Screening of Phosphodiesterase-5 Inhibitors and Their Analogs in Dietary Supplements by Liquid Chromatography–Hybrid Ion Trap–Time of Flight Mass Spectrometry
Abstract
1. Introduction
2. Results and Discussion
2.1. HPLC–MS Method Development
2.2. Method Validation
2.3. MSn Analysis
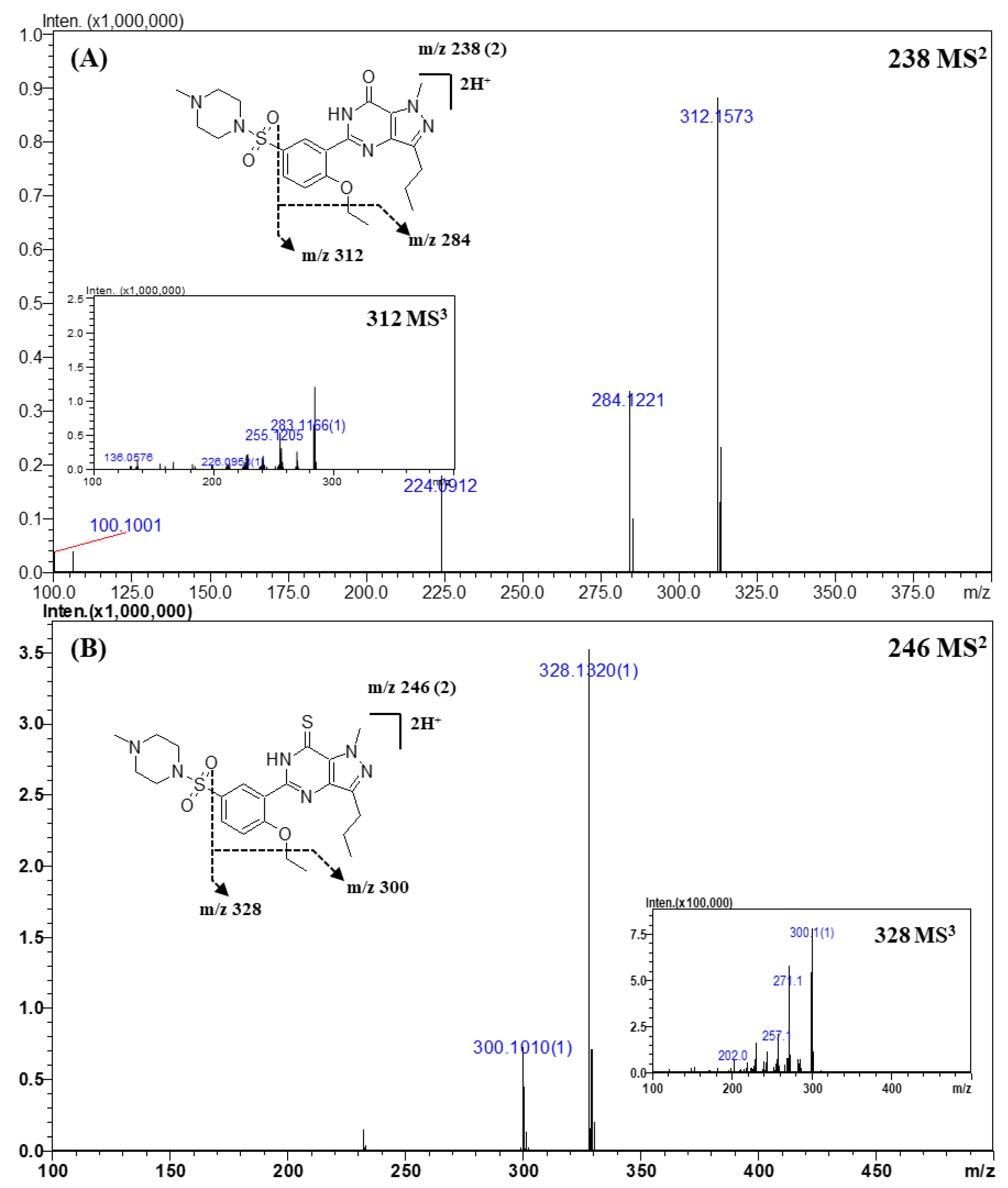
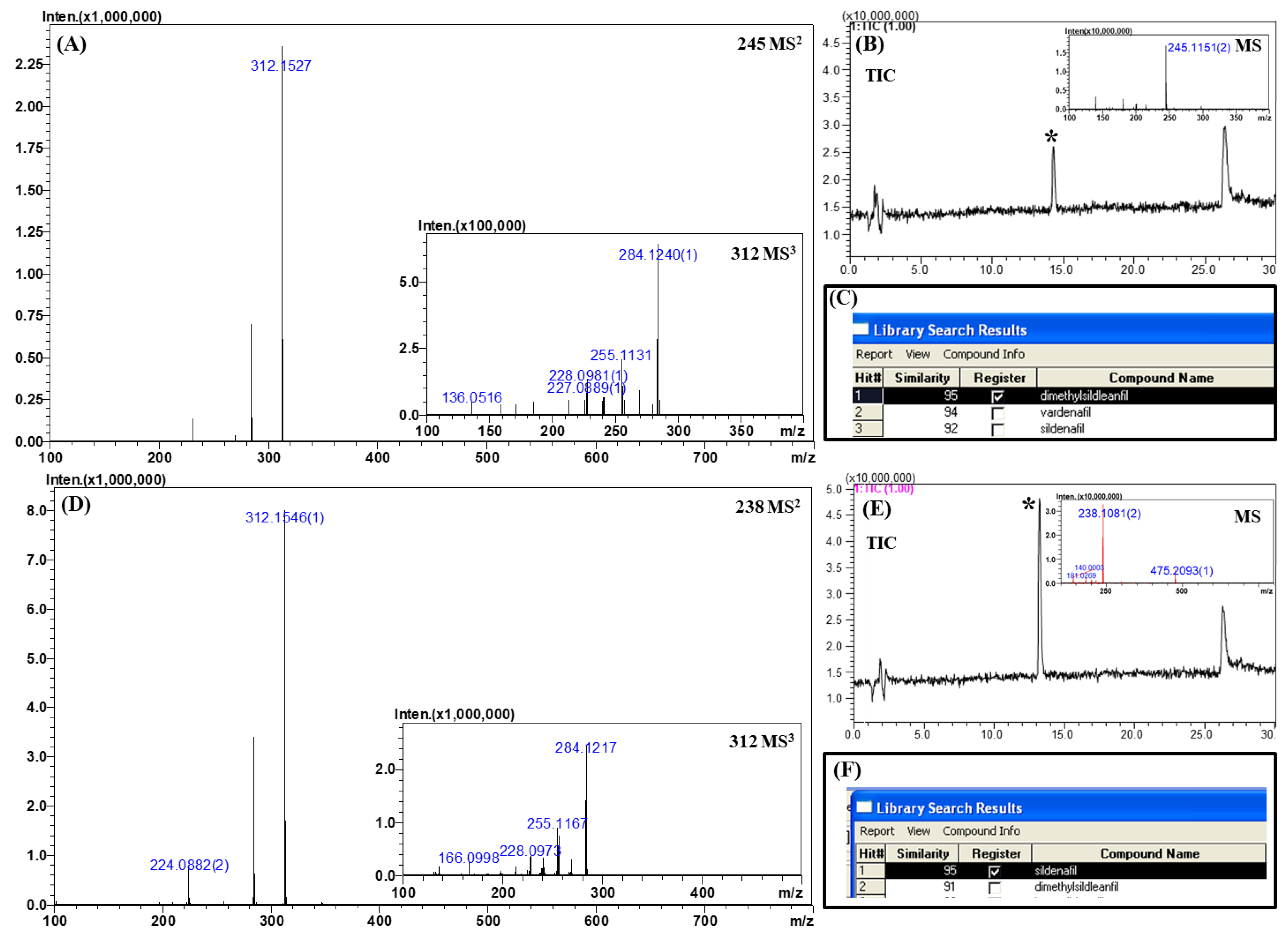
2.3.1. Sildenafil and Its Derivatives
2.3.2. Vardenafil and Its Derivatives
2.3.3. Tadalafil and Its Derivatives
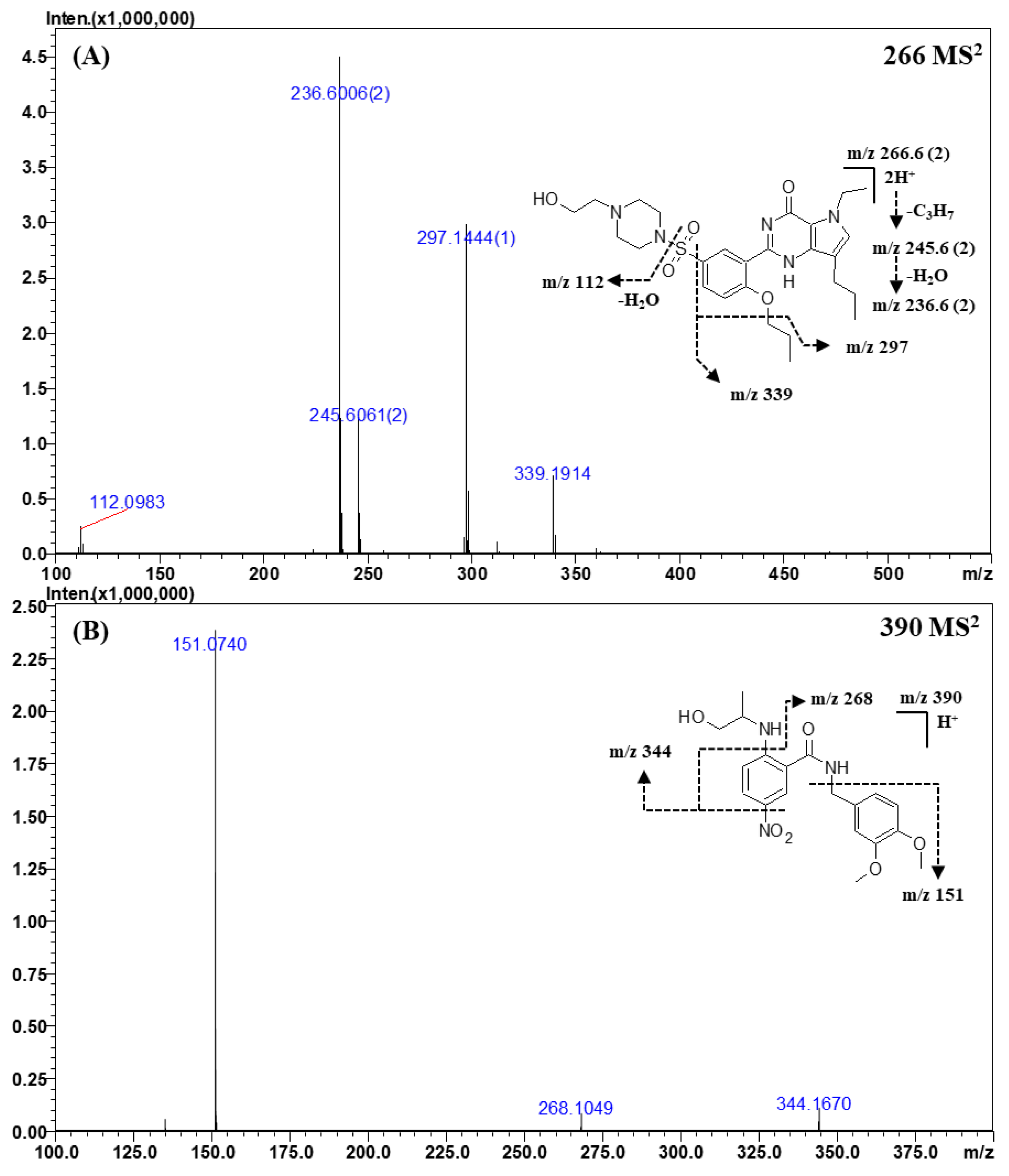
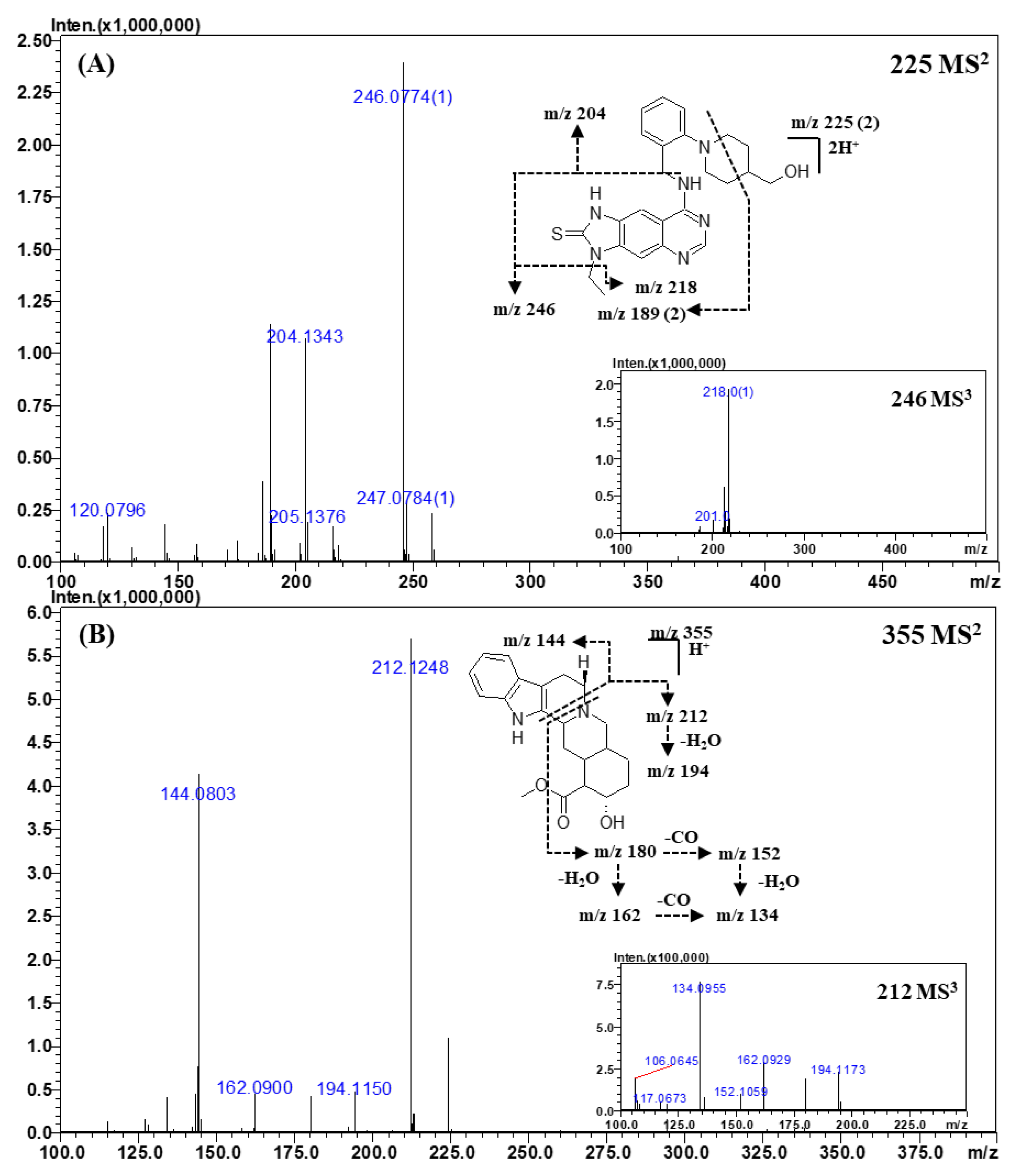
2.3.4. Other Classes
2.4. Analysis of Real Samples
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Sample Preparation
3.3. Instrumentation and Separation Conditions
3.4. MSn Analysis
3.5. Method Validation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rotella, D.P. Phosphodiesterase 5 inhibitors: Current status and potential applications. Nat. Rev. Drug Discov. 2002, 1, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Ke, H.; Wang, H. Crystal structure of phosphodiesterase’s and implications on substrate specificity and inhibitor selectivity. Curr. Top. Med. Chem. 2007, 7, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Prasad, B.; Savaliya, A.; Shah, R.; Gohil, V.; Kaur, A. Strategies for characterizing sildenafil, vardenafil, tadalafil and their analogues in herbal dietary supplements, and detecting counterfeit products containing these drugs. TrAC Trends Anal. Chem. 2009, 28, 13–28. [Google Scholar] [CrossRef]
- Lee, H.M.; Kim, C.S.; Jang, Y.M.; Kwon, S.W.; Lee, B.J. Separation and structural elucidation of a novel analogue of vardenafil included as an adulterant in a dietary supplement by liquid Chromatography-Electrospray ionization mass spectrometry, infrared spectroscopy and nuclear magnetic resonance spectroscopy. J. Pharm. Biomed. 2011, 54, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Venhuis, B.J.; de Kaste, D. Towards a decade of detecting new analogues of sildenafil, tadalafil and vardenafil in food supplements: A history, analytical aspects and health risks. J. Pharm. Biomed. 2012, 69, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.H.; Hong, M.K.; Kim, W.S.; Lee, Y.J.; Jeoung, Y.C. Identification of a new analogue of sildenafil added illegally to a functional food marketed for penile erectile dysfunction. Food Addit. Contam. 2003, 20, 793–796. [Google Scholar] [CrossRef] [PubMed]
- Lam, Y.-H.; Poon, W.-T.; Lai, C.-K.; Chan, A.Y.-W.; Mak, T.W.-L. Identification of a novel vardenafil analogue in herbal product. J. Pharm. Biomed. 2008, 46, 804–807. [Google Scholar] [CrossRef]
- Venhuis, B.J.; Zomer, G.; Hamzink, M.; Meiring, H.D.; Aubin, Y.; de Kaste, D. The identification of a nitrosated prodrug of the PDE-5 inhibitor aildenafil in a dietary supplement: A Viagra with a pop. J. Pharm. Biomed. 2011, 54, 735–741. [Google Scholar] [CrossRef]
- Balayssac, S.; Gilard, V.; Zedde, C.; Martino, R.; Malet-Martino, M. Analysis of herbal dietary supplements for sexual performance enhancement: First characterization of Propoxyphenyl-Thiohydroxyhomosildenafil and identification of sildenafil, thiosildenafil, phentolamine and tetrahydropalmatine as adulterants. J. Pharm. Biomed. 2012, 63, 135–150. [Google Scholar] [CrossRef]
- Cai, Y.; Cai, T.-G.; Shi, Y.; Cheng, X.-L.; Ma, L.-Y.; Ma, S.-C.; Lin, R.-C.; Feng, W. Simultaneous determination of eight PDE5-Is potentially adulterated in herbal dietary supplements with TLC and HPLC-PDA-MS methods. J. Liq. Chromatogr. Relat. Technol. 2010, 33, 1287–1306. [Google Scholar] [CrossRef]
- Zou, P.; Oh, S.S.; Hou, P.; Low, M.Y.; Koh, H.L. Simultaneous determination of synthetic Phosphodiesterase-5 inhibitors found in a dietary supplement and Pre-Mixed bulk powders for dietary supplements using High-Performance liquid chromatography with diode array detection and liquid Chromatography–Electrospray ionization tandem mass spectrometry. J. Chromatogr. A 2006, 1104, 113–122. [Google Scholar] [PubMed]
- Kim, S.-H.; Kim, H.-J.; Son, J.-H.; Jeon, B.-W.; Jeong, E.-S.; Cha, E.-J.; Lee, J.-I. Simultaneous determination of synthetic Phosphodiesterase-5 inhibitors in dietary supplements by liquid Chromatography-High resolution/mass spectrometry. Mass Spectrom. Lett. 2012, 3, 50–53. [Google Scholar] [CrossRef]
- Clewell, A.; Qureshi, I.; Endres, J.; Horvath, J.; Financsek, I.; Neal-Kababick, J.; Jade, K.; Schauss, A.G. Toxicological evaluation of a dietary supplement formulated for male sexual health prior to market release. Regul. Toxicol. Pharm. 2010, 57, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Venhuis, B.J.; Barends, D.M.; Zwaagstra, M.E.; de Kaste, D. Recent Developments in Counterfeits and Imitations of Viagra, Cialis and Levitra: A 2005–2006 Update; RIVM Report 37003001/2007; National Institute for Public-Health and the Environment: Utrecht, The Netherlands, 2007. [Google Scholar]
- Pissarnitski, D. Phosphodiesterase 5 (PDE 5) inhibitors for the treatment of male erectile disorder: Attaining selectivity versus PDE6. Med. Res. Rev. 2006, 26, 369–395. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.M.; Lee, B.J. A novel approach to simultaneous screening and confirmation of regulated pharmaceutical compounds in dietary supplements by LC/MS/MS with an Information-Dependent acquisition method. Food Addit. Contam. A 2011, 28, 396–407. [Google Scholar] [CrossRef]
- Savaliya, A.A.; Shah, R.P.; Prasad, B.; Singh, S. Screening of Indian aphrodisiac ayurvedic/herbal healthcare products for adulteration with sildenafil, tadalafil and/or vardenafil using LC/PDA and extracted ion LC-MS/TOF. J. Pharm. Biomed. 2010, 52, 406–409. [Google Scholar] [CrossRef]
- Oh, S.S.; Zou, P.; Low, M.Y.; Koh, H.L. Detection of sildenafil analogues in herbal products for erectile dysfunction. J. Toxicol. Environ. Health A 2006, 69, 1951–1958. [Google Scholar]
- Park, M.; Ahn, S. Quantitative analysis of sildenafil and tadalafil in various fake drugs recently distributed in Korea. J. Forensic Sci. 2012, 57, 1637–1640. [Google Scholar] [CrossRef]
- Song, F.; El-Demerdash, A.; Lee, S.J. Screening for multiple phosphodiesterase type 5 inhibitor drugs in dietary supplement materials by flow injection mass spectrometry and their quantification by liquid chromatography tandem mass spectrometry. J. Pharm. Biomed. 2012, 70, 40–46. [Google Scholar] [CrossRef]
- Fryčák, P.; Hartmanová, L.; Lorencová, I.; Lemr, K. Screening of synthetic Phosphodiesterase-5 inhibitors in herbal dietary supplements using Transmission-Mode desorption electrospray and High-Resolution mass spectrometry. J. Mass Spectrom. 2016, 51, 358–362. [Google Scholar] [CrossRef]
- Mokhtar, S.U.; Chin, S.T.; Kee, C.L.; Low, M.Y.; Drummer, O.H.; Marriott, P.J. Rapid determination of sildenafil and its analogues in dietary supplements using gas Chromatography–Triple quadrupole mass spectrometry. J. Pharm. Biomed. 2016, 121, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Bujang, N.B.; Chee, C.F.; Heh, C.H.; Rahman, N.A.; Buckle, M.J.C. Phosphodiesterase-5 inhibitors and their analogues as adulterants of herbal and food products: Analysis of the Malaysian market, 2014–2016. Food Addit. Contam. A 2017, 34, 1101–1109. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Wang, Z.; Xie, J.; Zeng, G.; Chen, W. Phosphodiesterase-5 inhibitors in Chinese tonic liquors by liquid chromatography coupled with quadrupole time of flight mass spectrometry. Food Addit. Contam. B 2018, 11, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-B.; Zheng, J.; Li, J.-J.; Yu, H.-Y.; Li, Q.-Y.; Xu, L.-H.; Liu, M.-J.; Xian, R.-Q.; Sun, Y.-E.; Liu, B.-J. Simultaneous analysis of 23 illegal adulterated aphrodisiac chemical ingredients in health foods and Chinese traditional patent medicines by ultrahigh performance liquid chromatography coupled with quadrupole Time-Of-Flight mass spectrometry. J. Food Drug Anal. 2018, 26, 1138–1153. [Google Scholar] [CrossRef] [PubMed]
- Jiru, M.; Stranska-Zachariasova, M.; Dzuman, Z.; Hurkova, K.; Tomaniova, M.; Stepan, R.; Cuhra, P.; Hajslova, J. Analysis of phosphodiesterase type 5 inhibitors as possible adulterants of Botanical-Based dietary supplements: Extensive survey of preparations available at the Czech market. J. Pharm. Biomed. 2019, 164, 713–724. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, H.N.; Park, S.; Lee, Y.-M.; Kang, H. Development of a specific fragment Pattern-Based Quadrupole-Orbitrap mass spectrometry method to screen adulterated products of Phosphodiesterase-5 inhibitors and their analogues. Sci. Justice 2019, 59, 433–441. [Google Scholar] [CrossRef]
- Yusop, A.Y.M.; Xiao, L.; Fu, S. Determination of phosphodiesterase 5 (PDE5) inhibitors in instant coffee premixes using liquid chromatography–high-resolution mass spectrometry (LC–HRMS). Talanta 2019, 204, 36–43. [Google Scholar] [CrossRef]
- Shi, S.; Wu, Y.; Zhou, M.; Cheng, Q. Simultaneous analysis of 31 Anti-Impotence compounds potentially illegally added to Herbal-Based dietary supplements by Ultra-High-Performance liquid chromatography coupled with quadrupole Time-Of-Flight mass spectrometry. J. Chromatogr. B 2020, 1144, 122077. [Google Scholar] [CrossRef]
- Park, H.J.; Jeong, H.K.; Chang, M.I.; Im, M.H.; Jeong, J.Y.; Choi, D.M.; Park, K.; Hong, M.K.; Youm, J.; Han, S.B.; et al. Structure determination of new analogues of vardenafil and sildenafil in dietary supplements. Food Addit. Contam. 2007, 24, 122–129. [Google Scholar] [CrossRef]
- Gratz, S.R.; Gamble, B.M.; Flurer, R.A. Accurate mass measurement using Fourier transform ion cyclotron resonance mass spectrometry for structure elucidation of designer drug analogs of tadalafil, vardenafil and sildenafil in herbal and pharmaceutical matrices. Rapid Commun. Mass Spectrom. 2006, 20, 2317–2327. [Google Scholar] [CrossRef]
- Inoue, S.; Mitsunori, S.M.; Ogasawara, M.; Osamu, E.; Gen, S. Simultaneous determination of medicinal ingredients in So-Called Health-Promoting food using liquid chromatography tandem mass spectrometry with a pentafluorophenyl stationary phase. J. Health Sci. 2009, 55, 183–191. [Google Scholar] [CrossRef][Green Version]
- Ge, X.; Low, M.-Y.; Zou, P.; Lin, L.; Yin, S.O.S.; Bloodworth, B.C.; Koh, H.-L. Structural elucidation of a PDE-5 inhibitor detected as an adulterant in a health supplement. J. Pharm. Biomed. 2008, 48, 1070–1075. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, T.; Takahashi, K.; Saijo, M.; Ishii, T.; Nagata, T.; Kurihara, M.; Haishima, Y.; Goda, Y.; Kawahara, N. Isolation and structural elucidation of cyclopentynafil and N-Octylnortadalafil found in a dietary supplement. Chem. Pharm. Bull. 2009, 57, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Reepmeyer, J.C.; d’Avignon, D.A. Structure elucidation of thioketone analogues of sildenafil detected as adulterants in herbal aphrodisiacs. J. Pharm. Biomed. 2009, 49, 145–150. [Google Scholar] [CrossRef]
- Zou, P.; Hou, P.; Oh, S.S.-Y.; Chong, Y.M.; Bloodworth, B.C.; Low, M.-Y.; Koh, H.-L. Isolation and identification of thiohomosildenafil and thiosildenafil in health supplements. J. Pharm. Biomed. 2008, 47, 279–284. [Google Scholar] [CrossRef]
- Hou, P.; Zou, P.; Low, M.Y.; Chan, E.; Koh, H.L. Structural identification of a new acetildenafil analogue from Pre-Mixed bulk powder intended as a dietary supplement. Food Addit. Contam. 2006, 23, 870–875. [Google Scholar] [CrossRef]
- Zou, P.; Hou, P.; Low, M.Y.; Koh, H.L. Structural elucidation of a tadalafil analogue found as an adulterant of a herbal product. Food Addit. Contam. 2006, 23, 446–451. [Google Scholar] [CrossRef]
- Schwartz, J.C.; Senko, M.W.; Syka, J.E. A Two-Dimensional quadrupole ion trap mass spectrometer. J. Am. Soc. Mass Spectrom. 2002, 13, 659–669. [Google Scholar] [CrossRef]
- David, H.; Russell, R.D.E. High-Resolution mass spectrometry and accurate mass measurements with emphasis on the characterization of peptides and proteins by Matrix-Assisted laser desorption/ionization Time-Of-Flight mass spectrometry. J. Mass Spectrom. 1997, 32, 263–276. [Google Scholar]
- Gadgil, H.S.; Pipes, G.D.; Dillon, T.M.; Treuheit, M.J.; Bondarenko, P.V. Improving mass accuracy of high performance liquid chromatography/electrospray ionization Time-Of-Flight mass spectrometry of intact antibodies. J. Am. Soc. Mass Spectrom. 2006, 17, 867–872. [Google Scholar] [CrossRef]
- Dafang, Z.; Xie, J.; Shuqiu, Z.; Lu, S. Study of the electrospray ionization tandem mass spectrometry of sildenafil derivatives. Rapid Commun. Mass Spectrom. 2002, 16, 1836–1843. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |


| Compound | Retention Time (min) | Elemental Composition | Measured Mass | Theoretical Mass | ∆m (mDa) | Mass Error (ppm ± SD a) |
|---|---|---|---|---|---|---|
| Sildenafil | 12.6 | C22H30N6O4S | 475.2125 | 475.2122 | 0.30 | 0.63 ± 2.21 |
| Carbodenafil | 9.3 | C24H32N6O3 | 453.2599 | 453.2609 | −1.02 | −2.26 ± 0.54 |
| Demethylhongdenafil | 9.3 | C24H32N6O3 | 453.2618 | 453.2609 | 0.94 | 2.08 ± 3.68 |
| Hydroxyhongdenafil | 9.9 | C25H34N6O4 | 483.2728 | 483.2714 | 1.41 | 2.91 ± 1.70 |
| Hongdenafil | 10.6 | C25H34N6O3 | 467.2753 | 467.2765 | −1.19 | −2.55 ± 3.09 |
| Hydroxyhomosildenafil | 10.9 | C23H32N6O5S | 505.2215 | 505.2228 | −1.26 | −2.49 ± 2.36 |
| Piperidinohongdenafil | 11.2 | C24H31N5O3 | 438.2505 | 438.2500 | 0.48 | 1.10 ± 1.47 |
| Homosildenafil | 13.0 | C23H32N6O4S | 489.2278 | 489.2279 | −0.06 | −0.12 ± 0.42 |
| Dimethylsildenafil | 13.7 | C23H32N6O4S | 489.2291 | 489.2279 | 1.24 | 2.54 ± 0.95 |
| Udenafil | 15.0 | C25H36N6O4S | 517.2567 | 517.2592 | −2.52 | −4.48 ± 0.63 |
| Cyclopentinafil | 16.2 | C26H36N6O4S | 529.2591 | 529.2592 | −0.09 | −0.17 ± 0.52 |
| Thiosildenafil | 23.6 | C22H30N6O3S2 | 491.1882 | 491.1893 | −1.09 | −2.22 ± 0.94 |
| Hydroxythiohomosildenafil | 23.7 | C23H32N6O4S2 | 521.1956 | 521.1999 | −4.32 | −8.30 ± 2.15 |
| Thiohomosildenafil | 24.4 | C23H32N6O3S2 | 505.2013 | 505.2050 | −3.73 | −7.37 ± 2.56 |
| Dimethylthiosildenafil | 25.1 | C23H32N6O3S2 | 505.2018 | 505.2050 | −3.23 | −6.38 ± 2.42 |
| Oxohongdenafil | 28.9 | C25H32N6O4 | 481.2569 | 481.2558 | 1.11 | 2.30 ± 1.22 |
| Benzylsildenafil | 30.6 | C28H34N6O4S | 551.2403 | 551.2435 | −3.19 | −5.79 ± 1.20 |
| Hydroxychlorodenafil | 40.5 | C19H23N4O3Cl | 391.1528 | 391.1531 | −0.28 | −0.72 ± 1.60 |
| Chlorodenafil | 48.4 | C19H21N4O3Cl | 389.1377 | 389.1375 | 0.17 | 0.43 ± 1.10 |
| Nitrodenafil | 49.6 | C17H19N5O4 | 358.1512 | 358.1510 | 0.17 | 0.47 ± 1.59 |
| Nor-neosildenafil | 51.7 | C22H29N5O4S | 460.2014 | 460.2013 | 0.08 | 0.18 ± 1.72 |
| Dichlorodenafil | 53.6 | C19H20N4O2Cl2 | 407.1038 | 407.1036 | 0.20 | 0.49 ± 1.81 |
| Vardenafil | 11.1 | C23H32N6O4S | 489.2296 | 489.2279 | 1.67 | 3.42 ± 0.86 |
| Acetylvardenafil | 9.3 | C25H34N6O3 | 467.2758 | 467.2765 | −0.69 | −1.48 ± 5.47 |
| Hydroxyvardenafil | 10.9 | C23H32N6O5S | 505.2209 | 505.2228 | −1.86 | −3.68 ± 0.84 |
| Nor-neovardenafil | 23.9 | C18H20N4O4 | 357.1557 | 357.1557 | −0.02 | −0.05 ± 0.86 |
| Desulfovardenafil | 37.1 | C17H20N4O2 | 313.1651 | 313.1659 | −0.80 | −2.55 ± 0.88 |
| Pseudovardenafil | 48.5 | C22H29N5O4S | 460.2013 | 460.2013 | −0.05 | −0.11 ± 0.64 |
| Tadalafil | 29.8 | C22H19N3O4 | 390.1449 | 390.1448 | 0.10 | 0.26 ± 1.42 |
| Aminotadalafil | 24.4 | C21H18N4O4 | 391.1410 | 391.1401 | 0.88 | 2.26 ± 1.57 |
| Acetaminotadalafil | 24.6 | C23H20N4O5 | 433.1502 | 433.1506 | −0.42 | −0.96 ± 1.81 |
| Demethyltadalafil | 25.5 | C21H17N3O4 | 376.1293 | 376.1292 | 0.07 | 0.18 ± 1.36 |
| Chloropretadalafil | 48.5 | C22H19N2O5Cl | 427.1031 | 427.1055 | −2.42 | −5.66 ± 1.62 |
| N-Octyltadalafil | 53.1 | C29H33N3O4 | 488.2559 | 488.2544 | 1.48 | 3.04 ± 3.23 |
| Yohimbine | 6.4 | C21H26N2O3 | 532.2580 | 532.2588 | −0.82 | −1.55 ± 0.97 |
| Mirodenafil | 22.4 | C26H37N5O5S | 355.2012 | 355.2016 | −0.43 | −1.22 ± 1.73 |
| Thioquinapiperifil | 10.8 | C24H28N6OS | 449.2098 | 449.2118 | −1.96 | −4.36 ± 2.06 |
| Xanthoanthrafil | 30.2 | C19H23N3O6 | 390.1660 | 390.1660 | −0.02 | −0.04 ± 1.94 |
| IS b (Phenolphthalein) | 27.7 | C20H14O4 | 319.0934 | 319.0965 | −3.10 | 9.72 |
| Compound | Equation | Range (μg/mL) | R2 |
|---|---|---|---|
| Sildenafil | y = 0.169 x + 0.006 | 0.30–0.80 | 0.993 |
| Carbodenafil | y = 0.430 x − 0.008 | 0.16–0.43 | 0.997 |
| Hongdenafil | y = 0.783 x + 0.037 | 0.38–1.00 | 0.997 |
| Hydroxyhongdenafil | y = 0.506 x + 0.170 | 0.30–0.80 | 0.994 |
| Demethylhongdenafil | y = 0.403 x + 0.020 | 0.32–0.85 | 0.996 |
| Piperidinohongdenafil | y = 0.504 x + 0.052 | 0.28–0.74 | 0.996 |
| Hydroxyhomosildenafil | y = 0.490 x + 0.074 | 0.74–1.98 | 0.994 |
| Homosildenafil | y = 0.321 x + 0.025 | 0.20–0.55 | 0.998 |
| Dimethylsildenafil | y = 0.409 x + 0.030 | 0.30–0.80 | 0.995 |
| Udenafil | y = 0.504 x + 0.133 | 0.30–0.80 | 0.999 |
| Cyclopentinafil | y = 0.389 x + 0.040 | 0.30–0.80 | 0.997 |
| Thiosildenafil | y = 0.165 x + 0.026 | 1.20–3.20 | 0.996 |
| Hydroxythiohomosildenafil | y = 0.198 x + 0.003 | 0.75–2.00 | 0.998 |
| Thiohomosildenafil | y = 0.252 x + 0.007 | 0.15–0.40 | 0.993 |
| Dimethylthiosildenafil | y = 0.255 x − 0.010 | 0.18–0.47 | 0.995 |
| Oxohongdenafil | y = 0.237 x + 0.034 | 0.60–1.60 | 0.993 |
| Benzylsildenafil | y = 0.157 x + 0.010 | 0.31–0.84 | 0.993 |
| Hydroxychlorodenafil | y = 0.302 x + 0.011 | 0.25–0.66 | 0.998 |
| Chlorodenafil | y = 0.341 x + 0.386 | 1.50–4.00 | 0.990 |
| Nitrodenafil | y = 0.444 x + 0.016 | 0.15–0.40 | 0.994 |
| Nor-neosildenafil | y = 0.356 x + 0.020 | 0.20–0.54 | 0.993 |
| Dichlorodenafil | y = 0.107 x + 0.017 | 0.38–1.00 | 0.992 |
| Vardenafil | y = 0.381 x + 0.014 | 0.15–0.40 | 0.999 |
| Acetylvardenafil | y = 0.501 x + 0.046 | 0.31–0.83 | 0.992 |
| Hydroxyvardenafil | y = 0.610 x + 0.055 | 0.75–2.00 | 0.998 |
| Nor-neovardenafil | y = 0.729 x + 0.027 | 0.17–0.45 | 0.993 |
| Desulfovardenafil | y = 1.289 x + 0.052 | 0.15–0.40 | 0.991 |
| Pseudovardenafil | y = 0.912 x + 0.045 | 0.15–0.40 | 0.992 |
| Tadalafil | y = 0.090 x + 0.015 | 0.70–1.87 | 0.997 |
| Aminotadalafil | y = 0.048 x + 0.018 | 1.20–3.20 | 0.997 |
| Acetaminotadalafil | y = 0.108 x + 0.022 | 0.38–1.00 | 0.990 |
| Demethyltadalafil | y = 0.088 x + 0.015 | 0.75–2.00 | 0.993 |
| Chloropretadalafil | y = 0.027 x + 0.044 | 1.20–3.20 | 0.992 |
| N-Octyltadalafil | y = 0.043 x + 0.019 | 0.75–2.00 | 0.996 |
| Yohimbine | y = 0.539 x + 0.028 | 0.13–0.34 | 0.995 |
| Mirodenafil | y = 0.939 x + 0.004 | 0.17–0.45 | 0.991 |
| Thioquinapiperifil | y = 0.198 x − 0.016 | 0.15–0.40 | 0.994 |
| Xanthoanthrafil | y = 0.176 x + 0.020 | 0.60–1.60 | 0.992 |
| Compound | Intra-Day (n = 5) | Inter-Day (n = 5) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Accuracy (%) | Precision (CV %) | Accuracy (%) | Precision (CV %) | |||||||||
| L a | M b | H c | L | M | H | L | M | H | L | M | H | |
| Sildenafil | 104.2 | 103.7 | 101.6 | 5.5 | 3.1 | 3.9 | 99.6 | 103.4 | 100.1 | 2.8 | 3.6 | 1.9 |
| Carbodenafil | 103.7 | 101.6 | 99.7 | 4.8 | 2.5 | 5.2 | 100.9 | 101.7 | 96.8 | 4.4 | 5.1 | 4.8 |
| Hongdenafil | 106.0 | 103.8 | 99.2 | 4.0 | 3.4 | 3.1 | 97.1 | 103.2 | 98.9 | 5.9 | 4.3 | 4.1 |
| Hydroxyhongdenafil | 101.2 | 98.6 | 97.6 | 4.2 | 2.2 | 2.0 | 94.1 | 98.4 | 98.2 | 5.3 | 3.3 | 2.0 |
| Demethylhongdenafil | 102.4 | 99.6 | 98.7 | 5.2 | 2.3 | 5.5 | 94.4 | 99.5 | 96.9 | 4.2 | 3.9 | 2.5 |
| Piperidinohongdenafil | 104.0 | 103.4 | 96.7 | 7.1 | 2.8 | 2.8 | 93.0 | 102.0 | 97.6 | 5.3 | 4.4 | 1.8 |
| Hydroxyhomosildenafil | 107.1 | 104.7 | 101.8 | 6.7 | 0.7 | 3.4 | 94.5 | 99.9 | 98.3 | 5.3 | 5.3 | 3.5 |
| Homosildenafil | 102.5 | 103.6 | 102.1 | 4.3 | 3.1 | 2.9 | 94.5 | 105.1 | 97.8 | 3.7 | 2.1 | 3.3 |
| Dimethylsildenafil | 105.6 | 104.7 | 102.0 | 7.8 | 1.1 | 1.7 | 96.0 | 101.1 | 100.0 | 1.2 | 3.3 | 3.3 |
| Udenafil | 98.9 | 104.7 | 104.7 | 3.3 | 3.0 | 1.4 | 93.8 | 103.6 | 100.7 | 6.5 | 2.2 | 6.1 |
| Cyclopentinafil | 100.2 | 100.9 | 102.2 | 4.1 | 1.5 | 2.3 | 95.4 | 102.7 | 98.7 | 5.4 | 6.1 | 3.2 |
| Thiosildenafil | 105.3 | 105.2 | 104.3 | 7.5 | 2.8 | 2.7 | 93.3 | 100.6 | 102.2 | 4.9 | 7.3 | 2.0 |
| Hydroxythiohomosildenafil | 102.9 | 104.2 | 102.5 | 5.2 | 2.5 | 2.7 | 97.1 | 104.4 | 101.9 | 4.6 | 1.6 | 5.4 |
| Thiohomosildenafil | 103.9 | 103.7 | 101.4 | 9.2 | 4.0 | 4.0 | 96.0 | 99.0 | 97.8 | 7.9 | 4.9 | 6.3 |
| Dimethylthiosildenafil | 99.5 | 96.5 | 99.9 | 3.7 | 6.7 | 7.6 | 96.2 | 101.2 | 97.0 | 8.4 | 6.8 | 6.1 |
| Oxohongdenafil | 98.0 | 101.8 | 99.9 | 2.3 | 2.1 | 2.4 | 91.3 | 100.4 | 99.6 | 4.0 | 3.4 | 1.4 |
| Benzylsildenafil | 96.2 | 96.6 | 99.3 | 3.1 | 3.5 | 5.6 | 91.8 | 99.7 | 97.7 | 4.3 | 3.4 | 3.2 |
| Hydroxychlorodenafil | 92.5 | 98.1 | 100.1 | 1.4 | 2.2 | 3.1 | 91.1 | 99.1 | 97.3 | 3.1 | 3.7 | 2.7 |
| Chlorodenafil | 97.0 | 103.3 | 102.2 | 4.6 | 4.5 | 1.9 | 89.7 | 103.0 | 97.8 | 7.8 | 4.2 | 3.4 |
| Nitrodenafil | 98.3 | 99.8 | 102.9 | 3.7 | 3.0 | 4.2 | 89.7 | 101.0 | 100.6 | 9.3 | 5.7 | 3.3 |
| Nor-neosildenafil | 105.1 | 105.3 | 100.0 | 6.5 | 1.4 | 2.9 | 92.3 | 102.3 | 97.4 | 3.0 | 3.2 | 1.9 |
| Dichlorodenafil | 93.5 | 100.7 | 101.5 | 2.6 | 6.2 | 4.8 | 90.9 | 103.4 | 97.8 | 4.1 | 6.3 | 3.4 |
| Vardenafil | 100.8 | 98.7 | 96.4 | 4.4 | 3.0 | 3.7 | 93.0 | 100.4 | 95.8 | 4.7 | 4.4 | 2.9 |
| Acetylvardenafil | 102.1 | 103.0 | 99.7 | 5.0 | 4.7 | 3.5 | 96.0 | 103.6 | 101.0 | 4.1 | 2.9 | 5.2 |
| Hydroxyvardenafil | 104.9 | 102.6 | 98.7 | 5.8 | 1.9 | 3.1 | 96.6 | 100.6 | 97.9 | 5.9 | 5.3 | 2.1 |
| Nor-neovardenafil | 87.8 | 96.6 | 98.1 | 5.9 | 3.6 | 1.3 | 97.0 | 103.1 | 97.7 | 8.7 | 5.9 | 1.6 |
| Desulfovardenafil | 86.7 | 94.7 | 96.5 | 5.2 | 3.3 | 2.6 | 91.1 | 100.9 | 97.4 | 3.2 | 5.4 | 1.8 |
| Pseudovardenafil | 101.0 | 101.2 | 100.5 | 3.0 | 1.2 | 2.8 | 91.3 | 101.0 | 98.5 | 7.3 | 4.1 | 3.2 |
| Tadalafil | 94.2 | 94.3 | 95.7 | 3.1 | 2.5 | 3.4 | 91.9 | 96.6 | 99.1 | 7.9 | 5.6 | 3.4 |
| Aminotadalafil | 98.4 | 97.7 | 98.7 | 3.2 | 3.9 | 4.7 | 92.6 | 102.3 | 99.9 | 5.4 | 3.5 | 1.3 |
| Acetaminotadalafil | 99.2 | 100.2 | 101.3 | 4.6 | 6.6 | 4.2 | 90.3 | 99.4 | 100.3 | 7.2 | 5.7 | 3.2 |
| Demethyltadalafil | 92.1 | 100.7 | 102.4 | 4.5 | 2.4 | 2.6 | 89.8 | 102.7 | 101.0 | 5.1 | 2.0 | 3.5 |
| Chloropretadalafil | 106.7 | 105.6 | 105.0 | 2.6 | 2.2 | 3.5 | 98.0 | 105.6 | 105.7 | 10.5 | 2.9 | 2.1 |
| N-Octyltadalafil | 107.8 | 106.0 | 98.1 | 7.9 | 0.6 | 4.8 | 95.4 | 103.9 | 97.9 | 6.5 | 5.7 | 1.8 |
| Yohimbine | 106.1 | 104.0 | 101.3 | 5.6 | 3.4 | 3.6 | 92.6 | 101.2 | 97.0 | 6.3 | 5.2 | 2.1 |
| Mirodenafil | 107.1 | 100.2 | 95.9 | 5.1 | 1.3 | 3.2 | 96.2 | 101.2 | 95.8 | 5.2 | 4.1 | 4.4 |
| Thioquinapiperifil | 112.0 | 105.0 | 104.4 | 5.1 | 2.9 | 1.9 | 104.4 | 99.3 | 100.0 | 3.3 | 7.5 | 3.6 |
| Xanthoanthrafil | 91.8 | 99.5 | 102.3 | 3.0 | 2.1 | 4.6 | 92.6 | 99.3 | 98.4 | 4.9 | 4.4 | 2.1 |
| Compound | Elemental Composition | Measured Mass | Theoretical Mass | ∆m (mDa) | Error (ppm) |
|---|---|---|---|---|---|
| Sildenafil | C22H30N6O4S a | 238.1087 b | 238.1100 b | −1.3 | −5.46 |
| C17H20N4O4S | 377.1263 | 377.1278 | −1.5 | −3.98 | |
| C17H19N4O2 | 312.1573 | 312.1581 | −0.8 | −2.56 | |
| C15H15N4O2 | 284.1221 | 284.1268 | −4.7 | −16.54 | |
| Thiosildenafil | C22H30N6O3S2 a | 246.0977 b | 246.0983 b | −0.6 | −2.50 |
| C17H19N4OS | 328.1344 | 328.1352 | −0.8 | −2.44 | |
| C15H15N4OS | 300.1022 | 300.1039 | −1.7 | −5.66 | |
| Vardenafil | C23H32N6O4S a | 245.1168 b | 245.1176 b | −0.8 | −3.26 |
| C17H19N4O2 | 312.1555 | 312.1581 | −2.6 | −8.33 | |
| C15H15N4O2 | 284.1253 | 284.1268 | −1.5 | −5.28 | |
| C8H10N2O | 151.0877 | 151.0866 | 1.1 | 7.28 | |
| Tadalafil | C22H19N3O4 a | 390.1454 | 390.1448 | 0.6 | 1.54 |
| C15H13N3O2 | 268.1050 | 268.1081 | −3.1 | −11.56 | |
| C17H11NO2 | 262.0866 | 262.0863 | 0.3 | 1.14 | |
| C14H13N3O | 240.1099 | 240.1131 | −3.2 | −13.33 | |
| C12H8N2O | 197.0707 | 197.0709 | −0.2 | −1.01 | |
| C11H8N2 | 169.0736 | 169.0760 | −2.4 | −14.19 | |
| C8H6O2 | 135.0451 | 135.0441 | 1.0 | 7.40 | |
| Mirodenafil | C26H37N5O5S a | 266.6326 b | 266.6330 b | −0.4 | −1.50 |
| C20H24N3O2 | 339.1914 | 339.1941 | −2.7 | −7.96 | |
| C17H18N3O2 | 297.1444 | 297.1472 | −2.8 | −9.42 | |
| C23H31N5O5S | 245.6061 b | 245.6096 b | −3.5 | −14.25 | |
| C23H29N5O4S | 236.6006 b | 236.6043 b | −3.7 | −15.64 | |
| Xanthoanthrafil | C19H23N3O6 | 390.1660 | 390.1660 | 0.0 | 0.00 |
| C9H10O2 | 151.0740 | 151.0754 | −1.4 | 18.17 | |
| Thioquinapiperifil | C24H28N6OS a | 225.1093 b | 225.1095 b | −0.2 | −0.89 |
| C11H11N5S | 246.0774 | 246.0808 | −3.4 | −13.82 | |
| C9H7N5S | 218.0493 | 218.0495 | −0.2 | −0.92 | |
| C13H17NO | 204.1343 | 204.1383 | −4.0 | −19.59 | |
| Yohimbine | C21H26N2O3 a | 355.2017 | 355.2016 | 0.1 | 0.28 |
| C11H17NO3 | 212.1248 | 212.1281 | −3.3 | −15.56 | |
| C11H15NO2 | 194.1150 | 194.1176 | −2.6 | −13.39 | |
| C10H9N | 144.0803 | 144.0808 | −0.5 | −3.47 | |
| C10H13NO2 | 180.1015 | 180.1019 | −0.4 | −2.22 | |
| C10H11NO | 162.0900 | 162.0913 | −1.3 | −8.02 | |
| C9H11N | 134.0955 | 134.0964 | −0.9 | −6.71 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, U.; Cho, H.-D.; Kang, M.H.; Suh, J.H.; Eom, H.Y.; Kim, J.; Seo, S.; Kim, G.; Koo, H.R.; Ha, N.; et al. Screening of Phosphodiesterase-5 Inhibitors and Their Analogs in Dietary Supplements by Liquid Chromatography–Hybrid Ion Trap–Time of Flight Mass Spectrometry. Molecules 2020, 25, 2734. https://doi.org/10.3390/molecules25122734
Kim U, Cho H-D, Kang MH, Suh JH, Eom HY, Kim J, Seo S, Kim G, Koo HR, Ha N, et al. Screening of Phosphodiesterase-5 Inhibitors and Their Analogs in Dietary Supplements by Liquid Chromatography–Hybrid Ion Trap–Time of Flight Mass Spectrometry. Molecules. 2020; 25(12):2734. https://doi.org/10.3390/molecules25122734
Chicago/Turabian StyleKim, Unyong, Hyun-Deok Cho, Myung Hee Kang, Joon Hyuk Suh, Han Young Eom, Junghyun Kim, Sumin Seo, Gunwoo Kim, Hye Ryoung Koo, Nary Ha, and et al. 2020. "Screening of Phosphodiesterase-5 Inhibitors and Their Analogs in Dietary Supplements by Liquid Chromatography–Hybrid Ion Trap–Time of Flight Mass Spectrometry" Molecules 25, no. 12: 2734. https://doi.org/10.3390/molecules25122734
APA StyleKim, U., Cho, H.-D., Kang, M. H., Suh, J. H., Eom, H. Y., Kim, J., Seo, S., Kim, G., Koo, H. R., Ha, N., Song, U. T., & Han, S. B. (2020). Screening of Phosphodiesterase-5 Inhibitors and Their Analogs in Dietary Supplements by Liquid Chromatography–Hybrid Ion Trap–Time of Flight Mass Spectrometry. Molecules, 25(12), 2734. https://doi.org/10.3390/molecules25122734




