane Clusters
2.1.2. Features of Thermodynamic Hydricity in Homologous Series
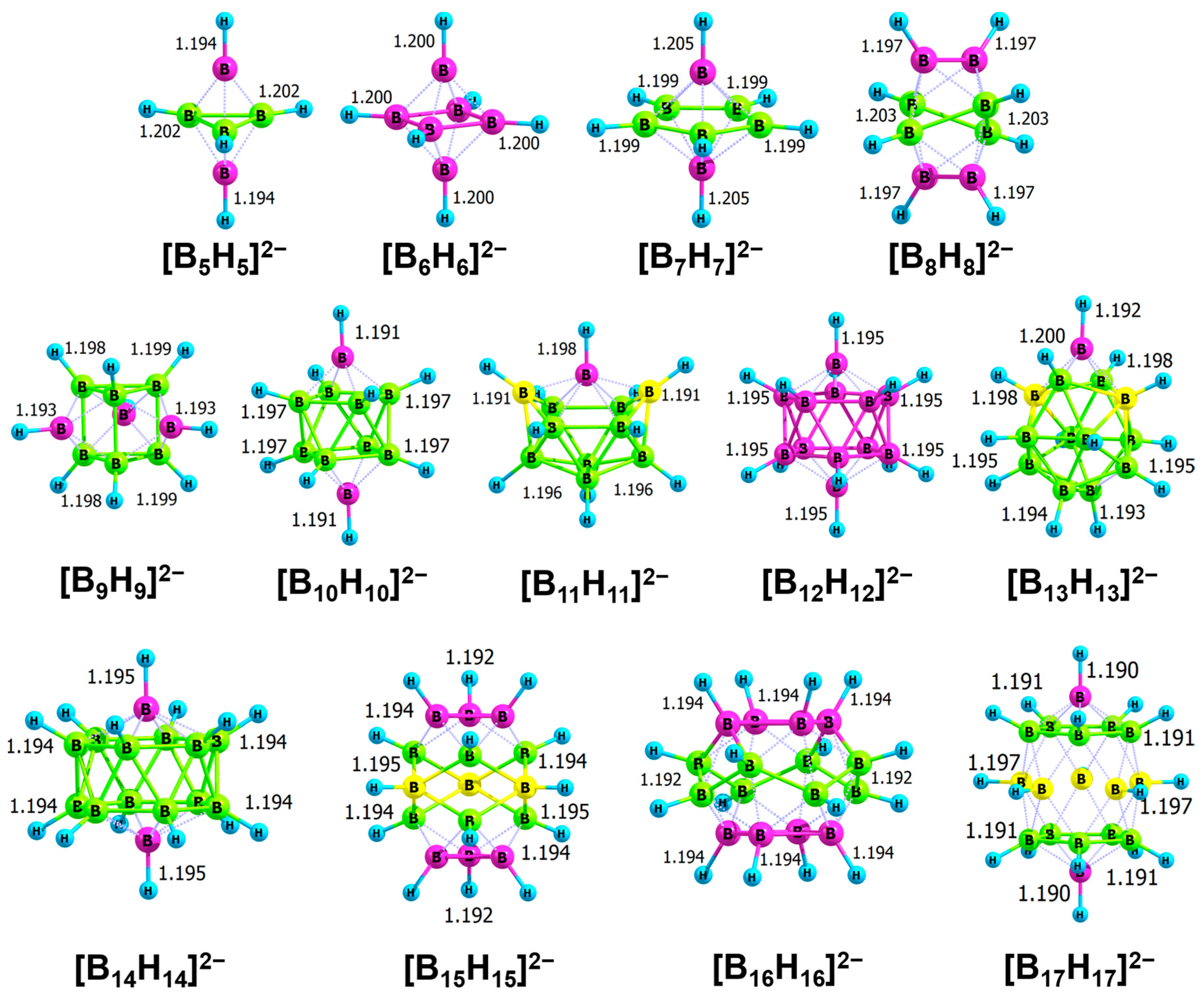
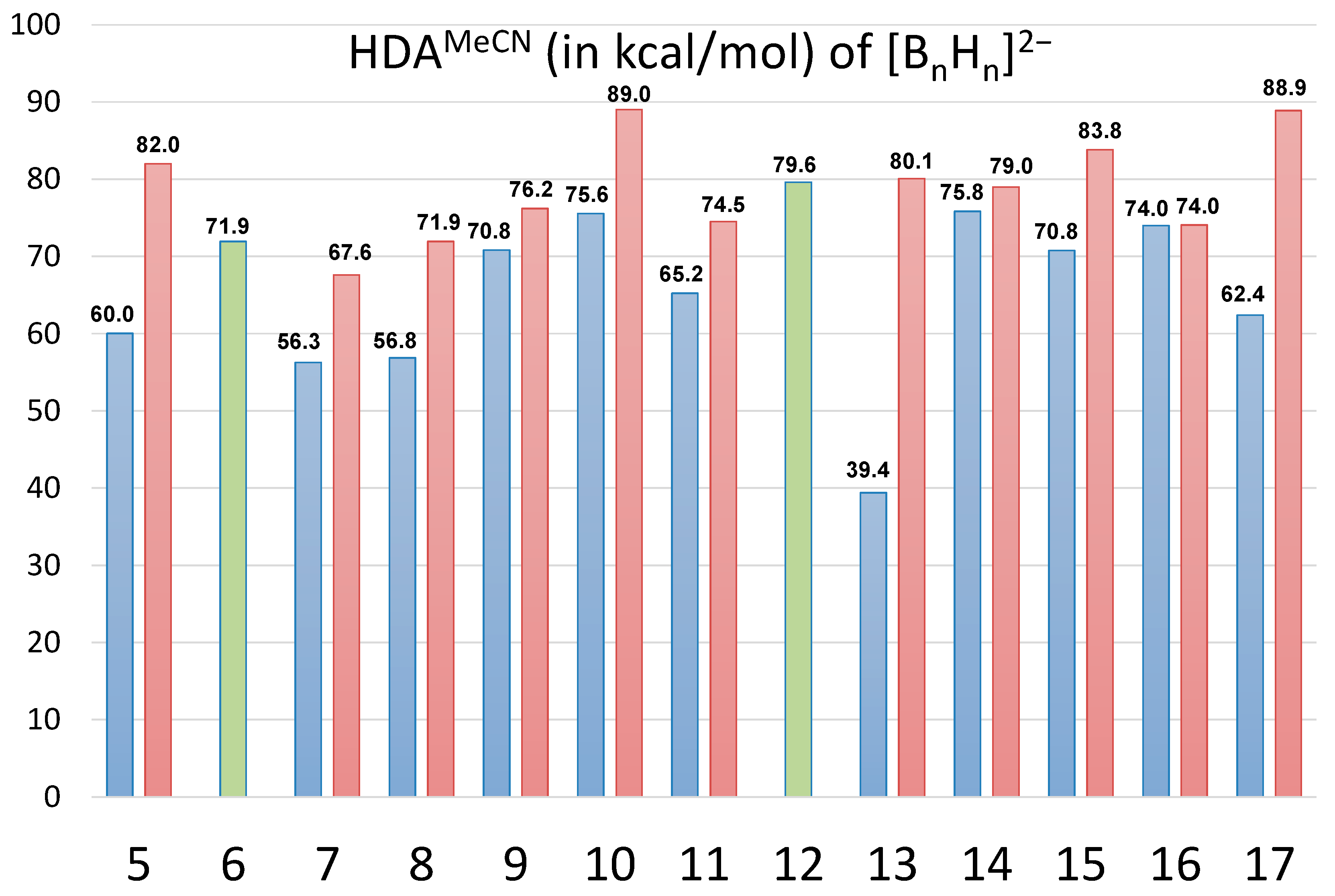
2.2. Thermodynamic Hydricity of Polyhedral Closo-Boranes
3. Materials and Methods
Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Greenwood, N.N. Electron-Deficient Boranes as Novel Electron-Donor Ligands. In ACS Symposium Series; American Chemical Society (ACS): Washington, WA, USA, 1994; Volume 565, pp. 333–345. [Google Scholar]
- Greenwood, N.N. The concept of boranes as ligands. Co-ord. Chem. Rev. 2002, 226, 61–69. [Google Scholar] [CrossRef]
- Alexandrova, A.N.; Boldyrev, A.I.; Zhai, H.-J.; Wang, L.-S. All-boron aromatic clusters as potential new inorganic ligands and building blocks in chemistry. Co-ord. Chem. Rev. 2006, 250, 2811–2866. [Google Scholar] [CrossRef]
- Besora, M.; Lledós, A. Coordination Modes and Hydride Exchange Dynamics in Transition Metal Tetrahydroborate Complexe. In Contemporary Metal Boron Chemistry I: Borylenes, Boryls, Borane Sigma-complexes, and Borohydrides; Springer: Berlin/Heidelberg, Germany, 2008; Volume 130, pp. 149–202. [Google Scholar]
- Hansen, B.R.S.; Paskevicius, M.; Li, H.-W.; Akiba, E.; Jensen, T.R. Metal boranes: Progress and applications. Coord. Chem. Rev. 2016, 323, 60–70. [Google Scholar] [CrossRef]
- Mohtadi, R.; Remhof, A.; Jena, P. Complex metal borohydrides: Multifunctional materials for energy storage and conversion. J. Phys. Condens. Matter 2016, 28, 353001. [Google Scholar] [CrossRef] [PubMed]
- Roll, M.F. Ionic borohydride clusters for the next generation of boron thin-films: Nano-building blocks for electrochemical and refractory materials. J. Mater. Res. 2016, 31, 2736–2748. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, S.; Dewhurst, R.D.; Ignat’Ev, N.V.; Finze, M.; Braunschweig, H. Boron: Its Role in Energy-Related Processes and Applications. Angew. Chem. Int. Ed. 2020, 59, 8800–8816. [Google Scholar] [CrossRef]
- Lu, Z.; Ciucci, F. Metal Borohydrides as Electrolytes for Solid-State Li, Na, Mg, and Ca Batteries: A First-Principles Study. Chem. Mater. 2017, 29, 9308–9319. [Google Scholar] [CrossRef]
- Guzik, M.N.; Mohtadi, R.; Sartori, S. Lightweight complex metal hydrides for Li-, Na-, and Mg-based batteries. J. Mater. Res. 2019, 34, 877–904. [Google Scholar] [CrossRef]
- Huang, S.; Qi, X.; Zhang, W.; Liu, T.; Zhang, Q. Exploring Sustainable Rocket Fuels: [Imidazolyl−Amine−BH2]+-Cation-Based Ionic Liquids as Replacements for Toxic Hydrazine Derivatives. Chem. Asian J. 2015, 10, 2725–2732. [Google Scholar] [CrossRef]
- Huang, S.; Qi, X.; Liu, T.; Wang, K.; Zhang, W.; Li, J.; Zhang, Q. Towards Safer Rocket Fuels: Hypergolic Imidazolylidene-Borane Compounds as Replacements for Hydrazine Derivatives. Chem. Eur. J. 2016, 22, 10187–10193. [Google Scholar] [CrossRef]
- Liu, T.; Qi, X.; Huang, S.; Jiang, L.; Li, J.; Tang, C.; Zhang, Q. Exploiting hydrophobic borohydride-rich ionic liquids as faster-igniting rocket fuels. Chem. Commun. 2016, 52, 2031–2034. [Google Scholar] [CrossRef] [PubMed]
- Ley, M.B.; Jepsen, L.H.; Lee, Y.-S.; Cho, Y.W.; Von Colbe, J.B.; Dornheim, M.; Rokni, M.M.; Jensen, J.O.; Sloth, M.; Filinchuk, Y.; et al. Complex hydrides for hydrogen storage—new perspectives. Mater. Today 2014, 17, 122–128. [Google Scholar] [CrossRef]
- Mohtadi, R.; Orimo, S.-I. The renaissance of hydrides as energy materials. Nat. Rev. Mater. 2016, 2, 16091. [Google Scholar] [CrossRef]
- Callini, E.; Atakli, Z.; Özlem, K.; Hauback, B.C.; Orimo, S.-I.; Jensen, C.; Dornheim, M.; Grant, D.M.; Cho, Y.W.; Chen, P.; et al. Complex and liquid hydrides for energy storage. Appl. Phys. A 2016, 122, 353. [Google Scholar] [CrossRef]
- Fisher, S.P.; Tomich, A.W.; Lovera, S.O.; Kleinsasser, J.F.; Guo, J.; Asay, M.J.; Nelson, H.M.; Lavallo, V. Nonclassical Applications of closo-Carborane Anions: From Main Group Chemistry and Catalysis to Energy Storage. Chem. Rev. 2019, 119, 8262–8290. [Google Scholar] [CrossRef] [PubMed]
- Hawthorne, M.F. New horizons for therapy based on the boron neutron capture reaction. Mol. Med. Today 1998, 4, 174–181. [Google Scholar] [CrossRef]
- Barth, R.F.; Coderre, J.A.; Vicente, M.G.H.; Blue, T.E. Boron Neutron Capture Therapy of Cancer: Current Status and Future Prospects. Clin. Cancer Res. 2005, 11, 3987–4002. [Google Scholar] [CrossRef]
- Issa, F.; Kassiou, M.; Rendina, L. Boron in Drug Discovery: Carboranes as Unique Pharmacophores in Biologically Active Compounds. Chem. Rev. 2011, 111, 5701–5722. [Google Scholar] [CrossRef]
- Scholz, M.S.; Hey-Hawkins, E. Carbaboranes as Pharmacophores: Properties, Synthesis, and Application Strategies. Chem. Rev. 2011, 111, 7035–7062. [Google Scholar] [CrossRef]
- Detlef, G. Boron clusters in medicinal chemistry: Perspectives and problems. Pure Appl. Chem. 2015, 87, 173–179. [Google Scholar]
- Leśnikowski, Z. Challenges and Opportunities for the Application of Boron Clusters in Drug Design. J. Med. Chem. 2016, 59, 7738–7758. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yan, H.; Zhao, Q. Carboranes as a Tool to Tune Phosphorescence. Chem. Eur. J. 2016, 47, 1888–1898. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Thilagar, P. Boron clusters in luminescent materials. Chem. Commun. 2016, 52, 1070–1093. [Google Scholar] [CrossRef] [PubMed]
- Nunez, R.; Tarrés, M.; Ferrer-Ugalde, A.; De Biani, F.F.; Teixidor, F. Electrochemistry and Photoluminescence of Icosahedral Carboranes, Boranes, Metallacarboranes, and Their Derivatives. Chem. Rev. 2016, 116, 14307–14378. [Google Scholar] [CrossRef] [PubMed]
- Kaszyński, P.; Douglass, A.G. Organic derivatives of closo-boranes: A new class of liquid crystal materials. J. Organomet. Chem. 1999, 581, 28–38. [Google Scholar] [CrossRef]
- Ringstrand, B.; Kaszynski, P. Functionalization of the [closo-1-CB9H10]− Anion for the Construction of New Classes of Liquid Crystals. Acc. Chem. Res. 2013, 46, 214–225. [Google Scholar] [CrossRef]
- Han, Y.-F.; Jin, G.-X. Half-Sandwich Iridium- and Rhodium-based Organometallic Architectures: Rational Design, Synthesis, Characterization, and Applications. Acc. Chem. Res. 2014, 47, 3571–3579. [Google Scholar] [CrossRef]
- Housecroft, C.E. Carboranes as guests, counterions and linkers in coordination polymers and networks. J. Organomet. Chem. 2015, 798, 218–228. [Google Scholar] [CrossRef]
- Wiedner, E.S.; Chambers, M.B.; Pitman, C.L.; Bullock, R.M.; Miller, A.J.M.; Appel, A.M. Thermodynamic Hydricity of Transition Metal Hydrides. Chem. Rev. 2016, 116, 8655–8692. [Google Scholar] [CrossRef]
- Heiden, Z.M.; Lathem, A.P. Establishing the Hydride Donor Abilities of Main Group Hydrides. Organometallics 2015, 34, 1818–1827. [Google Scholar] [CrossRef]
- Alherz, A.; Lim, C.-H.; Hynes, J.T.; Musgrave, C.B. Predicting Hydride Donor Strength via Quantum Chemical Calculations of Hydride Transfer Activation Free Energy. J. Phys. Chem. B 2018, 122, 1278–1288. [Google Scholar] [CrossRef] [PubMed]
- Golub, I.E.; Filippov, O.A.; Belkova, N.V.; Epstein, L.M.; Shubina, E.S. Hydride donating abilities of the tetracoordinated boron hydrides. J. Organomet. Chem. 2018, 865, 247–256. [Google Scholar] [CrossRef]
- Lathem, A.P.; Treich, N.R.; Heiden, Z.M. Establishing the Steric Bulk of Main Group Hydrides in Reduction Reactions. Isr. J. Chem. 2015, 55, 226–234. [Google Scholar] [CrossRef]
- Sivaev, I.B.; Bregadze, V.I. Lewis acidity of boron compounds. Coord. Chem. Rev. 2014, 270, 75–88. [Google Scholar] [CrossRef]
- Sivaev, I.B.; Prikaznov, A.V.; Anufriev, S.A. On relative electronic effects of polyhedral boron hydrides. J. Organomet. Chem. 2013, 747, 254–256. [Google Scholar] [CrossRef]
- Greenwood, N.N.; Greatrex, R. Kinetics and mechanism of the thermolysis and photolysis of binary boranes. Pure Appl. Chem. 1987, 59, 857–868. [Google Scholar] [CrossRef]
- Hwang, S.-J.; Bowman, R.; Reiter, J.W.; Soloveichik, G.L.; Zhao, J.-C.; Kabbour, H.; Ahn, C.C. Rijssenbeek NMR Confirmation for Formation of [B12H12]2− Complexes during Hydrogen Desorption from Metal Borohydrides. J. Phys. Chem. C 2008, 112, 3164–3169. [Google Scholar] [CrossRef]
- Ozolins, V.; Majzoub, E.H.; Wolverton, C. First-Principles Prediction of Thermodynamically Reversible Hydrogen Storage Reactions in the Li-Mg-Ca-B-H System. J. Am. Chem. Soc. 2009, 131, 230–237. [Google Scholar] [CrossRef]
- Zhang, Y.; Majzoub, E.; Ozolins, V.; Wolverton, C. Theoretical prediction of different decomposition paths for Ca(BH4)2 and Mg(BH4)2. Phys. Rev. B 2010, 82, 174107. [Google Scholar] [CrossRef]
- Friedrichs, O.; Remhof, A.; Hwang, S.-J.; Züttel, A. Role of Li2B12H for the Formation and Decomposition of LiBH4. Chem. Mater. 2010, 22, 3265–3268. [Google Scholar] [CrossRef]
- Zhang, Y.; Majzoub, E.; Ozolins, V.; Wolverton, C. Theoretical Prediction of Metastable Intermediates in the Decomposition of Mg(BH4)2. J. Phys. Chem. C 2012, 116, 10522–10528. [Google Scholar] [CrossRef]
- Pitt, M.; Paskevicius, M.; Brown, D.H.; Sheppard, D.A.; Buckley, C.E. Thermal Stability of Li2B12H12 and its Role in the Decomposition of LiBH4. J. Am. Chem. Soc. 2013, 135, 6930–6941. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Remhof, A.; Rentsch, D.; Lee, Y.-S.; Whan Cho, Y.; Zuttel, A. Is Y2(B12H12)3 the main intermediate in the decomposition process of Y(BH4)3? Chem. Commun. 2013, 49, 5234–5236. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Remhof, A.; Rentsch, D.; Züttel, A. The role of MgB12H12 in the hydrogen desorption process of Mg(BH4)2. Chem. Commun. 2015, 51, 700–702. [Google Scholar] [CrossRef] [PubMed]
- Schouwink, P.; Sadikin, Y.; van Beek, W.; Černý, R. Experimental observation of polymerization from BH4− to B12H122− in mixed-anion A3BH4B12H12 (A = Rb+, Cs+). Int. J. Hydrogen Energy 2015, 40, 10902–10907. [Google Scholar] [CrossRef]
- Huang, Z.-Q.; Chen, W.-C.; Chuang, F.-C.; Majzoub, E.H.; Ozolins, V. First-principles calculated decomposition pathways for LiBH4 nanoclusters. Sci. Rep. 2016, 6, 26056. [Google Scholar] [CrossRef] [PubMed]
- McKee, M.L. Deconvoluting the Reaction Path from B10H14 Plus BH4− to B12H122−. Can Theory Make a Contribution? In Boron: The Fifth Element; Hnyk, D., McKee, M., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 121–138. [Google Scholar]
- Sethio, D.; Daku, L.M.L.; Hagemann, H.; Kraka, E. Quantitative Assessment of B−B−B, B−Hb−B, and B−Ht Bonds: From BH3 to B12H122−. ChemPhysChem 2019, 20, 1967–1977. [Google Scholar] [CrossRef]
- Remmel, R.J.; Johnson, H.D.; Jaworiwsky, I.S.; Shore, S.G. Preparation and nuclear magnetic resonance studies of the stereochemically nonrigid anions nonahydrotetraborate(1-)dodecahydropentaborate(1-), undecahydrohexaborate(1-), and dodecahydroheptaborate(1-). Improved syntheses of pentaborane(11) and hexaborane(12). J. Am. Chem. Soc. 1975, 97, 5395–5403. [Google Scholar] [CrossRef]
- Liu, X.-R.; Chen, X.-M.; Zhang, J.; Jensen, T.R.; Chen, X. The interconversion between THF·B3H7 and B3H8−: An efficient synthetic method for MB3H8 (M = Li and Na). Dalton Trans. 2019, 48, 5140–5143. [Google Scholar] [CrossRef]
- Fernández, E.; Whiting, A. Synthesis and Application of Organoboron Compounds; Springer International Publishing: Cham, Switzerland, 2015; Volume 49. [Google Scholar]
- Leach, J.B.; Toft, M.A.; Himpsl, F.L.; Shore, S.G. New, systematic, good yield syntheses of boron hydrides: Preparation of tetraborane(10) and pentaborane(11). A practical conversion of pentaborane(9) to decaborane(14). J. Am. Chem. Soc. 1981, 103, 988–989. [Google Scholar] [CrossRef]
- Toft, M.A.; Leach, J.B.; Himpsl, F.L.; Shore, S.G. New systematic syntheses of boron hydrides via hydride ion abstraction reactions: Preparation of B2H6, B4H10, B5H11, and B10H14. Inorg. Chem. 1982, 21, 1952–1957. [Google Scholar] [CrossRef]
- Nguyen, M.T.; Matus, M.H.; Dixon, D.A. Heats of Formation of Boron Hydride Anions and Dianions and Their Ammonium Salts [BnHmy−][NH4+]y with y = 1−2. Inorg. Chem. 2007, 46, 7561–7570. [Google Scholar] [CrossRef] [PubMed]
- Ilic, S.; Alherz, A.; Musgrave, C.B.; Glusac, K.D. Thermodynamic and kinetic hydricities of metal-free hydrides. Chem. Soc. Rev. 2018, 47, 2809–2836. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-M.; Ma, N.; Zhang, Q.-F.; Wang, J.; Feng, X.; Wei, C.; Wang, L.-S.; Zhang, J.; Chen, X. Elucidation of the Formation Mechanisms of the Octahydrotriborate Anion (B3H8-) through the Nucleophilicity of the B-H Bond. J. Am. Chem. Soc. 2018, 140, 6718–6726. [Google Scholar] [CrossRef] [PubMed]
- Shore, S.G. Studies of the smaller boron hydrides and their derivatives. Pure Appl. Chem. 1977, 49, 717–732. [Google Scholar] [CrossRef]
- Marks, T.J.; Kolb, J.R. Covalent transition metal, lanthanide, and actinide tetrahydroborate complexes. Chem. Rev. 1977, 77, 263–293. [Google Scholar] [CrossRef]
- Makhaev, V.D. Structural and dynamic properties of tetrahydroborate complexes. Russ. Chem. Rev. 2000, 69, 727–746. [Google Scholar] [CrossRef]
- Visseaux, M.; Bonnet, F. Borohydride complexes of rare earths, and their applications in various organic transformations. Co-ord. Chem. Rev. 2011, 255, 374–420. [Google Scholar] [CrossRef]
- Golub, I.E.; Filippov, O.A.; Gutsul, E.I.; Belkova, N.V.; Epstein, L.M.; Rossin, A.; Peruzzini, M.; Shubina, E.S. Dimerization Mechanism of Bis(triphenylphosphine)copper(I) Tetrahydroborate: Proton Transfer via a Dihydrogen Bond. Inorg. Chem. 2012, 51, 6486–6497. [Google Scholar] [CrossRef]
- Belkova, N.V.; Bakhmutova-Albert, E.V.; Gutsul, E.I.; Bakhmutov, V.I.; Golub, I.E.; Filippov, O.A.; Epstein, L.M.; Peruzzini, M.; Rossin, A.; Zanobini, F.; et al. Dihydrogen Bonding in Complex (PP3)RuH(η1-BH4) Featuring Two Proton-Accepting Hydride Sites: Experimental and Theoretical Studies. Inorg. Chem. 2013, 53, 1080–1090. [Google Scholar] [CrossRef]
- Golub, I.E.; Filippov, O.A.; Belkova, N.V.; Epstein, L.M.; Rossin, A.; Peruzzini, M.; Shubina, E.S. Two pathways of proton transfer reaction to (triphos)Cu(η1-BH4) via a dihydrogen bond [triphos = 1,1,1-tris(diphenylphosphinomethyl)ethane]. Dalton Trans. 2016, 45, 9127–9135. [Google Scholar] [CrossRef] [PubMed]
- Belkova, N.V.; Golub, I.E.; Gutsul, E.I.; Lyssenko, K.A.; Peregudov, A.S.; Makhaev, V.D.; Filippov, O.A.; Epstein, L.M.; Rossin, A.; Peruzzini, M.; et al. Binuclear Copper(I) Borohydride Complex Containing Bridging Bis(diphenylphosphino) Methane Ligands: Polymorphic Structures of [(µ2-dppm)2Cu2(η2-BH4)2] Dichloromethane Solvate. Crystals 2017, 7, 318. [Google Scholar] [CrossRef]
- Golub, I.E.; Filippov, O.A.; Belkova, N.V.; Gutsul, E.I.; Epstein, L.M.; Rossin, A.; Peruzzini, M.; Shubina, E.S. Competition between the Hydride Ligands of Two Types in Proton Transfer to [{κ3-P-CH3C(CH2CH2PPh2)3}RuH(η2-BH4)]. Eur. J. Inorg. Chem. 2017, 2017, 4673–4682. [Google Scholar] [CrossRef]
- Safronov, S.V.; Gutsul, E.I.; Golub, I.E.; Dolgushin, F.M.; Nelubina, Y.V.; Filippov, O.A.; Epstein, L.M.; Peregudov, A.S.; Belkova, N.V.; Shubina, E.S.; et al. Synthesis, structural properties and reactivity of ruthenocene-based pincer Pd(II) tetrahydroborate. Dalton Trans. 2019, 48, 12720–12729. [Google Scholar] [CrossRef]
- Grebenik, P.D.; Green, M.L.H.; Kelland, M.A.; Leach, J.B.; Mountford, P.; Stringer, G.; Walker, N.M.; Wong, L.-L. Formation of three-vertex metallaboranes from monoborane precursors: X-ray crystal structures of the molybdenum and ruthenium complexes [Mo(η-C5H5)2H(η2-B2H5)] and [Ru(η-C5Me5)(PMe3)(η2-B2H7)]. J. Chem. Soc. Chem. Commun. 1988, 12, 799. [Google Scholar] [CrossRef]
- Green, M.L.H.; Leach, J.B.; Kelland, M.A. Synthesis and Interconversion of Some Small Ruthenaboranes: Reaction of a Ruthenium Borohydride with Pentaborane(9) to Form Larger Ruthenaboranes. Organometallics 2007, 26, 4031–4037. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Misra, N. Designing new electrolytic salts for Lithium Ion Batteries using superhalogen anions. Polyhedron 2016, 117, 422–426. [Google Scholar] [CrossRef]
- Alcaraz, G.; Sabo-Etienne, S. Coordination and Dehydrogenation of Amine-Boranes at Metal Centers. Angew. Chem. Int. Ed. 2010, 49, 7170–7179. [Google Scholar] [CrossRef]
- Titov, A.A.; Guseva, E.A.; Smol’Yakov, A.F.; Dolgushin, F.M.; Filippov, O.A.; Golub, I.E.; Krylova, A.I.; Babakhina, G.M.; Epstein, L.M.; Shubina, E.S. Complexation of trimeric copper(i) and silver(i) 3,5-bis(trifluoromethyl)pyrazolates with amine-borane. Russ. Chem. Bull. 2013, 62, 1829–1834. [Google Scholar] [CrossRef]
- Johnson, H.C.; Hooper, T.N.; Weller, A.S. The Catalytic Dehydrocoupling of Amine–Boranes and Phosphine–Boranes. In Topics in Organometallic Chemistry; Springer International Publishing: Cham, Switzerland, 2015; Volume 49, pp. 153–220. [Google Scholar]
- Rossin, A.; Peruzzini, M. Ammonia–Borane and Amine–Borane Dehydrogenation Mediated by Complex Metal Hydrides. Chem. Rev. 2016, 116, 8848–8872. [Google Scholar] [CrossRef]
- Todisco, S.; Luconi, L.; Giambastiani, G.; Rossin, A.; Peruzzini, M.; Golub, I.E.; Filippov, O.A.; Belkova, N.V.; Shubina, E.S. Ammonia Borane Dehydrogenation Catalyzed by (κ4-EP3)Co(H) [EP3 = E(CH2CH2PPh2)3; E = N, P] and H2 Evolution from Their Interaction with NH Acids. Inorg. Chem. 2017, 56, 4296–4307. [Google Scholar] [CrossRef] [PubMed]
- Colebatch, A.L.; Weller, A.S. Amine–Borane Dehydropolymerization: Challenges and Opportunities. Chem. Eur. J. 2018, 25, 1379–1390. [Google Scholar] [CrossRef] [PubMed]
- McKee, M.L.; Lipscomb, W.N. Symmetry lowering in boranes B3H9 and B4H12. Inorg. Chem. 1985, 24, 2317–2319. [Google Scholar] [CrossRef]
- Duke, B.; Gauld, J.W.; Schaefer, H.F. Ab Initio Characterization of a Triborane(9) Isomer with a Pentacoordinated Central Boron Atom. J. Am. Chem. Soc. 1995, 117, 7753–7755. [Google Scholar] [CrossRef]
- Korkin, A.A.; Schleyer, P.V.R.; McKee, M.L. Theoretical ab Initio Study of Neutral and Charged B3Hn (n = 3–9) Species. Importance of Aromaticity in Determining the Structural Preferences. Inorg. Chem. 1995, 34, 961–977. [Google Scholar] [CrossRef]
- Parshall, G.W. Borane Complexes of Transition Metals. J. Am. Chem. Soc. 1964, 86, 361–364. [Google Scholar] [CrossRef]
- Aldridge, S.; Shang, M.; Fehlner, T.P. Synthesis of Novel Molybdaboranes from (η5-C5R5)MoCln Precursors (R = H, Me; n = 1, 2, 4). J. Am. Chem. Soc. 1998, 120, 2586–2598. [Google Scholar] [CrossRef]
- Weller, A.S.; Shang, M.; Fehlner, T.P. Synthesis of Mono- and Ditungstaboranes from Reaction of Cp*WCl4and [Cp*WCl2]2 with BH3·thf or LiBH4(Cp* = η5-C5Me5). Control of Reaction Pathway by Choice of Monoboron Reagent and Oxidation State of Metal Center. Organometallics 1999, 18, 53–64. [Google Scholar] [CrossRef]
- Geetharani, K.; Tussupbayev, S.; Borowka, J.; Holthausen, M.C.; Ghosh, S. A Mechanistic Study of the Utilization of arachno-Diruthenaborane [(Cp*RuCO)2B2H6] as an Active Alkyne-Cyclotrimerization Catalyst. Chem. Eur. J. 2012, 18, 8482–8489. [Google Scholar] [CrossRef]
- Sharmila, D.; Mondal, B.; Ramalakshmi, R.; Kundu, S.; Varghese, B.; Ghosh, S. First-Row Transition-Metal-Diborane and -Borylene Complexes. Chem. Eur. J. 2015, 21, 5074–5083. [Google Scholar] [CrossRef]
- Prakash, R.; Bakthavachalam, K.; Varghese, B.; Ghosh, S. Chlorination of the terminal hydrogen atoms in the hydrogen-rich group 5 dimetallaboranes (Cp*M)2(B2H6)2 (M = Nb, Ta). J. Organomet. Chem. 2017, 846, 372–378. [Google Scholar] [CrossRef]
- Prakash, R.; Pradhan, A.N.; Jash, M.; Kahlal, S.; Cordier, M.; Roisnel, T.; Halet, J.-F.; Ghosh, S. Diborane(6) and Its Analogues Stabilized by Mono-, Bi-, and Trinuclear Group 7 Templates: Combined Experimental and Theoretical Studies. Inorg. Chem. 2020, 59, 1917–1927. [Google Scholar] [CrossRef] [PubMed]
- Haridas, A.; Kar, S.; Raghavendra, B.; Roisnel, T.; Dorcet, V.; Ghosh, S. B–H Functionalization of Hydrogen-Rich [(Cp*V)2(B2H6)2]: Synthesis and Structures of [(Cp*V)2(B2X2)2H8] (X = Cl, SePh; Cp* = η5-C5Me5). Organometallics 2019, 39, 58–65. [Google Scholar] [CrossRef]
- Godfroid, R.A.; Hill, T.G.; Onak, T.P.; Shore, S.G. Formation of [BH3]2− and [B2H6]2− From the Homogeneous Reduction of B2H6. J. Am. Chem. Soc. 1994, 116, 12107–12108. [Google Scholar] [CrossRef]
- Lippard, S.J.; Melmed, K.M. Transition metal borohydride complexes. III. Structure of octahydrotriboratobis(triphenylphosphine)copper(I). Inorg. Chem. 1969, 8, 2755–2762. [Google Scholar] [CrossRef]
- Ghosh, S.; Beatty, A.M.; Fehlner, T.P. The Reaction of Cp*ReH6, Cp* = C5Me5, with Monoborane to Yield a Novel Rhenaborane. Synthesis and Characterization of arachno-Cp*ReH3B3H8. Collect. Czechoslov. Chem. Commun. 2002, 67, 808–812. [Google Scholar] [CrossRef]
- Kim, D.Y.; You, Y.; Girolami, G.S. Synthesis and crystal structures of two (cyclopentadienyl)titanium(III) hydroborate complexes, [Cp∗TiCl(BH4)]2 and Cp2Ti(B3H8). J. Organomet. Chem. 2008, 693, 981–986. [Google Scholar] [CrossRef]
- Ramalakshmi, R.; Bhattacharyya, M.; Rao, C.E.; Ghosh, S. Synthesis, structure and chemistry of low-boron containing molybdaborane: Arachno -[Cp*Mo(CO)2B3H8 ]. J. Organomet. Chem. 2015, 792, 31–36. [Google Scholar] [CrossRef]
- Joseph, B.; Saha, K.; Prakash, R.; Nandi, C.; Roisnel, T.; Ghosh, S. Chalcogenolato-bridged dinuclear half sandwich complexes of ruthenium and iridium. Inorganica Chim. Acta 2018, 483, 106–110. [Google Scholar] [CrossRef]
- Titov, L.V.; Levicheva, M.D.; Psikha, S.B. Synthesis and Thermal Decomposition of Magnesium, Calcium, and Strontium Octahydrotriborates Solvated with Diglyme. Russ. J. Inorg. Chem. 1984, 29, 386–389. [Google Scholar]
- Rice, J.K.; Caldwell, N.J.; Nelson, H.H. Gas-phase reaction kinetics of boron monohydride. J. Phys. Chem. 1989, 93, 3600–3605. [Google Scholar] [CrossRef]
- Garland, N.L.; Stanton, C.T.; Fleming, J.W.; Baronavski, A.P.; Nelson, H.H. Boron monohydride reaction kinetics studied with a high-temperature reactor. J. Phys. Chem. 1990, 94, 4952–4956. [Google Scholar] [CrossRef]
- Ruscic, B.; Schwarz, M.; Berkowitz, J. Molecular structure and thermal stability of B2H4 and B2H+4 species. J. Chem. Phys. 1989, 91, 4576–4581. [Google Scholar] [CrossRef]
- Reddy, K.H.K.; Jemmis, E.D. Stabilization of diborane(4) by transition metal fragments and a novel metal to π Dewar–Chatt–Duncanson model of back donation. Dalton Trans. 2013, 42, 10633. [Google Scholar] [CrossRef] [PubMed]
- Kameda, M.; Kodama, G. Cleavage reaction of pentaborane(9). Formation of a new hypho triborane adduct. Inorg. Chem. 1980, 19, 2288–2292. [Google Scholar] [CrossRef]
- Snow, S.A.; Shimoi, M.; Ostler, C.D.; Thompson, B.K.; Kodama, G.; Parry, R.W. Metal complexes of the neutral borane adduct (B2H4∙2P(CH3)3). Inorg. Chem. 1984, 23, 511–512. [Google Scholar] [CrossRef]
- Katoh, K.; Shimoi, M.; Ogino, H. Syntheses and structures of [M(CO)5{B2H4∙2P(CH3)3}] and [M(CO)4{B2H4∙2P(CH3)3}] (M = chromium, molybdenum, tungsten). Inorg. Chem. 1992, 31, 670–675. [Google Scholar] [CrossRef]
- Parry, R.; Kodama, G. Coordination compounds formed using three-center hydrogen bridge bonds: An extension of the Lewis donor—acceptor coordinate bond. Co-ord. Chem. Rev. 1993, 128, 245–260. [Google Scholar] [CrossRef]
- Hata, M.; Kawano, Y.; Shimoi, M. Synthesis and Structure of a Dichromatetraborane Derivative [{(OC)4Cr}2(η4-H,H‘,H‘‘,H‘‘‘-BH2BH2·PMe2CH2PMe2)]. Inorg. Chem. 1998, 37, 4482–4483. [Google Scholar] [CrossRef]
- Shimoi, M.; Katoh, K.; Tobita, H.; Ogino, H. Syntheses and properties of bis{bis(trimethylphosphine)tetrahydrodiboron}copper(1+) halide (halide = chloride, iodide) and x-ray crystal structure of the iodide. Inorg. Chem. 1990, 29, 814–817. [Google Scholar] [CrossRef]
- Ring, M.A.; Witucki, E.F.; Greenough, R.C. Tautomerism exchange in B3H7.N(CH3)3 and B3H7.-THF. Inorg. Chem. 1967, 6, 395–396. [Google Scholar] [CrossRef]
- Dewkett, W.; Beall, H.; Bushweller, C. Quadrupolar relaxation and intramolecular exchange in (C6H5CH2)2NCH3(B3H7). Inorg. Nucl. Chem. Lett. 1971, 7, 633–636. [Google Scholar] [CrossRef]
- Driess, M.; Nöth, H. Molecular Clusters of the Main Group Elements; Wiley-VCH: Weinheim, Germany, 2008. [Google Scholar]
- Nordman, C.E.; Lipscomb, W.N. The molecular structure of B4H10. J. Am. Chem. Soc. 1953, 75, 4116–4117. [Google Scholar] [CrossRef]
- Brain, P.T.; Morrison, C.A.; Parsons, S.; Rankin, D.W.H. Tetraborane(10), B4H10: Structures in gaseous and crystalline phases. J. Chem. Soc. Dalton Trans. 1996, 24, 4589. [Google Scholar] [CrossRef]
- Kaufmann, E.; Schleyer, P.v.R. Dilithiodiborane(6) (Li2B2H4). An experimentally viable species with a B=B double bond. Planar no-bond-double-bond isomers with pentacoordinate boron? Inorg. Chem. 1988, 27, 3987–3992. [Google Scholar] [CrossRef]
- Muetterties, E.L.; Kane, A.R. Metalloboranes. VI. A B3H72− derivative of platinum. A possible π-allyl analog based on boron. J. Am. Chem. Soc. 1971, 93, 1041–1042. [Google Scholar] [CrossRef]
- Guggenberger, L.J.; Kane, A.R.; Muetterties, E.L. Metalloboranes. VII. Synthesis and chemistry of π-borallyl complexes and the crystal structure of [(CH3)2PC6H5]2PtB3H7. J. Am. Chem. Soc. 1972, 94, 5665–5673. [Google Scholar] [CrossRef]
- DiPasquale, A.; Lei, X.; Fehlner, T.P. Metallaborane Reactivity. Complexities of Cobalt Carbonyl Fragment Addition to 1,2-{Cp*RuH}2B3H7, Cp* = η5-C5Me5, and Characterization of a Diruthenium Analogue of Pentaborane(11) 1,2-{Cp*Ru}2(CO)2B3H7. Organometallics 2001, 20, 5044–5049. [Google Scholar] [CrossRef]
- Qu, Z.-W.; Li, Z.-S.; Ding, Y.-H.; Sun, C.-C. Theoretical Study of the Gas-Phase Reaction of Diborane(3) Anion B2H3-with CO2. J. Phys. Chem. A 2000, 104, 11952–11960. [Google Scholar] [CrossRef]
- Guermoune, A.; Jarid, A.; Ouassas, A.; Chafiq, S.; Es-Sofi, A. DFT and MP2 investigation of the B2H3− anion potential energy surface. Chem. Phys. Lett. 2004, 399, 190–195. [Google Scholar] [CrossRef]
- Krempp, M.; Damrauer, R.; DePuy, C.H.; Keheyan, Y. Gas-Phase Ion Chemistry of Boron Hydride Anions. J. Am. Chem. Soc. 1994, 116, 3629–3630. [Google Scholar] [CrossRef]
- Shore, S.G.; Johnson, H.D. Deprotonation of tetraborane(10) by ammonia. The temperature-dependent boron-11 nuclear magnetic resonance spectrum of B4H9−. J. Am. Chem. Soc. 1970, 92, 7586–7587. [Google Scholar] [CrossRef]
- Shore, S.G.; Parry, R.W. The crystalline compound ammonia-borane,1 H3NBH3. J. Am. Chem. Soc. 1955, 77, 6084–6085. [Google Scholar] [CrossRef]
- Boocock, S.K.; Toft, M.A.; Inkrott, K.E.; Hsu, L.Y.; Huffman, J.C.; Folting, K.; Shore, S.G. Irida-, rhoda-, nickela-, and cuprapentaboranes derived from the arachno-[B4H9]- ion. Crystal structure of [Ir(η4-B4H9)(CO){P(CH3)2C6H5}2], an analog of arachno-B5H11 and [Ir(η4-C4H6)(CO){P(CH3)2C6H5}2]+. Inorg. Chem. 1984, 23, 3084–3091. [Google Scholar] [CrossRef]
- Kawano, Y.; Matsumoto, H.; Shimoi, M. Syntheses of Diruthenaborane Clusters, ({Cp*Ru(µ-H)}2B3H7), ({(Cp*Ru)2(µ-H)}B4H9), and ((Cp*Ru)2(µ-H)(PMe3)(µ-η4-B2H5)). Chem. Lett. 1999, 28, 489–490. [Google Scholar] [CrossRef]
- Lenczyk, C.; Roy, D.K.; Oberdorf, K.; Nitsch, J.; Dewhurst, R.D.; Radacki, K.; Halet, J.-F.; Marder, T.B.; Bickelhaupt, F.M.; Braunschweig, H. Toward Transition-Metal-Templated Construction of Arylated B4 Chains by Dihydroborane Dehydrocoupling. Chem. Eur. J. 2019, 25, 16544–16549. [Google Scholar] [CrossRef] [PubMed]
- Hertz, R.K.; Denniston, M.L.; Shore, S.G. Preparation and characterization of B2H4∙2P(CH3)3. Inorg. Chem. 1978, 17, 2673–2674. [Google Scholar] [CrossRef]
- Dodds, A.R.; Kodama, G. Isolation and characterization of trimethylamine-tetraborane(8). Inorg. Chem. 1979, 18, 1465–1470. [Google Scholar] [CrossRef]
- Kameda, M.; Shimoi, M.; Kodama, G. Tetraborane(8) adducts of strongly basic phosphines. Inorg. Chem. 1984, 23, 3705–3709. [Google Scholar] [CrossRef]
- Snow, S.A.; Kodama, G. Novel coordination of a neutral borane adduct to nickel(0). Formation of Ni(CO)2[B2H4∙2P(CH3)3]. Inorg. Chem. 1985, 24, 795–796. [Google Scholar] [CrossRef]
- Shimoi, M.; Katoh, K.; Kawano, Y.; Kodama, G.; Ogino, H. Fluxional behavior of chromium and tungsten complexes of monodentate bis(trimethylphosphine)diborane(4), [M(CO)5(η1-B2H4·2PMe3)] (M = Cr, W):. J. Organomet. Chem. 2002, 659, 102–106. [Google Scholar] [CrossRef]
- Schleyer, P.V.R.; Najafian, K.; Mebel, A.M. The Large closo-Borane Dianions, BnHn2− (n = 13–17) Are Aromatic, Why Are They Unknown? Inorg. Chem. 1998, 37, 6765–6772. [Google Scholar] [CrossRef] [PubMed]
- Derecskei-Kovacs, A.; Dunlap, B.I.; Lipscomb, W.N.; Lowrey, A.; Marynick, D.S.; Massa, L. Quantum Chemical Studies of Boron Fullerene Analogs. Inorg. Chem. 1994, 33, 5617–5619. [Google Scholar] [CrossRef]
- Wang, Z.-X.; Schleyer, P.V.R. A “Sea Urchin” Family of Boranes and Carboranes: The 6m + 2n Electron Rule. J. Am. Chem. Soc. 2003, 125, 10484–10485. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnarajan, M.M.; Hoffmann, R.; Pancharatna, P.D.; Jemmis, E.D. Magic Electron Counts and Bonding in Tubular Boranes. Inorg. Chem. 2003, 42, 4650–4659. [Google Scholar] [CrossRef] [PubMed]
- Pancharatna, P.; Marutheeswaran, S.; Austeria, M.P.; Balakrishnarajan, M.M. Deltahedra with holes: Structural preferences of supraicosahedral boranes. Polyhedron 2013, 63, 55–59. [Google Scholar] [CrossRef]
- Shen, Y.-F.; Xu, C.; Cheng, L. Deciphering chemical bonding in BnHn2−(n = 2–17): Flexible multicenter bonding. RSC Adv. 2017, 7, 36755–36764. [Google Scholar] [CrossRef]
- Wade, K. The structural significance of the number of skeletal bonding electron-pairs in carboranes, the higher boranes and borane anions, and various transition-metal carbonyl cluster compounds. J. Chem. Soc. D 1971, 792–793. [Google Scholar] [CrossRef]
- Mark, A.F.; Ken, W. Evolving patterns in boron cluster chemistry. Pure Appl. Chem. 2003, 75, 1315–1323. [Google Scholar]
- Bicerano, J.; Marynick, D.S.; Lipscomb, W.N. Large closo boron hydrides. Inorg. Chem. 1978, 17, 2041–2042. [Google Scholar] [CrossRef]
- Bregadze, V.I.; Timofeev, S.V.; Sivaev, I.B.; Lobanova, I.A. Substitution reactions at boron atoms in metallacarboranes. Russ. Chem. Rev. 2004, 73, 433–453. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06 functionals and 12 other functionals. Theor. Chem. Accounts 2008, 119, 525. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- Adrienko, G.A. Chemcraft, Version 1.8 (build 574b). ChemCraft-Graphical Program for Visualisation of Quantum Chemistry Computations. Available online: http://www.chemcraftprog.com (accessed on 24 June 2020).
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Waldie, K.M.; Ostericher, A.L.; Reineke, M.H.; Sasayama, A.F.; Kubiak, C.P. Hydricity of Transition-Metal Hydrides: Thermodynamic Considerations for CO2 Reduction. ACS Catal. 2018, 8, 1313–1324. [Google Scholar] [CrossRef]
- Pitzer, K.S. Electron Deficient Molecules. I. The Principles of Hydroboron Structures. J. Am. Chem. Soc. 1945, 67, 1126–1132. [Google Scholar] [CrossRef]
- Goebbert, D.J.; Hernandez, H.; Francisco, J.S.; Wenthold, P.G. The Binding Energy and Bonding in Dialane. J. Am. Chem. Soc. 2005, 127, 11684–11689. [Google Scholar] [CrossRef]
- Kühl, O. The coordination chemistry of the proton. Chem. Soc. Rev. 2011, 40, 1235–1246. [Google Scholar] [CrossRef]
- Kaese, M.S.T.; Budy, B.S.H.; Bolte, M.; Lerner, H.-W.; Wagner, M. Deprotonation of a Seemingly Hydridic Diborane(6) To Build a B−B Bond. Angew. Chem. Int. Ed. 2017, 56, 7546–7550. [Google Scholar] [CrossRef]
- King, R.B. Three-dimensional aromaticity in polyhedral boranes and related molecules. Chem. Rev. 2001, 101, 1119–1152. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
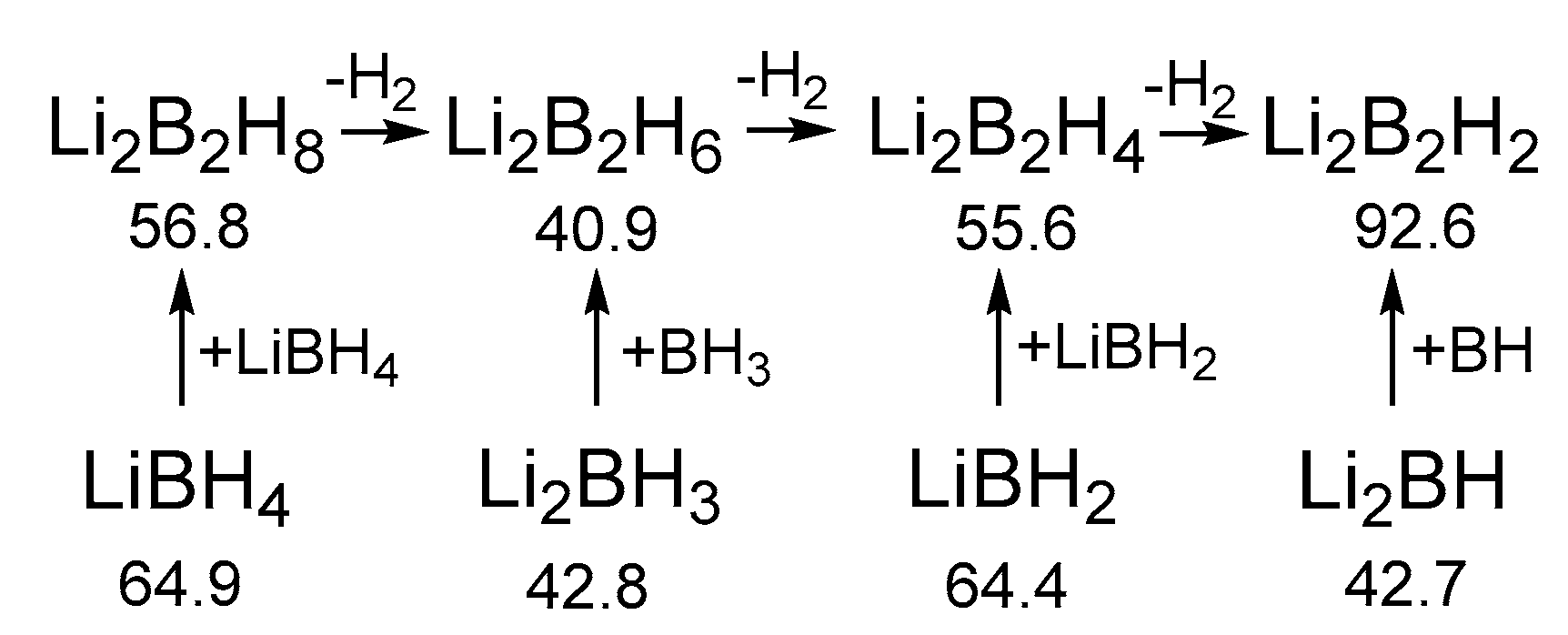
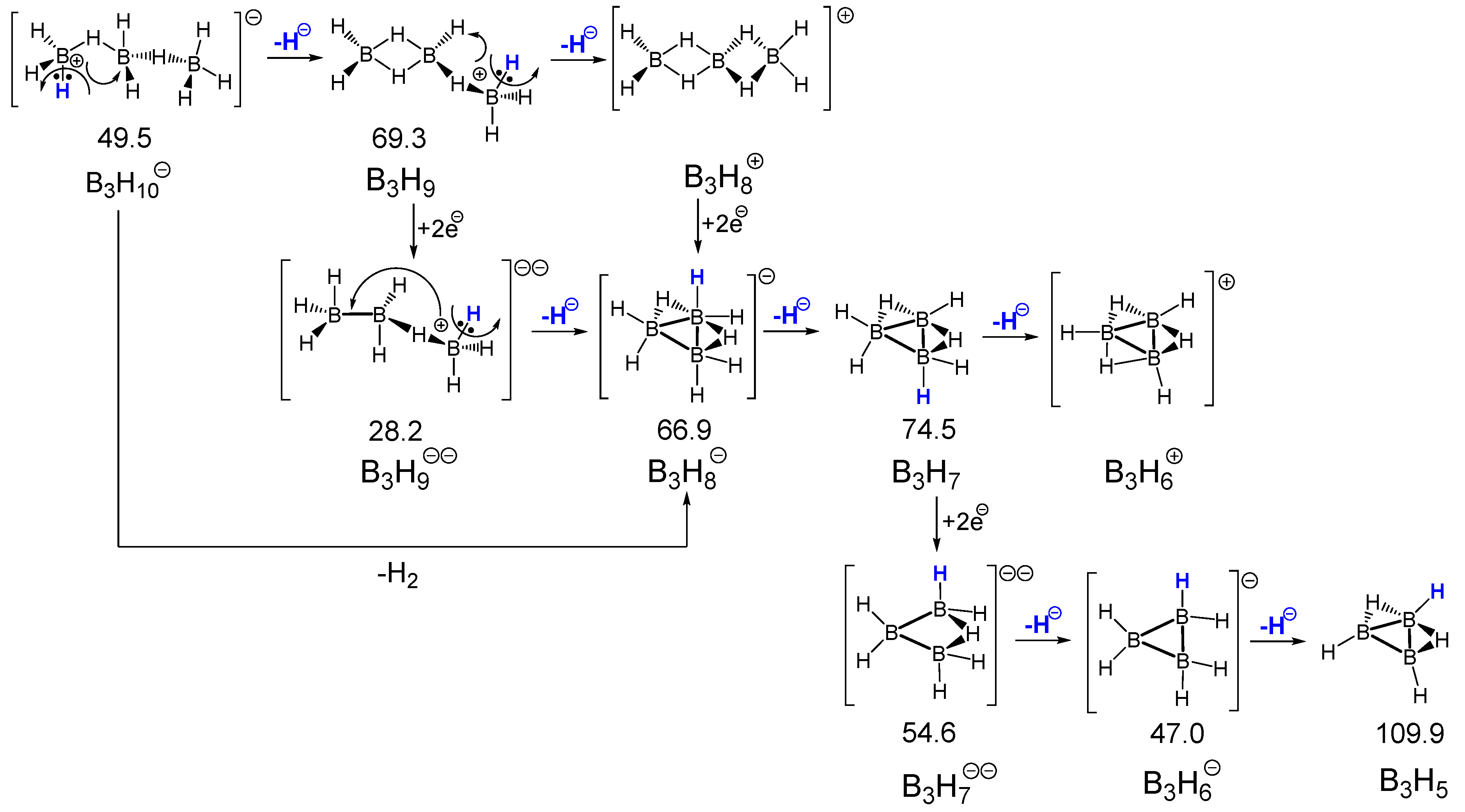
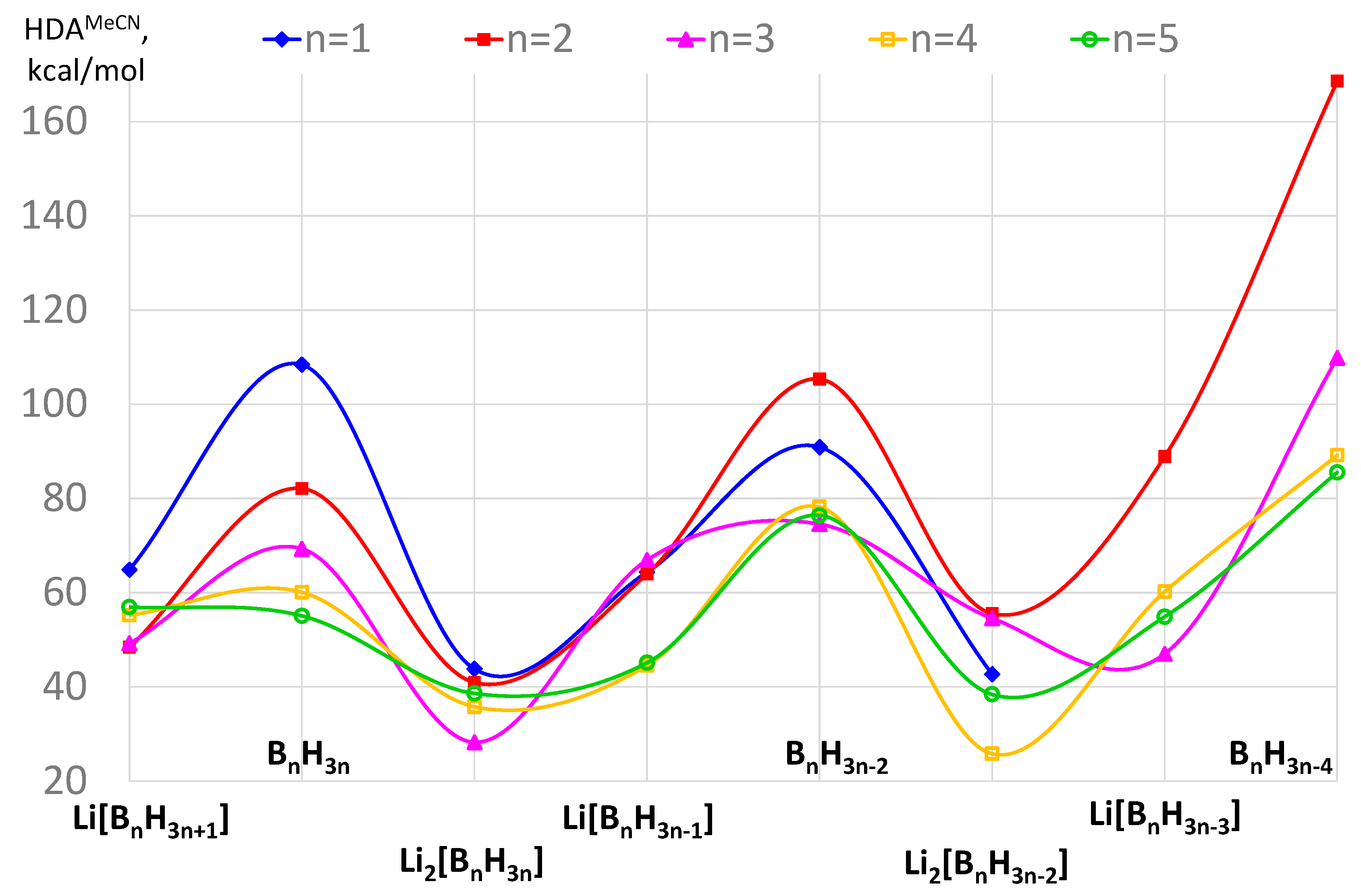
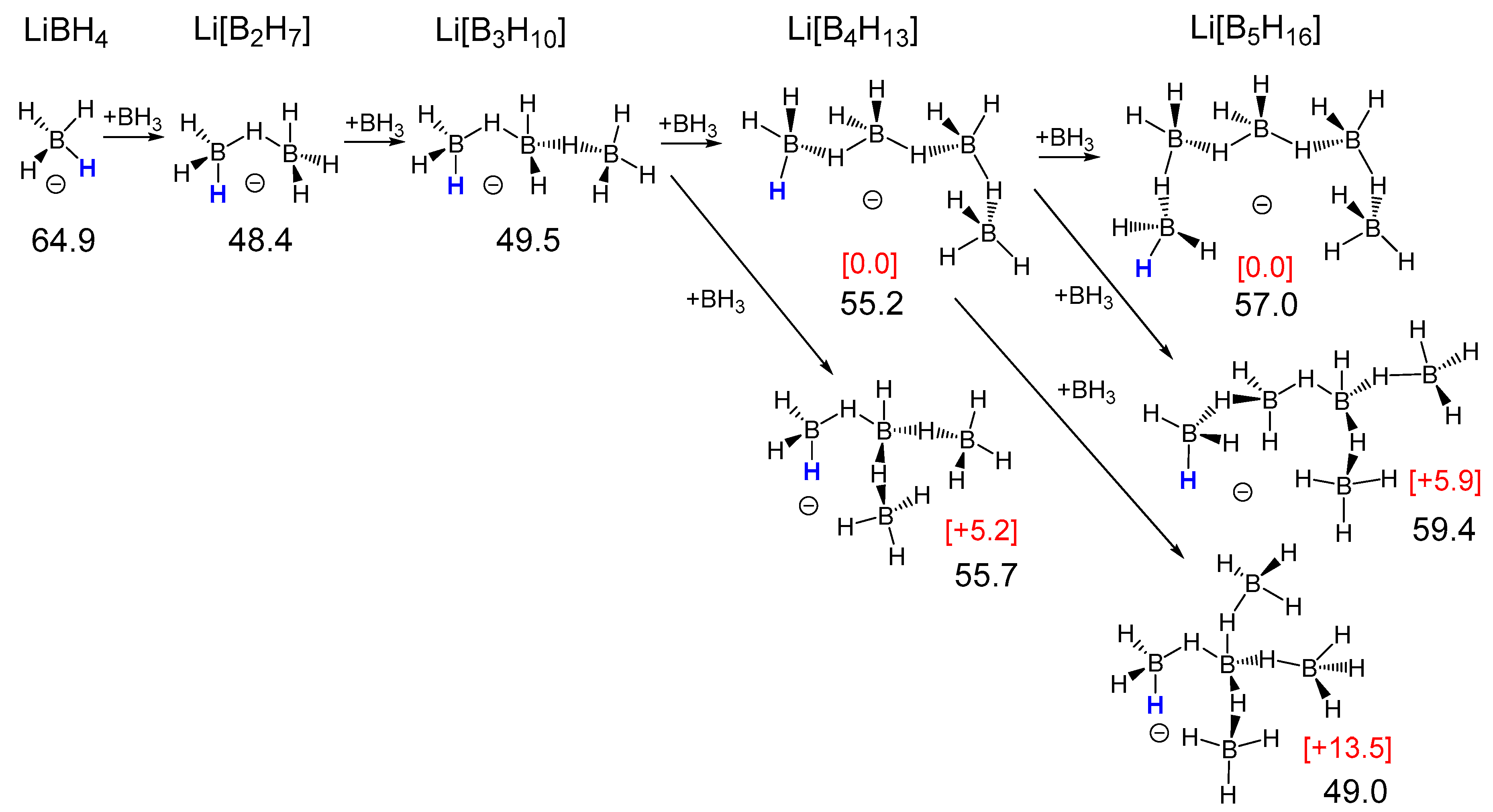
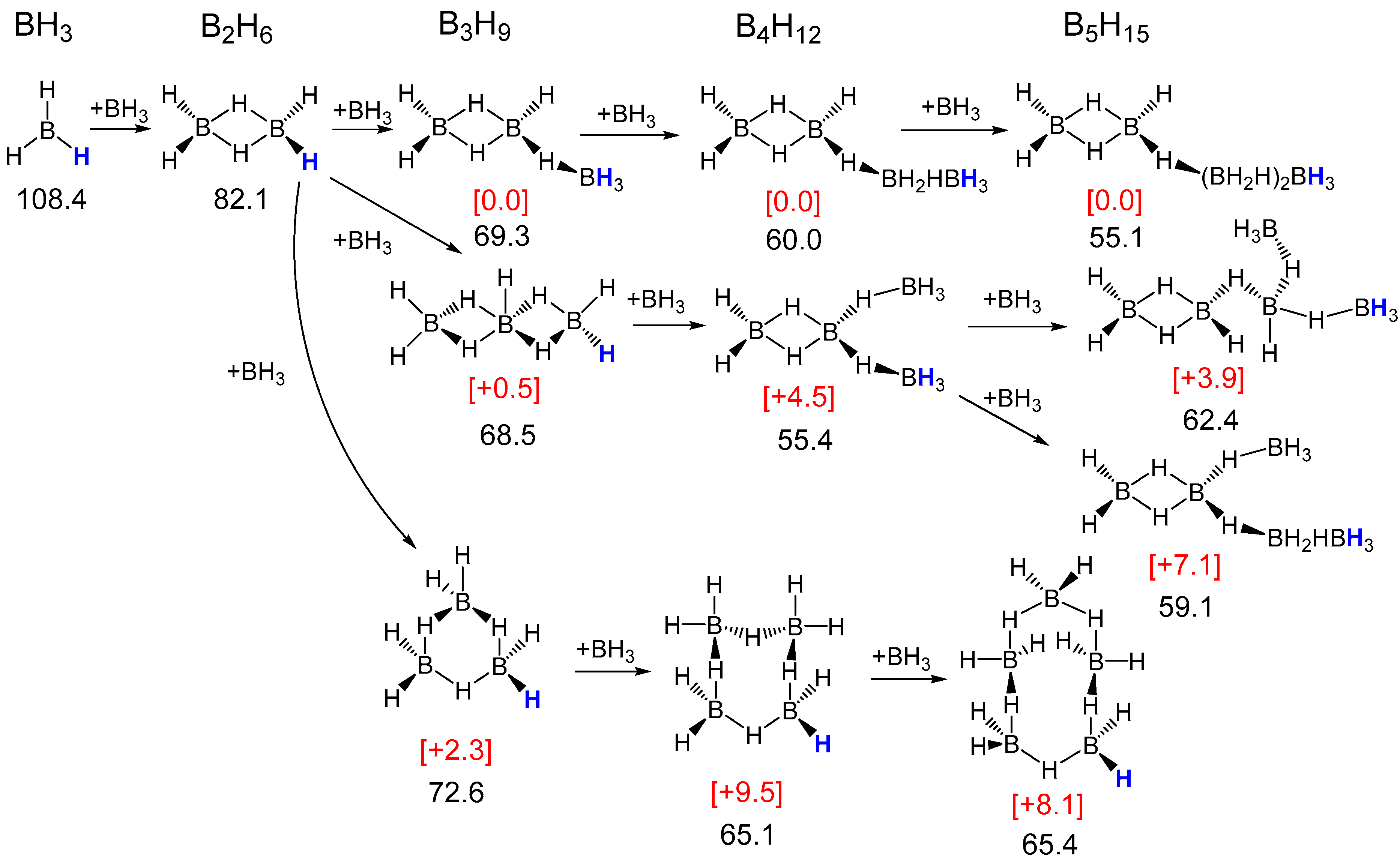
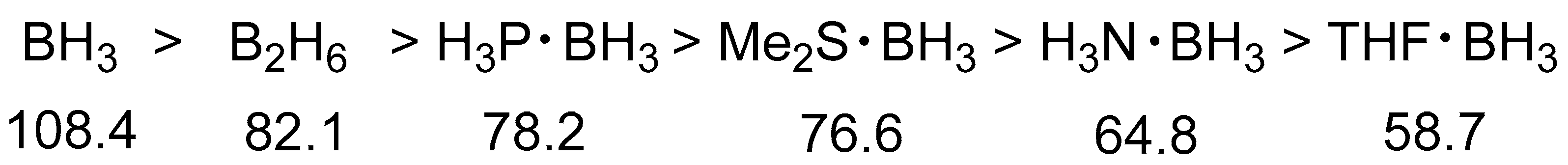
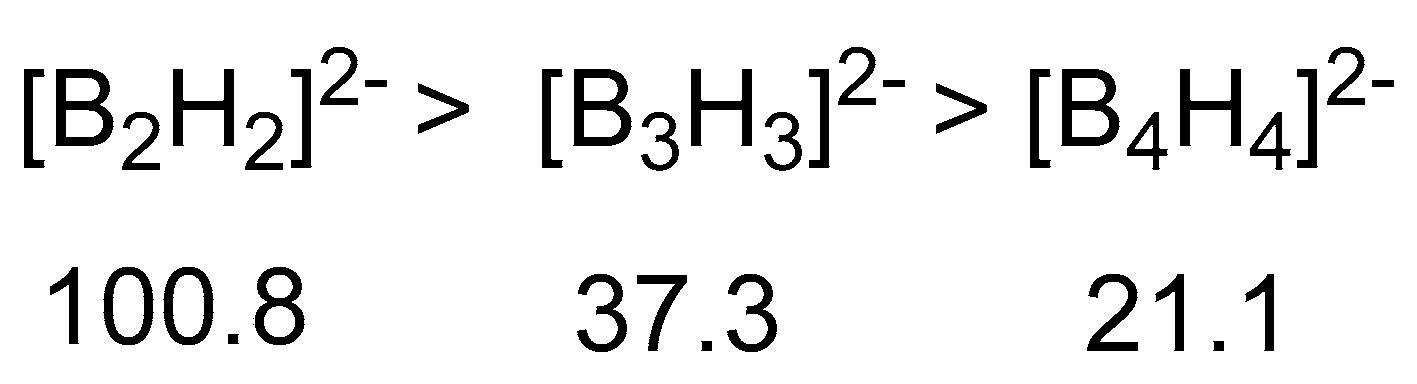
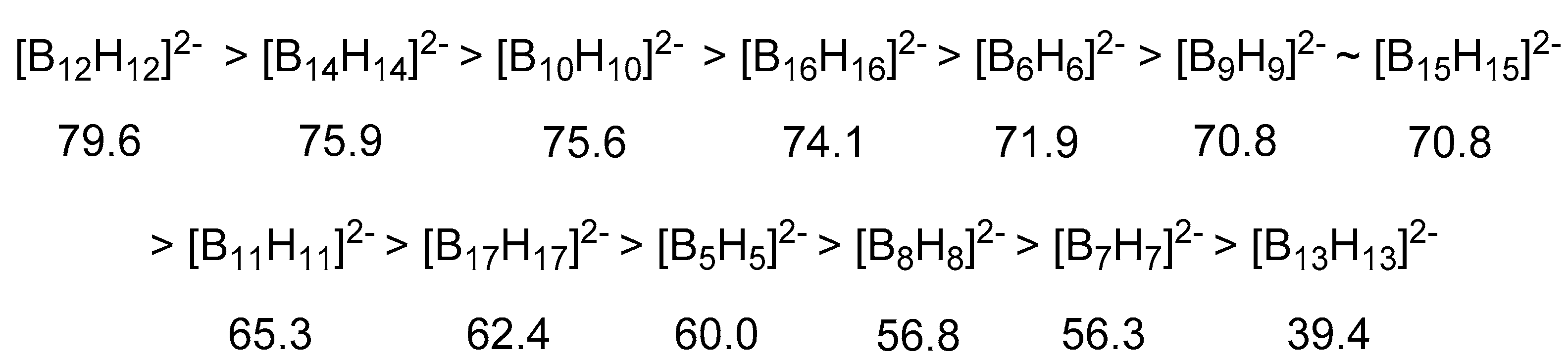
| n | Li[BnH3n+1] | BnH3n | Li2[BnH3n] | Li[BnH3n−1] | BnH3n−2 | Li2[BnH3n−2] | Li[BnH3n−3] | BnH3n−4 |
|---|---|---|---|---|---|---|---|---|
| 1 | LiBH4 | BH3 | Li2BH3 | LiBH2 | BH | Li2BH | - | - |
| 64.9 | 108.4 | 43.8 | 64.4 | 90.9 | 42.7 | |||
| 2 | Li[B2H7] | B2H6 | Li2[B2H6] | Li[B2H5] | B2H4 | Li2[B2H4] | Li[B2H3] | B2H2 |
| 48.4 | 82.1 | 40.9 | 63.9 | 105.4 | 55.6 | 88.9 | 168.6 | |
| 3 | Li[B3H10] | B3H9 | Li2[B3H9] | Li[B3H8] | B3H7 | Li2[B3H7] | Li[B3H6] | B3H5 |
| 49.2 | 69.3 | 28.2 | 66.9 | 74.5 | 54.6 | 47.0 | 109.8 | |
| 4 | Li[B4H13] | B4H12 | Li2[B4H12] | Li[B4H11] | B4H10 | Li2[B4H10] | Li[B4H9] | B4H8 |
| 55.2 | 60.1 | 35.8 | 44.5 | 78.2 | 25.8 | 60.2 | 89.2 | |
| 5 | Li[B5H16] | B5H15 | Li2[B5H15] | Li[B5H14] | B5H13 | Li2[B5H13] | Li[B5H12] | B5H11 |
| 57.0 | 55.1 | 38.6 | 45.1 | 76.4 | 38.4 | 54.9 | 85.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golub, I.E.; Filippov, O.A.; Kulikova, V.A.; Belkova, N.V.; Epstein, L.M.; Shubina, E.S. Thermodynamic Hydricity of Small Borane Clusters and Polyhedral closo-Boranes. Molecules 2020, 25, 2920. https://doi.org/10.3390/molecules25122920
Golub IE, Filippov OA, Kulikova VA, Belkova NV, Epstein LM, Shubina ES. Thermodynamic Hydricity of Small Borane Clusters and Polyhedral closo-Boranes. Molecules. 2020; 25(12):2920. https://doi.org/10.3390/molecules25122920
Chicago/Turabian StyleGolub, Igor E., Oleg A. Filippov, Vasilisa A. Kulikova, Natalia V. Belkova, Lina M. Epstein, and Elena S. Shubina. 2020. "Thermodynamic Hydricity of Small Borane Clusters and Polyhedral closo-Boranes" Molecules 25, no. 12: 2920. https://doi.org/10.3390/molecules25122920
APA StyleGolub, I. E., Filippov, O. A., Kulikova, V. A., Belkova, N. V., Epstein, L. M., & Shubina, E. S. (2020). Thermodynamic Hydricity of Small Borane Clusters and Polyhedral closo-Boranes. Molecules, 25(12), 2920. https://doi.org/10.3390/molecules25122920