Abstract
Sabah snake grass or Clinacanthus nutans has drawn public interest having significant economic benefits attributable to the presence of phytochemicals and several interesting bioactive constituents that may differ according to harvesting age and harvesting frequency. The current study was aimed to evaluate the effect of harvesting age and harvesting frequency towards herbal yield, antioxidant activities, phytochemicals synthesis, and bioactive compounds of C. nutans. A factorial randomized completely block design with five replications was used to illustrate the relationship between herbal yield, DPPH (2, 2-diphenyl-1-picrylhydrazyl) and ferric reducing antioxidant power (FRAP) assays, total phenolic and flavonoid content affected by harvesting age (week 8, 12, and 16 after transplanting), and harvesting frequency (harvest 1, 2, and 3). The bioactive compounds by HPLC were also determined to describe the interaction effect between both harvesting age and harvesting frequency. The yield, antioxidant activities, and phytochemical contents were gradually increased as the plant grew, with the highest recorded during week 16. However, the synthesis and activities of phytochemicals were reduced in subsequent harvests despite the increment of the herbal yield. All bioactive compounds were found to be influenced insignificantly and significantly by harvesting age and harvesting frequency, respectively, specifically to shaftoside, iso-orientin, and orientin. Among all constituents, shaftoside was the main compound at various harvesting ages and harvesting frequencies. These results indicated that harvesting at week 16 with 1st harvest frequency might enhance the yield while sustaining the high synthesis of polyphenols and antioxidant activities of C. nutans.
1. Introduction
A wide number of studies in current years has been focusing on the secondary metabolites due to the nature of antioxidant that potentially modulates human metabolism. These metabolites, which include saponins, tannins, anthocyanins, lignin, phenolics, and flavonoids, have been practically used by more than 80% of world populations to prevent and treat various health problems, such as cancer, diabetes, and high blood pressure [1]. Flavonoids, as the largest family having a low molecular structure of phenolic secondary metabolites, are well known to be associated with great length of pharmacological activities, which widely spread throughout the natural plants, including most the medicinal plants [2]. These compounds are receiving a number of considerable attention due to documented protective role against many diseases, which may be attributed to the biological effects of antioxidant activity against reactive oxygen species [3]. Due to the presence of this compound, medicinal plants have been utilized extensively in the world of pharmaceuticals as an alternative regiment against various diseases.
Native to tropical Asia countries, Clinacanthus nutans or commonly known as Sabah snake grass is well-reputed in Thai folklore medicine due to its ability to cure various ailments, such as skin inflammation and snake bites. For the past few decades, the research for C. nutans has been progressively done in a rapid trend. Broad-spectrum of pharmacological activities, which includes antioxidant [4], antiviral (herpes simplex virus (HSV), varicella-zoster virus, papillomavirus, and dengue virus) [5], anti-inflammatory [6], antibacterial [7,8,9], analgesic [10,11,12], antivenom [13,14,15], immunomodulating [16,17], neuroprotective [18,19,20], antidiabetic [21,22,23], α-glucosidase inhibitory [24], lipid-elevated inhibition [25], plasmid DNA protective [26], anti-angiogenic [27], anticancer [28], anti-proliferative [28], and antitumor as the latest biological action identified [29], has been very well reported in previous studies. Owing to significant economic benefits towards human health, the demand for this herb has been increased in recent years.
Although these constituents offer many substantial effects in human welfares, the biosynthesis of polyphenolic compounds attributed the biological activities may often modulate by various factors, which include cultivars, genotypes, environmental conditions, such as light intensity, relative humidity, and cultivation practices. Harvesting application as part of the cultivation practices has been known to have a significant role in influencing the quantitative and qualitative aspects in plants, resulting in an increment in growth, yield, and value of crops [30,31,32,33,34,35]. It is indeed shown that age-related factor has effectively enhanced the quantity and quality of the plant, specifically in phytochemical content and antioxidant activities in several crops [36,37,38,39]. However, repetitive and continuous harvesting has led to the degradation of these compounds [40,41,42]. These reported studies have essentially addressed to the agricultural and operational process done for C. nutans in Malaysia as farmers often repeatedly ratoon the plants for better yield increment, overlooking the effect of the practices on the quality aspect of C. nutans itself. Studies covering the response of phytochemical synthesis and bioactive compounds remain deficient despite the numerous findings regarding its beneficial aspects. As valuable natural resources, it is desirable to undertake further studies, gathering relevant information through harvesting management as alternative ways. Since phytochemicals’ content vary according to age and continuous harvesting, it is necessary to harvest the medicinal plants of a particular age in particular harvesting frequencies to sustain the medicinal potency of the plant. Therefore, this current study was aimed to evaluate the influence of interaction between harvesting age and harvesting frequencies towards the plant’s performances in synthesizing phytochemicals as well as bioactive constituents of C. nutans.
2. Results and Discussions
2.1. Herbal Yield
Both harvesting age and harvesting frequency significantly interacted with each other on the herbal production (Table 1, Appendix A). The highest production was recorded at week 16, while the lowest recorded at week 8. Similar results were obtained at different levels of harvesting frequency, where the plants that were harvested repeatedly via cutting produced more herbal yield.

Table 1.
Herbal yield, total phenolic, and total flavonoid content of the methanolic extracts of C. nutans at varying levels of harvesting age and harvesting frequency.
Under age-related harvesting, the manifested yield can be driven by many factors as plants constantly experience various stresses throughout the growth period [43]. These stresses lead to growth alteration in the shape and size of the plants to be well-adapted to environmental changes. It was presumed that light acquirement for photosynthesis has forced the assimilates produced by the leaves to be accumulated in the developing sink (new leaves), consequently leading to high herbal yield [44]. The mechanism may equally apply to the harvesting frequency. According to Marasha et al. [42], cutting operations in frequent harvesting may enhance the vegetative growth and yield of crops. A limitation of resource supply and changes in plant size via complete detachment of the aboveground part of plants possibly led the plant to shift its carbohydrates reservation to the leaf (dominant sink) for light acquisition, clarifying the increment of herbal yield at various harvesting frequencies (Table 1).
2.2. Total Phenolic Content (TPC) and Total Flavonoid Contents (TFC)
As shown in Table 1, TFC and TPC of C. nutans were significantly influenced by harvesting age and harvesting frequency (p ≤ 0.05, Appendix B). From the table shown, the concentration of TPC, which ranged between 5.82 and 10.84 mg GAE/g DW, was much slightly lower than the concentration of TFC, which ranged between 4.31 and 7.32 mg QE/g DW for both treatments. These results were in line with a number of studies signifying that flavonoid is a part of the phenolic compounds, making the quantity to be generally lower compared to phenolic contents. Under age-related, both TPC and TFC of C. nutans were responded proportionally with time (p ≤ 0.01, respectively) with the highest TPC and TFC recorded during week 16 of harvesting (average value: 10.84 mg GAE/g DW and 7.32 mg QE/g DW, respectively), statistically followed by week 12 (average value: 6.87 mg GAE/g DW and 6.21 mg QE/g DW, respectively) and week 8 (average value: 6.17 mg GAE/g DW and 4.31 mg QE/g DW, respectively) containing the lowest value. The production of TFC and TPC of Sabah snake grass, however, degraded following the number of harvesting frequency in a linear manner (p ≤ 0.01, respectively), with the lowest concentrations recorded during third harvesting frequency (average value: 5.82 mg GAE/g DW and 4.69 mg QE/g DW, respectively), followed by second (average value: 7.75 mg GAE/g DW and 6.02 mg QE/g DW, respectively) and first harvesting frequency (average value: 10.30 mg GAE/g DW and 7.13 mg QE/g DW, respectively). Although no interaction was analyzed for TFC between both treatments, the production of both TFC and TPC in C. nutans shared a similar pattern where both polyphenolics were enhanced significantly as the plant grew but substantially reduced as the plant was repeatedly harvested.
In a different study on C. nutans itself, similar results were also obtained by Ghasemzadeh et al. [37], who confirmed that the production of phytochemicals remained increased until the age of 6 months, which then decreased until the age of 1 year. Sharing the same family to C. nutans, Andrographis paniculata (Hempedu bumi) also displays an increment in the content of andrographolide until a certain point of harvesting stage and then decreases afterward due to the senescence of leaves during reproductive phase [33]. Current results on reduction in phytochemicals at different harvesting frequencies were in agreement with Toit [45] and Hue [41], stating the limitation of substrates for synthesizing the secondary metabolites as the probable cause. Marasha [42] explained that repetitive harvesting might help to stimulate and improve the vegetative growth of the plant. However, rapid regrowth of plant following the above-ground removal would require a high amount of carbohydrates acquisition, which restricted the substrate for carbon-based secondary metabolites (CBSM), explaining the degradation of phytochemicals at varying levels of harvesting frequency. Consistency of previous findings and current results justified that the phenolic and flavonoid content of C. nutans could highly be influenced by harvesting age and harvesting frequency.
2.3. Radical Scavenging Activity (DPPH) and Ferric Reducing Power Assay (FRAP)
In vitro, the specific antioxidant activity can be analyzed by different methods, yielding information on the effectiveness of phenolics to neutralize free radicals. Among in vitro assays, the DPPH-based technique is, furthermore, quick, simple, relatively straightforward to perform, and inexpensive compared to other assays. However, antioxidant activity should not be deduced depending on a single antioxidant test model. In this experiment, samples were subjected to both DPPH (2, 2-diphenyl-1-picrylhydrazyl) and ferric reducing antioxidant power (FRAP) assays to estimate the capacity of C. nutans in scavenging the free radicals and measure the comparison data obtained between these assays.
Pearson correlation analysis in Figure 1 demonstrated a highly significant and positive correlation in linear form between the DPPH and FRAP results. This finding was undoubtedly in line with a number of previous studies [46,47,48,49,50,51], considering these two assays displayed a complementary mechanistic basis [52], and supposedly believing that conducting two or more assays could enhance the overall evaluation of the antioxidant activity of plant extracts as each assay demonstrated various antioxidant performances [53]. Although these assays vary in mode of actions, both assays considerably exhibit a high degree of redundancy on account of sharing akin mechanism. As such, the selection of an assay before running the analysis is important as each assay has its own pros and cons. Compared to DPPH, FRAP is simple, rapid, and inexpensive [54], yet issues, such as color interferences in extracts and slow development of color, have been reported upon utilizing FRAP as an antioxidant assay [55,56,57]. ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) and Oxygen Radical Absorbance Capacity (ORAC) assays should be in favor of future studies to analyze the antioxidant action in C. nutans.
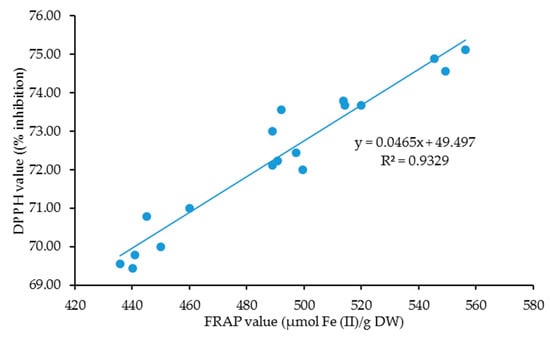
Figure 1.
The correlation between DPPH value (% inhibition) and FRAP value (µmol Fe (II)/g DW) for a combination of both harvesting age and harvesting frequency.
The DPPH scavenging activity and FRAP activity of C. nutans at different levels of harvesting age and harvesting frequency are presented in Table 2. Based on Table 2, the DPPH activity varied significantly and independently to the respective harvesting age and harvesting frequency (p < 0.05, Appendix B). In comparison to DPPH, FRAP activity exhibited a substantial effect and interaction effect at varying levels of harvesting age and harvesting frequency. Both DPPH and FRAP activities displayed a similar trend, where both of these antioxidant activities were increased simultaneously with harvesting age, with the highest DPPH and FRAP value observed at week 16, followed statistically by week 12 and week 8. Similar to phytochemicals, both DPPH and FRAP activities were declined with a number of harvesting frequency, having the lowest value recorded during third harvesting, while the first harvesting frequency contained the highest antioxidant activities. Although the trend exerted by these antioxidant activities was similar, the changes in value could be seen in the FRAP assay compared to DPPH. In addition, the pattern shown by FRAP activity was in line with phenolic contents, suggesting that FRAP assay favored the phenolic compounds since both phenolic and FRAP activities were interactively and substantially affected by harvesting age and harvesting frequencies. The occurrence of antioxidant activities in C. nutans has been very well affirmed by many studies [58,59,60,61]. Pannagtech [61] noted that C. nutans had the capability of inhibiting the oxidative stress in the 2,2′-azobis (amidinopropane) dihydrochloride (AAPH)-induced red blood cell lysis, which was attributed to the exertion of free radical scavenging activity and ferric reducing antioxidant power in plants.

Table 2.
DPPH and FRAP activities of the methanolic extracts of C. nutans at varying levels of harvesting age and harvesting frequency.
2.4. Bioactive Compounds (C-Glycosyl Flavone)
All known compounds displayed a good constancy with the corresponding standard solution in terms of retention times, confirming the validity of the results (Figure 2). The C-glycosyl flavone compounds at different levels of harvesting age and harvesting frequency are shown in Table 3. Briefly, current results presented below revealed that the shaftoside was the main flavone compound as it was constantly detected at all levels of both treatments, ranging from 5.32 µg/g to 7.39 µg/g for harvesting age and 11.27 µg/g to 3.20 µg/g for harvesting frequency. The remaining identified flavone compounds were detected in small amount, ranging from highest recorded in iso-orientin (harvesting age: 1.59–2.31 µg/g, harvesting frequency: 0.49–3.07 µg/g), followed statistically by orientin (harvesting age: 0.56–0.66 µg/g, harvesting frequency: 0.23–1.09 µg/g), iso-vitexin (harvesting age: 0.45–0.65 µg/g, harvesting frequency: 0.36–0.83 µg/g), and vitexin as the lowest amount presented (harvesting age: 0.09–0.32 µg/g, harvesting frequency: 0.23–0.51 µg/g). Although all compounds were present at most levels of treatments, a significant variation could be observed only in three compounds, namely, shaftoside, iso-orientin, and orientin, specifically for harvesting frequency (p < 0.05, Table 3, Appendix B). An abundant amount of shaftoside, in the current study, was consistent with the results of Chelyn et al. [62], where the largest amount of shaftoside was found in all locations, namely, Taiping, Perak, Kota Tinggi, Johor and Sendayan, and Negeri Sembilan. Stalikas [63] explained that the C-glycosyl flavone was favorable for the chemical markers due to the wide range of structural diversity. Since shaftoside was found to be abundant at all levels of harvesting age and harvesting frequency, it was suggested that shaftoside would be a possible chemical marker for C. nutans raw materials.
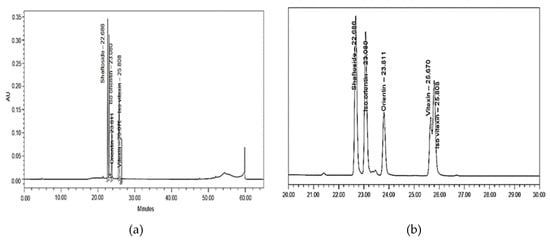
Figure 2.
HPLC chromatogram of the standard solution in (a) full and (b) zoom. Shaftoside (RT: 22.686), iso-orientin (RT: 23.080), orientin (RT: 23.811), vitexin (RT: 25.670), and iso-vitexin (RT: 25.808). RT refers to retention time.

Table 3.
Quantification of bioactive compounds of C. nutans at varying levels of harvesting age and harvesting frequency.
2.5. The Correlation between Herbal Yield, Antioxidant Activities, and Identified Polyphenolic Compounds
To date, it is acknowledged that phytochemicals are the major contributors to the activity of antioxidants in most potent medicinal and aromatic plants. Since there was a distinct demonstration in both treatments in this study, correlation data were divided into two tables. As can be seen in Table 4, for harvesting age, the herbal yield of C. nutans was significantly and positively correlated with phytochemicals and antioxidants. Under frequency-related harvesting, however, the yield showed a negative relationship with phytochemicals and antioxidants (Table 5). Results also showed that the DPPH activity was significantly correlated with FRAP activity in both harvesting age and harvesting frequency. The same results were also applied to total phenolics and total flavonoids, where both of these phytochemical contents were correlated significantly with both DPPH activity and FRAP activity.

Table 4.
Pearson correlation of herbal yield, phytochemicals, antioxidants, and bioactive compounds for the harvesting age of C. nutans.

Table 5.
Pearson correlation of herbal yield, phytochemicals, antioxidants, and bioactive compounds for the harvesting frequency of C. nutans.
In relation to the C-glycosyl flavone, a significant correlation between these constituents, phytochemicals, and antioxidant activities was only noticeable in the harvesting frequency and not in than harvesting age. There were three known compounds that demonstrated a significant correlation between phytochemicals and antioxidant activities, namely, shaftoside, iso-orientin, and orientin (Table 5). These correlations indeed elucidated the exhibition of antioxidants in C. nutans, which were greatly due to these identified compounds. Although the literature lacks the correlation between these compounds with antioxidant capacity, it is agreeable that phenylpropanoid glycosides possess high antioxidant power [64]. The phenylpropanoid pathway is an important biosynthesis of secondary metabolites in medicinal plants derived from phenylalanine and tyrosine [65]. A common metabolic fate, glycosylation, is a process that is known to regulate flavonoid’s structural stability [66]. Glycosylation is not only highly correlated with plant taxonomy [67], it is also recognized as the major regulator of phenylpropanoid’s availability, stability, toxic potential, and biological activity in plants [68]. The uncorrelated data between antioxidants and these constituents were shown at different levels of harvesting age, feasibly due to several factors. Seeing that this study was undertaken in an open field, various changes in exogenous sources, such as light, temperatures, soil nutrition, and textures, occurring throughout the plant growth process, presumably altered or disrupted the synthesis and structure of bioactive compounds [68]. Managing both negative and positive interactions with the environment, the plants constantly synthesize intricate and dynamic mixtures of secondary metabolites to counter explicit environmental conditions [69,70,71,72].
The relationship, reported between phytochemicals and antioxidant activities, is similar in several studies [37,73,74]. Flavonoids, as part of the phytochemicals, act as antioxidants, contributing to most of the biological activities in higher plants, including medicinal plants. Phytochemicals tend to respond simultaneously and linearly with antioxidants by direct correlation, indicating the responsibility of phytochemicals for the antioxidant activities exerted in plants [75,76,77]. This will indirectly affect the known individual of polyphenolic compounds, which can be seen in kaempferol and quercetin, while other constituents show poor correlation results [37]. The consistency of current results with the previous findings implied that the identified constituents, known as shaftoside, iso-orientin, and orientin flavone compound, could be the key, determining the antioxidant activities in C. nutans at different levels of harvesting frequency.
3. Materials and Methods
3.1. Standards and Chemicals
All chemicals were analytical and HPLC (HPLC analysis)-reagent grade. The chemicals included methanol (Systerm ®, ChemAR, Shah Alam, Malaysia), Folin-Ciocalteau reagent (Sigma-Aldrich, St. Louis, MO, USA), sodium carbonate (Sigma-Aldrich, St. Louis, MO, USA), gallic acid (Sigma-Aldrich, St. Louis, MO, USA), sodium nitrite (Systerm ®, ChemAR, Shah Alam, Malaysia), aluminium chloride (Systerm ®, ChemAR, Shah Alam, Malaysia), sodium hydroxide (Systerm ®, ChemAR, Shah Alam, Malaysia), quercetin (Sigma-Aldrich, St. Louis, MO, USA), 2,2-diphenyl-1-picrylhydrazyl (DPPH) (Sigma-Aldrich, St. Louis, MO, USA), sodium acetate buffer (Sigma-Aldrich, St. Louis, MO, USA), 2,4,6-tri [2-pyridyl]-s-triazine (TPTZ) (Sigma-Aldrich, St. Louis, MO, USA), iron (III) chloride (Systerm ®, ChemPur, Shah Alam, Malaysia), hydrochloric acid (Sigma-Aldrich, St. Louis, MO, USA), ferrous sulphate (Sigma-Aldrich, St. Louis, MO, USA), methanol HPLC grade (Sigma Aldrich, St. Louis, MO, USA), shaftoside (Sigma-Aldrich, St. Louis, MO, USA), orientin (HPLC grade, Sigma-Aldrich, St. Louis, MO, USA), iso-orientin (HPLC, Sigma-Aldrich, St. Louis, MO, USA), vitexin (Sigma-Aldrich, St. Louis, MO, USA), isovitexin (HPLC grade, Sigma-Aldrich, St. Louis, MO, USA), Milli Q water, distilled water, orthophosphoric acid (HPLC grade, Sigma-Aldrich, St. Louis, MO, USA), acetonitrile (HPLC grade, Sigma-Aldrich, St. Louis, MO, USA).
3.2. Planting Materials, Experimental Field, and Treatments
This study was carried out in open field conditions at Kompleks Ladang Bersepadu, Ladang 10, Universiti Putra Malaysia (UPM), Serdang, Selangor, Malaysia. The soil of the experimental area was clay loamy in texture, slightly acidic (pH 5.82), with good cation exchange capacity (8.21 cmol/kg). The soil nutrients were as follows: C (1.8%), N (0.12%), P (18.48 µg/g), K (141.5 µg/g), Ca (705.1 µg/g), and Mg (83.4 µg/g). The plants were propagated through stem cuttings and prepared in polyethylene bags filled with topsoil. The stems were then arranged and left aside under shading areas, allowing them to grow for a month until they grew evenly with daily irrigation. When propagated stems reached 90% uniformity in height, the stems were transplanted to the prepared field plots arranged by factorial randomized complete block design with five replications. The plants were harvested according to the treatments consisting of three levels of harvesting age (weeks 8, 12, and 16) and harvesting frequency (harvest 1, 2, and 3). Leaves were separated from the stems and oven-dried at 35 °C. Once dried, the samples were weighed, blended into powdered form, and stored at −20 °C for further analysis.
3.3. Extraction Method
The 0.5 g of every ground sample was extracted with 15 mL aqueous methanol (methanol: water, 80–20 v/v) for 3 h at room temperature in an orbital shaker. The extracts were separated from the residues by filtering through Whatman No.1 filter paper. The extracts were then transferred into airtight amber vials and stored at −80 °C until used for analysis.
3.4. Determination of Total Phenolic Content (TPC)
The content of phenolics, from the extract of C. nutans, was evaluated by using the Folin–Ciocalteau reagent-based assay, according to Barku [74] with slight modification. A 5 mL of Folin–Ciocalteau reagent was added to the 200 µL of samples, and the solutions were allowed to stand for 10 min at 25 °C. Later, 4 mL of sodium carbonate was added into the solutions, and the solutions were kept in total darkness for 20 min at room temperature. The absorbance reading of the blue color mixture was measured at 765 nm using an ultraviolet-visible (UV) spectrophotometer (Shimadzu, Kyoto, Japan). Gallic acid was used as a standard for the calibration curve, and the samples were expressed as mg GAE/g plant dry weight.
3.5. Determination of Total Flavonoid Content (TFC)
The flavonoid content of C. nutans was evaluated according to aluminum chloride assay [37]. The 1 mL of each extract was transferred into 10-mL volumetric flasks containing 4 mL of 80% methanol. Then, 0.3 mL of sodium nitrite (NaNO2) solution (1:5, w/v) was added to the flasks and allowed to stand for 6 min at room temperature. Next, 0.3 mL of aluminum chloride (AlCl3) solution (1:10, w/v) was added into the mixtures and kept for 6 min at room temperature. Finally, 2 mL of sodium hydroxide (NaOH) solution (1 M) was mixed and kept for 10 min at room temperature. The absorbance reading of yellow mixtures was measured at 510 nm by ultraviolet-visible (UV) spectrophotometer (Shimadzu, Kyoto, Japan). Quercetin was used as a standard for the calibration curve, and the samples were expressed as mg quercetin/g plant dry weight.
3.6. DPPH Radical Scavenging Activity Assay
The antioxidant activity of the aqueous methanol extract of C. nutans was evaluated using 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical, based on the electron transfer reaction between DPPH reagent and the plant extract according to the method [77]. A 0.2 mM solution of DPPH in pure methanol was prepared, and 2 mL of this solution was added to 2 mL of all extracts at various concentrations. The mixtures were shaken gently and allowed to stand for 30 min at room temperature. The absorbance for both positive control (DPPH solution) and samples was measured at 517 nm against methanol as a blank using an ultraviolet-visible (UV) spectrophotometer (Shimadzu, Kyoto, Japan). The inhibition percentage of the absorbance was calculated as follows:
Inhibition % = [(absorbance of control - absorbance of sample)/absorbance of control)] × 100
3.7. Ferric Reducing Antioxidant Power Assay (FRAP)
The ability to reduce ferric ions of the aqueous methanol extract was evaluated using the method described by Benzie and Strain [78]. The working FRAP reagent with the ratio of 10:1:1 was developed daily from 300 mM sodium acetate buffer, 10 mM 2,4,6-tri [2-pyridyl]-s-triazine (TPTZ) in 40 mM hydrochloric acid (37%), and 20 mM FeCl3. The pH value of the buffer was checked and maintained at pH 3.6. A ferrous sulfate (FeSO4·7H2O) was used as a standard, and a linear curve was prepared for various concentrations. For the analysis, 200 µL of the extract was added to 3 mL freshly prepared FRAP solution, and the reaction mixture was incubated for 30 min at 37 °C in the water bath. Then, the absorbance of the sample readings was recorded at 593 nm using an ultraviolet-visible (UV) spectrophotometer (Shimadzu, Kyoto, Japan). The solvent without sample was taken as a blank, and the FRAP value was calculated from the differences in the absorbance of a sample and a blank. The FRAP value based on the ability to reduce ferric ions of the extract was expressed as µM(Fe(II)/g dry mass.
3.8. Separation and Analysis of Bioactive Compounds (C-Glycosyl Flavone) by HPLC
The determination of the bioactive compounds of C. nutans was done, based on Chelyn [62], with slight modifications. The methanolic extract of C. nutans was prepared by adding methanol 80% (10 mL) to 0.5 g of powdered leaves. The mixtures were then vortexed for 10 min to dissolve the samples and sonicated for 30 min at 45 °C in an ultrasonic bath. Then, the samples were filtered using Whatman no. 1 filter paper, and the collected supernatant was subjected to HPLC analysis. The standard stock solutions of shaftoside, orientin, iso-orientin, vitexin, and iso-vitexin were prepared in HPLC grade methanol and stored at 4 °C. HPLC analysis was performed on Waters Alliance (E2695 and 2998 PAD Detector). The chromatographic separation was performed using C18 (250 × 4.6 mm, 5 µm). The solvent system consisted of mixtures of Milli Q water with orthophosphoric acid at pH 2.5 (solvent A) and absolute acetonitrile HPLC grade (solvent B). The solvents were degassed before delivering into the system. Samples for HPLC analysis were filtered through a 0.45 µm membrane filter. The flow rate and the injection volume were 0.8 mL/min and 20 µL, respectively. The signal was monitored at 280 nm. The gradient HPLC condition was as follows: 0–10 min 5–10% B; 10–40 min 10–40% B; 40–48 min 40–100% B; 48–55 min 100% B; 55–58 min 100–5% B; 58–64 min 5% B. A linear relationship between absorbance and concentration of standard was prepared based on the Beer-Lambert law. The quantification of compounds in samples was done based on a linear regression equation of standard, Y = aX ± b, where X was the concentration of phenolic, and Y was the peak height of phenolic obtained from HPLC. HPLC chromatogram for all standard solutions with retention time is shown in Figure 2.
3.9. Statistical Analysis
Collected data from the study were presented as mean ± SD of five replications, and means were analyzed with the analysis of variance (ANOVA) by statistical analysis system (SAS, Ver. 9.3, Cary, NC, USA). Means were compared using Duncan multiple range test (DMRT) at p < 0.05.
4. Conclusions
Generally, the results obtained from this study indicated that the yield, phytochemicals, antioxidant activities, and bioactive constituents of Clinacanthus nutans were changing according to varying levels of harvesting age and harvesting intervals. Plant harvested at week 16 during first harvesting frequency certainly produced the highest phenolic, flavonoids, and antioxidant activities, and then decreased with repeating harvesting. Of all known constituents, the shaftoside flavone compound was suggested as a chemical marker because it was the major compound found at all levels of harvesting age and harvesting frequency. C. nutans was considered to be a valuable resource, and it exhibited a good natural antioxidant.
However, the present scientific study was quite insufficient, preliminary, and fundamentally oriented. More sophisticated evaluation and pathway analyses with other biological and therapeutic effects due to biotic and abiotic factors should be highlighted for the next experiment. It would also be beneficial to have more experimental studies that could describe the correlation of the isolated phytochemicals from C. nutans using multiple and latest software analysis.
Regardless, the information obtained from this study would be greatly beneficial not only for the researchers but also for the farmers who are interested in cultivating the plants. It should be highlighted that continuous cutting of plants has a substantial negative effect on the quality despite the increment on the yield. Quality and quantity aspects should be in equilibrium balance when it comes to operating agricultural practices of any crop in order to produce high-quality plant-based products.
Author Contributions
Study designs, fieldwork, laboratory work, and writing of the first draft were performed by N.M.A.A.S. under the supervision of S.A. and Y.A. The first draft of the paper was reviewed by Y.A., S.A., R.A.H.B., and M.H. All authors reviewed and approved the final version. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Agriculture and Food Industry (Malaysia), grant number NRGS NH 0513A017.
Acknowledgments
The authors would like to acknowledge former project leader (Izham Ahmad) and all staff of the Laboratory of Physiology in the Department of Crop Science and Institute of Bioscience, Universiti Putra Malaysia, for help and guidance in order to accomplish this project.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
The mean square of main effect and two-way interaction ANOVA herbal yield of C. nutans in different levels of harvesting age and harvesting frequency.
| Source of Variations | d.f | Leaf Dry Weight |
| Block | 4 | 10.29 |
| Harvesting age (HA) | 2 | 3752.88 ** |
| Harvesting frequency (HF) | 2 | 2731.08 ** |
| Interaction (HA × HF) | 4 | 200.75 ** |
| Error | 32 | 3.73 |
| Total | 44 | |
| Contrast | ||
| Linear (HA) | 1 | 2482.52 ** |
| Quadratic (HA) | 1 | 18.94 * |
| Linear (HF) | 1 | 1820.61 ** |
| Quadratic (HF) | 1 | 0.02 |
| * and ** are significantly difference at p < 0.05 and p < 0.01 respectively. | ||
Appendix B
The mean square of main effect and two-way interaction ANOVA for phytochemicals, antioxidants and bioactive compounds of C. nutans in different levels of harvesting age and harvesting frequency.
| Source of Variations | d.f | Phytochemicals and Antioxidant Activity | C-glycosyl flavone (µg/g) | |||||||
| DPPH activity (%) | FRAP Activity (Fe (II)/g) | Total Phenolic Content (mg quercetin/g DW) | Total Flavonoid Content (mg GAE/g DW) | Shaftoside | Isoorientin | Orientin | Isovitexin | Vitexin | ||
| Block | 4 | 1.05 | 616.55 | 0.12 | 0.94 | 0.84 | 1.83 | 0.22 | 0.72 | 0.18 |
| Harvesting age (HA) | 2 | 49.85 ** | 10034.29 ** | 95.17 ** | 34.73 ** | 7.03 | 0.82 | 0.03 | 0.28 | 0.17 |
| Harvesting frequency (HF) | 2 | 97.23 ** | 25343.55 ** | 75.63 ** | 22.47 ** | 77.16 * | 14.39 * | 1.71 | 0.94 | 0.59 |
| Interaction (HA × HF) | 4 | 1.38 | 1301.28 * | 3.62 ** | 0.04 | 40.53 | 0.24 | 0.13 | 0.48 | 0.34 |
| Error | 32 | 0.59 | 371.59 | 0.18 | 0.61 | 17.42 | 1.59 | 0.09 | 0.56 | 0.20 |
| Total | 44 | |||||||||
| Contrast | ||||||||||
| Linear (HA) | 1 | 33.20 ** | 6621.36 * | 54.57 * | 22.68 * | 2.55 | 0.355 | 0.01 | 0.25 | 0.11 |
| Quadratic (HA) | 1 | 0.03 | 67.14 | 8.83 ** | 0.50 | 2.16 | 0.00 | 0 | 0.24 | 0.01 |
| Linear (HF) | 1 | 64.77 ** | 16759.20 ** | 50.04 ** | 14.91 ** | 51.10 * | 11.32 * | 1.13 * | 0.33 | 0.11 |
| Quadratic (HF) | 1 | 0.05 | 137.13 | 0.33 * | 0.04 | 0.3 | 2.78 | 0.02 | 0.3 | 0.01 |
| * and ** are significantly difference at p < 0.05 and p < 0.01 respectively. | ||||||||||
References
- Zhang, X.; World Health Organization. Traditional Medicine Strategy. 2002–2005. Available online: http://apps.who.int/medicinedocs/en/d/Js4928e/ (accessed on 22 March 2017).
- Saxena, M.; Saxena, J.; Nema, R.; Singh, D.; Gupta, A. Phytochemistry of medicinal plant. J. Pharmacogn. Phytochem. 2013, 1. [Google Scholar]
- Schijlen, E.G.W.M.; Ric de Vos, C.H.; Tunen, A.J.V.; Bovy, A.G. Modification of flavonoid biosynthesis in crop plants. Phytochemistry 2004, 65, 2631–2648. [Google Scholar] [CrossRef] [PubMed]
- Wanikiat, P.; Panthong, A.; Sujayanon, P.; Yoosok, C.; Rossi, A.G.; Reutrakul, V. The anti-inflammatory effects and the inhibition of neutrophil responsiveness by Barleria lupulina and Clinacanthus nutans extracts. J. Ethnopharmacol. 2007, 116, 234–244. [Google Scholar] [CrossRef]
- Kunsorn, P.; Ruangrungsi, N.; Lipipun, V.; Khanboon, A.; Rungsihirunrat, K. The identities and anti-herpes simplex virus activity of Clinacanthus nutans and Clinacanthus siamensis. Asian Pac. J. Trop. Biomed. 2013, 3, 284–290. [Google Scholar] [CrossRef]
- Shuyprom, A. Chemical composition investigation of the Clinacanthus nutans (Burm. F.) lindau leaves. Master’s Thesis, Suranaree University of Technology, Ratchasima, Thailand, 2004. [Google Scholar]
- Ho, S.Y.; Tiew, W.P.; Madhavan, P.; Abdul Shukkoor, M.S.; Akowuah, G.A. Phytochemical analysis and antibacterial activity of methanolic extract of Clinacanthus nutans leaf. Int. J. Drug Dev. Res. 2013, 5, 349–355. [Google Scholar]
- Wong, F.-C.; Yong, A.-L.; Ong, H.-C.; Chai, T.-T. Evaluation of the antibacterial activities of selected medicinal plants and determination of their phenolic constituents. ScienceAsia 2013, 39, 591–595. [Google Scholar]
- Tinh, T.D.D. Biological activities of Clinacanthus nutans (Burm. F) Lindau Extracts. Ph.D. Thesis, International University HCMC, Ho Chi Minh, Vietnam, 2014. [Google Scholar]
- Abdul Rahim, M.H.; Zakaria, Z.A.; Mohd Sani, M.H. Methanolic extract of Clinacanthus nutans exerts antinociceptive activity via the opioid/nitric oxide-mediated, but cGMP-independent, pathways. Evid.-Based Complementary Altern. Med. 2016, 1–11. [Google Scholar]
- Kittisiripornkul, S.; Witthayasat, K. The Antiinflammatory Action and Toxicological Studies of Extracts from Clinacanthus Nutans. Ph.D. Thesis, Mahidol University, Bangkok, Thailand, 1984. [Google Scholar]
- Satatyavivad, J.; Bunyaprephatsara, N.; Kitisiripornkul, S.; Tanasomwang, W. Analgesic and anti-inflammatory activities of extract of Clinacanthus nutans (Burm.F.) lindau. Thai J. Pharm. Sci. 1996, 3, 7–17. [Google Scholar]
- Uawonggul, N.; Chaveerach, A.; Thammasirirak, S.; Arkaravichien, T.; Chuachan, C.; Daduang, S. Screening of plants acting against Heterometrus laoticus scorpion venomactivity on fibroblast cell lysis. J. Ethnopharmacol. 2006, 103, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Uawonggul, N.; Thammasirirak, S.; Chaveerach, A.; Chuachan, C.; Daduang, J.; Daduang, S. Plant extract activities against the fibroblast cell lysis by honey bee venom. J. Med. Plants Res. 2011, 5, 1978–1986. [Google Scholar]
- Thongharb, C.; Tejasen, P. The effect of Slaed Pang Porn (Clinacanthus nutans) on Thailand cobra venom (Naja naja siamensis). Thai J. Pharm. Sci. 1977, 2, 1057–1063. [Google Scholar]
- Charerntantanakul, W.; Kawaree, R. Effects of medicinal plants extracts on interleukin-10 and tumor necrosis factor alpha gene expressions in porcine peripheral blood mononuclear cells. Chiang Mai Vet. J. 2010, 8, 93–103. [Google Scholar]
- Sriwanthana, B.; Chavalittumrong, P.; Chompuk, L. Effect of Clinacanthus nutans on human cell-mediatedimmune response in vitro. Thail. J. Pharm. Sci. 1996, 20, 261–267. [Google Scholar]
- Tan, C.S.-H.; Ho, C.F.-Y.; Heng, S.-S. Clinacanthus nutans extracts modulate epigenetic link to cytosolic phospholipase A2 expression in SH-SY5Y cells and primary cortical neurons. Neuromolecular Med. 2016, 18, 441–452. [Google Scholar] [CrossRef]
- Tsai, H.-D.; Wu, J.-S.; Kao, M.-H. Clinacanthus nutans protects cortical neurons against hypoxia-induced toxicity by downregulating HDAC1/6. Neuromolecular Med. 2016, 18, 274–282. [Google Scholar] [CrossRef]
- Wu, J.-S.; Kao, M.-H.; Tsai, H.-D. Clinacanthus nutans mitigates neuronal apoptosis and ischemic brain damage through augmenting the C/EBPβ-driven PPAR-γ transcription. Mol. Neurobiol. 2017, 1–14. [Google Scholar]
- Wong, F.C.; Yong, A.L.; Ting, E.P.S. Antioxidant, metal chelating, anti-glucosidase activities and phytochemical analysis of selected tropical medicinal plants. Iran. J. Pharm. Res. 2014, 13, 1407–1413. [Google Scholar]
- Alam, M.A.; Zaidul, I.S.M.; Ghafoor, K. Identification of bioactive compounds with GC–Q-TOF–MS in the extracts from Clinacanthus nutans using subcritical carbon dioxide extraction. Sep. Sci. Technol. (Phila.) 2017, 52, 852–863. [Google Scholar] [CrossRef]
- Lee, S.Y.; Mediani, A.; Nur Ashikin, A.H.; Azliana, A.B.S.; Abas, F. Antioxidant and α-glucosidase inhibitory activities of the leaf and stem of selected traditional medicinal plants. Int. Food Res. J. 2014, 21, 165–172. [Google Scholar]
- Khoo, L.W.; Mediani, A.; Zolkeflee, N.K.Z. Phytochemical diversity of Clinacanthus nutans extracts and their bioactivity correlations elucidated by NMR based metabolomics. Phytochem. Lett. 2015, 14, 123–133. [Google Scholar] [CrossRef]
- Ong, S.L.; Paneerchelvan, S.; Lai, H.Y.; Rao, N.K. In vitro lipase inhibitory effect of thirty-two selected plants in Malaysia. Asian J. Pharm. Clin. Res. 2014, 7, 19–24. [Google Scholar]
- Yuann, J.M.P.; Wang, J.S.; Jian, H.L.; Lin, C.C.; Liang, J.Y. Effects of Clinacanthus nutans (Burm. f) lindau leaf extracts on protection of plasmid DNA from riboflavin photoreaction. Mc-Trans. Biotechnol. 2012, 4, 45–58. [Google Scholar]
- Ng, C.T.; Fong, L.Y.; Tan, J.J.; Rajab, N.F.; Abas, F.; Shaari, K.; Chan, K.M.; Juliana, F.; Yong, Y.K. Water extract of Clinacanthus nutans leaves exhibits in vitro, ex vivo and in vivo antiangiogenic activities in endothelial cell via suppression of cell proliferation. Bmc Complementary Altern. Med. 2018, 18, 210. [Google Scholar] [CrossRef]
- Yong, Y.K.; Tan, J.J.; Teh, S.S.; Mah, S.H.; Ee, G.C.L.; Chiong, H.S.; Ahmad, Z. Clinacanthus nutans extracts are antioxidant with antiproliferative effect on cultured human cancer cell lines. Evid.-Based Complementary Altern. Med. 2013. [Google Scholar] [CrossRef]
- Abd Rahman, N.M.A.N.; Nurliyana, M.Y.; Natasha Nur Afiqah, M.N.F.; Osman, M.A.; Hamid, M.; Mohd Lila, M.A. Antitumor and antioxidant effects of Clinacanthus nutans Lindau in 4 T1 tumor-bearing mice. Bmc Complementary Altern. Med. 2019, 19, 340. [Google Scholar] [CrossRef]
- Amaglo, N.K.; Timpo, G.M.; Ellis, W.O.; Bennett, R.N. Effect of spacing and harvest frequency on the growth and leaf yield of moringa (Moringa oleifera Lam), a leafy vegetable crop. Ghana J. Hortic. 2006, 33–40. [Google Scholar]
- Brum, O.B.; Lopez, S.; Garcia, R.; Andres, S.; Calleja, A. Influence of harvest season, cutting frequency and nitrogen fertilization of mountain meadows on yield, floristic composition and protein content of herbage. Rev. Bras. De Zootec. 2009, 38, 594–604. [Google Scholar] [CrossRef][Green Version]
- Golpavar, A.R. Determination of the best harvesting times to obtain maximum dry herbage, essential oil and thymol yield in garden thyme (Thymus vulgaris L.). Int. J. Life Sci. Med Res. 2011, 1, 1–4. [Google Scholar]
- Kumar, S.; Kumar, A. Spatial and harvesting influence on growth, yield, quality, and economic potential of Kalmegh (Andrographis paniculata Wall Ex. Nees). J. Agric. Rural Dev. Trop. Subtrop. 2013, 114, 69–76. [Google Scholar]
- Olasoji, J.O.; Aluko, A.O.; Adeniyan, S.O.; Olosunde, A.A.; Okoh, J.O. Effect of time of harvest on physiological maturity and kenaf (Hibiscus canabinus) seed quality. Afr. J. Plant Sci. 2012, 6, 282–289. [Google Scholar] [CrossRef]
- Mequanent, Y.; Ayele, N. The effect of harvesting age on maturity indices of quality parameters of sugarcane varieties at Metahara sugar estate in cool season. J. Agric. Nat. Resour. Sci. 2014, 1, 232–237. [Google Scholar]
- Zigene, Z.D.; Kassahun, B.M.; Ketaw, T.T. Effects of harvesting age and spacing on leaf yield and essential oil yield of rosemary (Rosmarinus officinalis L.). Afr. J. Plant Sci. Biotechnol. 2012, 6, 9–12. [Google Scholar]
- Ghasemzadeh, A.; Nasiri, A.; Jaafar, H.Z.E.; Baghdadi, A.; Ahmad, I. Changes in phytochemical synthesis, chalcone synthase activity and pharmaceutical qualities of Sabah snake grass (Clinacanthus nutans L.) in relation to plant age. Molecules 2014, 19, 17632–17648. [Google Scholar] [CrossRef]
- Hagos, H.; Worku, W.; Takele, A. Effect of drying off period and harvest age on quality and yield of ratoon cane (Saccharium officinarium L.). Adv. Crop Sci. Technol. 2014, 2. [Google Scholar] [CrossRef]
- Gebremeskel, H.; Woldetsadik, K. Effect of plant spacing and harvesting age on growth, biomass and oil yield of rose-scented geranium (Pelargonium Graveolens L. Herit). Basic Res. J. 2015, 4, 94–101. [Google Scholar]
- Maudu, M.; Mudau, F.N.; Mariga, I.K. The effect of pruning on growth and chemical composition of cultivated Bush tea (Athrixia phylicoides D.C). J. Med. Plants Res. 2010, 4, 2353–2358. [Google Scholar] [CrossRef]
- Hue, K.T.; Van, D.T.T.; Ledin, I.; Wredle, E.; Sporndly, E. Effect of harvesting frequency, variety and leaf maturity on nutrient composition, hydrogen cyanide content and cassava foliage yield. Asian-Australas. J. Anim. Sci. 2012, 25, 1691–1700. [Google Scholar] [CrossRef]
- Marasha, N.; Mariga, I.K.; Ngezimana, W.; Mudau, F.N. Effect of pruning on carbohydrate dynamics of herbal and medicinal plant species: Prospects leading to research on the influence of pruning on productivity and biochemical composition of Bush tea (Athrixia phylicoides D.C.). Afr. J. Agric. Res. 2013, 8, 3528–3533. [Google Scholar] [CrossRef][Green Version]
- Francini, A.; Sebastiani, L. Abiotic stress effects on performance of horticultural crops. Horticulturae 2019, 5, 67. [Google Scholar] [CrossRef]
- Smith, M.R.; Rao, I.M.; Merchant, A. Source-sink relationships in crop plants and their influence on yield development and nutritional quality. Front. Plant Sci. 2018, 9, 1889. [Google Scholar] [CrossRef]
- Du Toit, J.T.; Bryant, J.P.; Frisby, K. Regrowth and palatability of Acacia shoots following pruning by African savanna browsers. Ecology 1990, 71, 149–154. [Google Scholar] [CrossRef]
- Dudonné, S.; Vitrac, X.; Coutière, P.; Woillez, M.; Mérillon, J.-M. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Jiang, L.; Ye, S.; Ye, Y.; Ren, F. Systematic evaluation of antioxidant capacities of the ethanolic extract of different tissues of jujube (Ziziphus jujuba Mill.) from China. Food Chem. Toxicol. 2010, 48, 1461–1465. [Google Scholar] [CrossRef]
- Qader, S.W.; Abdulla, M.A.; Chua, L.S.; Najim, N.; Zain, M.M.; Hamdan, S. Antioxidant, total phenolic content and cytotoxicity evaluation of selected Malaysian plants. Molecules 2011, 16, 3433–3443. [Google Scholar] [CrossRef] [PubMed]
- Tai, Z.; Cai, L.; Dai, L.; Dong, L.; Wang, M.; Yang, Y.; Cao, Q.; Ding, Z. Antioxidant activity and chemical constituents of edible flower of Sophora viciifolia. Food Chem. 2011, 126, 1648–1654. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.-X.; Chen, J.-W. Commercial quality, major bioactive compound content and antioxidant capacity of 12 cultivars of loquat (Eriobotrya japonica Lindl.) fruits. J. Sci. Food Agric. 2011, 91, 1057–1063. [Google Scholar] [CrossRef]
- Szydłowska-Czerniak, A.; Bartkowiak-Broda, I.; Karlović, I.; Karlovits, G.; Szłyk, E. Antioxidant capacity, total phenolics, glucosinolates and colour parameters of rapeseed cultivars. Food Chem. 2011, 127, 556–563. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 54, 1841–1856. [Google Scholar] [CrossRef]
- Clarke, G.; Ting, K.N.; Wiart, C.; Fry, J. High correlation of 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging, ferric reducing activity potential and total phenolics content indicates redundancy in use of all there assays to screen for antioxidant activity of extracts of plants from the Malaysian rainforest. Antioxidants 2013, 2, 1–10. [Google Scholar]
- Shalaby, E.A.; Shanab, S.M.M. Antioxidant compounds, assays of determination and mode of action. Afr. J. Pharm. Pharmacol. 2013, 7, 528–539. [Google Scholar] [CrossRef]
- Ozgen, M.; Reese, R.N.; Tulio, A.Z., Jr.; Scheerens, J.C.; Miller, A.R. Modified 2,2-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) method to measure antioxidant capacity of selected small fruits and comparison to ferric reducing antioxidant power (FRAP) and 2,2′-diphenyl-1-picrylhydrazyl (DPPH) methods. J. Agric. Food Chem. 2006, 54, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Stratil, P.; Klejdus, B.; Kuban, V. Determination of total content of phenlic compounds and their antioxidant activity in vegetables—Evaluation of spectrophotometric methods. J. Agric. Food Chem. 2006, 54, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Halvorsen, B.L.; Blomhoff, R. Validation of a quantitative assay for the total content of lipophilic and hydrophilic antioxidants in foods. Food Chem. 2011, 127, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Raya, K.B.; Ahmad, S.H.; Farhana, S.F.; Mohammad, M.; Tajidin, N.E.; Parvez, A. Changes in phytochemical contents in different parts of Clinacanthus nutans (Burm. f.) Lindau due to storage duration. Bragantia Camp. 2015, 74, 445–452. [Google Scholar] [CrossRef]
- Sulaiman, I.S.C.; Basri, M.; Chan, K.W.; Ashari, S.E.; Masoumi, H.R.F.; Ismail, M. In vitro antioxidant, cytotoxic and phytochemical studies of Clinacanthus nutans Lindau leaf extracts. Afr. J. Pharm. Pharmacol. 2015, 9, 861–874. [Google Scholar] [CrossRef]
- Arullappan, S.; Rajamanickam, P.; Thevar, N.; Kodimani, C.C. In vitro screening of cytotoxic, antimicrobial and antioxidant activities of Clinacanthus nutans (Acanthaceae) leaf extracts. Trop. J. Pharm. Res. 2014, 13, 1455–1461. [Google Scholar] [CrossRef]
- Pannangpetch, P.; Laupattarakasem, P.; Kukongviriyapan, V.; Kukongviriyapan, U.; Kongyingyoes, B.; Aromdee, C. Anti-oxidant activity and protective effect against oxidative haemolysis of Clinacanthus nutans (Burm.f) Lindau. Songklanakarin J. Sci. Technol. 2007, 29, 1–9. [Google Scholar]
- Chelyn, J.L.; Omar, M.H.; Yousuf, N.S.A.M.; Ranggasamy, R.; Wasiman, M.I.; Ismail, Z. Analysis of flavone c-glycosides in the leaves of Clinacanthus nutans (Burm. f.) lindau by HPTLC and HPLC-UV/DAD. Sci. World J. 2014, 6. [Google Scholar] [CrossRef]
- Stalikas, C.D. Extraction, separation, and detection methods for phenolic acids and flavonoids. J. Sep. Sci. 2007, 30, 3268–3295. [Google Scholar] [CrossRef]
- Sliumpaite, I.; Venskutanis, P.R.; Murkovic, M.; Pukalskas, A. Antioxidant properties and polyphenolics composition of common hedge hyssop (Gratiola oficinalis L.). J. Funct. Foods 2013, 5, 1927–1937. [Google Scholar] [CrossRef]
- Roy, J.L.; Huss, B.; Creach, A.; Hawkins, S.; Neutelings, G. Glycosylation is a major regulator of phenylpropanoid availability and biological activity in plants. Front. Plants Sci. 2016, 7, 735. [Google Scholar]
- Rawat, P.; Kumar, M.; Sharan, K.; Chattopadhyay, N.; Maurya, R. Ulmosides A and B: Flavonoid 6-C-glycosides from Ulmus wallichiana, stimulating osteoblast differentiation assessed by alkaline phosphatase. Bioorg. Med. Chem. Lett. 2009, 19, 4684–4687. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liang, Z.; Liao, X.; Zhou, C.; Xie, Z.; Zhu, S.; Wei, G.; Huang, Y. Identification of C-glycosyl flavones by high performance liquid chromatography electrospray ionization mass spectrometry and quantification of five main C-glycosyl flavones in Flickingeria fimbriata. Bmc Chem. 2019, 13, 94. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.Z.; Harnly, J.M. A screening method for the identification of glycosylated flavonoids and other phenolic compounds using a standard analytical approach for all plant materials. J. Agric. Food Chem. 2007, 55, 1084–1096. [Google Scholar] [CrossRef]
- Cheynier, V.; Comte, G.; Davies, K.M.; Lattanzio, V.; Martens, S. Plant phenolics: Recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiol. Biochem. 2013, 72, 1–20. [Google Scholar] [CrossRef]
- Mierziak, J.; Kostyn, K.; Kulma, A. Flavonoids as important molecules of plant interactions with the environment. Molecules 2014, 19, 16240–16265. [Google Scholar] [CrossRef]
- Holbein, J.; Grundler, F.M.; Siddique, S. Plant basal resistance to nematodes: An update. J. Exp. Bot. 2016, 67, 2049–2061. [Google Scholar] [CrossRef]
- Ishihara, H.; Tohge, T.; Viehover, P.; Fernie, A.R.; Weisshar, B.; Stracke, R. Natural variation in flavonol accumulation in Arabidopsis is determined by the flavonol glucosyltransferase BGLU6. J. Exp. Bot. 2016, 67, 1505–1517. [Google Scholar] [CrossRef]
- Ibrahim, M.H.; Jaafar, H.Z.E.; Karimi, E.; Ghasemzadeh, A. Primary, secondary metabolites, photosynthetic capacity and antioxidant activity of the malaysian herb Kacip fatimah (Labisia Pumila Benth) exposed to potassium fertilization under greenhouse conditions. Int. J. Mol. Sci. 2012, 13, 15321–15342. [Google Scholar] [CrossRef]
- Barku, V.Y.A.; Boye, A.; Ayaba, S. Phytochemical screening and assessment of wound healing activity of the leaves of Anogeissus leiocarpus. Eur. J. Exp. Biol. 2013, 3, 18–25. [Google Scholar]
- Kim, D.O.; Jeong, S.W.; Lee, C.Y. Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem. 2003, 81, 321–326. [Google Scholar] [CrossRef]
- Djeridane, A.; Yousfi, M.; Nadjemi, B.; Boutassouna, D.; Stocker, P.; Vidal, N. Antioxidant activity of some algerian medicinal plants extracts containing phenolic compounds. Food Chem. 2006, 97, 654–660. [Google Scholar] [CrossRef]
- Oliveira, M.D.; Cipolatti, E.P.; Furlong, E.B.; Soares, L.D.S. Phenolic compounds and antioxidant activity in fermented rice (Oryza sativa) bran. Ciência Tecnol. De Aliment. Camp. 2012, 32, 531–537. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (frap) as a measure of ‘‘antioxidant power’’: The frap assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the sabah snake grass are available from the authors. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).