Activation of Peracetic Acid with Lanthanum Cobaltite Perovskite for Sulfamethoxazole Degradation under a Neutral pH: The Contribution of Organic Radicals
Abstract
1. Introduction
2. Results
2.1. Characterization of LaCoO3
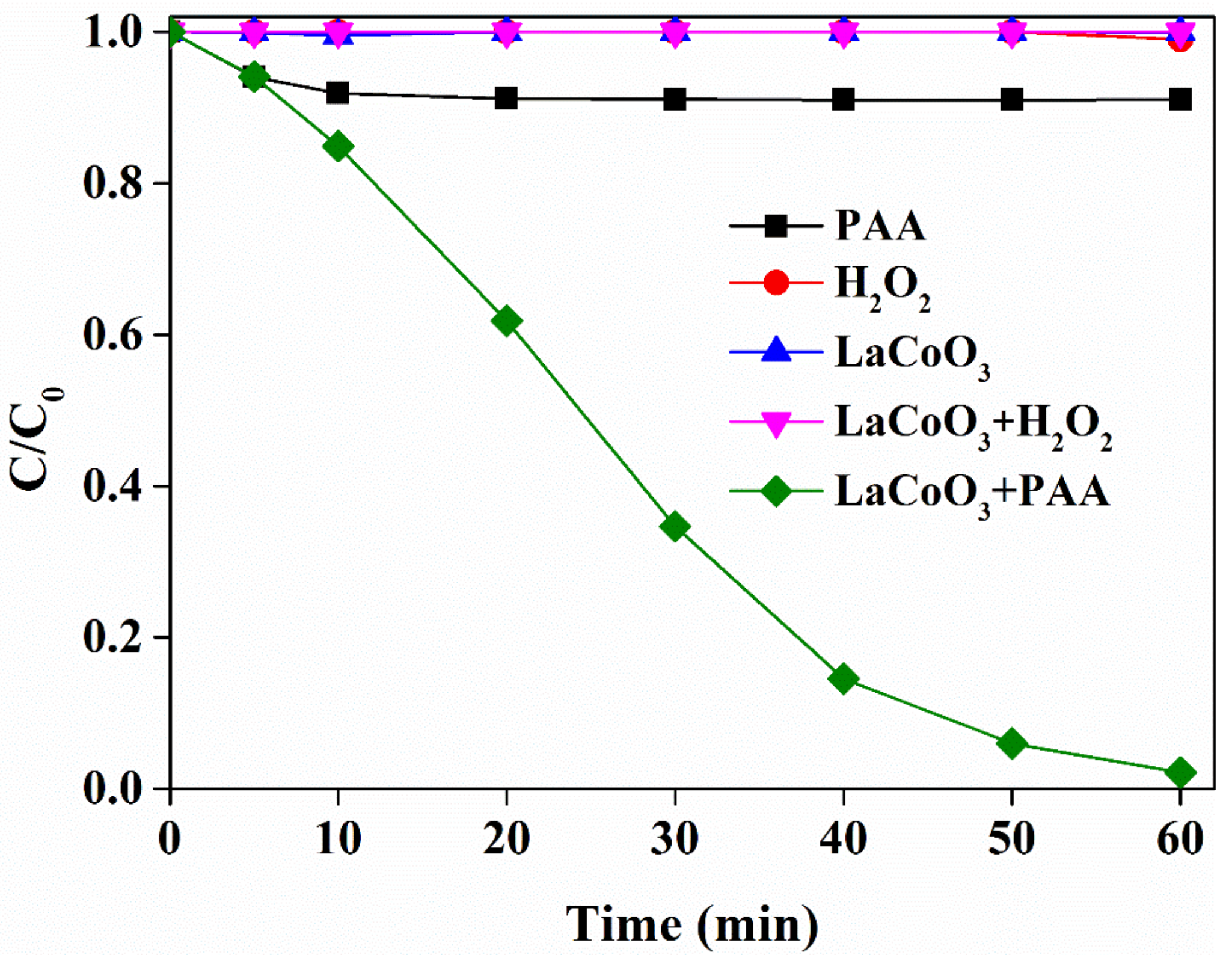
2.2. Degradation of SMX by PAA Activated with LaCoO3
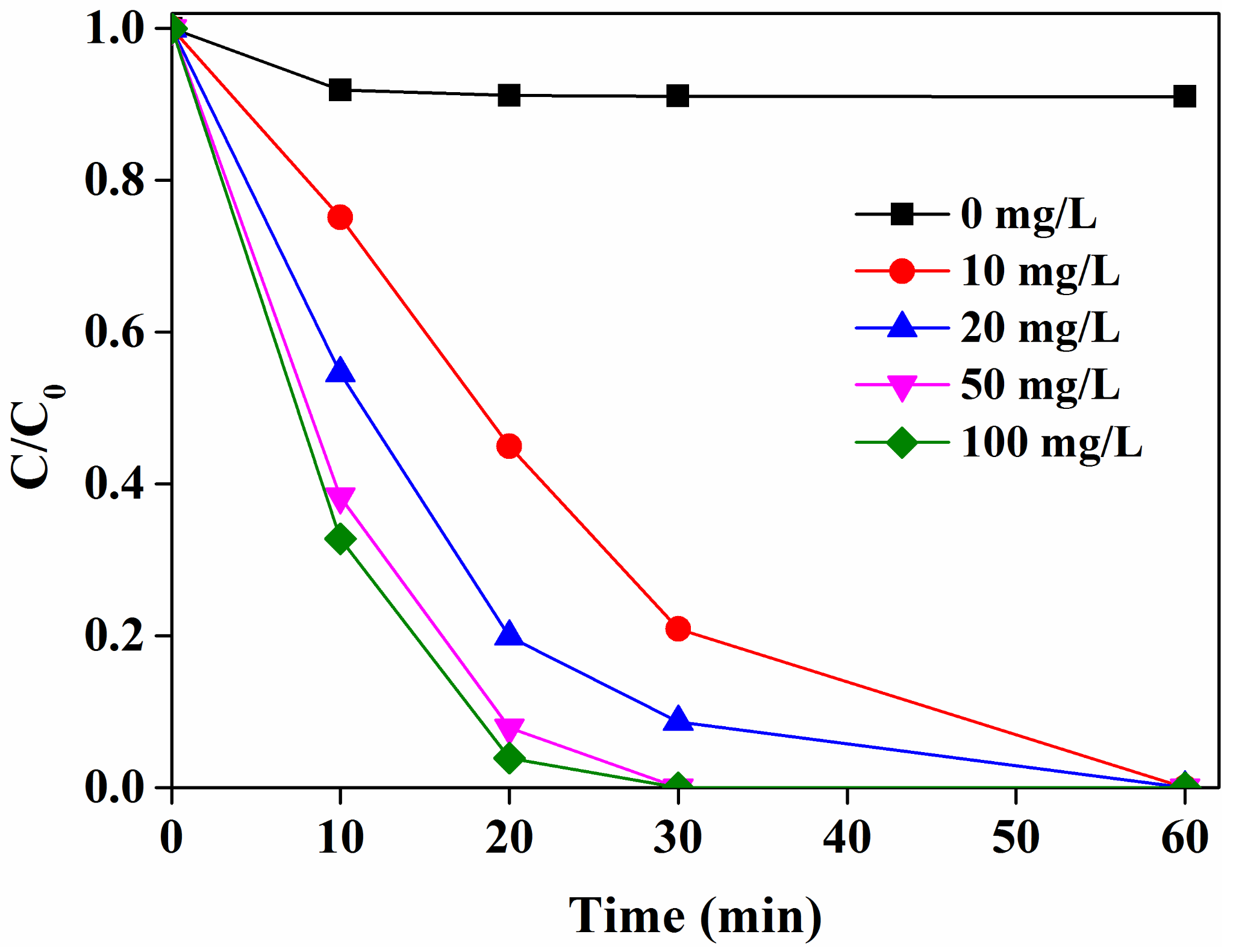
2.3. Effects of Catalyst Loadings
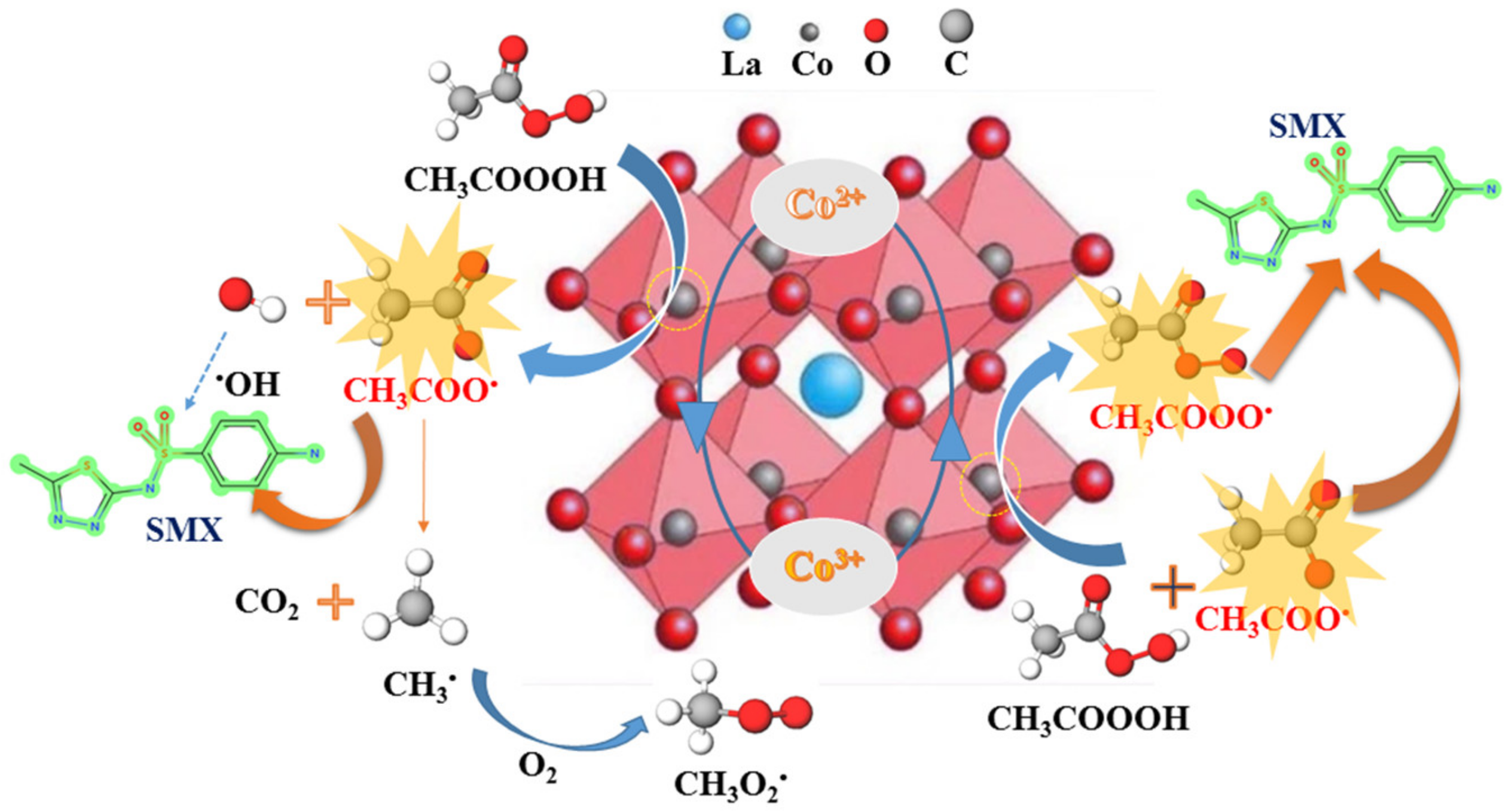
2.4. Activation Mechanism
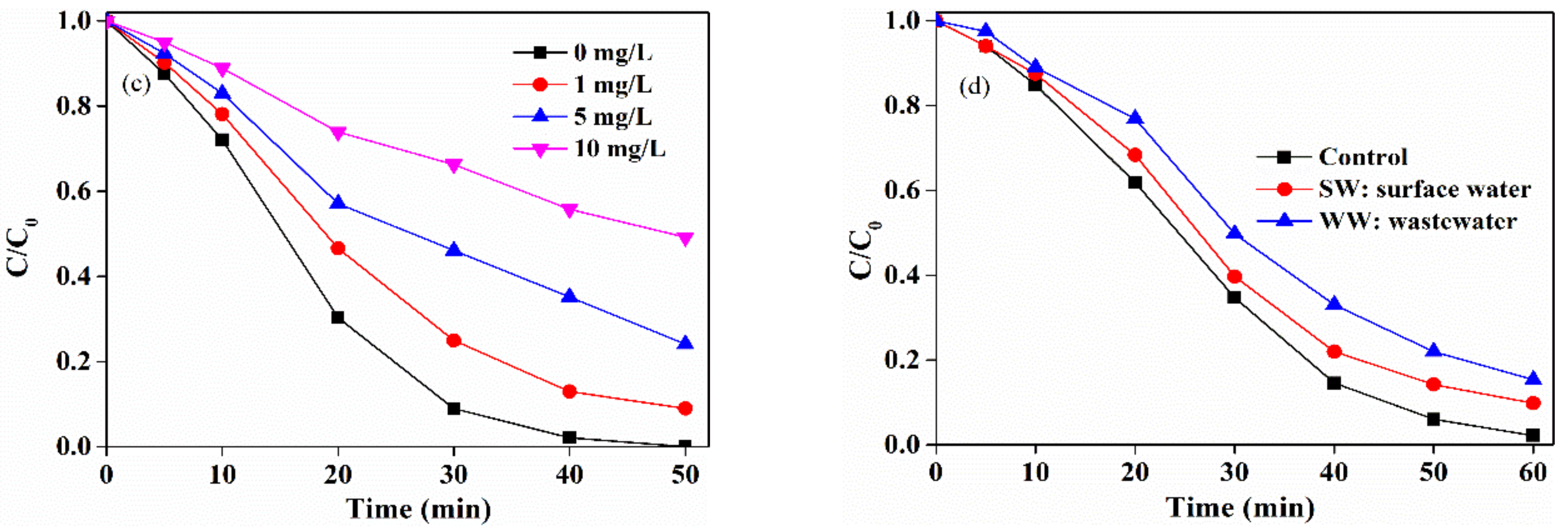
2.5. Effects of Water Matrices
2.6. Reusability of LaCoO3
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Preparation and Characterization of LaCoO3
3.3. Experimental Procedures
3.4. Analytical Methods
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Oturan, M.A.; Aaron, J.-J. Advanced Oxidation Processes in Water Wastewater Treatment Principles and Applications A Review. Crit. Rev. Environ. Sci. Tech. 2014, 44, 2577–2641. [Google Scholar] [CrossRef]
- Zhou, D.; Chen, L.; Li, J.; Wu, F. Transition metal catalyzed sulfite auto-oxidation systems for oxidative decontamination in waters: A state-of-the-art minireview. Chem. Eng. J. 2018, 346, 726–738. [Google Scholar] [CrossRef]
- Xie, P.; Ma, J.; Liu, W.; Zou, J.; Yue, S.; Li, X.; Wiesner, M.R.; Fang, J. Removal of 2-MIB and geosmin using UV/persulfate: Contributions of hydroxyl and sulfate radicals. Water Res. 2015, 69, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.; You, M.; Pan, F.; Liu, M.; Yang, P.; Xia, D.; Li, Q.; Wang, Y.; Fu, J. CuFe2O4@GO nanocomposite as an effective and recoverable catalyst of peroxymonosulfate activation for degradation of aqueous dye pollutants. Chin. Chem. Lett. 2019, 30, 2216–2220. [Google Scholar] [CrossRef]
- Ma, M.; Chen, L.; Zhao, J.; Liu, W.; Ji, H. Efficient activation of peroxymonosulfate by hollow cobalt hydroxide for degradation of ibuprofen and theoretical study. Chin. Chem. Lett. 2019, 30, 2191–2195. [Google Scholar] [CrossRef]
- Liu, T.; Yin, K.; Liu, C.; Luo, J.; Crittenden, J.; Zhang, W.; Luo, S.; He, Q.; Deng, Y.; Liu, H. The role of reactive oxygen species and carbonate radical in oxcarbazepine degradation via UV, UV/H2O2: Kinetics, mechanisms and toxicity evaluation. Water Res. 2018, 147, 204–213. [Google Scholar] [CrossRef]
- Glaze, W.H.; Kang, J.W.; Chapin, D.H. The Chemistry of Water Treatment Processes Involving Ozone, Hydrogen Peroxide and Ultraviolet Radiation. Ozone Sci. Eng. 1987, 9, 335–352. [Google Scholar] [CrossRef]
- Glaze, W.H.; Kang, J.W. Advanced oxidation processes. Description of a kinetic model for the oxidation of hazardous materials in aqueous media with ozone and hydrogen peroxide in a semibatch reactor. Ind. Eng. Chem. Res. 1989, 28, 1573–1580. [Google Scholar] [CrossRef]
- Duan, P.; Qi, Y.; Feng, S.; Peng, X.; Wang, W.; Yue, Y.; Shang, Y.; Li, Y.; Gao, B.; Xu, X. Enhanced degradation of clothianidin in peroxymonosulfate/catalyst system via core-shell FeMn @ N-C and phosphate surrounding. Appl. Catal. B Environ. 2020, 267, 118717. [Google Scholar] [CrossRef]
- Rehman, F.; Sayed, M.; Khan, J.A.; Shah, N.S.; Khan, H.M.; Dionysiou, D.D. Oxidative removal of brilliant green by UV/S2O82−, UV/HSO5− and UV/H2O2 processes in aqueous media: A comparative study. J. Hazard. Mater. 2018, 357, 506–514. [Google Scholar] [CrossRef]
- Oh, W.-D.; Dong, Z.; Lim, T.-T. Generation of sulfate radical through heterogeneous catalysis for organic contaminants removal: Current development, challenges and prospects. Appl. Catal. B Environ. 2016, 194, 169–201. [Google Scholar] [CrossRef]
- Haag, W.R.; Yao, C.C.D. Rate constants for reaction of hydroxyl radicals with several drinking water contaminants. Environ. Sci. Tech. 1992, 26, 1005–1013. [Google Scholar] [CrossRef]
- Chen, J.B.; Fang, C.; Xia, W.J.; Huang, T.Y.; Huang, C.H. Selective Transformation of β-Lactam Antibiotics by Peroxymonosulfate: Reaction Kinetics and Non-Radical Mechanism. Environ. Sci. 2018, 52, 1461–1470. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.Y.; Song, L. Degradation of tetracycline by peroxymonosulfate activated with zero-valent iron: Performance, intermediates, toxicity and mechanism. Chem. Eng. J. 2019, 364, 45–56. [Google Scholar] [CrossRef]
- Tsitonaki, A.; Petri, B.; Crimi, M.; Mosbk, H.; Siegrist, R.L.; Bjerg, P.L. In Situ Chemical Oxidation of Contaminated Soil and Groundwater Using Persulfate: A Review. Crit. Rev. Environ. Sci. Tech. 2010, 40, 55–91. [Google Scholar] [CrossRef]
- Kitis, M. Disinfection of wastewater with peracetic acid: A review. Environ. Int. 2004, 30, 47–55. [Google Scholar] [CrossRef]
- Chen, S.; Cai, M.Q.; Liu, Y.; Zhang, L.; Feng, L. Effects of water matrices on the degradation of naproxen by reactive radicals in the UV/peracetic acid process. Water Res. 2019, 150, 153–161. [Google Scholar] [CrossRef]
- Zhang, C.; Brown, P.J.B.; Hu, Z. Thermodynamic properties of an emerging chemical disinfectant, peracetic acid. Sci. Total. Environ. 2018, 621, 948–959. [Google Scholar] [CrossRef]
- Cai, M.; Sun, P.; Zhang, L.; Huang, C.H. UV/Peracetic Acid for Degradation of Pharmaceuticals and Reactive Species Evaluation. Environ Sci Technol. 2017, 51, 14217–14224. [Google Scholar] [CrossRef]
- Lee, W.N.; Huang, C.H. Formation of disinfection byproducts in wash water and lettuce by washing with sodium hypochlorite and peracetic acid sanitizers. Food Chem. X. 2019, 1, 100003. [Google Scholar] [CrossRef]
- Chhetri, R.K.; Baun, A.; Andersen, H.R. Acute toxicity and risk evaluation of the CSO disinfectants performic acid, peracetic acid, chlorine dioxide and their by-products hydrogen peroxide and chlorite. Sci. Total. Environ. 2019, 677, 1–8. [Google Scholar] [CrossRef]
- Shah, A.D.; Liu, Z.Q.; Salhi, E.; Höfer, T.; Werschkun, B.; von Gunten, U. Formation of disinfection by-products during ballast water treatment with ozone, chlorine, and peracetic acid: Influence of water quality parameters. Environ. Sci. Water Res. Tech. 2015, 1, 465–480. [Google Scholar]
- Zhao, X.; Zhang, T.; Zhou, Y.; Liu, D. Preparation of peracetic acid from hydrogen peroxide. J. Mol. Catal. A Chem. 2007, 271, 246–252. [Google Scholar] [CrossRef]
- Sun, P.Z.; Zhang, T.; Mejia-Tickner, B.; Zhang, R.; Cai, M.; Huang, C.H. Rapid Disinfection by Peracetic Acid Combined with UV Irradiation. Environ. Sci. Tech. Lett. 2018, 5, 400–404. [Google Scholar] [CrossRef]
- Du, P.; Liu, W.; Cao, H.; Zhao, H.; Huang, C.H. Oxidation of amino acids by peracetic acid: Reaction kinetics, pathways and theoretical calculations. Water Res X. 2018, 1, 100002. [Google Scholar] [CrossRef]
- Zhang, K.; Zhou, X.; Du, P.; Zhang, T.; Cai, M.; Sun, P.; Huang, C.H. Oxidation of beta-lactam antibiotics by peracetic acid: Reaction kinetics, product and pathway evaluation. Water Res. 2017, 123, 153–161. [Google Scholar] [CrossRef]
- Ina, E.V.R.; Makarova, K.; Golovina, E.A.; As, H.V.; Virkutyte, J. Free Radical Reaction Pathway, Thermochemistry of Peracetic Acid Homolysis, and Its Application for Phenol Degradation: Spectroscopic Study and Quantum Chemistry Calculations. Environ. Sci. Tech. 2010, 44, 6815–6821. [Google Scholar]
- Rothbart, S.; Ember, E.E.; Van, E.R. Mechanistic studies on the oxidative degradation of Orange II by peracetic acid catalyzed by simple manganese(ii) salts. Tuning the lifetime of the catalyst. New J. Chem. 2012, 36, 732–748. [Google Scholar] [CrossRef]
- Rokhina, E.V.; Makarova, K.; Lahtinen, M.; Golovina, E.A. Ultrasound-assisted MnO2 catalyzed homolysis of peracetic acid for phenol degradation: The assessment of process chemistry and kinetics. Chem. Eng. J. 2013, 221, 476–486. [Google Scholar] [CrossRef]
- Luukkonen, T.; Pehkonen, S.O. Peracids in water treatment: A critical review. Crit. Rev. Environ. Sci. Tech. 2016, 47, 1–39. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, J.; Xiong, B.; Bai, F.; Wang, S.; Wan, Y.; Zhang, L.; Xie, P.; Wiesner, M.R. Application of Cobalt/Peracetic Acid to Degrade Sulfamethoxazole at Neutral Condition: Efficiency and Mechanisms. Environ. Sci. Tech. 2020, 54, 464–475. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Mukhopadhyay, M.; Murthy, Z.V.P. Degradation of 4-Chlorophenol in Wastewater by Organic Oxidants. Ind.Eng.Chem.Res. 2010, 49, 3094–3098. [Google Scholar] [CrossRef]
- Zhou, F.; Lu, C.; Yao, Y.; Sun, L.; Gong, F.; Li, D.; Pei, K.; Lu, W.; Chen, W. Activated carbon fibers as an effective metal-free catalyst for peracetic acid activation: Implications for the removal of organic pollutants. Chem. Eng. J. 2015, 281, 953–960. [Google Scholar] [CrossRef]
- Kim, J.; Zhang, T.; Liu, W.; Du, P.; Dobson, J.T.; Huang, C.H. Advanced Oxidation Process with Peracetic Acid and Fe(II) for Contaminant Degradation. Environ. Sci. Technol. 2019, 53, 13312–13322. [Google Scholar] [CrossRef]
- Sun, H.Q.; Kwan, C.K.; Suvorova, A.; Ming, H.; Moses, A. Catalytic oxidation of organic pollutants on pristine and surface nitrogen-modified carbon nanotubes with sulfate radicals. Appl. Catal. B Environ. 2014, 154–155, 134–141. [Google Scholar] [CrossRef]
- Guo, H.C.; Zhou, X.F.; Zhang, Y.L.; Yao, Q.F.; Qian, Y.J.; Chu, H.Q.; Chen, J.B. Carbamazepine degradation by heterogeneous activation of peroxymonosulfate with lanthanum cobaltite perovskite: Performance, mechanism and toxicity. J. Environ. Sci. 2020, 91, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ren, J.; Wang, Y.; Zhang, F.; Liu, X.; Guo, Y.; Lu, G. Nanocasted Synthesis of Mesoporous LaCoO3 Perovskite with Extremely High Surface Area and Excellent Activity in Methane Combustion. J. Phys. Chem. C. 2008, 112, 15293–15298. [Google Scholar] [CrossRef]
- Dacquin, J.P.; Dujardin, C.; Granger, P. Surface reconstruction of supported Pd on LaCoO3: Consequences on the catalytic properties in the decomposition of N2O. J. Catal. 2008, 253, 37–49. [Google Scholar] [CrossRef]
- Pang, X.; Guo, Y.; Zhang, Y.; Xu, B.; Qi, F. LaCoO3 perovskite oxide activation of peroxymonosulfate for aqueous 2-phenyl-5-sulfobenzimidazole degradation: Effect of synthetic method and the reaction mechanism. Chem. Eng. J. 2016, 304, 897–907. [Google Scholar] [CrossRef]
- Taran, O.P.; Ayusheev, A.B.; Ogorodnikova, O.L.; Prosvirin, I.P.; Isupova, L.A.; Parmon, V.N. Perovskite-like catalysts LaBO3 (B = Cu, Fe, Mn, Co, Ni) for wet peroxide oxidation of phenol. Appl. Catal. B Environ. 2016, 180, 86–93. [Google Scholar] [CrossRef]
- Li, C.X.; Wu, J.E.; Peng, W.; Fang, Z.F.; Liu, J. Peroxymonosulfate activation for efficient sulfamethoxazole degradation by Fe3O4/β-FeOOH nanocomposites: Coexistence of radical and non-radical reactions. Chem. Eng. J. 2019, 365, 904–914. [Google Scholar] [CrossRef]
- Wang, S.Z.; Xu, L.J.; Wang, J.L. Nitrogen-doped graphene as peroxymonosulfate activator and electron transfer mediator for the enhanced degradation of sulfamethoxazole. Chem. Eng. J. 2019, 375, 122041. [Google Scholar] [CrossRef]
- Gkika, C.; Petala, A.; Frontistis, Z. Heterogeneous activation of persulfate by lanthanum strontium cobaltite for sulfamethoxazole degradation. Catal. Today. 2020. [Google Scholar] [CrossRef]
- Oh, W.D.; Chang, V.W.C.; Lim, T.T. A comprehensive performance evaluation of heterogeneous bi2fe4o9/peroxymonosulfate system for sulfamethoxazole degradation. Environ. Sci. Pollut. Res. 2017, 26, 1026–1035. [Google Scholar] [CrossRef] [PubMed]
- Lalas, K.; Petala, A.; Frontistis, Z. Sulfamethoxazole degradation by the CuOx/persulfate system. Catal. Today. 2020. [Google Scholar] [CrossRef]
- Yan, J.F.; Li, J.; Peng, J.L. Efficient degradation of sulfamethoxazole by the CuO@Al2O3 (EPC) coupled PMS system: Optimization, degradation pathways and toxicity evaluation. Chem. Eng. J. 2019, 359, 1097–1110. [Google Scholar] [CrossRef]
- Hammouda, H.; Kalliola, S.; Sillanpaa, S.; Safaei, M.; Zhao, Z. Degradation and mineralization of phenol in aqueous medium by heterogeneous monopersulfate activation on nanostructured cobalt based-perovskite catalysts ACoO(3) (A = La, Ba, Sr and Ce): Characterization, kinetics and mechanism study. Appl. Catal. B Environ. 2017, 215, 60–73. [Google Scholar] [CrossRef]
- Duan, X.G.; Su, C.; Miao, J.; Zhong, Y.; Shao, Z.; Wang, S.; Sun, H. Insights into perovskite-catalyzed peroxymonosulfate activation: Maneuverable cobalt sites for promoted evolution of sulfate radicals. Appl. Catal. B Environ. 2018, 220, 626–634. [Google Scholar] [CrossRef]
- Hill, R.T. Decomposition of peracetic acid catalyzed by cobalt(II) and vanadium(V). Can. J. Chem. 1998, 76, 1064–1069. [Google Scholar]
- Kim, J.; Du, P.; Liu, W.; Luo, C.; Zhao, H.; Huang, C.H. Cobalt/Peracetic Acid: Advanced Oxidation of Aromatic Organic Compounds by Acetylperoxyl Radicals. Environ. Sci. Technol. 2020, 54, 5268–5278. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Moores, A.; Ghoshal, S. Transformation of Fluorotelomer Sulfonate by Cobalt(II)-Activated Peroxymonosulfate. Environ. Sci. Technol. 2020, 54, 4631–4640. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the lanthanum cobaltite perovskite are available from the authors. |




| Sample | TN (mg/L) | TP (mg/L) | pH | UV254 | TOC (mg/L) | Cl− (mg/L) |
|---|---|---|---|---|---|---|
| SW | 0.81 | 0.07 | 7.8 | 0.13 | 3.1 | 32.6 |
| WW | 11.1 | 0.84 | 7.1 | 0.41 | 10.6 | 92.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Wu, H.; Zhang, L.; Liang, B.; Sun, X.; Chen, J. Activation of Peracetic Acid with Lanthanum Cobaltite Perovskite for Sulfamethoxazole Degradation under a Neutral pH: The Contribution of Organic Radicals. Molecules 2020, 25, 2725. https://doi.org/10.3390/molecules25122725
Zhou X, Wu H, Zhang L, Liang B, Sun X, Chen J. Activation of Peracetic Acid with Lanthanum Cobaltite Perovskite for Sulfamethoxazole Degradation under a Neutral pH: The Contribution of Organic Radicals. Molecules. 2020; 25(12):2725. https://doi.org/10.3390/molecules25122725
Chicago/Turabian StyleZhou, Xuefei, Haowei Wu, Longlong Zhang, Bowen Liang, Xiaoqi Sun, and Jiabin Chen. 2020. "Activation of Peracetic Acid with Lanthanum Cobaltite Perovskite for Sulfamethoxazole Degradation under a Neutral pH: The Contribution of Organic Radicals" Molecules 25, no. 12: 2725. https://doi.org/10.3390/molecules25122725
APA StyleZhou, X., Wu, H., Zhang, L., Liang, B., Sun, X., & Chen, J. (2020). Activation of Peracetic Acid with Lanthanum Cobaltite Perovskite for Sulfamethoxazole Degradation under a Neutral pH: The Contribution of Organic Radicals. Molecules, 25(12), 2725. https://doi.org/10.3390/molecules25122725





