Chiral Aspects of Local Anesthetics
Abstract
1. Introduction
- Anesthetics of the ester type (procaine, tetracaine, oxyprocaine, proxymetacaine)
- Amide- and anilide-type anesthetics (cinchocaine, procaine)
- Anesthetics related to basic ketones (falicaine) and basic ethers (fomocaine)
2. Mechanism of the Action of Local Anesthetics
3. Chirality in the Pharmacodynamics and Toxicity of Local Anesthetics
3.1. Cocaine
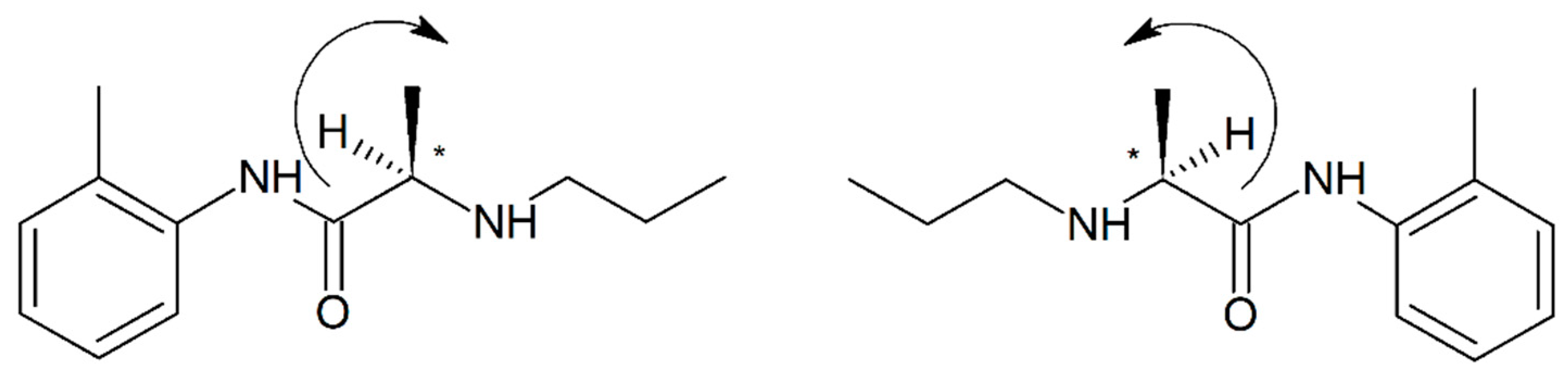
3.2. Mepivacaine
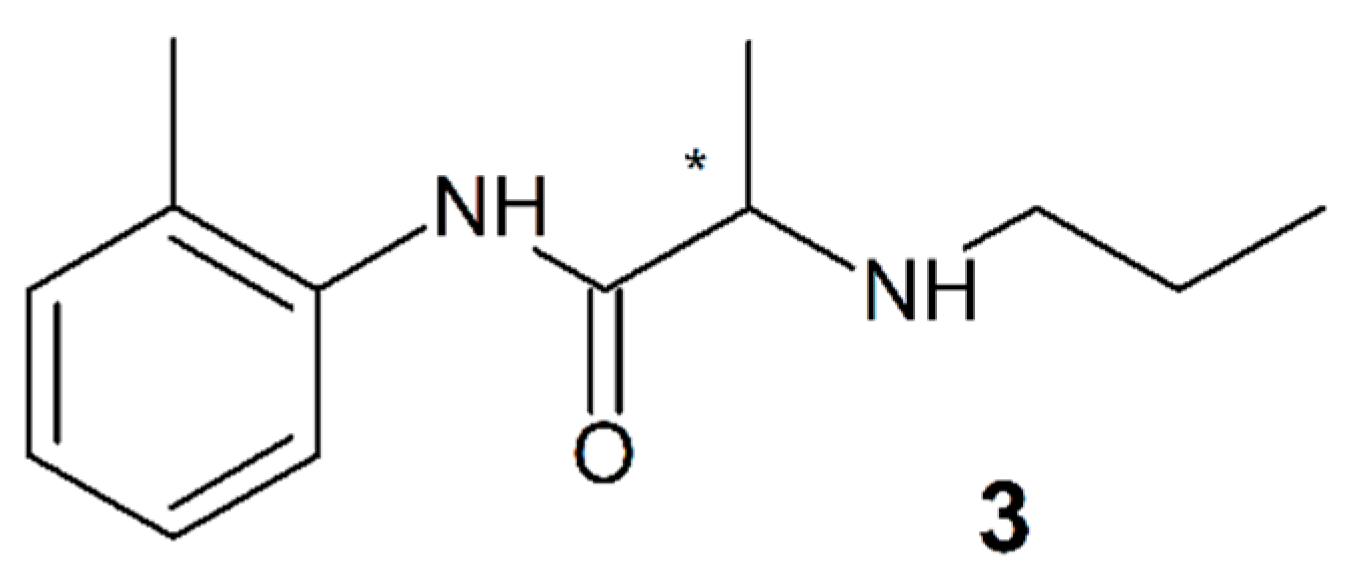
3.3. Prilocaine
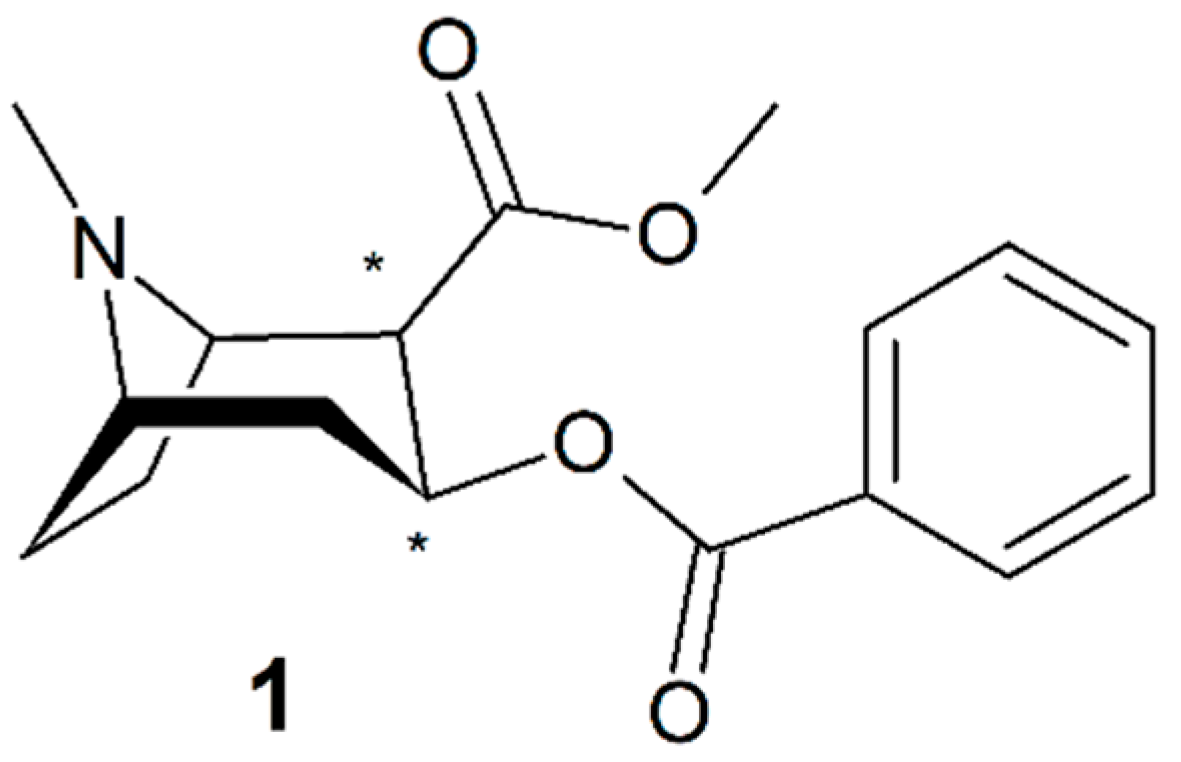
3.4. Bupivacaine and Levobupivacaine
3.5. Ropivacaine
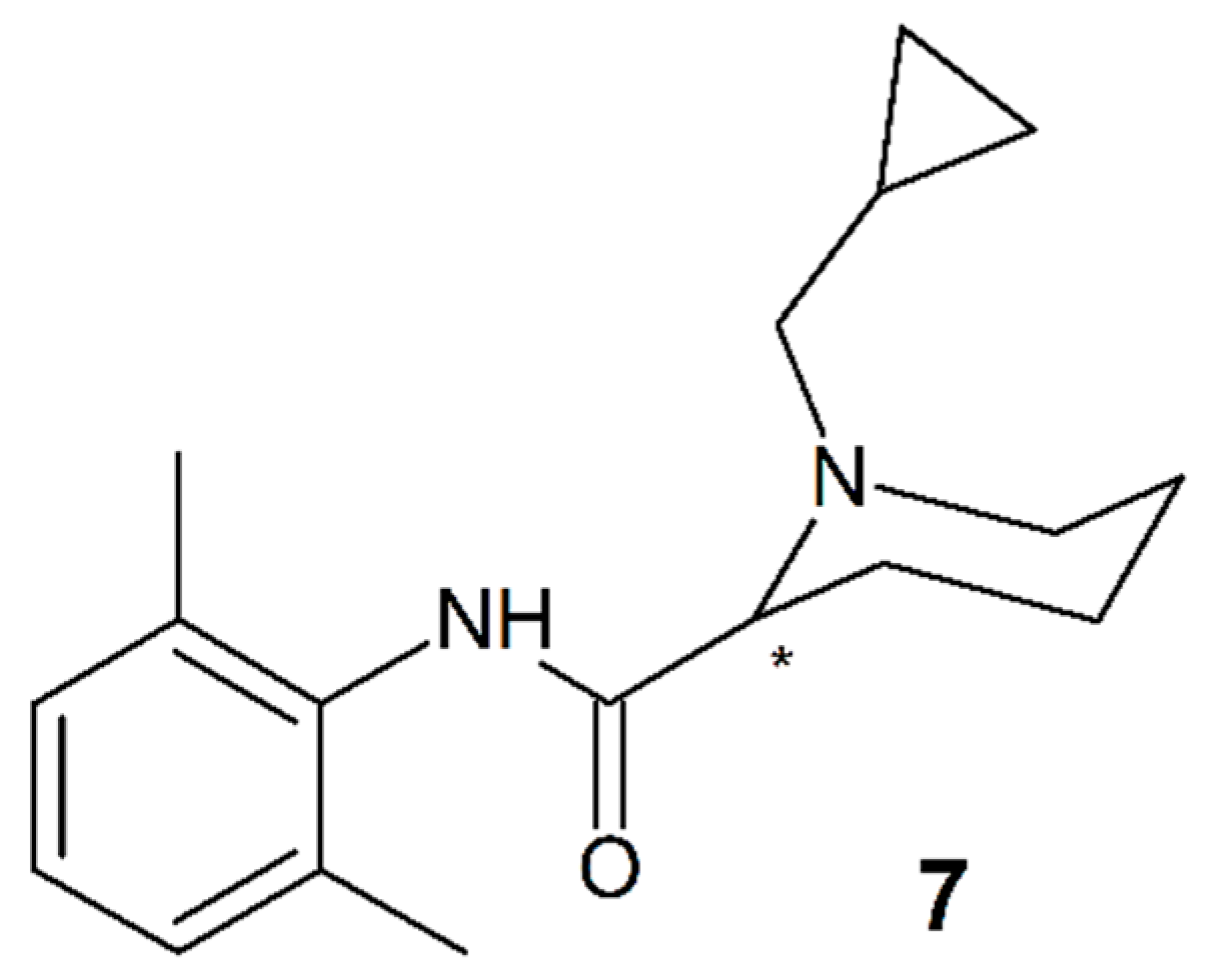
3.6. IQB-9302
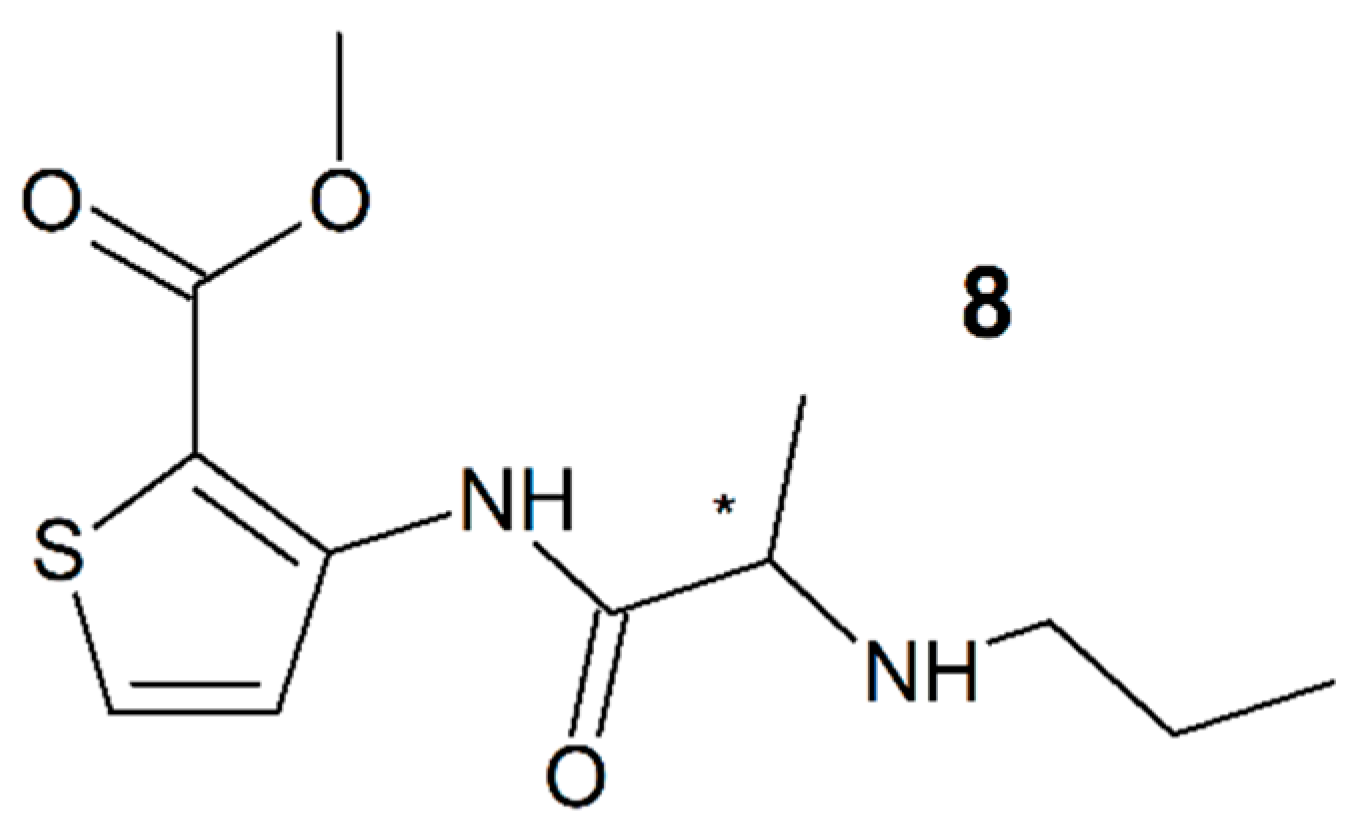
3.7. Articaine
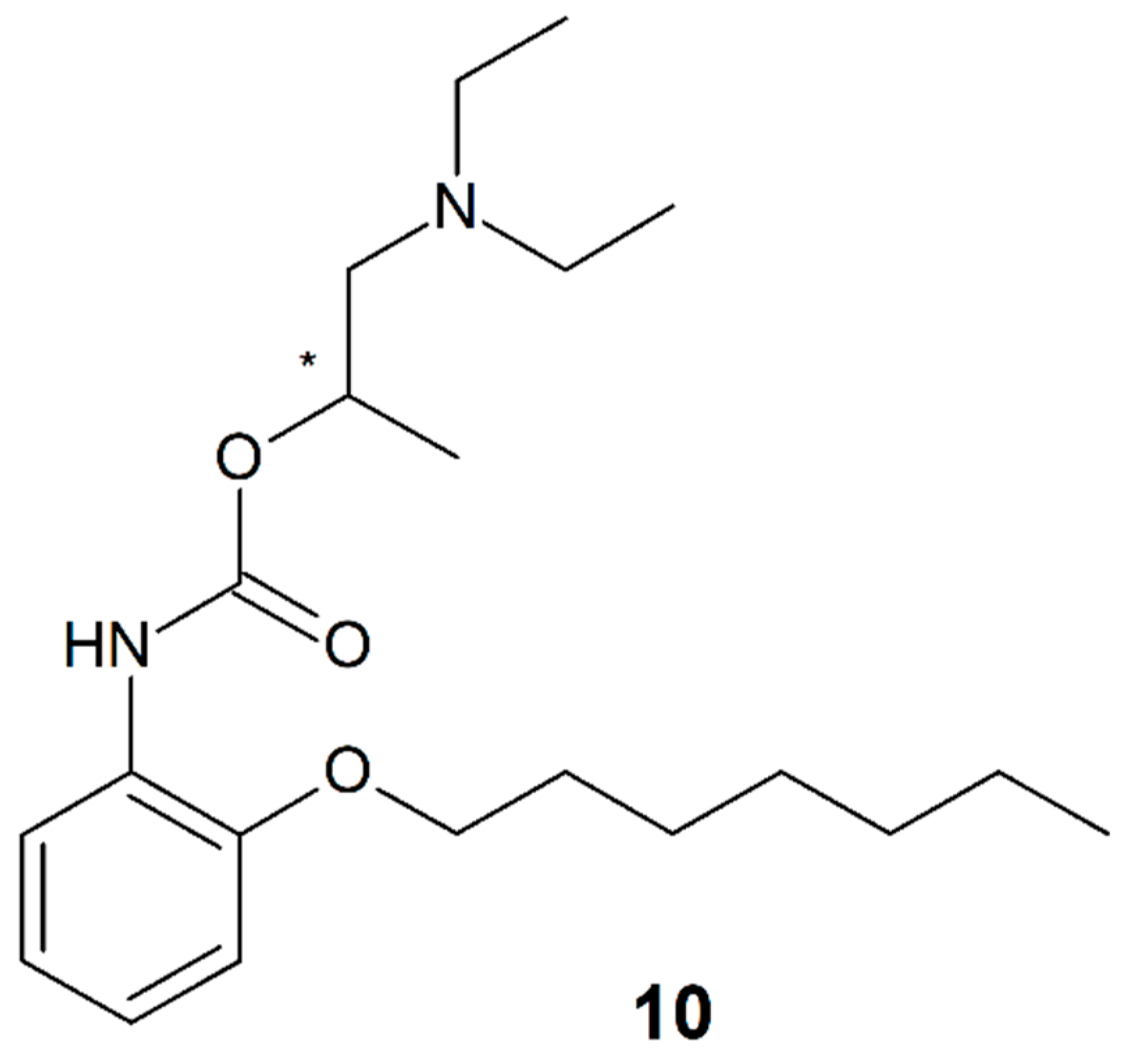
3.8. Etidocaine
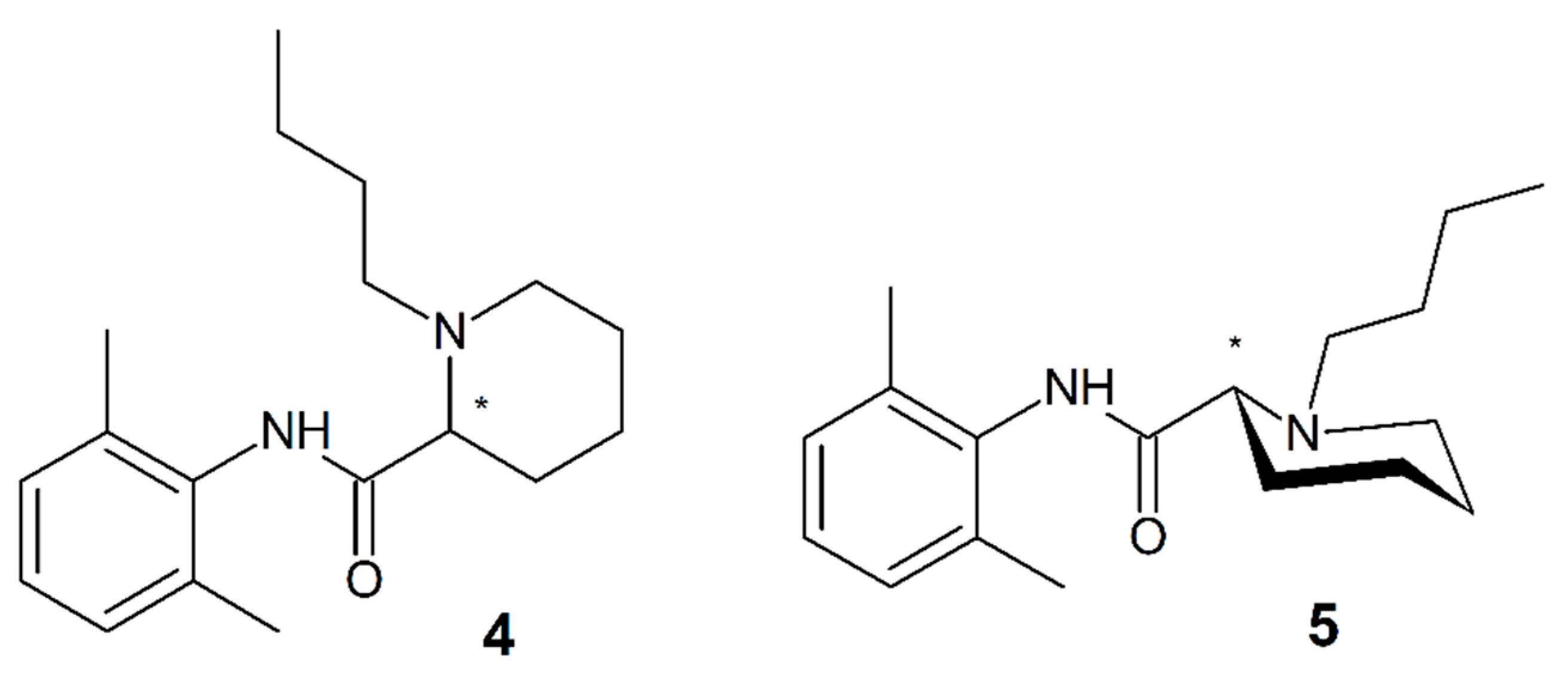
3.9. Carbizocaine
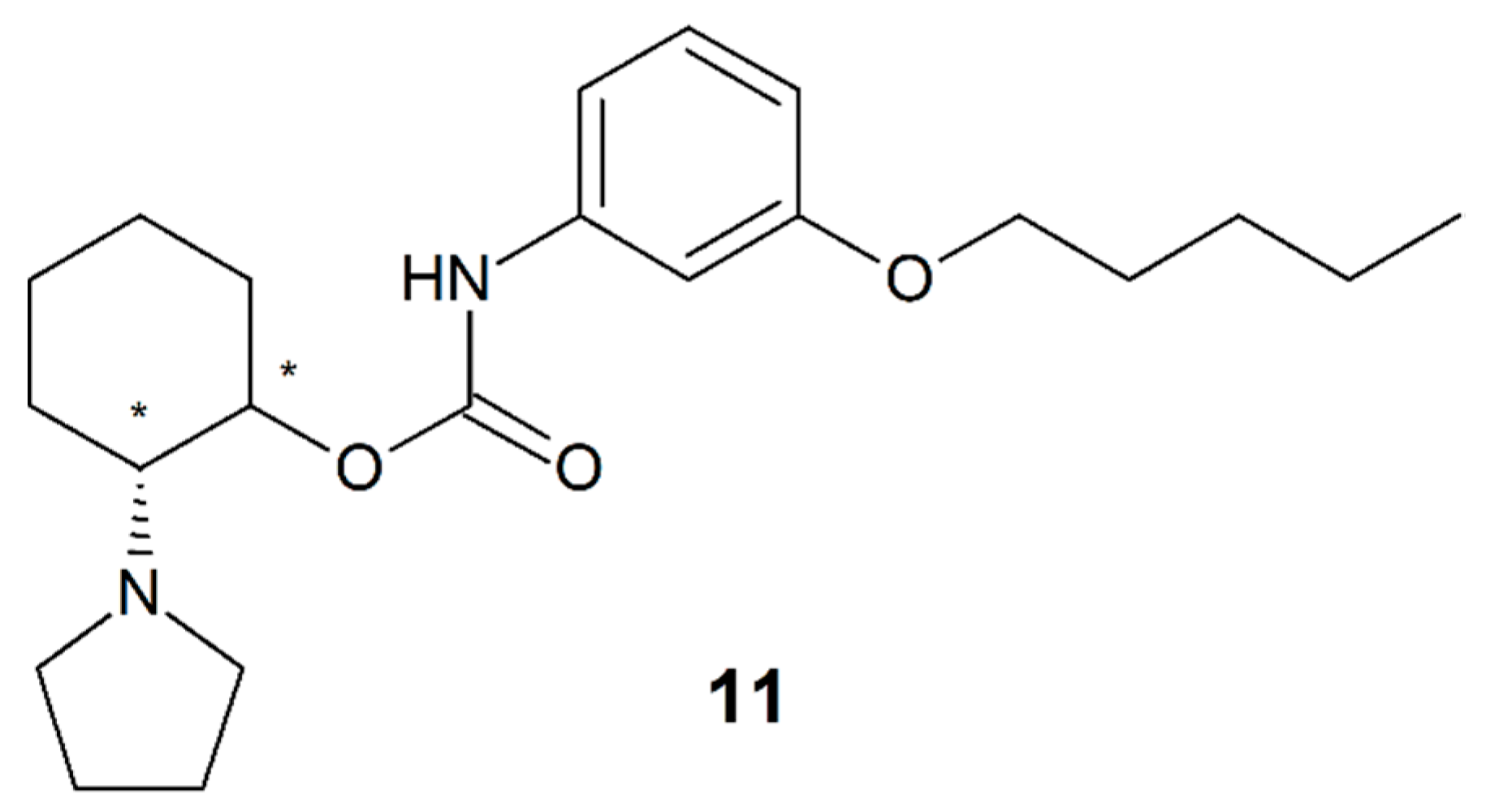
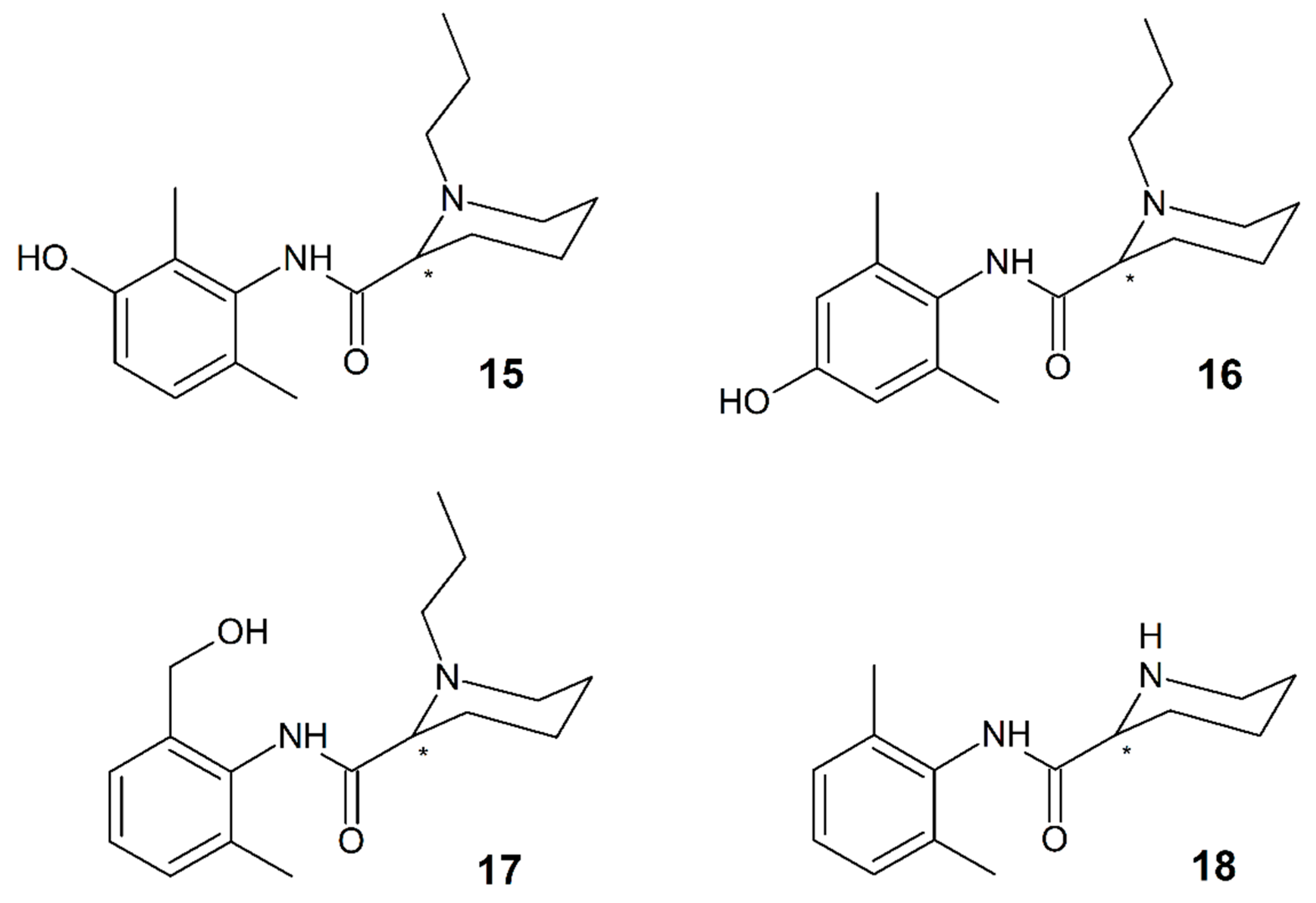
3.10. Pentacaine
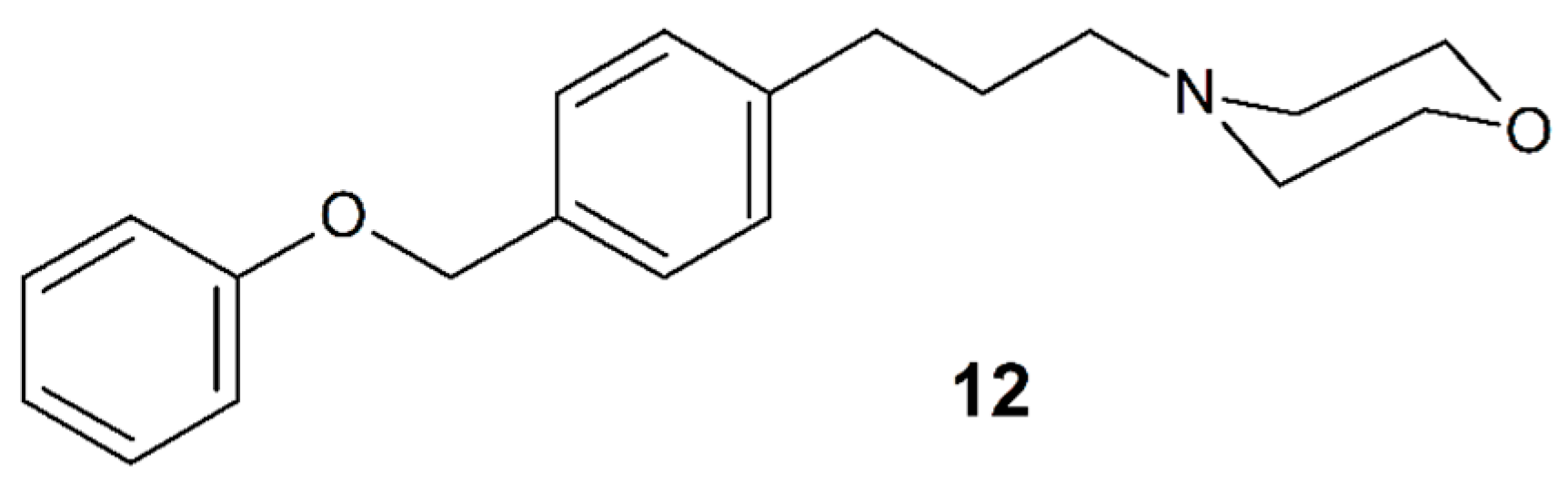
3.11. Fomocaine
4. Stereoselectivity in the Pharmacokinetics of Local Anesthetics
4.1. Bupivacaine (4)
4.2. Prilocaine (3)
4.3. Mepivacaine (2)
4.4. Ropivacaine (6)
5. Stereoselective Analysis
5.1. High-Performance Liquid Chromatography
5.2. Capillary Electrophoresis
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mosby’s Medical Dictionary, 8th ed.; Elsevier: St. Louis, MO, USA, 2009.
- Lofgrön, N. Studies on Local Anestetics. Xylocaine a New Drug; Haeggstroms: Stockholm, Sweden, 1948. [Google Scholar]
- Heavner, J.E. Local anesthetics. Curr. Opin. Anaesthesiol. 2007, 20, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Whiteside, J.B.; Wildsmith, J.A.W. Developments in local anaesthetic drugs. Br. J. Anaesth. 2001, 87, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Steinhilber, D.; Schubert-Zsilavecz, M.; Roth, H. Medizinische Chemie. Targets, Arzneistoffe, Chemische Biologie, 2nd ed.; Deutscher Apotheker Verlag: Stuttgart, Germany, 2010. [Google Scholar]
- Brooks, W.H.; Guida, W.C.; Daniel, K.G. The significance of chirality in drug design and development. Curr. Top. Med. Chem. 2011, 11, 760–770. [Google Scholar] [CrossRef] [PubMed]
- Hutt, A.J.; Valentová, J. The chiral switch: The development of single enantiomer drugs from racemates. Acta Fac. Pharm. Univ. Comen. 2003, 50, 7–23. [Google Scholar]
- Hutt, A.J.; O’Grady, J. Drug chirality: A consideration of the significance of the stereochemistry of antimicrobial agents. Antimicrob. Chemother. 1996, 37, 7–32. [Google Scholar] [CrossRef]
- Lane, R.M.; Baker, G.B. Chirality and drugs used in psychiatry: Nice to know or need to know? Cell. Mol. Neurobiol. 1999, 19, 355–372. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, H.; Zhang, Q.; Zhang, P. Chirality in metal-based anticancer agents. Dalton Trans. 2018, 47, 4017–4026. [Google Scholar] [CrossRef]
- Ranade, V.V.; Somberg, J.C. Chiral cardiovascular drugs: An overview. Am. J. 2005, 12, 439–459. [Google Scholar] [CrossRef]
- Čižmáriková, R.; Habala, L.; Valentová, J.; Markuliak, M. Survey of pharmacological activity and pharmacokinetics of selected β-adrenergic blockers in regard to their stereochemistry. Appl. Sci. 2019, 9, 625. [Google Scholar] [CrossRef]
- Cahn, R.S.; Ingold, C.K.; Prelog, V. Specification of molecular chirality. Angew. Chem. Int. Ed. 1966, 5, 385–415. [Google Scholar] [CrossRef]
- Local Anesthesia Drugs Market Perspective, Comprehensive Analysis Along with Major Segments and Forecast, 2020–2026. Market Insights Reports. 2020. Available online: www.marketinsightsreports.com/reports/04252005472/impact-of-covid-19-outbreak-on-local-anesthesia-drugs-global-market-research-report-2020 (accessed on 29 May 2020).
- Local Anesthesia Drugs Market Size, Share & Trends Analysis Report by Product (Bupivacaine, Ropivacaine, Lidocaine, Chloroprocaine, Prilocaine, Benzocaine), By Application, And Segment Forecasts, 2018–2025. Grand View Research. 2018. Available online: https://www.grandviewresearch.com/industry-analysis/local-anesthesia-drugs-market (accessed on 29 May 2020).
- Levobupivacaine Market–Global Industry Analysis, Size, Share, Growth, Trends, and Forecast, 2020–2027. Atlantic Market Research. 2020. Available online: www.atlanticmarketresearch.com/levobupivacaine-market (accessed on 29 May 2020).
- Fozzard, H.A.; Lee, P.J.; Lipkind, G.M. Mechanism of local anesthetic drug action on voltage-gated sodium channels. Curr. Pharm. Des. 2005, 11, 2671–2686. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, H.; Mizogami, M. Interaction of local anesthetics with biomembranes consisting of phospholipids and cholesterol: Mechanistic and clinical implications for anesthetic and cardiotoxic effects. Anesth. Res. Pr. 2013, 2013, 18. [Google Scholar] [CrossRef] [PubMed]
- Strichartz, G.R.; Ritchie, J.M. The action of local anesthetics on ion channels of excitable tissues. In Local Anesthetics; Strichartz, G.R., Ed.; Springer: Berlin, Germany, 1987; pp. 21–52. [Google Scholar]
- Valenzuela, C.; Moreno, C.; de la Cruz, A.; Macias, A.; Prieto, A.; Conzalez, T. Stereoselective interactions between local anesthetics and ion channels. Chirality 2012, 24, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Nau, C.; Strichartz, G.R. Drug chirality in anesthesia. Anesthesiology 2002, 97, 497–502. [Google Scholar]
- Mitra, S.; Chopra, P. Chirality and anaesthetic drugs: A review and an update. Indian J. Anaesth. 2011, 55, 556–562. [Google Scholar] [CrossRef]
- Burke, D.; Henderson, D.J. Chirality: A blueprint for the future. Br. J. Anaesth. 2002, 88, 563–576. [Google Scholar] [CrossRef]
- Tomin, J.; Živanov-Čurlis, J.; Popović, D.; Glogovac, S.; Bašić, D. Differences in local anesthetic effects of optically active isomers of local anesthetic compounds. Biotechnol. Biotechnol. Equip. 2006, 20, 9–14. [Google Scholar] [CrossRef]
- Calvey, T.N. Isomerism and anaesthetic drugs. Acta Anaesthesiol. Scand. Suppl. 1995, 106, 83–90. [Google Scholar] [CrossRef]
- Ekatodramis, G.; Borgeat, A. The enantiomers: Revolution or evolution. Curr. Top. Med. Chem. 2001, 1, 205–206. [Google Scholar] [CrossRef]
- Sidebotham, D.A.; Schug, S.A. Stereochemistry in anaesthesia. Clin. Exp. Pharm. Physiol. 1997, 24, 126–130. [Google Scholar] [CrossRef]
- Dewick, P.M. Essentials of Organic Chemistry: For Students of Pharmacy; John Wiley & Sons Ltd.: Chichester, UK, 2006. [Google Scholar]
- Gottlieb, R. Über die pharmakologische bedeutung des psicains als lokalanästhetikum. Münch. Med. Wschr. 1924, 71, 850–851. [Google Scholar]
- Schmidt, G.; Kalischer, B.; Wückel, B. Vergleichende untersuchungen über die wirkungen von normal-cocain und pseudococain. Naunyn Schmiedeberg’s Arch. Exp. Path. Pharmak. 1961, 240, 523–538. [Google Scholar]
- Katz, J.L.; Tirelli, E.; Witkin, J.M. Stereoselective effects of cocaine. Behav. Pharm. 1990, 1, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Tullar, B.F. Optical isomers of mepivacaine and bupivacaine. J. Med. Chem. 1971, 14, 891–892. [Google Scholar] [CrossRef] [PubMed]
- Luduena, F.P.; Bogsdo, E.F.; Tullar, B.T. Optical isomers of mepivacaine and bupivacaine. Arch. Int. Pharm 1972, 200, 359–369. [Google Scholar]
- Åkerman, B.; Person, H.; Tegnér, C. Local anaesthetic properties of the optically active isomers of prilocaine (Citanest). Acta Pharm. Toxicol. 1967, 25, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Foster, R.H.; Markham, A. Levobupivacaine: A review of its pharmacology and use as a local anaesthetic. Drugs 2000, 59, 551–579. [Google Scholar] [CrossRef] [PubMed]
- Cox, C.R.; Faccenda, K.A.; Gilhooly, C.; Bannister, J.; Scott, N.B.; Morrison, L.M. Extradural S(-)-bupivacaine: Comparison with racemic RS-bupivacaine. Brit. J. Anaesth. 1998, 80, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Derossi, R.; Miguel, G.L.; Frazílio, F.O.; Nunes, D.B.; Kassab, T.A. L-Bupivacaine 0.5% vs. racemic 0.5% bupivacaine for caudal epidural analgesia in horses. J. Vet. Pharm. 2005, 28, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Cox, C.R.; Checketts, M.R.; Mackenzie, N.; Scott, N.B.; Bannister, J. Comparison of S(-)-bupivacaine with racemic (RS)-bupivacaine in supraclavicular brachial plexus block. Br. J. Anaesth. 1998, 80, 594–598. [Google Scholar] [CrossRef]
- Mukozawa, M.; Takakura, K.; Mizogami, M. Direct vasocontractile activities of bupivacaine enantiomers on the isolated rat thoracic aorta. Anesth. Res. Pr. 2010, 2010. [Google Scholar] [CrossRef] [PubMed]
- Vanhoutte, F.; Vereecke, J.; Verbeke, N.; Carmeliet, E. Stereoselective effects of the enantiomers of bupivacaine on the electrophysiological properties of the guinea-pig papillary muscle. Br. J. Anaesth. 1991, 103, 1275–1281. [Google Scholar] [CrossRef]
- Tsuchiya, H.; Mizogami, M. R(+)-, Rac-, and S(-)-bupivacaine stereostructure-specifically interact with membrane lipids at cardiotoxically relevant concentrations. Anesth. Analg. 2012, 114, 310–312. [Google Scholar] [CrossRef] [PubMed]
- Nau, C.; Vogel, W.; Hempelmann, G.; Bräu, M.E. Stereoselectivity of bupivacaine in local anesthetic-sensitive ion channels of peripheral nerve. Anesthesiology 1999, 91, 786–795. [Google Scholar] [CrossRef] [PubMed]
- Kanai, Y.; Katsuki, H.; Takasaki, M. Comparisons of the anesthetic potency and intracellular concentrations of S(-) and R(+) bupivacaine and ropivacaine in crayfish giant axon in vitro. Anesth. Analg. 2000, 90, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Kanai, Y.; Tateyama, S.; Nakamura, T.; Kasaba, T.; Takasaki, M. Effects of levobupivacaine, bupivacaine, and ropivacaine on tail-flick response and motor function in rats following epidural or intrathecal administration. Reg. Anesth. Pain Med. 1999, 24, 444–452. [Google Scholar] [CrossRef]
- Valenzuela, C.; Snyders, D.J.; Bennett, P.B.; Tamargo, J.; Hondeghem, L.M. Stereoselective block of cardiac sodium channels by bupivacaine in guinea pig ventricular myocytes. Circulation 1995, 92, 3014–3024. [Google Scholar] [CrossRef] [PubMed]
- González, T.; Longobardo, M.; Caballero, R.; Delpón, E.; Sinisterra, J.; Tamargo, J.; Valenzuela, C. Stereoselective effects of the enantiomers of a new local anaesthetic, IQB-9302, on a human cardiac potassium channel (Kv1.5). Br. J. Pharm. 2001, 132, 385–392. [Google Scholar]
- Shah, J.; Bhatt, K.A. A comparative study between levobupivacaine 0.5% plus lignocaine 2% or bupivacaine 0.5% plus lignocaine 2% for peribulbar block in cataract surgery. Anaesth. Pain Intensive Care 2019, 23, 192–197. [Google Scholar] [CrossRef]
- Yadev, I.; Gupta, S.; Dua, C.K. Comparative evaluation of clinical efficacy of intrathecal isobaric levobupivacaine and isobaric bupivacaine in patients undergoing infra-umbilical surgery. Anaesth. Pain Intensive Care 2016, 20, 303–308. [Google Scholar]
- Leone, S.; Di Cianni, S.; Casati, A.; Fanelli, G. Pharmacology, toxicology, and clinical use of new long acting local anesthetics, ropivacaine and levobupivacaine. Acta Biomed. 2008, 79, 92–105. [Google Scholar] [PubMed]
- Scott, D.B.; Fagan, D.; Bowler, G.M.; Bloomfield, P.; Lundh, R. Acute toxicity of ropivacaine compared with that of bupivacaine. Anesth. Analg. 1989, 69, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Casati, A.; Putzu, M. Bupivacaine, levobupivacaine and ropivacaine: Are they clinically different? Best Pr. Res. Clin. Anaesthesiol. 2005, 19, 247–268. [Google Scholar] [CrossRef] [PubMed]
- Bardsley, H.; Gristwood, R.; Baker, H.; Watson, N.; Nimmo, W. A comparison of the cardiovascular effects of levobupivacaine and rac-bupivacaine following intravenous administration to healthy volunteers. Br. J. Clin. Pharm. 1998, 46, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Mazoit, J.X.; Decaux, A.; Bouaziz, H.; Edouard, A. Comparative ventricular electrophysiologic effect of racemic bupivacaine, levobupivacaine, and ropivacaine on the isolated rabbit heart. Anesthesiology 2000, 93, 784–792. [Google Scholar] [CrossRef]
- Lygre, H.; Moe, G.; Nerdal, W.; Holmsen, H. Interaction of articaine hydrochloride with prokaryotic membrane lipids. Acta Odontol. Scand. 2009, 67, 1–7. [Google Scholar] [CrossRef]
- Steinkopf, S.; Hanekam, L.; Schaathun, M.; Budnjo, A.; Haug, B.E.; Nerdal, W. Interaction of local anaesthetic articaine enantiomers with brain lipids: A Langmuir monolayer study. Eur. J. Pharm. Sci. 2012, 47, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Malamed, S.F.; Gangnon, S.; Leblanc, D. Articaine hydrochloride: A study of the safety of a new amide local anesthetic. J. Am. Dent. Assoc. 2001, 132, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Albright, G.A. Cardiac arrest following regional anesthesia with etidocaine or bupivacaine. Anesthesiology 1979, 51, 285–287. [Google Scholar] [CrossRef] [PubMed]
- Lund, P.C.; Cwik, J.C.; Pagdanganan, R.T. Etidocaine—A new long-acting anesthetic agent: A clinical evaluation. Anesth. Analg. 1973, 52, 482–494. [Google Scholar] [CrossRef]
- Munson, E.S.; Tucker, W.K.; Ausinsch, B.; Malagodi, M.H. Etidocaine, bupivacaine, and lidocaine seizure thresholds in monkeys. Anesthesiology 1975, 42, 471–478. [Google Scholar] [CrossRef]
- Stankovicová, T.; Stolc, S.; Csöllei, J.; Benes, L. Local anesthetic effect of carbisocaine and its enantiomers. Pharmazie 1995, 50, 622–623. [Google Scholar] [PubMed]
- Beneš, L. Trapencaine hydrochloride. Drugs Fut. 1991, 16, 627–630. [Google Scholar] [CrossRef]
- Nosáľová, V.; Zaviacic, M.; Jakubovský, J.; Babulová, A.; Polák, S. Gastroprotective effect of pentacaine: Role of mast cells. Agents Actions 1988, 23, 283–285. [Google Scholar] [CrossRef]
- Nosáľová, V.; Juránek, I.; Babulová, A. Effect of trapencaine and some of its derivatives on gastric wall mucus in stressed rats. Pharmazie 1995, 50, 424–425. [Google Scholar]
- Nosáľová, V.; Ričicová, V.; Kupková, Z.; Beneš, L.; Babulová, A. Differences between gastric antiulcer effects of trapencaine enantiomers. Pharmazie 2003, 58, 657–660. [Google Scholar]
- Fleck, C.; Kämena, M.; Tschritter, L.; Kersten, L.; Seeling, A.; Glassl, P.; Oelschläger, H. Local anaesthetic effectivity and toxicity of fomocaine, five N-free fomocaine metabolites and two chiralic fomocaine derivatives in rats compared with procaine. Arzneimittelforschung 2001, 51, 451–458. [Google Scholar] [CrossRef]
- Oelschläger, H.; Glassl, P.; Seeling, A.; Wange, J.; Listing, M.; Jung, B. Synthesis and pharmacologic action of chiral fomocaine ((4-[2-methyl-3-(morpholin-4-yl)propyl)benzyl)-phenyl-ether and (4-(1-methyl-3-(morpholin-4-yl)propyl]benzyl)-phenyl-ether). Pharmazie 2001, 56, 620–625. [Google Scholar]
- Fleck, C.; Wennek-Klose, J.; Wange, J.; Oelschläger, H. Effects and toxicity of new fomocaine derivatives and of 2-hydroxypropyl-ß-cyclodextrin inclusion compounds in rats. Arzneimittelforschung 2004, 54, 265–274. [Google Scholar] [PubMed]
- Mazot, J.X.; Cao, L.S.; Samii, K. Binding of bupivacaine to human serum proteins, isolated albumin and isolated alpha-1-acid glycoprotein. Differences between the two enantiomers are partly due to cooperativity. J. Pharm. Exp. 1996, 276, 109–115. [Google Scholar]
- Martínez-Gómez, M.A.; Villanueva-Camañas, R.M.; Sagrado, S.; Medina-Hernández, M.J. Evaluation of enantioselective binding of basic drugs to plasma by ACE. Electrophoresis 2007, 28, 3056–3063. [Google Scholar] [CrossRef]
- Burm, A.G.; van der Meer, A.D.; van Kleef, J.W.; Zeijlmans, P.W.; Groen, K. Pharmacokinetics of the enantiomers of bupivacaine following intravenous administration of the racemate. Br. J. Clin. Pharm. 1994, 38, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Veering, B.T.; Burm, A.G.L.; Feyen, H.M.; Olieman, W.; Souverijn, J.H.M.; van Kleef, J.W. Pharmacokinetics of bupivacaine during postoperative epidural infusion: Enantioselectivity and role of protein binding. Anestesiology 2002, 96, 1062–1069. [Google Scholar] [CrossRef]
- Rutten, A.J.; Mather, L.E.; McLean, C.F. Cardiovascular effects and regional clearances of I.V. bupivacaine in sheep: Enantiomeric analysis. Br. J. Anaesth. 1991, 67, 247–256. [Google Scholar] [CrossRef][Green Version]
- Rutten, A.J.; Mather, L.E.; McLean, C.F.; Nancarrow, C. Tissue distribution of bupivacaine enantiomers in sheep. Chirality 1993, 5, 485–491. [Google Scholar] [CrossRef]
- Franquelo, C.; Toledo, A.; Manubens, J.; Valladares, J.E.; Cristòfol, C.; Arboix, M. Pharmacokinetics and pharmacologic effects of the S(-) isomer of bupivacaine after intravenous and epidural administration in dogs. Am. J. Vet. Res. 1999, 60, 832–835. [Google Scholar]
- Fawcett, J.P.; Kennedy, J.; Kumar, A.; Ledger, R.; Zacharias, M. Stereoselective urinary excretion of bupivacaine and its metabolites during epidural infusion. Chirality 1999, 11, 50–55. [Google Scholar] [CrossRef]
- Ledger, R. Nonaromatic hydroxylation of bupivacaine during continuous epidural infusion in man. J. Biochem. Biophys. Methods 2003, 57, 105–114. [Google Scholar] [CrossRef]
- Souza, M.C.O.; Marques, M.P.; Duarte, G.; Lanchote, V.L. Analysis of bupivacaine enantiomers in plasma as total and unbound concentrations using LC-MS/MS: Application in a pharmacokinetic study of a parturient with placental transfer. J. Pharm. Biomed. Anal. 2019, 164, 268–275. [Google Scholar] [CrossRef]
- Fawcett, J.P.; Kennedy, J.; Kumar, A.; Ledger, R. Comparative efficacy and pharmacokinetics of racemic bupivacaine and S-bupivacaine in third molar surgery. J. Pharm. Pharm. Sci. 2002, 5, 199–204. [Google Scholar]
- Herdewall, B.M.; Klinge, B.; Peterson, I.; Hulegal, G.; Abdel Rehim, M. Plasma levels of lidocaine, o-toluidine, and prilocaine after application of 8.5 g Oraqix® in patients with generalized periodontitis: Effect on blood methemoglobin and tolerability. Acta Odontol. Scand. 2003, 61, 230–234. [Google Scholar] [CrossRef]
- Copeland, S.E.; Ladd, L.A.; Gu, X.Q.; Mather, L.E. The effects of general anesthesia on whole body and regional pharmacokinetics of local anesthetics at toxic doses. Anesth. Analg. 2008, 106, 1440–1449. [Google Scholar] [CrossRef]
- Vree, T.B.; Beumer, E.M.C.; Lagerwerf, A.J.; Simon, M.A.M.; Gielen, M.J.M. Clinical pharmacokinetics of R(+)-and S(−)-mepivacaine after high doses of racemic mepivacaine with epinephrine in the combined psoas compartment/sciatic nerve block. Anesth. Analg. 1992, 75, 75–80. [Google Scholar] [CrossRef]
- Burm, A.G.; Cohen, I.M.; van Kleef, J.W.; Vletter, A.A.; Olieman, W.; Groen, K. Pharmacokinetics of the enantiomers of mepivacaine after intravenous administration of the racemate in volunteers. Anesth. Analg. 1997, 84, 85–89. [Google Scholar] [CrossRef]
- Mather, L.E. Disposition of mepivacaine and bupivacaine enantiomers in sheep. Br. J. Anest. 1991, 67, 239–246. [Google Scholar] [CrossRef][Green Version]
- Oda, Y.; Furuichi, K.; Tanaka, K.; Hiroi, T.; Imaoka, S.; Asada, A.; Fujimori, M.; Funae, Y. Metabolism of a new local anesthetic, ropivacaine, by human hepatic cytochrome P450. Anesthesiology 1995, 82, 214–220. [Google Scholar] [CrossRef]
- Ekström, G.; Gunnarsson, U.B. Ropivacaine, a new amide-type local anesthetic agent, is metabolized by cytochromes P450 1A and 3A in human liver microsomes. Drug Metab. Dispos. 1996, 24, 955–961. [Google Scholar]
- Gatley, S.J. Activities of the enantiomers of cocaine and some related compounds as substrates and inhibitors of plasma butyrylcholinesterase. Biochem. Pharm. 1991, 41, 1249–1254. [Google Scholar] [CrossRef]
- Siluveru, M.; Stewart, J.T. Enantiomeric HPLC separations of chiral local anesthetics using cellulose based chiral stationary phases. Anal. Lett. 1997, 30, 1167–1178. [Google Scholar] [CrossRef]
- Siluveru, M.; Stewart, J.T. Stereoselective determination of mepivacaine in human serum using a brush-type chiral stationary phase and solid-phase extraction. J. Chromatogr. B. 1997, 690, 359–362. [Google Scholar] [CrossRef]
- Rustichelli, C.; Ferioli, V.; Gamberini, G.; Stancanelli, R. Enantiomeric separation of local anaesthetic drug by HPLC on chiral stationary phases. Chromatographia 2001, 54, 731–736. [Google Scholar] [CrossRef]
- Hroboňová, K.; Lehotay, J.; Čižmárik, J.; Armstrong, D.W. In-vitro study of enzymatic hydrolysis of diperodon enantiomers in blood serum by two-dimensional HPLC. J. Pharm. Biomed. Anal. 2002, 30, 875–880. [Google Scholar] [CrossRef]
- Hroboňová, K.; Lehotay, J.; Čižmárik, J. HPLC enantioseparation of phenylcarbamic acid derivatives by using macrocyclic chiral stationary phases. Nova Biotechnol. Chim. 2016, 15, 12–22. [Google Scholar] [CrossRef]
- Čižmárik, J.; Lehotay, J.; Hromuľáková, K.; Pokorná, M.; Lacuška, M. HPLC separation of enantiomers of carbisocaine. Pharmazie 1997, 52, 402–403. [Google Scholar]
- Hroboňová, K.; Lehotay, J. Separation of enantiomers of phenylcarbamic acid derivatives by HPLC method. Čes. Slov. Farm. 2004, 53, 197–202. [Google Scholar]
- Hroboňová, K.; Lehotay, J.; Čižmárik, J.; Renčová, M.; Armstrong, D.W. Study of mechanism of enantioseparation. II. HPLC chiral analysis of alkoxysubstituted esters of phenylcarbamic acid. J. Liq. Chromatogr. Rel. Technol. 2002, 25, 1711–1720. [Google Scholar] [CrossRef]
- Rojkovičová, T.; Hroboňová, K.; Lehotay, J.; Čižmárik, J. Coupled column chromatography for separation and determination of enantiomers of phenylcarbamic acid derivatives in serum. Pharmazie 2003, 58, 108–110. [Google Scholar]
- Rojkovičová, T.; Lehotay, J.; Ďungelová, J.; Armstrong, D. Study of mechanism of enantioseparation. III. The influence of carbohydrate moieties of teicoplanin-bonded chiral stationary phase on the separation of some derivates of phenylcarbamic acid. J. Liq. Chromatogr. Relat. Technol. 2006, 25, 2723–2738. [Google Scholar]
- Ďungelová, J.; Lehotay, J.; Hroboňová, K.; Čižmárik, J.; Armstrong, D.W. Study of local anaesthetics. Part CLVIII. Chromatographic separation of some derivates of substituted phenylcarbamic acid on vancomycin-based stationary phase. J. Liq. Chromatogr. 2002, 25, 299–312. [Google Scholar]
- Van de Griend, S.C.E.; Gröningsson, K.; Westerlund, D. Chiral separation of local anaesthetics with capillary electrophoresis. Chromatographia 1996, 42, 263–268. [Google Scholar] [CrossRef]
- Siluveru, M.; Stewart, J.T. Stereoselective determination of R-(−)- and S-(+)-prilocaine in human serum by capillary electrophoresis using a derivatized cyclodextrin and ultraviolet detection. J. Chromatogr. B. 1997, 693, 205–210. [Google Scholar] [CrossRef]
- Jäverfalk, E.M.; Amini, A.; Westerlund, D.; Andrén, P.E. Chiral separation of local anaesthetics by a capillary electrophoresis/partial filling technique coupled on-line to micro-electrospray mass spectrometry. J. Mass Spectrom. 1998, 33, 183–186. [Google Scholar] [CrossRef]
- Al-nouti, Y.; Barlet, M.G. Comparison of local anesthetic-cyclodextrin non-covalent complexes using capillary electrophoresis and electrospray ionization mass spectrometry. J. Am. Soc. Mass. Spectr. 2002, 13, 928–935. [Google Scholar] [CrossRef][Green Version]
- Amini, A.; Wiersma, B.; Westerlud, D.; Paulsen-Sörman, U. Determination of the enantiomeric purity of S-ropivacaine by capillary electrophoresis with methyl-β-cyclodextrin as chiral selector using conventional and complete filling techniques. Eur. J. Pharm. Sci. 1999, 9, 17–24. [Google Scholar] [CrossRef]
- Cherkaoui, S.; Veuthey, J.L. Use of negatively charged cyclodextrins for the simultaneous enantioseparation of selected anesthetic drugs by capillary electrophoresis-mass spectrometry. J. Pharm. Biomed. Anal. 2002, 27, 615–626. [Google Scholar] [CrossRef]
- Li, Y.; Yu, Y.; Zhu, P.; Duan, G.; Li, Y.; Song, F. Chiral separation of bupivacaine hydrochloride by capillary electrophoresis with high frequency conductivity detection and its application to rabbit serum and pharmaceutical injection. Pharmazie 2012, 67, 25–30. [Google Scholar]
- Martinez-Pla, J.J.; Martin-Biosca, Y.; Sagrado, S.; Villanueva-Camanas, R.M.; Medina-Hernandez, M.J.J. A Chiral separation of bupivacaine enantiomers by capillary electrophoresis partial-filling technique with human serum albumin as chiral selector. J. Chromatogr. A 2004, 1048, 111–118. [Google Scholar] [CrossRef]
- Amini, A.; Beijersten, I.; Pettersson, C.; Westerlund, D. Enantiomeric separation of local anaesthetic drugs by micellar electrokinetic capillary chromatography with taurodeoxycholate as chiral selector. J. Chromatogr. A. 1996, 737, 301–313. [Google Scholar] [CrossRef]





| INN 1 | pKa | Partition Coeffic. 2 | Solubility pH 7.4 | Protein Binding (%) | Half-Life | Speed on Onset | Duration | Toxicity |
|---|---|---|---|---|---|---|---|---|
| Mepivacaine | 7.6 | 21 | Moderate | 78 | 2.0–3.0 h | Fast | Intermediate | Low |
| Prilocaine | 7.9 | 25 | Moderate | 6 | 1.25h | Slow | Intermediate | Low |
| Bupivacaine | 8.1 | 346 | Very good | 95 | 2.7–3.5 h | Slow | Long | High |
| Ropivacaine | 8.1 | 115 | Very good | 94 | 1.8 h | Slow | Long | Intermediate |
| Articaine | 7.8 | 32 | Moderate | 95 | 0.5 h | Very fast | Long | Low |
| Etidocaine | 7.7 | 800 | Very good | 94 | 2.7 h | Fast | Intermediate | Intermediate |
| Lidocaine | 7.9 | 43 | Moderate | 77 | 1.5–1.8 h | Fast | Intermediate | Low |
| Procaine | 8.9 | 1.7 | Low | 6 | 30–50 s | Slow | Short | Low |
| Tetracaine | 8.2 | 221 | Very good | 76 | 2–4 min | Fast | Short | Intermediate |
| Chiral Stationary Phase | Mobile Phase | Chiral Local Anesthetic | Ref. |
|---|---|---|---|
| Supelcosil LC-urea | Acetonitrile | Cocaine | [31] |
| Chiracel OD | Hexane/ethanol 99:1 v/v | Bupivacaine, mepivacaine, prilocaine | [87] |
| Chiracel OD | Hexane/propan-2-ol | Articaine | [89] |
| Chiracel OJ | Hexane/ethanol 98:2 v/v | Etidocaine | [87] |
| Sumichiral OA-4700 | Hexane/dichloroethane/methanol 85:10:5 v/v/v | Prilocaine | [88] |
| Chirobiotic V | 5% Acetonitrile in triethylammonium acetate | Articaine | [89] |
| Chirobiotic T | Methanol/acetonitrile/acetic acid/triethylamine 45:55:0.3:0.2 v/v/v/v | Diperodon, alkoxyphenylcarbamates | [90] |
| Chirobiotic TAG | Acetonitrile/methanol/acetic acid/triethylamine 80:20:0.3:0.2 v/v/v/v | Diperodon | [91] |
| β-CD | % Acetonitrile in water and 0.01% triethylamine | Carbisocaine | [92] |
| β-CD, Chirobiotic TAG | Methanol/acetic acid/diethylamine 100 v/17.49 mmol/0–9.57 mmol/L | Alkoxyphenylcarbamates | [96] |
| Chirex 3020 | 95% Hexane/ethanol (80:20, v/v) | Bupivacaine | [76] |
| Chiral Selector | Part of Background Electrolyte | Chiral Local Anesthetic | Reference |
|---|---|---|---|
| Heptakis(2,6-di-O-methyl- ß-CD) | Triethanolamine | Mepivacaine, ropivacaine, bupivacaine | [98] |
| Heptakis(2,6-di-O-methyl- ß-CD) | Phosphate buffer | Prilocaine | [99] |
| Methyl-ß-CD | Phosphate buffer | Bupivacaine, ropivacaine | [100] |
| methyl-ß-CD | Acetate buffer | Bupivacaine, ropivacaine | [102] |
| Carboxymethyl, sulfobutyl ether and sulfonated-β-CD | Tris–phosphate buffer | Bupivacaine, mepivacaine, prilocaine | [103] |
| Sulfobutyl ether | Acetate buffer | Bupivacaine | [104] |
| Local Anesthetic | Total Number of Stereoisomers | Most Active Isomer | More Toxic Isomer | Used as |
|---|---|---|---|---|
| Cocaine (1) | 8 | (1S,2S,3R,5R)-(+) | (1R,2R,3S,5S)-(−) | (1R,2R,3S,5S)-(−) |
| Mepivacaine (2) | 2 | (S)-(−) | NA | Racemate |
| Prilocaine (3) | 2 | Comparable | (R)-(−) | Racemate |
| Bupivacaine (4) | 2 | Comparable | (R)-(+) | (S)-(−) |
| Ropivacaine (6) | 2 | NA | (R)-(+) | (S)-(−) |
| Articaine (8) | 2 | NA | NA | Racemate |
| IQB-9302 (7) | 2 | NA | NA | Racemate |
| Etidocaine (9) | 2 | NA | NA | Racemate |
| Carbisocaine (10) | 2 | Comparable | NA | Racemate |
| Pentacaine (11) | 4 | NA | NA | Racemate |
| OG/5 (13) | 2 | NA | NA | Racemate |
| OG/3 (14) | 2 | NA | NA | Racemate |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Čižmáriková, R.; Čižmárik, J.; Valentová, J.; Habala, L.; Markuliak, M. Chiral Aspects of Local Anesthetics. Molecules 2020, 25, 2738. https://doi.org/10.3390/molecules25122738
Čižmáriková R, Čižmárik J, Valentová J, Habala L, Markuliak M. Chiral Aspects of Local Anesthetics. Molecules. 2020; 25(12):2738. https://doi.org/10.3390/molecules25122738
Chicago/Turabian StyleČižmáriková, Ružena, Jozef Čižmárik, Jindra Valentová, Ladislav Habala, and Mário Markuliak. 2020. "Chiral Aspects of Local Anesthetics" Molecules 25, no. 12: 2738. https://doi.org/10.3390/molecules25122738
APA StyleČižmáriková, R., Čižmárik, J., Valentová, J., Habala, L., & Markuliak, M. (2020). Chiral Aspects of Local Anesthetics. Molecules, 25(12), 2738. https://doi.org/10.3390/molecules25122738





