Optimization of Oyster (Crassostrea talienwhanensis) Protein Hydrolysates Using Response Surface Methodology
Abstract
1. Introduction
2. Results
2.1. Selection of Proteolytic Enzymes
2.2. Single-Factor Experiments
2.3. Optimization of Hydrolysis Conditions by BBD
2.4. Separation and Purification of the OP
3. Discussion
4. Material and Methods
4.1. Materials and Chemicals
4.2. Preparation of Oyster Protein Hydrolysates
4.3. Single-Factor Experiments
4.4. Optimization of Oyster Protein Hydrolysate Preparative Conditions
4.5. Degree of Hydrolysis
4.6. Hydroxyl-Radical-Scavenging Activity
4.7. Amino Acid Composition Analysis
4.8. Purification of Oyster Protein Hydrolysates
4.8.1. Ultrafiltration
4.8.2. Gel Filtration Chromatography
4.8.3. Identification of OP by Mass Spectrometry
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hao, G.; Zhang, C.; Cao, W.; Hao, J. Effects of intragastric administration of five oyster components on endurance exercise performance in mice. Pharm. Biol. 2014, 52, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Zha, G.; Chen, V.P.; Luk, W.K.; Zou, X.; Choi, R.C.; Tsim, K.W. Characterization of acetylcholinesterase in Hong Kong oyster (Crassostrea hongkongensis) from South China Sea. Chem.-Biol. Interact. 2013, 203, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.K.; He, H.L.; Wang, G.F.; Wu, H.; Zhou, B.C.; Chen, X.L.; Zhang, Y.Z. Oyster (Crassostrea gigas) Hydrolysates Produced on a Plant Scale Have Antitumor Activity and Immunostimulating Effects in BALB/c Mice. Mar. Drugs 2010, 8, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-Y.; Kim, H.-J.; Han, J.-S. Anti-inflammatory Effect of Oyster Shell Extract in LPS-stimulated Raw 264.7 Cells. Prev. Nutr. Food Sci. 2013, 18, 23–29. [Google Scholar] [CrossRef]
- Miao, J.; Liao, W.; Kang, M.; Jia, Y.; Wang, Q.; Duan, S.; Xiao, S.; Cao, Y.; Ji, H. Anti-fatigue and anti-oxidant activities of oyster (Ostrea rivularis) hydrolysate prepared by compound protease. Food Funct. 2018, 9, 6578–6586. [Google Scholar] [CrossRef]
- Wang, X.; Yu, H.; Xing, R.; Liu, S.; Chen, X.; Li, P. Effect and mechanism of oyster hydrolytic peptides on spatial learning and memory in mice. RSC Adv. 2018, 8, 6125–6135. [Google Scholar] [CrossRef]
- Acquah, C.; Stefano, E.D.; Udenigwe, C.C. Role of hydrophobicity in food peptide functionality and bioactivity. J. Food Bioact. 2018, 4, 88–98. [Google Scholar] [CrossRef]
- Nyo, M.K.; Nguyen, L.T. Value-Addition of Defatted Peanut Cake by Proteolysis: Effects of Proteases and Degree of Hydrolysis on Functional Properties and Antioxidant Capacity of Peptides. Waste Biomass Valorization 2019, 10, 1251–1259. [Google Scholar] [CrossRef]
- Kristinsson, H.G.; Rasco, B.A. Fish protein hydrolysates: Production, biochemical, and functional properties. Crit. Rev. Food Sci. 2000, 40, 43–81. [Google Scholar] [CrossRef]
- Fang, X.; Xie, N.; Chen, X.; Yu, H.; Chen, J. Optimization of antioxidant hydrolysate production from flying squid muscle protein using response surface methodology. Food Bioprod. Process. 2012, 90, 676–682. [Google Scholar] [CrossRef]
- Kim, S.K.; Kim, Y.T.; Byun, H.G.; Nam, K.S.; Joo, D.S.; Shahidi, F. Isolation and characterization of antioxidative peptides from gelatin hydrolysate of Alaska pollack skin. J. Agric. Food Chem. 2001, 49, 1984–1989. [Google Scholar] [CrossRef] [PubMed]
- You, L.; Regenstein, J.M.; Liu, R.H. Optimization of hydrolysis conditions for the production of antioxidant peptides from fish gelatin using response surface methodology. J. Food Sci. 2010, 75, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Aspmo, S.I.; Horn, S.J.; Eijsink, V.G.H. Enzymatic hydrolysis of Atlantic cod (Gadus morhua L.) viscera. Process Biochem. 2005, 40, 1957–1966. [Google Scholar] [CrossRef]
- Ren, J.; Zhao, M.; Shi, J.; Wang, J.; Jiang, Y.; Cui, C.; Kakuda, Y.; Xue, S.J. Optimization of antioxidant peptide production from grass carp sarcoplasmic protein using response surface methodology. LWT-Food Sci. Technol. 2008, 41, 1624–1632. [Google Scholar] [CrossRef]
- Korenaga, M.; Nishina, S.; Korenaga, K.; Tomiyama, Y.; Yoshioka, N.; Hara, Y.; Sasaki, Y.; Shimonaka, Y.; Hino, K. Branched-chain amino acids reduce hepatic iron accumulation and oxidative stress in hepatitis C virus polyprotein-expressing mice. Liver Int. 2015, 35, 1303–1314. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yu, H.; Xing, R.; Chen, X.; Liu, S.; Li, P. Optimization of the Extraction and Stability of Antioxidative Peptides from Mackerel (Pneumatophorus japonicus) Protein. BioMed Res. Int. 2017, 2017, 6837285. [Google Scholar] [CrossRef]
- Wang, X.; Yu, H.; Xing, R.; Chen, X.; Li, R.; Li, K.; Liu, S.; Li, P. Purification and identification of antioxidative peptides from mackerel (Pneumatophorus japonicus) protein. RSC Adv. 2018, 8, 20488–20498. [Google Scholar] [CrossRef]
- Bhaskar, N.; Mahendrakar, N.S. Protein hydrolysate from visceral waste proteins of Catla (Catla catla): Optimization of hydrolysis conditions for a commercial neutral protease. Bioresour. Technol. 2008, 99, 4105–4111. [Google Scholar] [CrossRef]
- Wang, X.; Yu, H.; Xing, R.; Chen, X.; Liu, S.; Li, P. Optimization of antioxidative peptides from mackerel (Pneumatophorus japonicus) viscera. Peerj 2018, 6, 1–21. [Google Scholar] [CrossRef]
- Zheng, Z.; Li, J.; Li, J.; Sun, H.; Liu, Y. Physicochemical and antioxidative characteristics of black bean protein hydrolysates obtained from different enzymes. Food Hydrocolloid. 2019, 97, 105222. [Google Scholar] [CrossRef]
- Tan, X.; Qi, L.; Fan, F.; Guo, Z.; Wang, Z.; Song, W.; Du, M. Analysis of volatile compounds and nutritional properties of enzymatic hydrolysate of protein from cod bone. Food Chem. 2018, 264, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Amiza, M.A.; Nurul Ashikin, S.; Faazaz, A.L. Optimization of enzymatic protein hydrolysis from silver catfish (Pangasius sp.) frame. Int. Food Res. J. 2011, 18, 775–781. [Google Scholar]
- Ovissipour, M.; Abedian Kenari, A.; Motamedzadegan, A.; Nazari, R.M. Optimization of Enzymatic Hydrolysis of Visceral Waste Proteins of Yellowfin Tuna (Thunnus albacares). Food Bioprocess Technol. 2010, 5, 696–705. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Y.; Peng, C.; Wang, J. Optimization of the preparation of fish protein anti-obesity hydrolysates using response surface methodology. Int. J. Mol. Sci. 2013, 14, 3124–3139. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Zhang, G.; Li, X.; Zhao, B.; Zhou, S. Enzymatic Hydrolysis of Protein from Small Yellow Croaker (Psendosciaena Polyactis) and Evaluation of Its Antioxidant Activity. J. Food Biochem. 2013, 37, 278–285. [Google Scholar] [CrossRef]
- Guerard, F.; Sumaya-Martinez, M.T.; Laroque, D.; Chabeaud, A.; Dufossé, L. Optimization of free radical scavenging activity by response surface methodology in the hydrolysis of shrimp processing discards. Process Biochem. 2007, 42, 1486–1491. [Google Scholar] [CrossRef]
- Feng, K.; Chen, W.; Sun, L.; Liu, J.; Zhao, Y.; Li, L.; Wang, Y.; Zhang, W. Optimization extraction, preliminary characterization and antioxidant activity in vitro of polysaccharides from Stachys sieboldii Miq. tubers. Carbohyd. Polym. 2015, 125, 45–52. [Google Scholar] [CrossRef]
- Ahn, C.B.; Je, J.Y.; Cho, Y.S. Antioxidant and anti-inflammatory peptide fraction from salmon byproduct protein hydrolysates by peptic hydrolysis. Food Res. Int. 2012, 49, 92–98. [Google Scholar] [CrossRef]
- Dong, X.P.; Zhu, B.W.; Zhao, H.X.; Zhou, D.Y.; Wu, H.T.; Yang, J.F.; Li, D.M.; Murata, Y. Preparation and in vitro antioxidant activity of enzymatic hydrolysates from oyster (Crassostrea talienwhannensis) meat. Int. J. Food Sci. Technol. 2010, 45, 978–984. [Google Scholar] [CrossRef]
- Hamzeh, A.; Rezaei, M.; Khodabandeh, S.; Motamedzadegan, A.; Noruzinia, M.; Mac Regenstein, J. Optimization of Antioxidant Peptides Production from the Mantle of Cuttlefish (Sepia pharaonis) Using RSM and Fractionation. J. Aquat. Food Prod. Technol. 2019, 28, 392–401. [Google Scholar] [CrossRef]
- Wali, A.; Wubulikasimu, A.; Yanhua, G.; Omar, A.; Arken, A.; Yili, A.; Aisa, H.A. Separation and Purification of Antioxidant Peptides from Enzymatically Prepared Scorpion (Buthus martensii Karsch) Protein Hydrolysates. Int. J. Pept. Res. Ther. 2019. [Google Scholar] [CrossRef]
- Noman, A.; Jiang, Q.; Xu, Y.; Ali, A.H.; Al-Bukhaiti, W.Q.; Abed, S.M.; Xia, W. Influence of Degree of Hydrolysis on Chemical Composition, Functional Properties, and Antioxidant Activities of Chinese Sturgeon (Acipenser sinensis) Hydrolysates Obtained by Using Alcalase 2.4 L. J. Aquat. Food Prod. Technol. 2019, 28, 583–597. [Google Scholar] [CrossRef]
- Zhang, J.B.; Zhao, Y.Q.; Wang, Y.M.; Chi, C.F.; Wang, B. Eight Collagen Peptides from Hydrolysate Fraction of Spanish Mackerel Skins: Isolation, Identification, and In Vitro Antioxidant Activity Evaluation. Mar. Drugs 2019, 17, 224. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.R.; Zhang, L.; Ding, D.G.; Chi, C.F.; Wang, B.; Huo, J.C. Preparation, Identification, and Activity Evaluation of Eight Antioxidant Peptides from Protein Hydrolysate of Hairtail (Trichiurus japonicas) Muscle. Mar. Drugs 2019, 17, 23. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.Y.; Wang, Y.M.; Li, L.; Chi, C.F.; Wang, B. Four Antioxidant Peptides from Protein Hydrolysate of Red Stingray (Dasyatis akajei) Cartilages: Isolation, Identification, and In Vitro Activity Evaluation. Mar. Drugs 2019, 17, 236. [Google Scholar] [CrossRef]
- Wang, L.; Sun, J.; Ding, S.; Qi, B. Isolation and identification of novel antioxidant and antimicrobial oligopeptides from enzymatically hydrolyzed anchovy fish meal. Process Biochem. 2018, 74, 148–155. [Google Scholar] [CrossRef]
- Umayaparvathi, S.; Arumugam, M.; Meenakshi, S.; Dräger, G.; Kirschning, A.; Balasubramanian, T. Purification and Characterization of Antioxidant Peptides from Oyster (Saccostrea cucullata) Hydrolysate and the Anticancer Activity of Hydrolysate on Human Colon Cancer Cell Lines. Int. J. Pept. Res. Ther. 2013, 20, 231–243. [Google Scholar] [CrossRef]
- Zhou, D.Y.; Zhu, B.W.; Qiao, L.; Wu, H.T.; Li, D.M.; Yang, J.F.; Murata, Y. In vitro antioxidant activity of enzymatic hydrolysates prepared from abalone (Haliotis discus hannai Ino) viscera. Food Bioprod. Process. 2012, 90, 148–154. [Google Scholar] [CrossRef]
- You, L.; Ren, J.; Yang, B.; Regenstein, J.; Zhao, M. Antifatigue activities of loach protein hydrolysates with different antioxidant activities. J. Agric. Food Chem. 2012, 60, 12324–12331. [Google Scholar] [CrossRef]
- Luo, F.L.; Xing, R.E.; Wang, X.Q.; Peng, Q.C.; Li, P.C. Proximate composition, amino acid and fatty acid profiles of marine snail Rapana venosa meat, visceral mass and operculum. J. Sci. Food Agric. 2017, 97, 5361–5368. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |


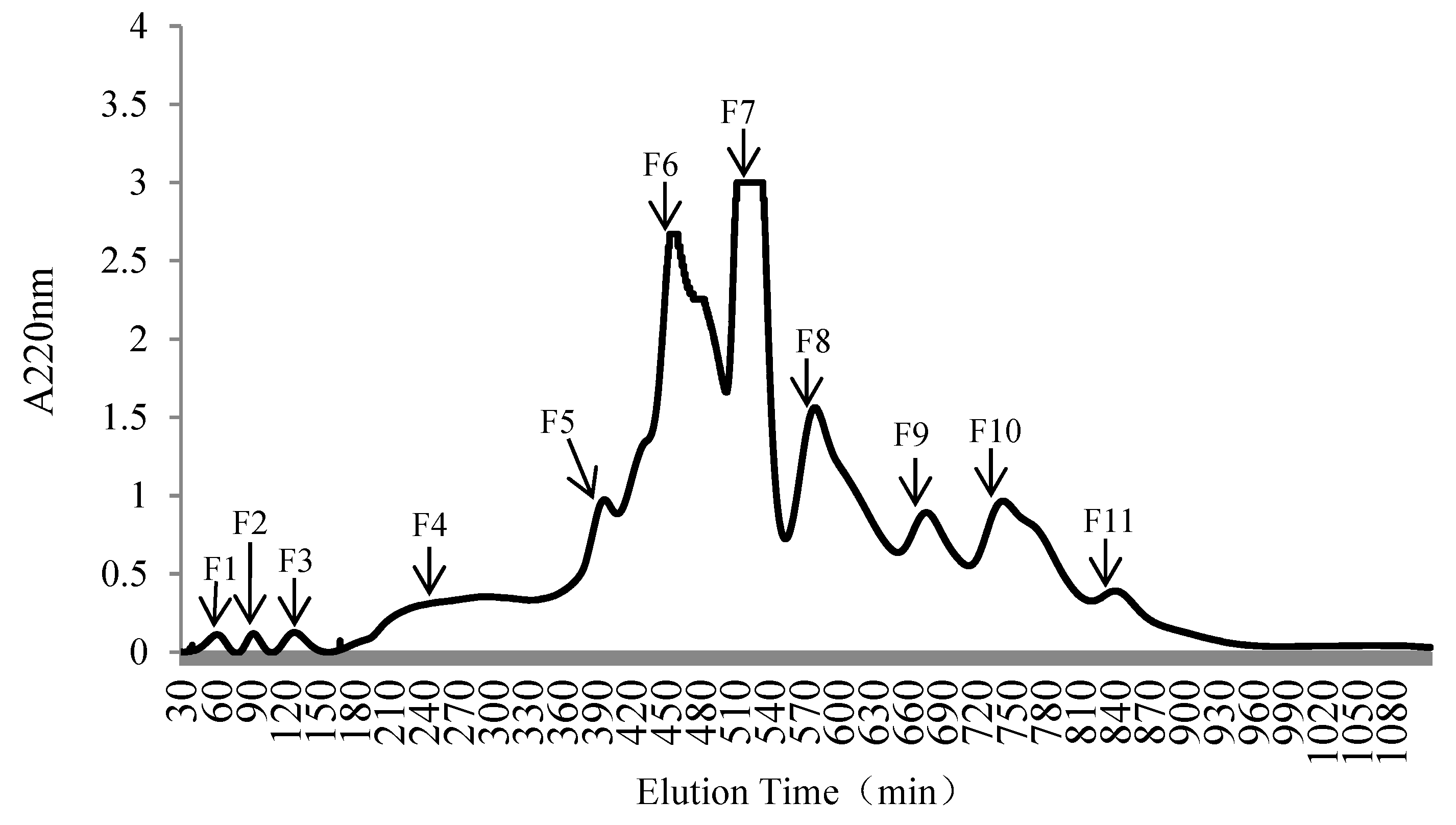
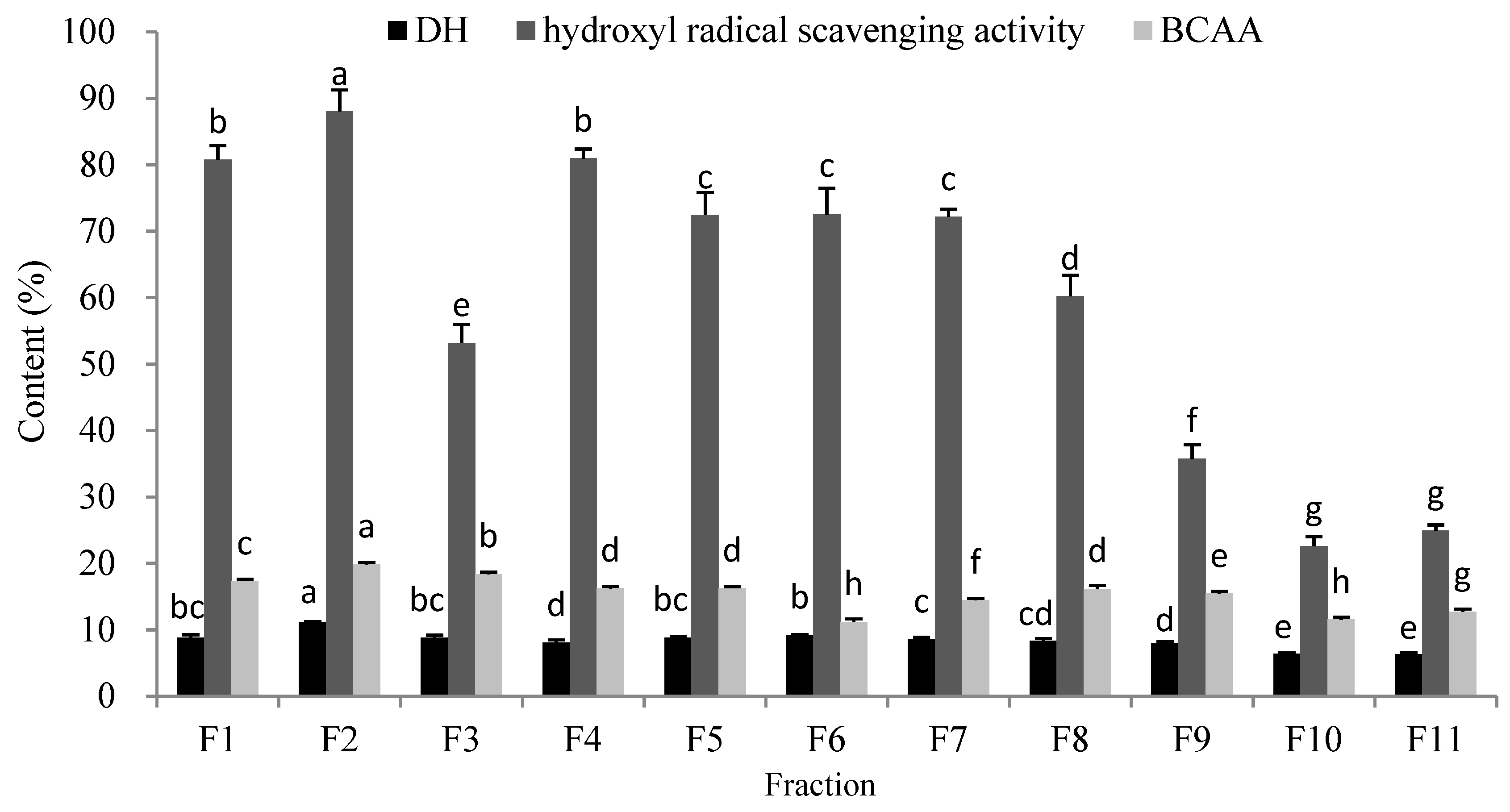
| Numbers | Response Values | A (U/g) | B | C (°C) | D (h) | E (w/v) |
|---|---|---|---|---|---|---|
| OP-1 | DH | 1500 | 8.0 | 40 | 6.0 | 8.0 |
| OP-2 | Hydroxyl radical Scavenging activity | 1500 | 8.5 | 55 | 5.0 | 6.0 |
| OP-3 | BCAA | 1500 | 8.0 | 50 | 6.0 | 6.0 |
| Numbers | A (U/g) | B | C (°C) | D (h) | E (w/v) | Y1: DH (%) | Y2: Hydroxyl-Radical-Scavenging Activity (%) | Y3: BCAA (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Y1/Y3 | Y2 | Y1 | Y2 | Y3 | Y1/Y3 | Y2 | Y1 | Y2/Y3 | |||||
| 1 | 1000 | 7 | 8 | 40 | 55 | 50 | 6 | 5 | 8 | 6 | 7.71 | 45.90 | 17.08 |
| 2 | 2000 | 7 | 8 | 40 | 55 | 50 | 6 | 5 | 8 | 6 | 8.05 | 49.21 | 17.55 |
| 3 | 1000 | 9 | 9 | 40 | 55 | 50 | 6 | 5 | 8 | 6 | 7.66 | 63.79 | 17.36 |
| 4 | 2000 | 9 | 9 | 40 | 55 | 50 | 6 | 5 | 8 | 6 | 8.85 | 62.33 | 17.35 |
| 5 | 1500 | 8 | 8.5 | 35 | 50 | 40 | 5 | 4 | 8 | 6 | 7.12 | 55.92 | 17.25 |
| 6 | 1500 | 8 | 8.5 | 45 | 60 | 60 | 5 | 4 | 8 | 6 | 7.08 | 58.91 | 17.19 |
| 7 | 1500 | 8 | 8.5 | 35 | 50 | 40 | 7 | 6 | 8 | 6 | 7.23 | 49.82 | 18.00 |
| 8 | 1500 | 8 | 8.5 | 45 | 60 | 60 | 7 | 6 | 8 | 6 | 7.05 | 55.10 | 17.16 |
| 9 | 1500 | 7 | 8 | 40 | 55 | 50 | 6 | 5 | 6 | 4 | 7.44 | 47.56 | 17.84 |
| 10 | 1500 | 9 | 9 | 40 | 55 | 50 | 6 | 5 | 6 | 4 | 6.95 | 57.08 | 17.72 |
| 11 | 1500 | 7 | 8 | 40 | 55 | 50 | 6 | 5 | 10 | 8 | 7.12 | 44.57 | 16.94 |
| 12 | 1500 | 9 | 9 | 40 | 55 | 50 | 6 | 5 | 10 | 8 | 8.51 | 54.40 | 17.62 |
| 13 | 1000 | 8 | 8.5 | 35 | 50 | 40 | 6 | 5 | 8 | 6 | 6.92 | 63.76 | 17.41 |
| 14 | 2000 | 8 | 8.5 | 35 | 50 | 40 | 6 | 5 | 8 | 6 | 7.45 | 62.22 | 17.35 |
| 15 | 1000 | 8 | 8.5 | 45 | 60 | 60 | 6 | 5 | 8 | 6 | 7.38 | 60.55 | 16.73 |
| 16 | 2000 | 8 | 8.5 | 45 | 60 | 60 | 6 | 5 | 8 | 6 | 7.62 | 64.99 | 17.30 |
| 17 | 1500 | 8 | 8.5 | 40 | 55 | 50 | 5 | 4 | 6 | 4 | 6.39 | 51.30 | 17.88 |
| 18 | 1500 | 8 | 8.5 | 40 | 55 | 50 | 7 | 6 | 6 | 4 | 6.93 | 52.97 | 17.95 |
| 19 | 1500 | 8 | 8.5 | 40 | 55 | 50 | 5 | 4 | 10 | 8 | 7.24 | 52.27 | 17.28 |
| 20 | 1500 | 8 | 8.5 | 40 | 55 | 50 | 7 | 6 | 10 | 8 | 7.05 | 36.76 | 17.87 |
| 21 | 1500 | 7 | 8 | 35 | 50 | 40 | 6 | 5 | 8 | 6 | 7.47 | 46.76 | 17.49 |
| 22 | 1500 | 9 | 9 | 35 | 50 | 40 | 6 | 5 | 8 | 6 | 7.20 | 70.85 | 17.65 |
| 23 | 1500 | 7 | 8 | 45 | 60 | 60 | 6 | 5 | 8 | 6 | 7.01 | 60.68 | 17.23 |
| 24 | 1500 | 9 | 9 | 45 | 60 | 60 | 6 | 5 | 8 | 6 | 7.65 | 62.57 | 17.08 |
| 25 | 1000 | 8 | 8.5 | 40 | 55 | 50 | 5 | 4 | 8 | 6 | 7.51 | 47.16 | 17.16 |
| 26 | 2000 | 8 | 8.5 | 40 | 55 | 50 | 5 | 4 | 8 | 6 | 8.20 | 56.98 | 17.34 |
| 27 | 1000 | 8 | 8.5 | 40 | 55 | 50 | 7 | 6 | 8 | 6 | 7.25 | 53.76 | 17.58 |
| 28 | 2000 | 8 | 8.5 | 40 | 55 | 50 | 7 | 6 | 8 | 6 | 8.69 | 45.21 | 17.50 |
| 29 | 1500 | 8 | 8.5 | 35 | 50 | 40 | 6 | 5 | 6 | 4 | 5.91 | 52.22 | 18.15 |
| 30 | 1500 | 8 | 8.5 | 45 | 60 | 60 | 6 | 5 | 6 | 4 | 6.27 | 53.13 | 17.17 |
| 31 | 1500 | 8 | 8.5 | 35 | 50 | 40 | 6 | 5 | 10 | 8 | 6.65 | 48.95 | 17.21 |
| 32 | 1500 | 8 | 8.5 | 45 | 60 | 60 | 6 | 5 | 10 | 8 | 6.75 | 49.81 | 17.28 |
| 33 | 1000 | 8 | 8.5 | 40 | 55 | 50 | 6 | 5 | 6 | 4 | 6.25 | 49.28 | 17.84 |
| 34 | 2000 | 8 | 8.5 | 40 | 55 | 50 | 6 | 5 | 6 | 4 | 7.06 | 62.71 | 17.23 |
| 35 | 1000 | 8 | 8.5 | 40 | 55 | 50 | 6 | 5 | 10 | 8 | 6.75 | 54.25 | 16.80 |
| 36 | 2000 | 8 | 8.5 | 40 | 55 | 50 | 6 | 5 | 10 | 8 | 7.54 | 40.93 | 17.73 |
| 37 | 1500 | 7 | 8 | 40 | 55 | 50 | 5 | 4 | 8 | 6 | 7.34 | 49.71 | 17.33 |
| 38 | 1500 | 9 | 9 | 40 | 55 | 50 | 5 | 4 | 8 | 6 | 8.15 | 51.79 | 17.36 |
| 39 | 1500 | 7 | 8 | 40 | 55 | 50 | 7 | 6 | 8 | 6 | 7.54 | 36.92 | 17.71 |
| 40 | 1500 | 9 | 9 | 40 | 55 | 50 | 7 | 6 | 8 | 6 | 7.88 | 60.65 | 17.83 |
| 41 | 1500 | 8 | 8.5 | 40 | 55 | 50 | 6 | 5 | 8 | 6 | 10.45 | 62.45 | 17.35 |
| 42 | 1500 | 8 | 8.5 | 40 | 55 | 50 | 6 | 5 | 8 | 6 | 10.34 | 60.60 | 17.49 |
| 43 | 1500 | 8 | 8.5 | 40 | 55 | 50 | 6 | 5 | 8 | 6 | 9.86 | 63.82 | 17.20 |
| 44 | 1500 | 8 | 8.5 | 40 | 55 | 50 | 6 | 5 | 8 | 6 | 9.93 | 66.14 | 17.23 |
| 45 | 1500 | 8 | 8.5 | 40 | 55 | 50 | 6 | 5 | 8 | 6 | 10.33 | 58.39 | 17.40 |
| 46 | 1500 | 8 | 8.5 | 40 | 55 | 50 | 6 | 5 | 8 | 6 | 9.89 | 65.88 | 17.49 |
| Response Values | Model | p-Value | Lack of Fit | Predicted R2 | Adj R2 | R2 |
|---|---|---|---|---|---|---|
| F-Value | Prob > F | p-Value | ||||
| DH (%) | 45.31 | <0.0001 | 0.6612 | 0.9086 | 0.9517 | 0.9732 |
| Hydroxyl-radical-scavenging activity (%) | 24.96 | <0.0001 | 0.4923 | 0.8314 | 0.9142 | 0.9523 |
| BCAA (%) | 48.61 | <0.0001 | 0.4359 | 0.9104 | 0.9549 | 0.9749 |
| Response Values | A (U/g) | B | C (℃) | D (h) | E (w/v) | Predicted Value | Experimental Value |
|---|---|---|---|---|---|---|---|
| DH | 1593.2 | 8.2 | 40.1 | 6.0 | 8.2 | 10.20 | 9.85 ± 0.76 |
| Hydroxyl-radical-scavenging activity | 1546.3 | 9.0 | 50.2 | 5.1 | 5.6 | 70.91 | 70.12 ± 2.37 |
| BCAA | 1323.8 | 8.3 | 41.7 | 6.7 | 4.8 | 18.15 | 17.88 ± 1.28 |
| No. | Sequence | Mass (Da) | Length | Parental Protein | Position |
|---|---|---|---|---|---|
| 1 | LAGELHQEQENYK | 1557.74 | 13 | K1QTC1 | 745–757 |
| 2 | AIDTIINQK | 1014.57 | 9 | K1R6Z7 | 223–231 |
| 3 | DSYVGDEAQSK | 1197.52 | 11 | Q8TA69;C4NY62; | 52–62 |
| 4 | PGTTEDEPVK | 1071.51 | 10 | K1Q5P0 | 498–507 |
| 5 | ETVIDTIQK | 1045.57 | 9 | K1RHA0 | 148–156 |
| 6 | DLESQLK | 831.43 | 7 | K1QRU8 | 989–995 |
| 7 | NAETELGETSQR | 1333.61 | 12 | K1QTC1 | 681–692 |
| 8 | EYDESGPSIVHR | 1387.64 | 12 | Q8TA69;C4NY62 | 362–373 |
| 9 | DSDLEGHPTPR | 1222.56 | 11 | K1RBC9 | 173–183 |
| 10 | HDNPGDLGDLH | 1188.52 | 11 | K1PY89;K1QLW5 | 128–138 |
| 11 | AQCEMEPNH | 1114.42 | 9 | K1PY89;K1QLW5 | 47–55 |
| 12 | ESAGIHETT | 943.42 | 9 | Q8TA69 | 271–279 |
| 13 | NTVLSGGTT | 848.42 | 9 | Q8TA69 | 297–305 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Yu, H.; Xing, R.; Liu, S.; Chen, X.; Li, P. Optimization of Oyster (Crassostrea talienwhanensis) Protein Hydrolysates Using Response Surface Methodology. Molecules 2020, 25, 2844. https://doi.org/10.3390/molecules25122844
Wang X, Yu H, Xing R, Liu S, Chen X, Li P. Optimization of Oyster (Crassostrea talienwhanensis) Protein Hydrolysates Using Response Surface Methodology. Molecules. 2020; 25(12):2844. https://doi.org/10.3390/molecules25122844
Chicago/Turabian StyleWang, Xueqin, Huahua Yu, Ronge Xing, Song Liu, Xiaolin Chen, and Pengcheng Li. 2020. "Optimization of Oyster (Crassostrea talienwhanensis) Protein Hydrolysates Using Response Surface Methodology" Molecules 25, no. 12: 2844. https://doi.org/10.3390/molecules25122844
APA StyleWang, X., Yu, H., Xing, R., Liu, S., Chen, X., & Li, P. (2020). Optimization of Oyster (Crassostrea talienwhanensis) Protein Hydrolysates Using Response Surface Methodology. Molecules, 25(12), 2844. https://doi.org/10.3390/molecules25122844







