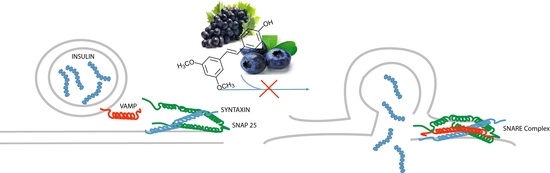
Determining the Effect of Pterostilbene on Insulin Secretion Using Chemoproteomics
Abstract
:1. Introduction
2. Results
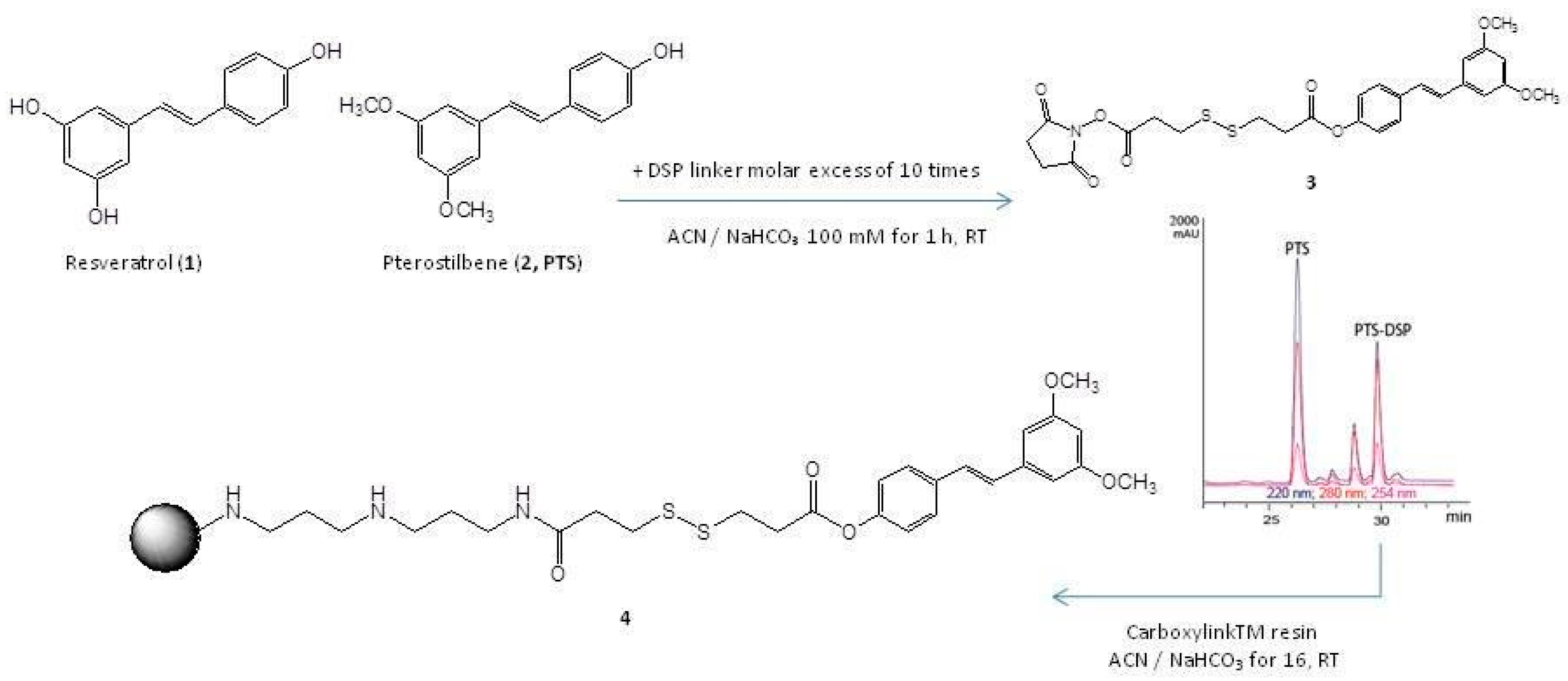
2.1. PTS Immobilization on a Solid Support
2.2. Identification of PTS Interactors by AP-MS
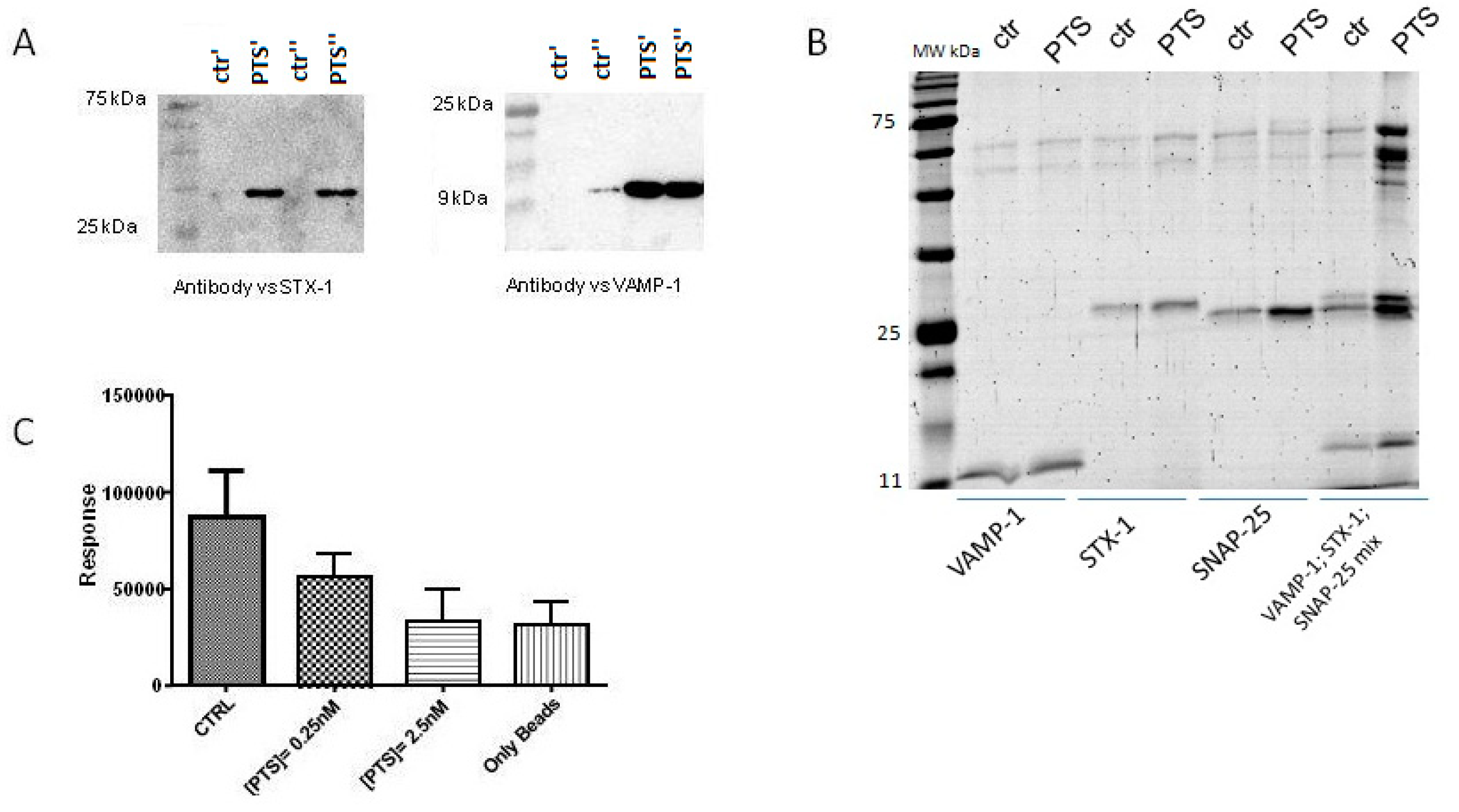
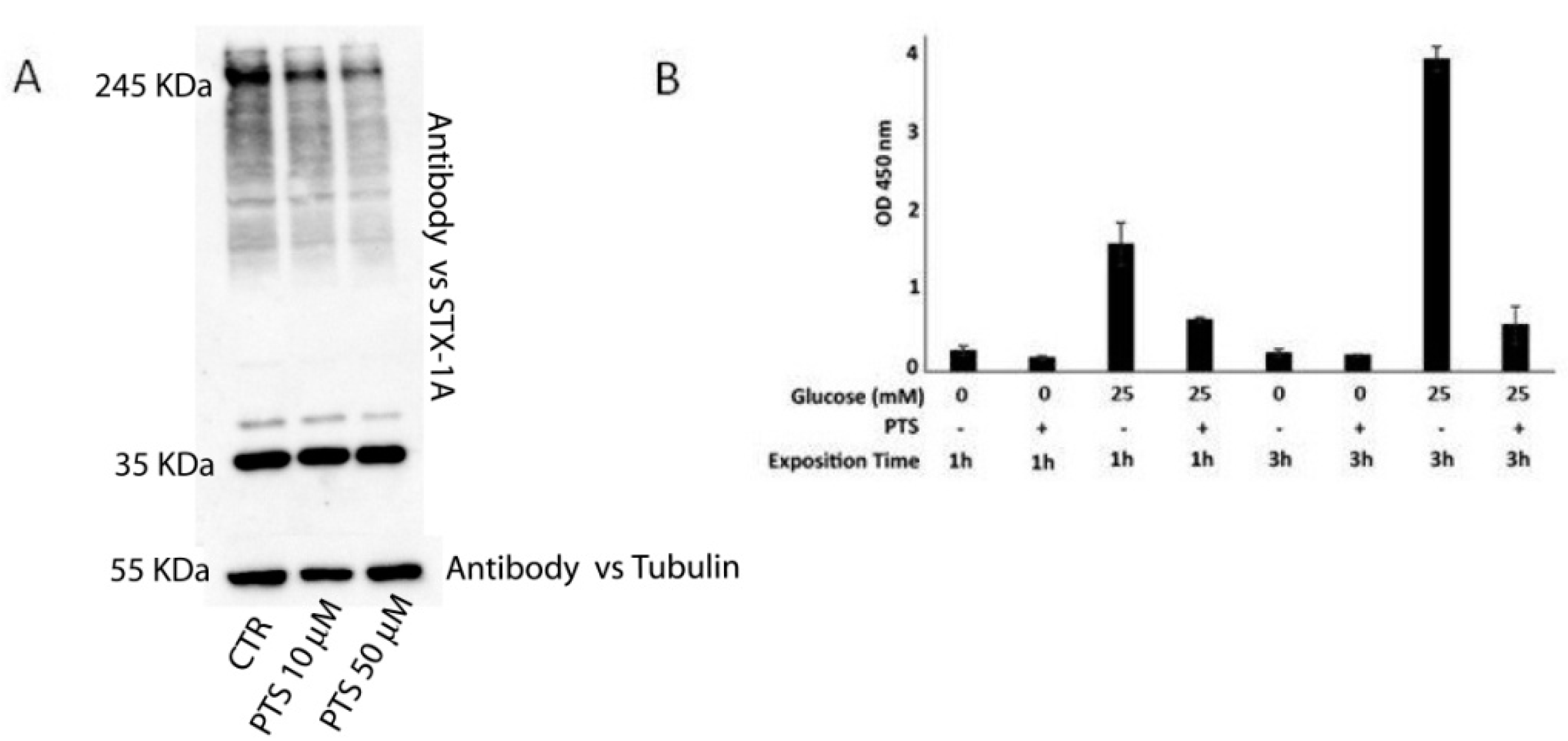
2.3. In Vitro and In Cell Assays
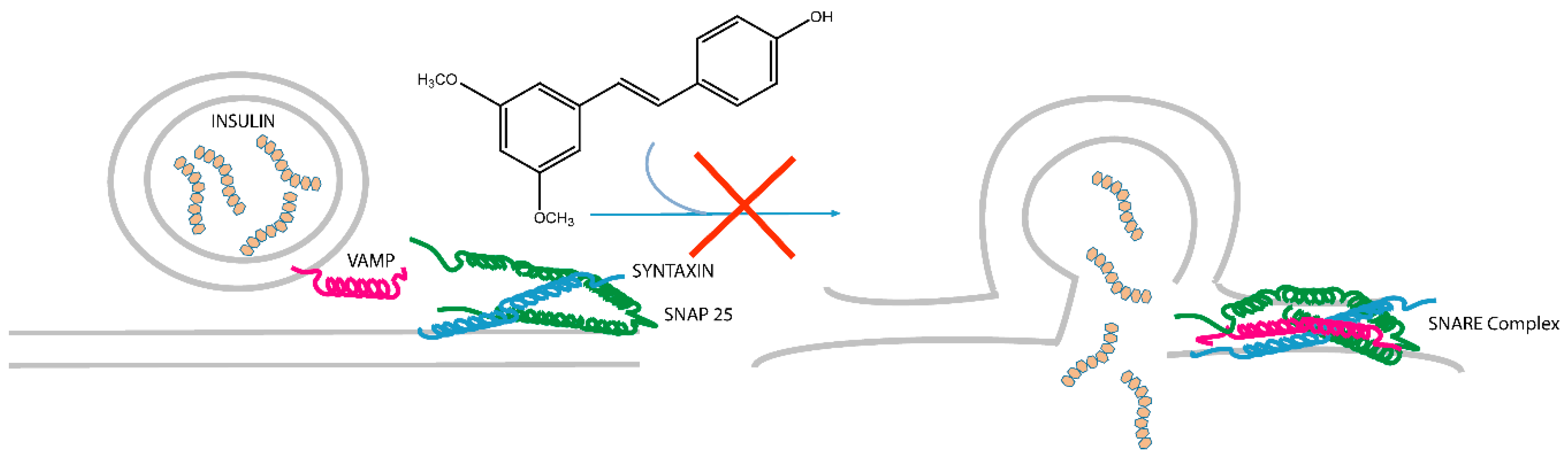
3. Discussion
4. Materials and Methods
4.1. PTS Immobilization on a Functional Matrix
4.2. Affinity Chromatography
4.3. Protein Identification by LCMSMS Analysis
4.4. Immunoblotting Analysis
4.5. Affinity Purification of Recombinant Proteins
4.6. Amplified Luminescent Proximity Homogeneous Assay
4.7. INS-1 832/13 Pancreatic Cell Culture
4.8. INS-1 832/13 Pancreatic Cell Assay
4.9. Western Blotting on INS-1 832/13 Lysate Treated with PTS
4.10. ELISA Assays
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Boue, S.M.; Cleveland, T.E.; Carter-Wientjes, C.; Shih, B.Y.; Bhatnagar, D.; McLachlan, J.M.; Burow, M.E. Phytoalexin-enriched functional foods. J. Agric. Food Chem. 2009, 57, 2614–2622. [Google Scholar] [CrossRef] [PubMed]
- de Sá Coutinho, D.; Pacheco, M.T.; Frozza, R.L.; Bernardi, A. Anti-Inflammatory effects of resveratrol: Mechanistic insights. Int. J. Mol. Sci. 2018, 19, 1812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiwari, V.; Chopra, K. Resveratrol abrogates alcohol-induced cognitive deficits by attenuating oxidative-nitrosative stress and inflammatory cascade in the adult rat brain. Neurochem. Int. 2013, 6, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Sang, S. Metabolism and pharmacokinetics of resveratrol and pterostilbene. Biofactors 2018, 1, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Perecko, T.; Drabikova, K.; Rackova, L.; Ciz, M.; Podborska, M.; Lojek, A.; Harmatha, J.; Smidrkal, J.; Nosal, R.; Jancinova, V. Molecular targets of the natural antioxidant pterostilbene: Effect on protein kinase C, caspase-3 and apoptosis in human neutrophils in vitro. Neuroendocrinol. Endocrinol. Lett. 2010, 2, 84–90. [Google Scholar]
- Rimando, A.; Cuendet, M.; Desmarchelier, C.; Mehta, R.; Pezzuto, J.M.; Duke, S. Cancer chemopreventive and antioxidant activities of pterostilbene, a naturally occurring analogue of resveratrol. J. Agric. Food Chem. 2002, 50, 3453–3457. [Google Scholar] [CrossRef]
- Hou, Y.; Li, N.; Xie, G.; Wang, J.Y.; Qing, J.; Congcong, L.; Xia, L.; Guoxun, T.; Yingzhan, W. Pterostilbene exerts anti-neuroinflammatory effect on lipopolysaccharide-activated microglia via inhibition of MAPK signalling pathways. J. Funct. Foods 2015, 6, 676–687. [Google Scholar] [CrossRef]
- Gomez-Zorita, S.; Belles, C.; Briot, A.; Fernández-Quintela, A.; Portillo, M.P.; Carpéné, C. Pterostilbene inhibits lipogenic activity similar to resveratrol or caffeine but differently modulates lipolysis in adipocytes. Phytother. Res. 2017, 8, 1273–1282. [Google Scholar] [CrossRef]
- De Filippis, B.; Ammazzalorso, A.; Fantacuzzi, M.; Giampietro, L.; Maccallini, C.; Amoroso, R. Anticancer activity of stilbene-based derivatives. ChemMedChem 2017, 12, 558–570. [Google Scholar] [CrossRef]
- Hsu, C.L.; Lin, Y.J.; Ho, C.T.; Yen, G.C. The inhibitory effect of pterostilbene on inflammatory responses during the interaction of 3T3-L1 adipocytes and RAW 264.7 macrophages. J. Agric. Food Chem. 2013, 61, 602–610. [Google Scholar] [CrossRef]
- Hsu, C.L.; Lin, Y.J.; Ho, C.T.; Yen, G.C. Inhibitory effects of garcinol and pterostilbene on cell proliferation and adipogenesis in 3T3-L1 cells. Food Funct. 2012, 3, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Park, E.S.; Lim, Y.; Hong, J.T.; Yoo, H.S.; Lee, C.K.; Pyo, M.Y.; Yun, Y.P. Pterostilbene, a natural dimethylated analog of resveratrol, inhibits rat aortic vascular smooth muscle cell proliferation by blocking Akt-dependent pathway. Vascul. Pharmacol. 2010, 53, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Rorsman, P.; Braun, M. Regulation of insulin secretion in human pancreatic islets. Ann. Rev. Physiol. 2013, 75, 155–179. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.D.; Corkey, B.E.; Istfan, N.W.; Apovian, C.M. Hyperinsulinemia: An early indicator of metabolic dysfunction. J. Endocr. Soc. 2019, 3, 1727–1747. [Google Scholar] [CrossRef] [PubMed]
- Kubota, K.; Funabashi, M.; Ogura, Y. Target deconvolution from phenotype-based drug discovery by using chemical proteomics approaches. Biochim. Biophys. Acta Proteins Proteom. 2019, 1867, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Margarucci, L.; Monti, M.C.; Cassiano, C.; Mozzicafreddo, M.; Angeletti, M.; Riccio, R.; Tosco, A.; Casapullo, A. Chemical proteomics-driven discovery of oleocanthal as an Hsp90 inhibitor. Chem. Commun. (Camb) 2013, 49, 5844–5846. [Google Scholar] [CrossRef]
- Shevchenko, A.; Tomas, H.; Havlis, J.; Olsen, J.V.; Mann, M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat. Protoc. 2007, 1, 2856–2860. [Google Scholar] [CrossRef]
- Fasshauer, D.; Otto, H.; Eliason, W.K.; Jahn, R.; Brunger, A.T. Structural changes are associated with soluble N-ethylmaleimide-sensitive fusion protein attachment protein receptor complex formation. J. Biol. Chem. 1997, 272, 28036–28041. [Google Scholar] [CrossRef] [Green Version]
- Yu, A.; Chen, N.; Scheller, R. Snare-mediated membrane fusion. Nat. Rev. 2001, 2, 99–108. [Google Scholar]
- Hou, J.C.; Min, L.; Pessin, J.E. Insulin granule biogenesis, trafficking and exocytosis. Vitam. Horm. 2009, 80, 473–506. [Google Scholar]
- Kubista, H.; Edelbauer, H.; Boehm, S. Evidence for structural and functional diversity among SDS-resistant SNARE complexes in neuroendocrine cells. J. Cell Sci. 2004, 29, 955–966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otto, H.; Hanson, P.I.; Jahn, R. Assembly and disassembly of a ternary complex of synaptobrevin, syntaxin, and SNAP-25 in the membrane of synaptic vesicles. Proc. Natl. Acad. Sci. USA 1997, 94, 6197–6201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matveeva, E.A.; Whiteheart, S.W.; Slevin, J.T. Accumulation of 7S SNARE complexes in hippocampal synaptosomes from chronically kindled rats. J. Neurochem. 2003, 84, 621–624. [Google Scholar] [CrossRef] [PubMed]
- Gaisano, H.Y. Recent new insights into the role of SNARE and associated proteins in insulin granule exocytosis. Diabetes Obes. Metab. 2017, 19, 115–123. [Google Scholar] [CrossRef] [Green Version]
- Straub, S.G.; Sharp, G.W. Glucose-stimulated signaling pathways in biphasic insulin secretion. Diabetes Metab. Res. Rev. 2002, 18, 451–463. [Google Scholar] [CrossRef]
- Tang, D.Q.; Cao, L.Z.; Burkhardt, B.R.; Xia, C.Q.; Litherland, S.A.; Atkinson, M.A.; Yang, L.J. In Vivo and In Vitro Characterization of insulin-producing cells obtained from murine bone marrow. Diabetes 2004, 53, 1721–1732. [Google Scholar] [CrossRef] [Green Version]
- Finicelli, M.; Squillaro, T.; Di Cristo, F.; Di Salle, A.; Melone, M.A.B.; Galderisi, U.; Peluso, G. Metabolic syndrome, mediterranean diet, and polyphenols: Evidence and perspectives. J. Cell. Phys. 2019, 234, 5807–5826. [Google Scholar] [CrossRef]
- Macut, D.; Bjekic-Macut, J.; Rahelic, D.; Doknic, M. Insulin and the polycystic ovary syndrome. Diabetes Res. Clin. Pr. 2017, 130, 163–170. [Google Scholar] [CrossRef]
- Kelly, C.T.; Mansoor, J.; Dohm, G.L.; Chapman, W.H.; Pender, J.R.; Pories, W.J. Hyperinsulinemic syndrome: The metabolic syndrome is broader than you think. Surgery 2014, 156, 405–411. [Google Scholar] [CrossRef]
- Lipson, K.L.; Fonseca, S.G.; Ishigaki, S.; Nguyen, L.X.; Foss, E.; Bortell, R.; Rossini, A.A.; Urano, F. Regulation of insulin biosynthesis in pancreatic beta cells by an endoplasmic reticulum resident protein kinase IRE1. Cell Metab. 2006, 4, 245–254. [Google Scholar] [CrossRef] [Green Version]
- Eletto, D.; Eletto, D.; Boyle, S.; Argon, Y. PDIA6 regulates insulin secretion by selectively inhibiting the RIDD activity of IRE1. FASEB J. 2016, 30, 653–665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCormack, D.; McFadden, D. A Review of Pterostilbene Antioxidant Activity and Disease Modification. Oxid. Med. Cell. Longev. 2013, 2013, 575482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Sample Availability: Not available. |
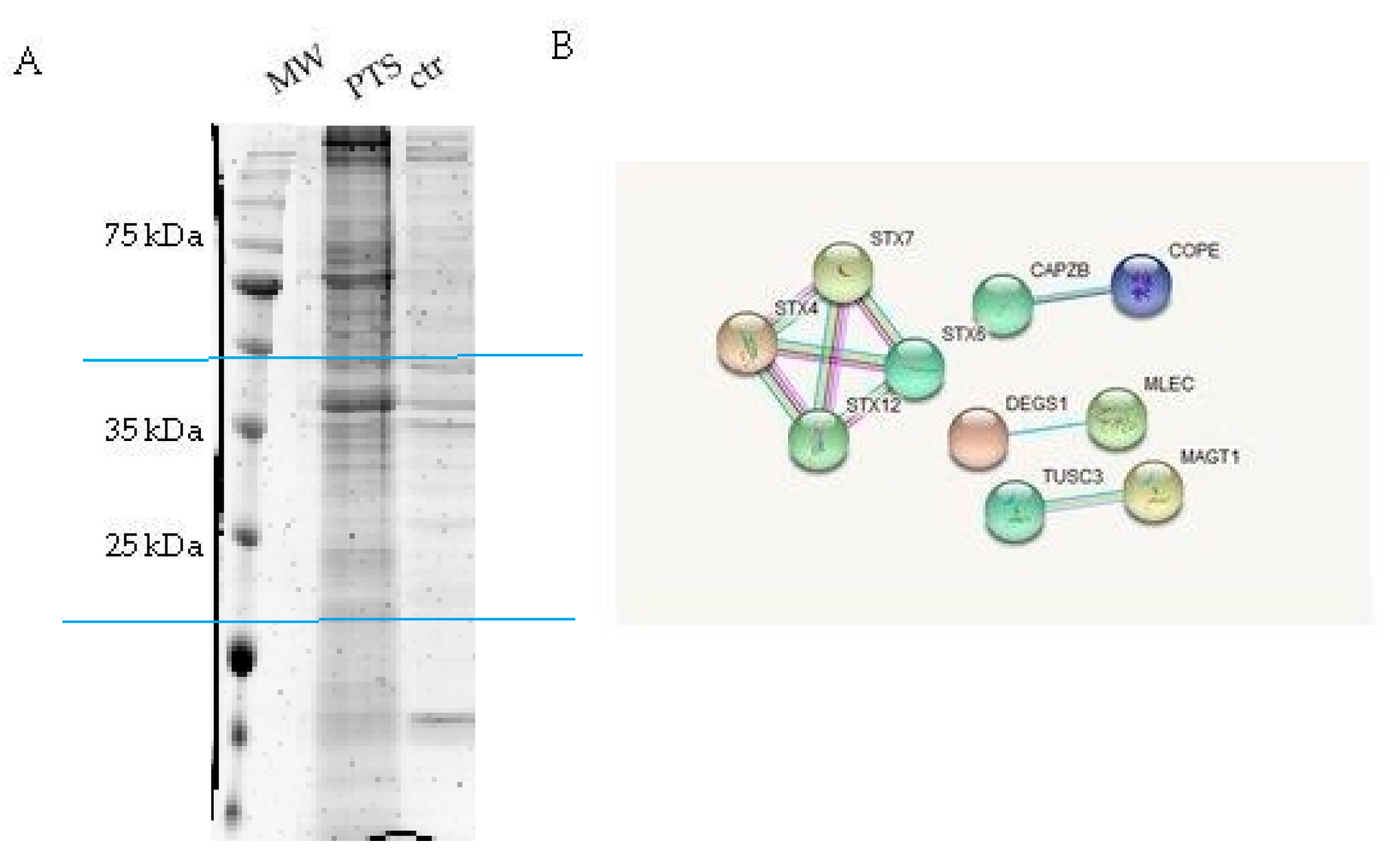
| Accession | Mass (Da) | Average Score | Average Matches | Description |
|---|---|---|---|---|
| SYPL1_HUMAN | 28889 | 90 | 11 | Synaptophysin-like protein 1 |
| EMD_HUMAN | 29033 | 306 | 10 | Emerin |
| STX6_HUMAN | 29215 | 111 | 3 | Syntaxin-6 |
| STX7_HUMAN | 29911 | 129 | 5 | Syntaxin-7 |
| CCHL_HUMAN | 30981 | 149 | 7 | Cytochrome c-type heme lyase |
| CAPZB_HUMAN | 31616 | 67 | 5 | F-actin-capping protein subunit beta |
| STX12_HUMAN | 31736 | 129 | 4 | Syntaxin-12 |
| AT1B3_HUMAN | 31834 | 157 | 6 | NA/K-transporting ATPase subunit beta-3 |
| VDAC2_HUMAN | 32060 | 756 | 35 | Voltage-dep. anion-selective channel-2 |
| PGAM5_HUMAN | 32213 | 108 | 10 | Serine/threonine-protein phosphatase PGAM5 |
| MLEC_HUMAN | 32385 | 653 | 20 | Malectin |
| MCAT_HUMAN | 33264 | 68 | 5 | Carnitine/acylcarnitine carrier protein |
| NB5R1_HUMAN | 34244 | 164 | 10 | NADH-cytochrome b5 reductase 1 |
| STX4_HUMAN | 34273 | 146 | 9 | Syntaxin-4 |
| TMX2_HUMAN | 34358 | 493 | 22 | Thioredoxin-related transmembrane -2 |
| DHB12_HUMAN | 34416 | 50 | 6 | Estradiol 17-beta-dehydrogenase 12 |
| DHRS1_HUMAN | 34458 | 132 | 4 | Dehydrogenase/reductase SDR family-1 |
| COPE_HUMAN | 34688 | 184 | 4 | Coatomer subunit epsilon |
| EMC2_HUMAN | 34982 | 394 | 14 | ER membrane protein complex subunit 2 |
| COQ9_HUMAN | 35658 | 199 | 10 | Ubiquinone biosynthesis protein COQ9 |
| PPP6_HUMAN | 35806 | 269 | 6 | Ser/thr-protein phosphatase 6 catalytic sub. |
| ECH1_HUMAN | 36136 | 131 | 4 | Delta(3,5)-Delta(2,4)-dienoyl-CoA isomerase |
| CIA30_HUMAN | 37797 | 96 | 7 | Complex I intermediate-associated protein 30 |
| DEGS1_HUMAN | 38012 | 180 | 15 | Sphingolipid delta(4)-desaturase DES1 |
| SCAM1_HUMAN | 38295 | 353 | 18 | Secretory carrier-associated membrane- 1 |
| MAGT1_HUMAN | 38411 | 120 | 5 | Magnesium transporter protein 1 |
| SCAM3_HUMAN | 38661 | 441 | 11 | Secretory carrier-associated membrane- 3 |
| LMA2L_HUMAN | 39913 | 109 | 5 | VIP36-like protein |
| TUSC3_HUMAN | 39993 | 192 | 5 | Tumor suppressor candidate 3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cassiano, C.; Eletto, D.; Tosco, A.; Riccio, R.; Monti, M.C.; Casapullo, A. Determining the Effect of Pterostilbene on Insulin Secretion Using Chemoproteomics. Molecules 2020, 25, 2885. https://doi.org/10.3390/molecules25122885
Cassiano C, Eletto D, Tosco A, Riccio R, Monti MC, Casapullo A. Determining the Effect of Pterostilbene on Insulin Secretion Using Chemoproteomics. Molecules. 2020; 25(12):2885. https://doi.org/10.3390/molecules25122885
Chicago/Turabian StyleCassiano, Chiara, Daniela Eletto, Alessandra Tosco, Raffaele Riccio, Maria Chiara Monti, and Agostino Casapullo. 2020. "Determining the Effect of Pterostilbene on Insulin Secretion Using Chemoproteomics" Molecules 25, no. 12: 2885. https://doi.org/10.3390/molecules25122885
APA StyleCassiano, C., Eletto, D., Tosco, A., Riccio, R., Monti, M. C., & Casapullo, A. (2020). Determining the Effect of Pterostilbene on Insulin Secretion Using Chemoproteomics. Molecules, 25(12), 2885. https://doi.org/10.3390/molecules25122885