Morphological, Physiochemical and Thermal Properties of Microcrystalline Cellulose (MCC) Extracted from Bamboo Fiber
Abstract
1. Introduction
2. Characterization
2.1. Chemical Changes Analysis during Procedure
2.2. Morphological, Particle Size and Elemental Analysis
2.3. Crystallinity Analysis
2.4. Thermal Analysis
3. Results and Discussion
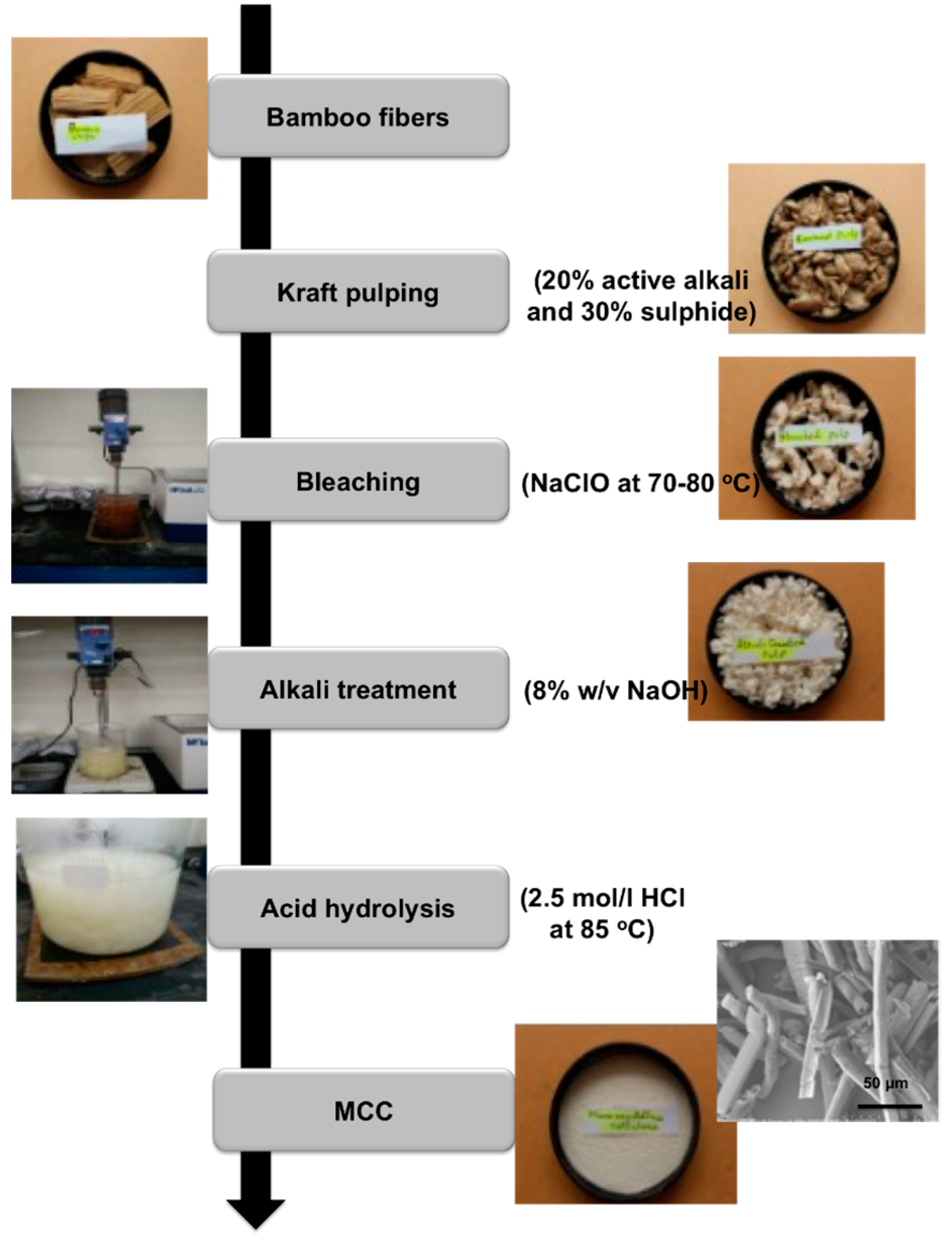
3.1. Visual Analysis, Yield and Physiochemical Categorization
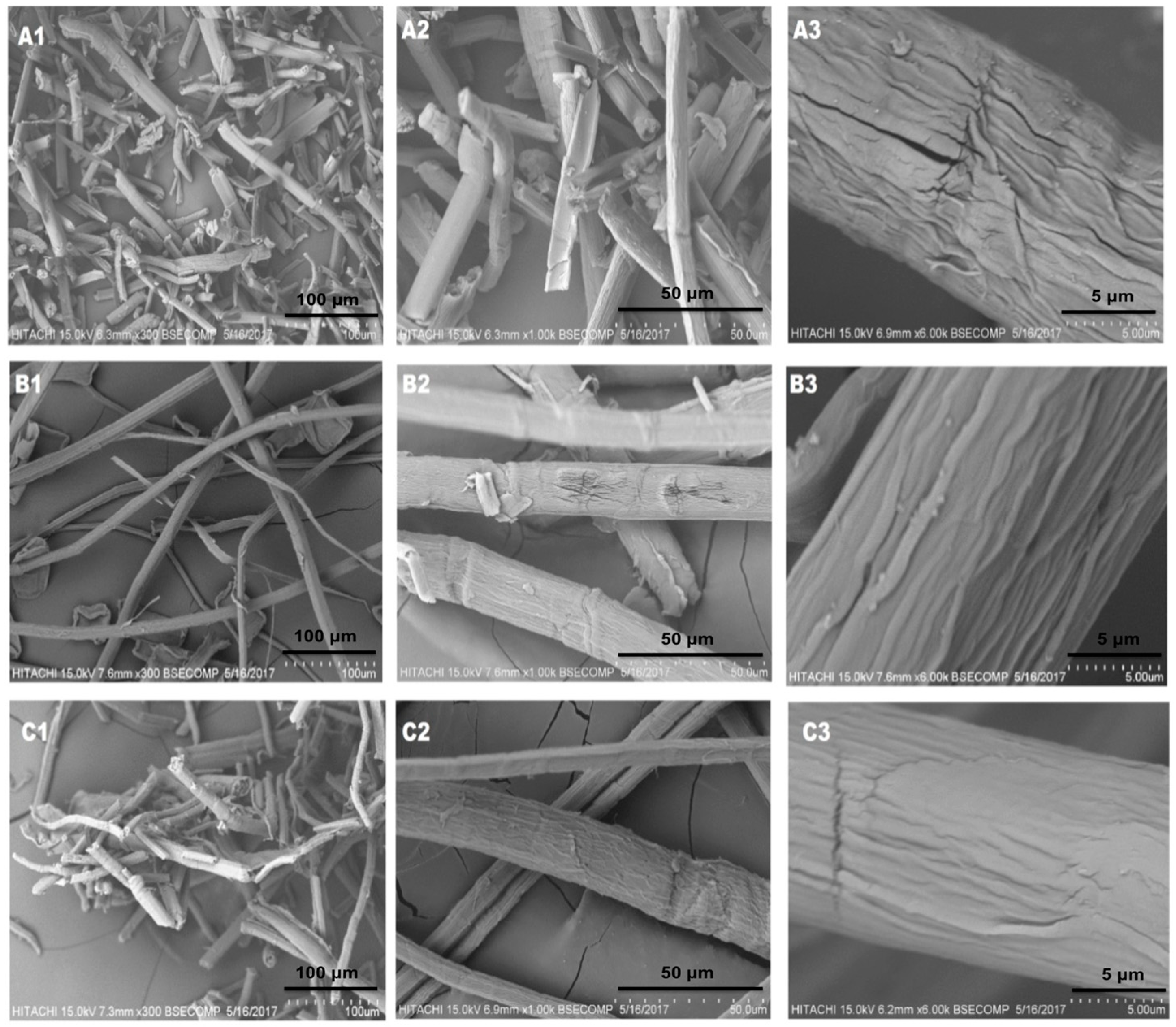
3.2. Analysis of Micro/Nano-Structured Morphology
3.3. FTIR Analysis of Intermediate and Final Products
3.4. Calculation of Crystallinity Using XRD
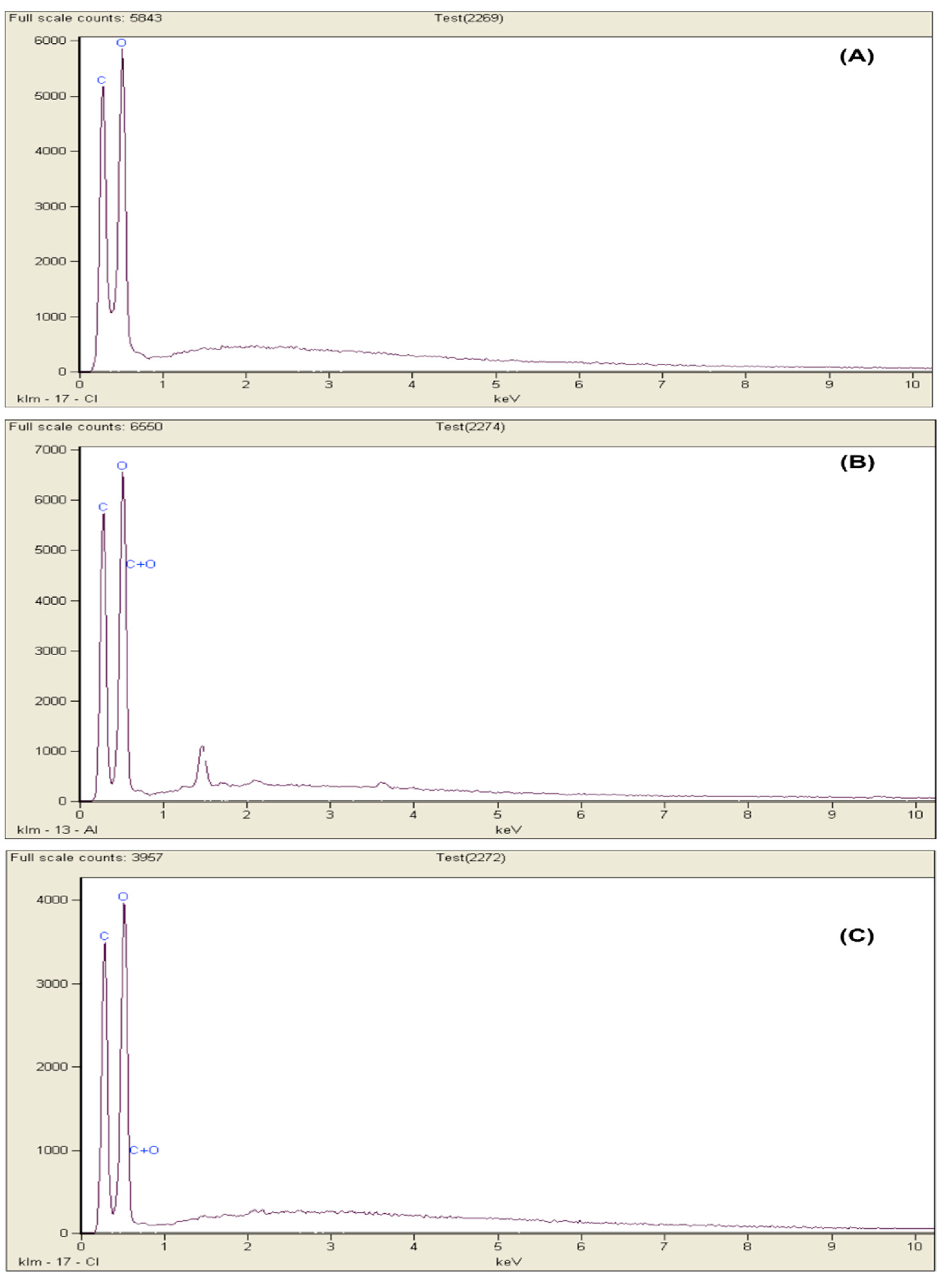
3.5. EDX Analysis
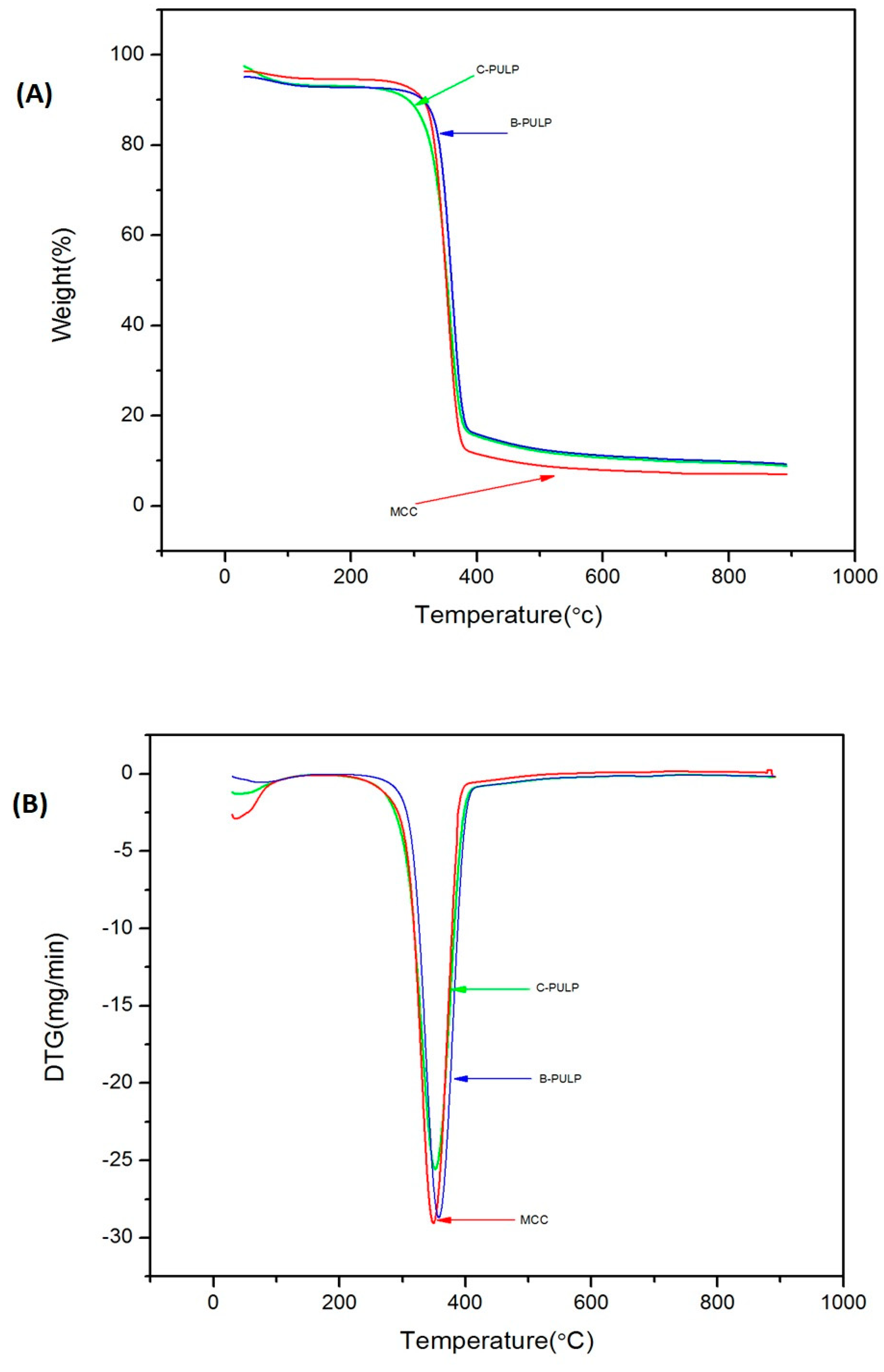
3.6. Analysis of Thermal Properties
4. Materials and Methods
4.1. Materials
4.2. Experiments
4.2.1. Kraft Pulping
4.2.2. Bleaching
4.2.3. Alkali Treatment
4.2.4. Acid Hydrolysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- McHargue, C.J.; Darby, J.B., Jr.; Yacamán, M.J.; Gasga, J.R. Synthesis and Properties of Advanced Materials; Springer: New York, NY, USA, 1997; ISBN 978-0-7923-9816-5. [Google Scholar]
- Somiya, S. Handbook of Advanced Ceramics: Materials, Applications, Processing, and Properties; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Abdul Khalil, H.P.S.; Bhat, I.U.H.; Jawaid, M.; Zaidon, A.; Hermawan, D.; Hadi, Y.S. Bamboo fibre reinforced biocomposites: A review. Mater. Des. 2012, 42, 353–368. [Google Scholar] [CrossRef]
- Jawaid, M.; Abdul Khalil, H.P.S. Cellulosic/synthetic fibre reinforced polymer hybrid composites: A review. Carbohydr. Polym. 2011, 86, 1–18. [Google Scholar] [CrossRef]
- Puglia, D.; Biagiotti, J.; Kenny, J.M. A Review on Natural Fibre-Based Composites—Part II. J. Nat. Fibers 2005, 1, 23–65. [Google Scholar] [CrossRef]
- El-Sakhawy, M.; Hassan, M.L. Physical and mechanical properties of microcrystalline cellulose prepared from agricultural residues. Carbohydr. Polym. 2007, 67, 1–10. [Google Scholar] [CrossRef]
- Trache, D.; Khimeche, K.; Mezroua, A.; Benziane, M. Physicochemical properties of microcrystalline nitrocellulose from Alfa grass fibres and its thermal stability. J. Therm. Anal. Calorim. 2016, 124, 1485–1496. [Google Scholar] [CrossRef]
- Fahma, F.; Iwamoto, S.; Hori, N.; Iwata, T.; Takemura, A. Isolation, preparation, and characterization of nanofibers from oil palm empty-fruit-bunch (OPEFB). Cellulose 2010, 17, 977–985. [Google Scholar] [CrossRef]
- Qi, H.; Lu, A.; Zheng, Q.; Yang, Q. Functional polymeric materials based on cellulose. Int. J. Polym. Sci. 2016, 2016. [Google Scholar] [CrossRef]
- Das, K.; Ray, D.; Bandyopadhyay, N.R.; Sengupta, S. Study of the properties of microcrystalline cellulose particles from different renewable resources by XRD, FTIR, nanoindentation, TGA and SEM. J. Polym. Environ. 2010, 18, 355–363. [Google Scholar] [CrossRef]
- Sun, C. (Calvin) True Density of Microcrystalline Cellulose. J. Pharm. Sci. 2005, 94, 2132–2134. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.; Gafur, M.A.; Sharafat, M.K.; Minami, H.; Miah, M.A.J.; Ahmad, H. Biocompatible microcrystalline cellulose particles from cotton wool and magnetization via a simple in situ co-precipitation method. Carbohydr. Polym. 2017, 170, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Schuh, V.; Allard, K.; Herrmann, K.; Gibis, M.; Kohlus, R.; Weiss, J. Impact of carboxymethyl cellulose (CMC) and microcrystalline cellulose (MCC) on functional characteristics of emulsified sausages. Meat Sci. 2013, 93, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Haafiz, M.K.M.; Hassan, A.; Zakaria, Z.; Inuwa, I.M. Isolation and characterization of cellulose nanowhiskers from oil palm biomass microcrystalline cellulose. Carbohydr. Polym. 2014, 103, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Bae, D.H.; Choi, H.J.; Choi, K.; Do Nam, J.; Islam, M.S.; Kao, N. Fabrication of phosphate microcrystalline rice husk based cellulose particles and their electrorheological response. Carbohydr. Polym. 2017, 165, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Winuprasith, T.; Suphantharika, M. Microfibrillated cellulose from mangosteen (Garcinia mangostana L.) rind: Preparation, characterization, and evaluation as an emulsion stabilizer. Food Hydrocoll. 2013, 32, 383–394. [Google Scholar] [CrossRef]
- Kian, L.K.; Jawaid, M.; Ariffin, H.; Alothman, O.Y. Isolation and characterization of microcrystalline cellulose from roselle fibers. Int. J. Biol. Macromol. 2017, 103, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Gratani, L.; Crescente, M.F.; Varone, L.; Fabrini, G.; Digiulio, E. Growth pattern and photosynthetic activity of different bamboo species growing in the Botanical Garden of Rome. Flora-Morphol. Distrib. Funct. Ecol. Plants 2008, 203, 77–84. [Google Scholar] [CrossRef]
- Grosser, D.; Liese, W. On the anatomy of Asian bamboos, with special reference to their vascular bundles. Wood Sci. Technol. 1971, 5, 290–312. [Google Scholar] [CrossRef]
- Van Dam, J.E.; Elbersen, H.W.; Montaño, C.M.D. Bamboo production for industrial utilization. In Perennial Grasses for Bioenergy and Bioproducts; Elsevier: Amsterdam, The Netherlands, 2018; pp. 175–216. [Google Scholar]
- Qiu, H.; Xu, J.; He, Z.; Long, L.; Yue, X. Bamboo as an Emerging Source of Raw Material for Household and Building Products. BioResources 2019, 14, 2465–2467. [Google Scholar]
- Haafiz, M.M.; Eichhorn, S.J.; Hassan, A.; Jawaid, M. Isolation and characterization of microcrystalline cellulose from oil palm biomass residue. Carbohydr. Polym. 2013, 93, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Li, L.-J.; Wang, Y.-P.; Wang, G.; Cheng, H.-T.; Han, X.-J. Evaluation of properties of natural bamboo fiber for application in summer textiles. J. Fiber Bioeng. Inform. 2010, 3, 94–99. [Google Scholar]
- Johar, N.; Ahmad, I.; Dufresne, A. Extraction, preparation and characterization of cellulose fibres and nanocrystals from rice husk. Ind. Crop. Prod. 2012, 37, 93–99. [Google Scholar] [CrossRef]
- Merci, A.; Urbano, A.; Grossmann, M.V.E.; Tischer, C.A.; Mali, S. Properties of microcrystalline cellulose extracted from soybean hulls by reactive extrusion. Food Res. Int. 2015, 73, 38–43. [Google Scholar] [CrossRef]
- Elanthikkal, S.; Gopalakrishnapanicker, U.; Varghese, S.; Guthrie, J.T. Cellulose microfibres produced from banana plant wastes: Isolation and characterization. Carbohydr. Polym. 2010, 80, 852–859. [Google Scholar] [CrossRef]
- Trache, D.; Donnot, A.; Khimeche, K.; Benelmir, R.; Brosse, N. Physico-chemical properties and thermal stability of microcrystalline cellulose isolated from Alfa fibres. Carbohydr. Polym. 2014, 104, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Mathew, A.P.; Oksman, K.; Karim, Z.; Liu, P.; Khan, S.A.; Naseri, N. Process scale up and characterization of wood cellulose nanocrystals hydrolysed using bioethanol pilot plant. Ind. Crop. Prod. 2014, 58, 212–219. [Google Scholar] [CrossRef]
- Gupta, P.K.; Uniyal, V.; Naithani, S. Polymorphic transformation of cellulose I to cellulose II by alkali pretreatment and urea as an additive. Carbohydr. Polym. 2013, 94, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Spagnol, C.; Rodrigues, F.H.; Pereira, A.G.; Fajardo, A.R.; Rubira, A.F.; Muniz, E.C. Superabsorbent hydrogel composite made of cellulose nanofibrils and chitosan-graft-poly (acrylic acid). Carbohydr. Polym. 2012, 87, 2038–2045. [Google Scholar] [CrossRef]
- Sofla, M.R.K.; Brown, R.J.; Tsuzuki, T.; Rainey, T.J. A comparison of cellulose nanocrystals and cellulose nanofibres extracted from bagasse using acid and ball milling methods. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 035004. [Google Scholar] [CrossRef]
- Ferrer, A.; Salas, C.; Rojas, O.J. Physical, thermal, chemical and rheological characterization of cellulosic microfibrils and microparticles produced from soybean hulls. Ind. Crop. Prod. 2016, 84, 337–343. [Google Scholar] [CrossRef]
- Jahan, M.S.; Saeed, A.; He, Z.; Ni, Y. Jute as raw material for the preparation of microcrystalline cellulose. Cellulose 2011, 18, 451–459. [Google Scholar] [CrossRef]
- Chowdhury, M.N.K.; Beg, M.D.H.; Khan, M.R.; Mina, M.F. Modification of oil palm empty fruit bunch fibers by nanoparticle impregnation and alkali treatment. Cellulose 2013, 20, 1477–1490. [Google Scholar] [CrossRef]
- Sonia, A.; Dasan, K.P. Chemical, morphology and thermal evaluation of cellulose microfibers obtained from Hibiscus sabdariffa. Carbohydr. Polym. 2013, 92, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Jonoobi, M.; Khazaeian, A.; Tahir, P.M.; Azry, S.S.; Oksman, K. Characteristics of cellulose nanofibers isolated from rubberwood and empty fruit bunches of oil palm using chemo-mechanical process. Cellulose 2011, 18, 1085–1095. [Google Scholar] [CrossRef]
- Hussin, M.H.; Pohan, N.A.; Garba, Z.N.; Kassim, M.J.; Rahim, A.A.; Brosse, N.; Yemloul, M.; Fazita, M.N.; Haafiz, M.M. Physicochemical of microcrystalline cellulose from oil palm fronds as potential methylene blue adsorbents. Int. J. Biol. Macromol. 2016, 92, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Razali, N.; Salit, M.S.; Jawaid, M.; Ishak, M.R.; Lazim, Y. A study on chemical composition, physical, tensile, morphological, and thermal properties of roselle fibre: Effect of fibre maturity. BioResources 2015, 10, 1803–1824. [Google Scholar] [CrossRef]
- Neto, W.P.F.; Silvério, H.A.; Dantas, N.O.; Pasquini, D. Extraction and characterization of cellulose nanocrystals from agro-industrial residue–Soy hulls. Ind. Crop. Prod. 2013, 42, 480–488. [Google Scholar] [CrossRef]
- Liu, D.; Song, J.; Anderson, D.P.; Chang, P.R.; Hua, Y. Bamboo fiber and its reinforced composites: Structure and properties. Cellulose 2012, 19, 1449–1480. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |


| Chemical Composition (%) | Coding of Materials Used in This Article | MCC Yield (%) | ||
|---|---|---|---|---|
| Cellulose | 54.61 | Cooked pulp | C-Pulp | 80 |
| Hemicellulose | 6.85 | Bleached pulp | B-Pulp | |
| Lignin | 20.85 | Microcrystalline cellulose | MCC | |
| Others | 17.69 | |||
| Samples | Tinitial (°C) a | Tfinal (°C) b | Wresidue (%) c |
|---|---|---|---|
| C-Pulp | 290 | 695 | 8.4 |
| B-pulp | 314 | 698 | 9.4 |
| MCC | 315 | 450 | 6.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rasheed, M.; Jawaid, M.; Karim, Z.; Abdullah, L.C. Morphological, Physiochemical and Thermal Properties of Microcrystalline Cellulose (MCC) Extracted from Bamboo Fiber. Molecules 2020, 25, 2824. https://doi.org/10.3390/molecules25122824
Rasheed M, Jawaid M, Karim Z, Abdullah LC. Morphological, Physiochemical and Thermal Properties of Microcrystalline Cellulose (MCC) Extracted from Bamboo Fiber. Molecules. 2020; 25(12):2824. https://doi.org/10.3390/molecules25122824
Chicago/Turabian StyleRasheed, Masrat, Mohammad Jawaid, Zoheb Karim, and Luqman Chuah Abdullah. 2020. "Morphological, Physiochemical and Thermal Properties of Microcrystalline Cellulose (MCC) Extracted from Bamboo Fiber" Molecules 25, no. 12: 2824. https://doi.org/10.3390/molecules25122824
APA StyleRasheed, M., Jawaid, M., Karim, Z., & Abdullah, L. C. (2020). Morphological, Physiochemical and Thermal Properties of Microcrystalline Cellulose (MCC) Extracted from Bamboo Fiber. Molecules, 25(12), 2824. https://doi.org/10.3390/molecules25122824








