Drug-Drug Interactions of Irinotecan, 5-Fluorouracil, Folinic Acid and Oxaliplatin and Its Activity in Colorectal Carcinoma Treatment
Abstract
1. Introduction
2. Results
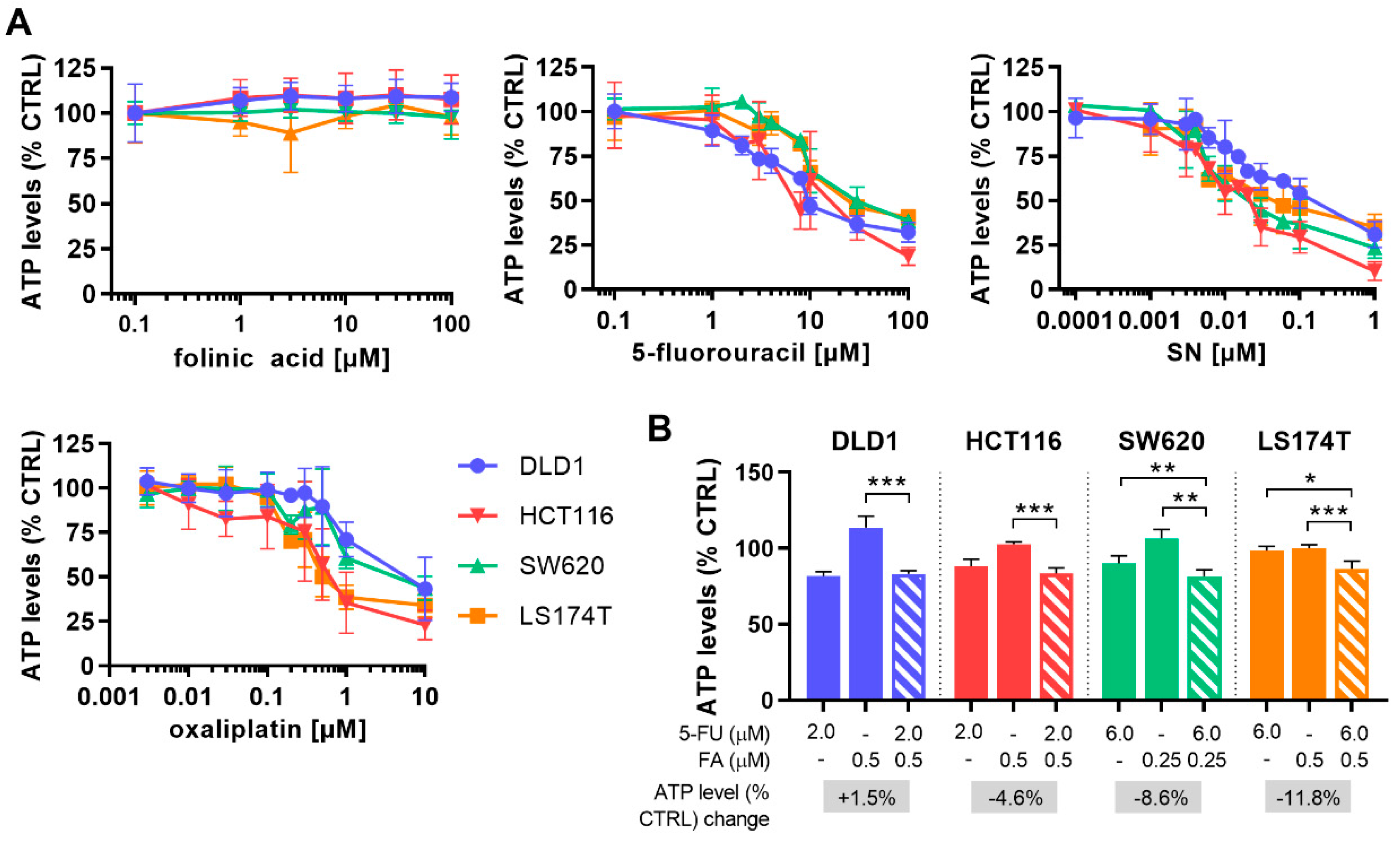
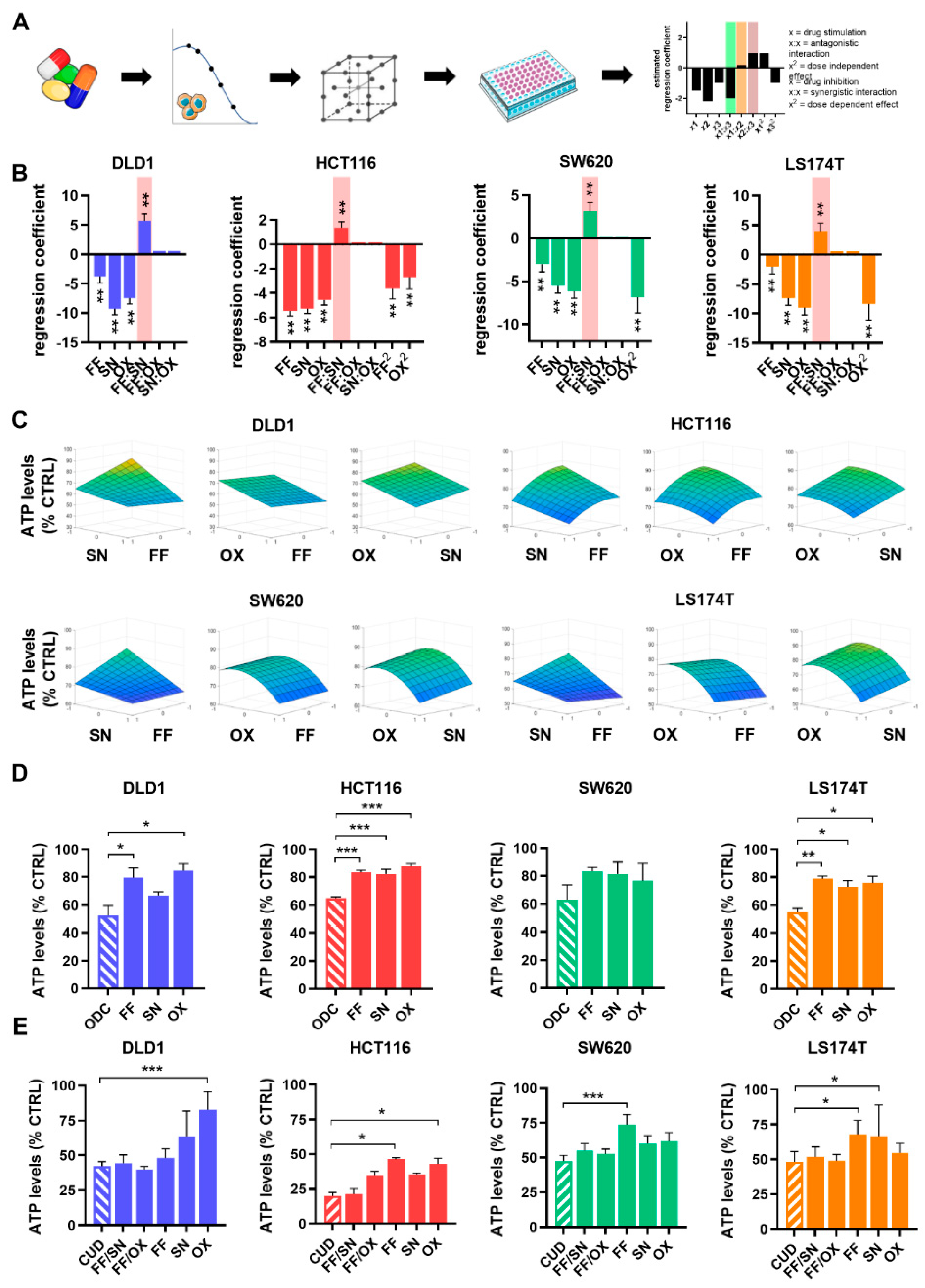
2.1. Optimization of Cell Line-Specific Low-Dose Drug Combinations
2.2. The Search for Optimal Drug Administration Sequence
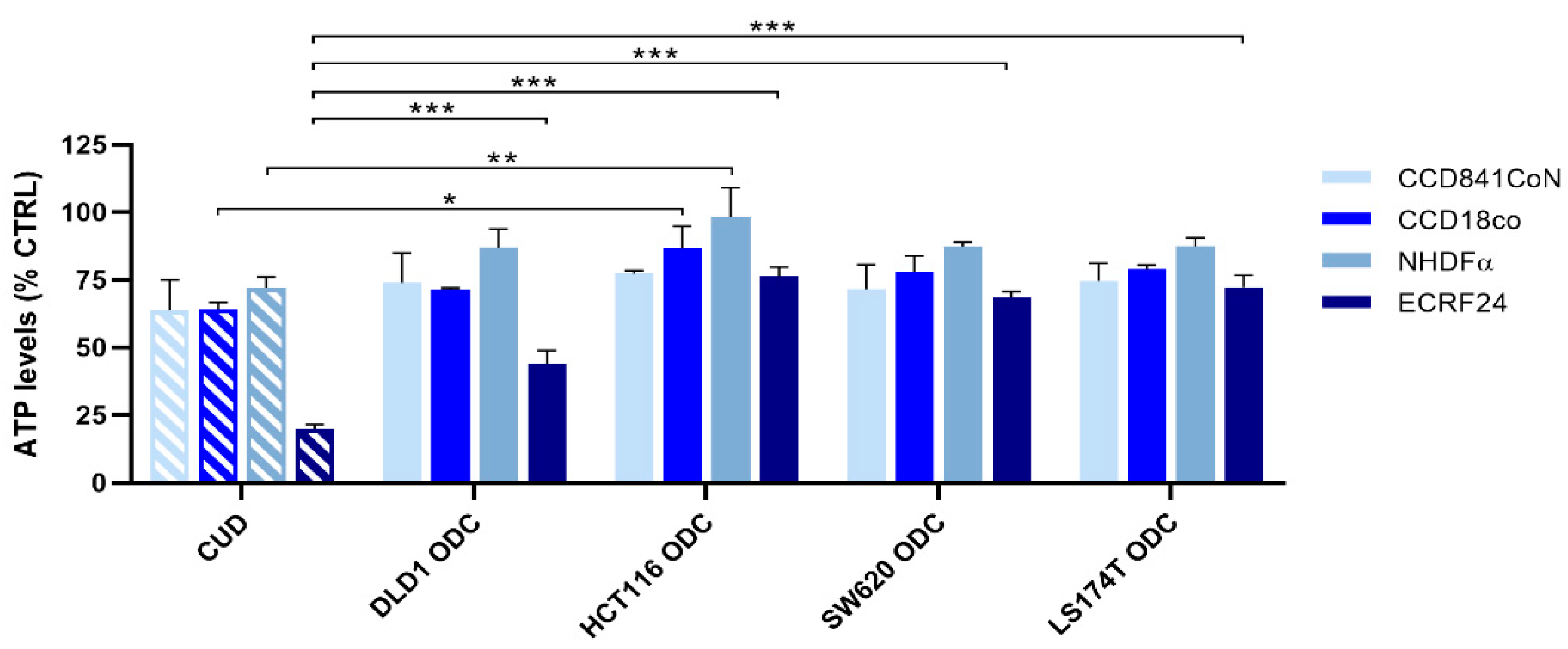
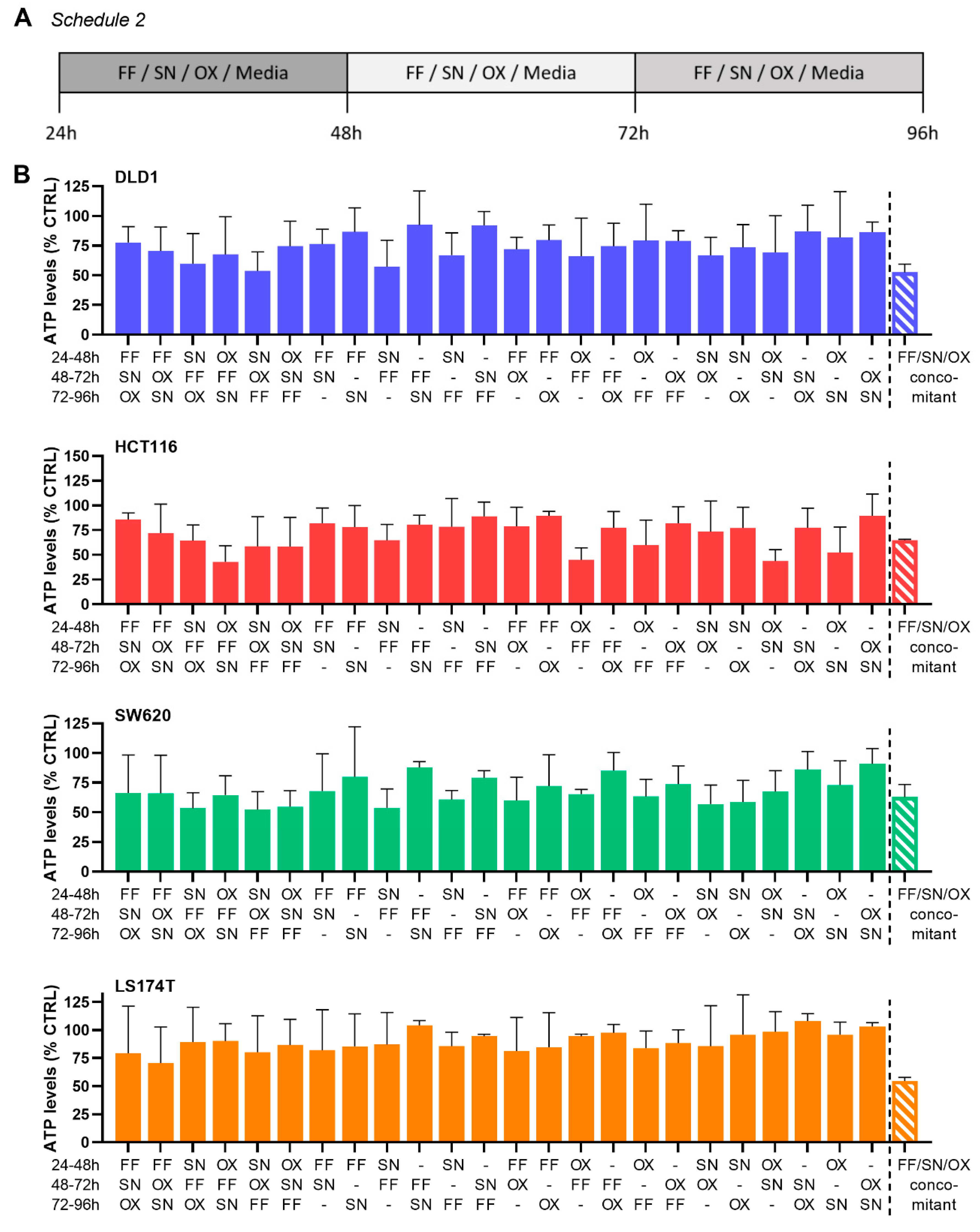
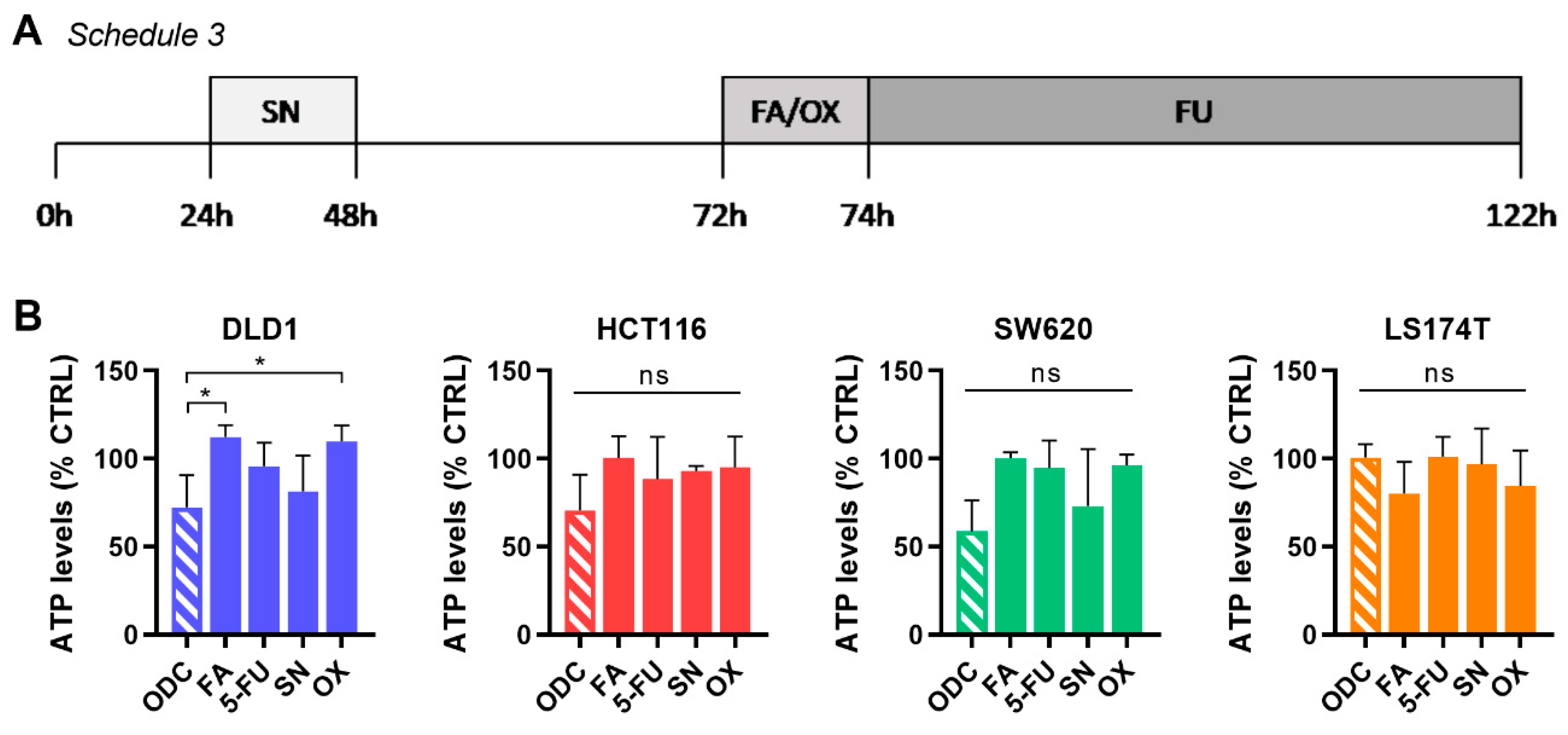
2.3. Integration of Cell Line-Specific ODCs in Clinically Used Drug Administration Schedules
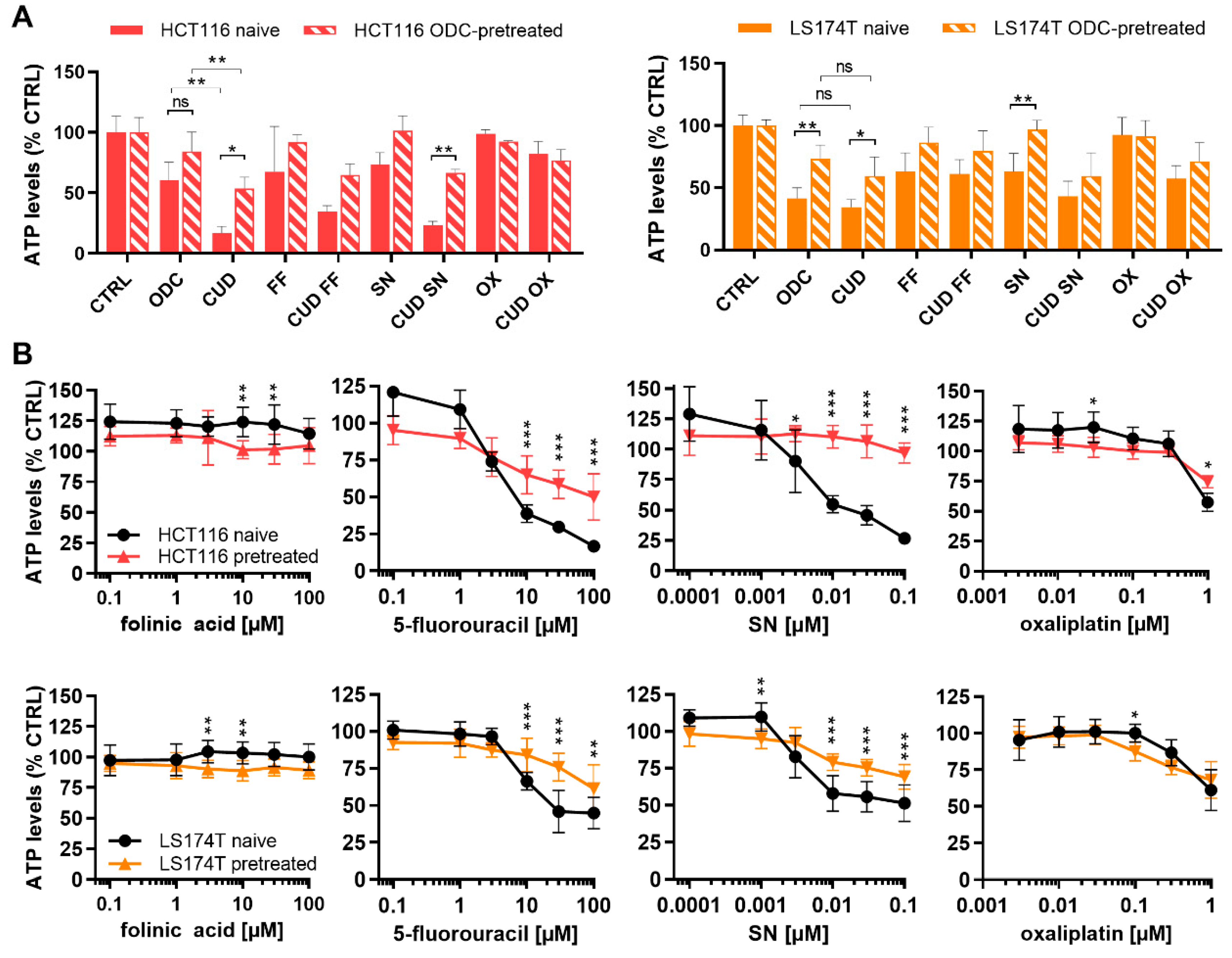
2.4. Chronically Pretreated Colorectal Carcinoma Cells Lose Sensitivity to FA/5-FU/OX/SN Combinations
3. Discussion
4. Materials and Methods
4.1. Cells and Cell Culture Conditions
4.2. Drugs, Treatments and Metabolic Activity Assay
4.3. Orthogonal Array Composite Design and Linear Regression Model
4.4. Calculation of Clinically Used Doses (CUD)
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Torre, L.A.; Soerjomataram, I.; Hayes, R.B.; Bray, F.; Weber, T.K.; Jemal, A. Global patterns and trends in colorectal cancer incidence in young adults. Gut 2019, 68, 2179–2185. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Guinney, J.; Dienstmann, R.; Wang, X.; de Reynies, A.; Schlicker, A.; Soneson, C.; Marisa, L.; Roepman, P.; Nyamundanda, G.; Angelino, P.; et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 2015, 21, 1350–1356. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Cervantes, A.; Adam, R.; Sobrero, A.; Van Krieken, J.H.; Aderka, D.; Aranda Aguilar, E.; Bardelli, A.; Benson, A.; Bodoky, G.; et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 2016, 27, 1386–1422. [Google Scholar] [CrossRef]
- Stintzing, S. Management of colorectal cancer. F1000Prime Rep. 2014, 6, 108. [Google Scholar] [CrossRef]
- Poston, G.J.; Tait, D.; O’Connell, S.; Bennett, A.; Berendse, S.; Guideline Development, G. Diagnosis and management of colorectal cancer: Summary of NICE guidance. BMJ 2011, 343, d6751. [Google Scholar] [CrossRef]
- McQuade, R.M.; Stojanovska, V.; Bornstein, J.C.; Nurgali, K. Colorectal Cancer Chemotherapy: The Evolution of Treatment and New Approaches. Curr. Med. Chem. 2017, 24, 1537–1557. [Google Scholar] [CrossRef]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-Fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef]
- Machover, D.; Goldschmidt, E.; Chollet, P.; Metzger, G.; Zittoun, J.; Marquet, J.; Vandenbulcke, J.M.; Misset, J.L.; Schwarzenberg, L.; Fourtillan, J.B.; et al. Treatment of advanced colorectal and gastric adenocarcinomas with 5-fluorouracil and high-dose folinic acid. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1986, 4, 685–696. [Google Scholar] [CrossRef] [PubMed]
- Mini, E.; Trave, F.; Rustum, Y.M.; Bertino, J.R. Enhancement of the antitumor effects of 5-fluorouracil by folinic acid. Pharmacol. Ther. 1990, 47, 1–19. [Google Scholar] [CrossRef]
- Martinez-Balibrea, E.; Martínez-Cardús, A.; Ginés, A.; de Porras, V.R.; Moutinho, C.; Layos, L.; Manzano, J.L.; Bugés, C.; Bystrup, S.; Esteller, M.; et al. Tumor-Related Molecular Mechanisms of Oxaliplatin Resistance. Mol. Cancer Ther. 2015, 14, 1767. [Google Scholar] [CrossRef]
- Voigt, W.; Matsui, S.; Yin, M.B.; Burhans, W.C.; Minderman, H.; Rustum, Y.M. Topoisomerase-I inhibitor SN-38 can induce DNA damage and chromosomal aberrations independent from DNA synthesis. Anticancer Res. 1998, 18, 3499–3505. [Google Scholar]
- Dai, J.; Chen, Y.; Gong, Y.; Wei, J.; Cui, X.; Yu, H.; Zhao, W.; Gu, D.; Chen, J. The efficacy and safety of irinotecan ± bevacizumab compared with oxaliplatin ± bevacizumab for metastatic colorectal cancer: A meta-analysis. Medicine (Baltimore) 2019, 98, e17384. [Google Scholar] [CrossRef]
- Ychou, M.; Rivoire, M.; Thezenas, S.; Quenet, F.; Delpero, J.R.; Rebischung, C.; Letoublon, C.; Guimbaud, R.; Francois, E.; Ducreux, M.; et al. A randomized phase II trial of three intensified chemotherapy regimens in first-line treatment of colorectal cancer patients with initially unresectable or not optimally resectable liver metastases. The METHEP trial. Ann. Surg. Oncol. 2013, 20, 4289–4297. [Google Scholar] [CrossRef]
- Kaczirek, K. ASCO 2016—Update colorectal liver metastases. Memo 2017, 10, 103–105. [Google Scholar] [CrossRef]
- Bachet, J.B.; Lucidarme, O.; Levache, C.B.; Barbier, E.; Raoul, J.L.; Lecomte, T.; Desauw, C.; Brocard, F.; Pernot, S.; Breysacher, G.; et al. FOLFIRINOX as induction treatment in rectal cancer patients with synchronous metastases: Results of the FFCD 1102 phase II trial. Eur. J. Cancer 2018, 104, 108–116. [Google Scholar] [CrossRef]
- Falcone, A.; Ricci, S.; Brunetti, I.; Pfanner, E.; Allegrini, G.; Barbara, C.; Crinò, L.; Benedetti, G.; Evangelista, W.; Fanchini, L.; et al. Phase III Trial of Infusional Fluorouracil, Leucovorin, Oxaliplatin, and Irinotecan (FOLFOXIRI) Compared With Infusional Fluorouracil, Leucovorin, and Irinotecan (FOLFIRI) As First-Line Treatment for Metastatic Colorectal Cancer: The Gruppo Oncologico Nord Ovest. J. Clin. Oncol. 2007, 25, 1670–1676. [Google Scholar] [CrossRef]
- Sunakawa, Y.; Schirripa, M.; Lenz, H.-J. Understanding the FOLFOXIRI-regimen to optimize treatment for metastatic colorectal cancer. Crit. Rev. Oncol. Hematol. 2016, 100, 117–126. [Google Scholar] [CrossRef]
- Masi, G.; Vasile, E.; Loupakis, F.; Cupini, S.; Fornaro, L.; Baldi, G.; Salvatore, L.; Cremolini, C.; Stasi, I.; Brunetti, I.; et al. Randomized trial of two induction chemotherapy regimens in metastatic colorectal cancer: An updated analysis. J. Natl. Cancer Inst. 2011, 103, 21–30. [Google Scholar] [CrossRef]
- Masi, G.; Cupini, S.; Marcucci, L.; Cerri, E.; Loupakis, F.; Allegrini, G.; Brunetti, I.M.; Pfanner, E.; Viti, M.; Goletti, O.; et al. Treatment with 5-fluorouracil/folinic acid, oxaliplatin, and irinotecan enables surgical resection of metastases in patients with initially unresectable metastatic colorectal cancer. Ann. Surg. Oncol. 2006, 13, 58–65. [Google Scholar] [CrossRef]
- Masi, G.; Loupakis, F.; Pollina, L.; Vasile, E.; Cupini, S.; Ricci, S.; Brunetti, I.M.; Ferraldeschi, R.; Naso, G.; Filipponi, F.; et al. Long-term outcome of initially unresectable metastatic colorectal cancer patients treated with 5-fluorouracil/leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) followed by radical surgery of metastases. Ann. Surg. 2009, 249, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Souglakos, J.; Androulakis, N.; Syrigos, K.; Polyzos, A.; Ziras, N.; Athanasiadis, A.; Kakolyris, S.; Tsousis, S.; Kouroussis, C.; Vamvakas, L.; et al. FOLFOXIRI (folinic acid, 5-fluorouracil, oxaliplatin and irinotecan) vs FOLFIRI (folinic acid, 5-fluorouracil and irinotecan) as first-line treatment in metastatic colorectal cancer (MCC): A multicentre randomised phase III trial from the Hellenic Oncology Research Group (HORG). Br. J. Cancer 2006, 94, 798–805. [Google Scholar] [CrossRef] [PubMed]
- Guion-Dusserre, J.-F.; Bertaut, A.; Ghiringhelli, F.; Vincent, J.; Quipourt, V.; Marilier, S.; Tharin, Z.; Bengrine-Lefevre, L. Folfirinox in elderly patients with pancreatic or colorectal cancer-tolerance and efficacy. World J. Gastroenterol. 2016, 22, 9378–9386. [Google Scholar] [CrossRef] [PubMed]
- Simkens, L.H.; van Tinteren, H.; May, A.; ten Tije, A.J.; Creemers, G.J.; Loosveld, O.J.; de Jongh, F.E.; Erdkamp, F.L.; Erjavec, Z.; van der Torren, A.M.; et al. Maintenance treatment with capecitabine and bevacizumab in metastatic colorectal cancer (CAIRO3): A phase 3 randomised controlled trial of the Dutch Colorectal Cancer Group. Lancet 2015, 385, 1843–1852. [Google Scholar] [CrossRef]
- Koeberle, D.; Betticher, D.C.; von Moos, R.; Dietrich, D.; Brauchli, P.; Baertschi, D.; Matter, K.; Winterhalder, R.; Borner, M.; Anchisi, S.; et al. Bevacizumab continuation versus no continuation after first-line chemotherapy plus bevacizumab in patients with metastatic colorectal cancer: A randomized phase III non-inferiority trial (SAKK 41/06). Ann. Oncol. 2015, 26, 709–714. [Google Scholar] [CrossRef]
- Braun, M.S.; Seymour, M.T. Balancing the efficacy and toxicity of chemotherapy in colorectal cancer. Ther. Adv. Med. Oncol. 2011, 3, 43–52. [Google Scholar] [CrossRef]
- Leal, F.; Ferreira, F.P.; Sasse, A.D. FOLFOXIRI Regimen for Metastatic Colorectal Cancer: A Systematic Review and Meta-Analysis. Clin. Color. Cancer 2017, 16, 405–409. [Google Scholar] [CrossRef]
- Weiss, A.; Berndsen, R.H.; Ding, X.; Ho, C.M.; Dyson, P.J.; van den Bergh, H.; Griffioen, A.W.; Nowak-Sliwinska, P. A streamlined search technology for identification of synergistic drug combinations. Sci. Rep. 2015, 5, 14508. [Google Scholar] [CrossRef]
- Berndsen, R.H.; Swier, N.; van Beijnum, J.R.; Nowak-Sliwinska, P. Colorectal Cancer Growth Retardation through Induction of Apoptosis, Using an Optimized Synergistic Cocktail of Axitinib, Erlotinib, and Dasatinib. Cancers 2019, 11, 1878. [Google Scholar] [CrossRef] [PubMed]
- Weiss, A.; Le Roux-Bourdieu, M.; Zoetemelk, M.; Ramzy, G.M.; Rausch, M.; Harry, D.; Miljkovic-Licina, M.; Falamaki, K.; Wehrle-Haller, B.; Meraldi, P.; et al. Identification of a Synergistic Multi-Drug Combination Active in Cancer Cells via the Prevention of Spindle Pole Clustering. Cancers 2019, 11, 1612. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, D.; Eide, P.W.; Eilertsen, I.A.; Danielsen, S.A.; Eknaes, M.; Hektoen, M.; Lind, G.E.; Lothe, R.A. Epigenetic and genetic features of 24 colon cancer cell lines. Oncogenesis 2013, 2, e71. [Google Scholar] [CrossRef]
- Chen, T.R.; Dorotinsky, C.S.; McGuire, L.J.; Macy, M.L.; Hay, R.J. DLD-1 and HCT-15 cell lines derived separately from colorectal carcinomas have totally different chromosome changes but the same genetic origin. Cancer Genet. Cytogenet. 1995, 81, 103–108. [Google Scholar] [CrossRef]
- Berg, K.C.G.; Eide, P.W.; Eilertsen, I.A.; Johannessen, B.; Bruun, J.; Danielsen, S.A.; Bjørnslett, M.; Meza-Zepeda, L.A.; Eknæs, M.; Lind, G.E.; et al. Multi-omics of 34 colorectal cancer cell lines—A resource for biomedical studies. Mol. Cancer 2017, 16, 116. [Google Scholar] [CrossRef]
- Tom, B.H.; Rutzky, L.P.; Jakstys, M.M.; Oyasu, R.; Kaye, C.I.; Kahan, B.D. Human colonic adenocarcinoma cells. I. Establishment and description of a new line. In Vitro 1976, 12, 180–191. [Google Scholar] [CrossRef]
- Brattain, M.G.; Fine, W.D.; Khaled, F.M.; Thompson, J.; Brattain, D.E. Heterogeneity of malignant cells from a human colonic carcinoma. Cancer Res. 1981, 41, 1751–1756. [Google Scholar]
- Al-Shyoukh, I.; Yu, F.; Feng, J.; Yan, K.; Dubinett, S.; Ho, C.M.; Shamma, J.S.; Sun, R. Systematic quantitative characterization of cellular responses induced by multiple signals. BMC Syst. Biol. 2011, 5, 88. [Google Scholar] [CrossRef]
- Ding, X.; Liu, W.; Weiss, A.; Li, Y.; Wong, I.; Griffioen, A.W.; van den Bergh, H.; Xu, H.; Nowak-Sliwinska, P.; Ho, C.M. Discovery of a low order drug-cell response surface for applications in personalized medicine. Phys. Biol. 2014, 11, 065003. [Google Scholar] [CrossRef]
- Nowak-Sliwinska, P.; Weiss, A.; Ding, X.; Dyson, P.J.; van den Bergh, H.; Griffioen, A.W.; Ho, C.M. Optimization of drug combinations using Feedback System Control. Nat. Protoc. 2016, 11, 302–315. [Google Scholar] [CrossRef]
- Edwards, M.S.; Chadda, S.D.; Zhao, Z.; Barber, B.L.; Sykes, D.P. A systematic review of treatment guidelines for metastatic colorectal cancer. Colorectal Dis. 2012, 14, e31–e47. [Google Scholar] [CrossRef] [PubMed]
- Fischel, J.L.; Rostagno, P.; Formento, P.; Dubreuil, A.; Etienne, M.C.; Milano, G. Ternary combination of irinotecan, fluorouracil-folinic acid and oxaliplatin: Results on human colon cancer cell lines. Br. J. Cancer 2001, 84, 579–585. [Google Scholar] [CrossRef][Green Version]
- Hammond, W.A.; Swaika, A.; Mody, K. Pharmacologic resistance in colorectal cancer: A review. Ther. Adv. Med. Oncol. 2016, 8, 57–84. [Google Scholar] [CrossRef]
- Mans, D.R.; Grivicich, I.; Peters, G.J.; Schwartsmann, G. Sequence-dependent growth inhibition and DNA damage formation by the irinotecan-5-fluorouracil combination in human colon carcinoma cell lines. Eur. J. Cancer 1999, 35, 1851–1861. [Google Scholar] [CrossRef]
- Guichard, S.; Cussac, D.; Hennebelle, I.; Bugat, R.; Canal, P. Sequence-dependent activity of the irinotecan-5FU combination in human colon-cancer model HT-29 in vitro and in vivo. Int. J. Cancer 1997, 73, 729–734. [Google Scholar] [CrossRef]
- Shibayama, Y.; Iwashita, Y.; Yoshikawa, Y.; Kondo, T.; Ikeda, R.; Takeda, Y.; Osada, T.; Sugawara, M.; Yamada, K.; Iseki, K. Effect of 5-fluorouracil treatment on SN-38 absorption from intestine in rats. Biol. Pharm. Bull. 2011, 34, 1418–1425. [Google Scholar] [CrossRef] [PubMed]
- Falcone, A.; Di Paolo, A.; Masi, G.; Allegrini, G.; Danesi, R.; Lencioni, M.; Pfanner, E.; Comis, S.; Del Tacca, M.; Conte, P. Sequence effect of irinotecan and fluorouracil treatment on pharmacokinetics and toxicity in chemotherapy-naive metastatic colorectal cancer patients. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2001, 19, 3456–3462. [Google Scholar] [CrossRef]
- Violette, S.; Poulain, L.; Dussaulx, E.; Pepin, D.; Faussat, A.M.; Chambaz, J.; Lacorte, J.M.; Staedel, C.; Lesuffleur, T. Resistance of colon cancer cells to long-term 5-fluorouracil exposure is correlated to the relative level of Bcl-2 and Bcl-X(L) in addition to Bax and p53 status. Int. J. Cancer 2002, 98, 498–504. [Google Scholar] [CrossRef]
- Shi, X.; Liu, S.; Kleeff, J.; Friess, H.; Buchler, M.W. Acquired resistance of pancreatic cancer cells towards 5-Fluorouracil and gemcitabine is associated with altered expression of apoptosis-regulating genes. Oncology 2002, 62, 354–362. [Google Scholar] [CrossRef]
- Piret, B.; Piette, J. Topoisomerase poisons activate the transcription factor NF-kappaB in ACH-2 and CEM cells. Nucleic Acids Res. 1996, 24, 4242–4248. [Google Scholar] [CrossRef]
- Xu, Y.; Villalona-Calero, M.A. Irinotecan: Mechanisms of tumor resistance and novel strategies for modulating its activity. Ann. Oncol. 2002, 13, 1841–1851. [Google Scholar] [CrossRef] [PubMed]
- van Triest, B.; Pinedo, H.M.; van Hensbergen, Y.; Smid, K.; Telleman, F.; Schoenmakers, P.S.; van der Wilt, C.L.; van Laar, J.A.; Noordhuis, P.; Jansen, G.; et al. Thymidylate synthase level as the main predictive parameter for sensitivity to 5-fluorouracil, but not for folate-based thymidylate synthase inhibitors, in 13 nonselected colon cancer cell lines. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 1999, 5, 643–654. [Google Scholar]
- Rose, M.G.; Farrell, M.P.; Schmitz, J.C. Thymidylate synthase: A critical target for cancer chemotherapy. Clin. Colorectal Cancer 2002, 1, 220–229. [Google Scholar] [CrossRef]
- Lu, K.; McGuire, J.J.; Slocum, H.K.; Rustum, Y.M. Mechanisms of acquired resistance to modulation of 5-fluorouracil by leucovorin in HCT-8 human ileocecal carcinoma cells. Biochem. Pharmacol. 1997, 53, 689–696. [Google Scholar] [CrossRef]
- Spears, C.P.; Gustavsson, B.G.; Berne, M.; Frosing, R.; Bernstein, L.; Hayes, A.A. Mechanisms of innate resistance to thymidylate synthase inhibition after 5-fluorouracil. Cancer Res. 1988, 48, 5894–5900. [Google Scholar]
- Conroy, T.; Hammel, P.; Hebbar, M.; Ben Abdelghani, M.; Wei, A.C.; Raoul, J.L.; Chone, L.; Francois, E.; Artru, P.; Biagi, J.J.; et al. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N. Engl. J. Med. 2018, 379, 2395–2406. [Google Scholar] [CrossRef] [PubMed]
- Tumor Subtypes in Subjects on FOLFIRINOX With Non-Metastatic Pancreatic Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT03977233 (accessed on 7 December 2019).
- Hara, M.H.; Reilly, E.M.; Rosemarie, M.; Varadhachary, G.; Wainberg, Z.A.; Ko, A.; Fisher, G.A.; Rahma, O.; Lyman, J.P.; Cabanski, C.R.; et al. Abstract CT004: A Phase Ib study of CD40 agonistic monoclonal antibody APX005M together with gemcitabine (Gem) and nab-paclitaxel (NP) with or without nivolumab (Nivo) in untreated metastatic ductal pancreatic adenocarcinoma (PDAC) patients. Cancer Res. 2019, 79, CT004. [Google Scholar] [CrossRef]
- Alistar, A.; Morris, B.B.; Desnoyer, R.; Klepin, H.D.; Hosseinzadeh, K.; Clark, C.; Cameron, A.; Leyendecker, J.; D’Agostino, R., Jr.; Topaloglu, U.; et al. Safety and tolerability of the first-in-class agent CPI-613 in combination with modified FOLFIRINOX in patients with metastatic pancreatic cancer: A single-centre, open-label, dose-escalation, phase 1 trial. Lancet Oncol. 2017, 18, 770–778. [Google Scholar] [CrossRef]
- Modest, D.P.; Pant, S.; Sartore-Bianchi, A. Treatment sequencing in metastatic colorectal cancer. Eur. J. Cancer 2019, 109, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.L.; Tang, C.W.; Feng, W.M.; Bao, Y.; Chai, Z.Z. Prolonged Capecitabine Chemotherapy Following Capecitabine and Oxaliplatin (CAPOX) Regimen Chemotherapy Failed to Improve Survival of Stage III Colorectal Cancer After Radical Resection. Med. Sci. Monit. 2019, 25, 4831–4836. [Google Scholar] [CrossRef]
- Twelves, C. Capecitabine as first-line treatment in colorectal cancer. Pooled data from two large, phase III trials. Eur. J. Cancer 2002, 38, 15–20. [Google Scholar] [CrossRef]
- A Study of Xeloda (Capecitabine) in Combination with Avastin + Short Course Chemotherapy in Patients with Metastatic Colorectal Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT00642603 (accessed on 7 December 2019).
- Moiseyenko, V.M.; Moiseyenko, F.V.; Yanus, G.A.; Kuligina, E.S.; Sokolenko, A.P.; Bizin, I.V.; Kudriavtsev, A.A.; Aleksakhina, S.N.; Volkov, N.M.; Chubenko, V.A.; et al. First-Line Cetuximab Monotherapy in KRAS/NRAS/BRAF Mutation-Negative Colorectal Cancer Patients. Clin. Drug Investig. 2018, 38, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Aljubran, A.; Elshenawy, M.A.; Kandil, M.; Zahir, M.N.; Shaheen, A.; Gad, A.; Alshaer, O.; Alzahrani, A.; Eldali, A.; Bazarbashi, S. Efficacy of Regorafenib in Metastatic Colorectal Cancer: A Multi-institutional Retrospective Study. Clin. Med. Insights Oncol. 2019, 13, 1179554918825447. [Google Scholar] [CrossRef] [PubMed]
- Samalin, E.; De La Fouchardiere, C.; Thézenas, S.; Sarabi, M.; Assenat, E.; Portales, F.; Carrere, S.; Rivoire, M.; Rouanet, P.; Bleuse, J.-P.; et al. Triplet chemotherapy (TC) with FOLFIRINOX regimen in metastatic colorectal cancer (mCRC): Experience of two French centres. J. Clin. Oncol. 2015, 33, 776. [Google Scholar] [CrossRef]
- Ganesh, K.; Stadler, Z.K.; Cercek, A.; Mendelsohn, R.B.; Shia, J.; Segal, N.H.; Diaz, L.A., Jr. Immunotherapy in colorectal cancer: Rationale, challenges and potential. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 361–375. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Tykodi, S.S.; Chow, L.Q.; Hwu, W.J.; Topalian, S.L.; Hwu, P.; Drake, C.G.; Camacho, L.H.; Kauh, J.; Odunsi, K.; et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 2012, 366, 2455–2465. [Google Scholar] [CrossRef]
- Overman, M.J.; McDermott, R.; Leach, J.L.; Lonardi, S.; Lenz, H.J.; Morse, M.A.; Desai, J.; Hill, A.; Axelson, M.; Moss, R.A.; et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): An open-label, multicentre, phase 2 study. Lancet Oncol. 2017, 18, 1182–1191. [Google Scholar] [CrossRef]
- Parente, P.; Parcesepe, P.; Covelli, C.; Olivieri, N.; Remo, A.; Pancione, M.; Latiano, T.P.; Graziano, P.; Maiello, E.; Giordano, G. Crosstalk between the Tumor Microenvironment and Immune System in Pancreatic Ductal Adenocarcinoma: Potential Targets for New Therapeutic Approaches. Gastroenterol. Res. Pract. 2018, 2018, 7530619. [Google Scholar] [CrossRef]
- Dosset, M.; Vargas, T.R.; Lagrange, A.; Boidot, R.; Végran, F.; Roussey, A.; Chalmin, F.; Dondaine, L.; Paul, C.; Marie-Joseph, E.L.; et al. PD-1/PD-L1 pathway: An adaptive immune resistance mechanism to immunogenic chemotherapy in colorectal cancer. OncoImmunology 2018, 7, e1433981. [Google Scholar] [CrossRef]
- Roxburgh, C.S.; Shia, J.; Vakiani, E.; Daniel, T.; Weiser, M.R. Potential immune priming of the tumor microenvironment with FOLFOX chemotherapy in locally advanced rectal cancer. OncoImmunology 2018, 7, e1435227. [Google Scholar] [CrossRef]
- Bencsikova, B.; Budinska, E.; Selingerova, I.; Pilatova, K.; Fedorova, L.; Greplova, K.; Nenutil, R.; Valik, D.; Obermannova, R.; Sheard, M.A.; et al. Circulating T cell subsets are associated with clinical outcome of anti-VEGF-based 1st-line treatment of metastatic colorectal cancer patients: A prospective study with focus on primary tumor sidedness. BMC Cancer 2019, 19, 687. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Kraus, S.G.; Quaney, M.J.; Daniels, M.A.; Mitchem, J.B.; Teixeiro, E. FOLFOX Chemotherapy Ameliorates CD8 T Lymphocyte Exhaustion and Enhances Checkpoint Blockade Efficacy in Colorectal Cancer. Front. Oncol. 2020, 10. [Google Scholar] [CrossRef]
- Nywening, T.M.; Wang-Gillam, A.; Sanford, D.E.; Belt, B.A.; Panni, R.Z.; Cusworth, B.M.; Toriola, A.T.; Nieman, R.K.; Worley, L.A.; Yano, M.; et al. Targeting tumour-associated macrophages with CCR2 inhibition in combination with FOLFIRINOX in patients with borderline resectable and locally advanced pancreatic cancer: A single-centre, open-label, dose-finding, non-randomised, phase 1b trial. Lancet Oncol. 2016, 17, 651–662. [Google Scholar] [CrossRef]
- Fontijn, R.; Hop, C.; Brinkman, H.J.; Slater, R.; Westerveld, A.; van Mourik, J.A.; Pannekoek, H. Maintenance of vascular endothelial cell-specific properties after immortalization with an amphotrophic replication-deficient retrovirus containing human papilloma virus 16 E6/E7 DNA. Exp. Cell Res. 1995, 216, 199–207. [Google Scholar] [CrossRef]
- Xu, H.; Jaynes, J.; Ding, X. Combining two-level and three-level orthogonal arrays for factor screening and response surface exploration. Stat. Sin. 2014, 24. [Google Scholar] [CrossRef]
- Weiss, A.; Ding, X.; van Beijnum, J.R.; Wong, I.; Wong, T.J.; Berndsen, R.H.; Dormond, O.; Dallinga, M.; Shen, L.; Schlingemann, R.O.; et al. Rapid optimization of drug combinations for the optimal angiostatic treatment of cancer. Angiogenesis 2015, 18, 233–244. [Google Scholar] [CrossRef]
- Greiner, P.O.; Zittoun, J.; Marquet, J.; Cheron, J.M. Pharmacokinetics of (−)-folinic acid after oral and intravenous administration of the racemate. Br. J. Clin. Pharmacol. 1989, 28, 289–295. [Google Scholar] [CrossRef]
- Kaldate, R.R.; Haregewoin, A.; Grier, C.E.; Hamilton, S.A.; McLeod, H.L. Modeling the 5-fluorouracil area under the curve versus dose relationship to develop a pharmacokinetic dosing algorithm for colorectal cancer patients receiving FOLFOX6. Oncol. Ogist. 2012, 17, 296–302. [Google Scholar] [CrossRef]
- Mathijssen, R.H.; Verweij, J.; Loos, W.J.; de Bruijn, P.; Nooter, K.; Sparreboom, A. Irinotecan pharmacokinetics-pharmacodynamics: The clinical relevance of prolonged exposure to SN-38. Br. J. Cancer 2002, 87, 144–150. [Google Scholar] [CrossRef]
- Burz, C.; Berindan-Neagoe, I.B.; Balacescu, O.; Tanaselia, C.; Ursu, M.; Gog, A.; Vlase, L.; Chintoanu, M.; Balacescu, L.; Leucuta, S.E.; et al. Clinical and pharmacokinetics study of oxaliplatin in colon cancer patients. J. Gastrointest. Liv. Dis. JGLD 2009, 18, 39–43. [Google Scholar]
- Innocenti, F.; Schilsky, R.L.; Ramirez, J.; Janisch, L.; Undevia, S.; House, L.K.; Das, S.; Wu, K.; Turcich, M.; Marsh, R.; et al. Dose-finding and pharmacokinetic study to optimize the dosing of irinotecan according to the UGT1A1 genotype of patients with cancer. J. Clin. Oncol. 2014, 32, 2328–2334. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
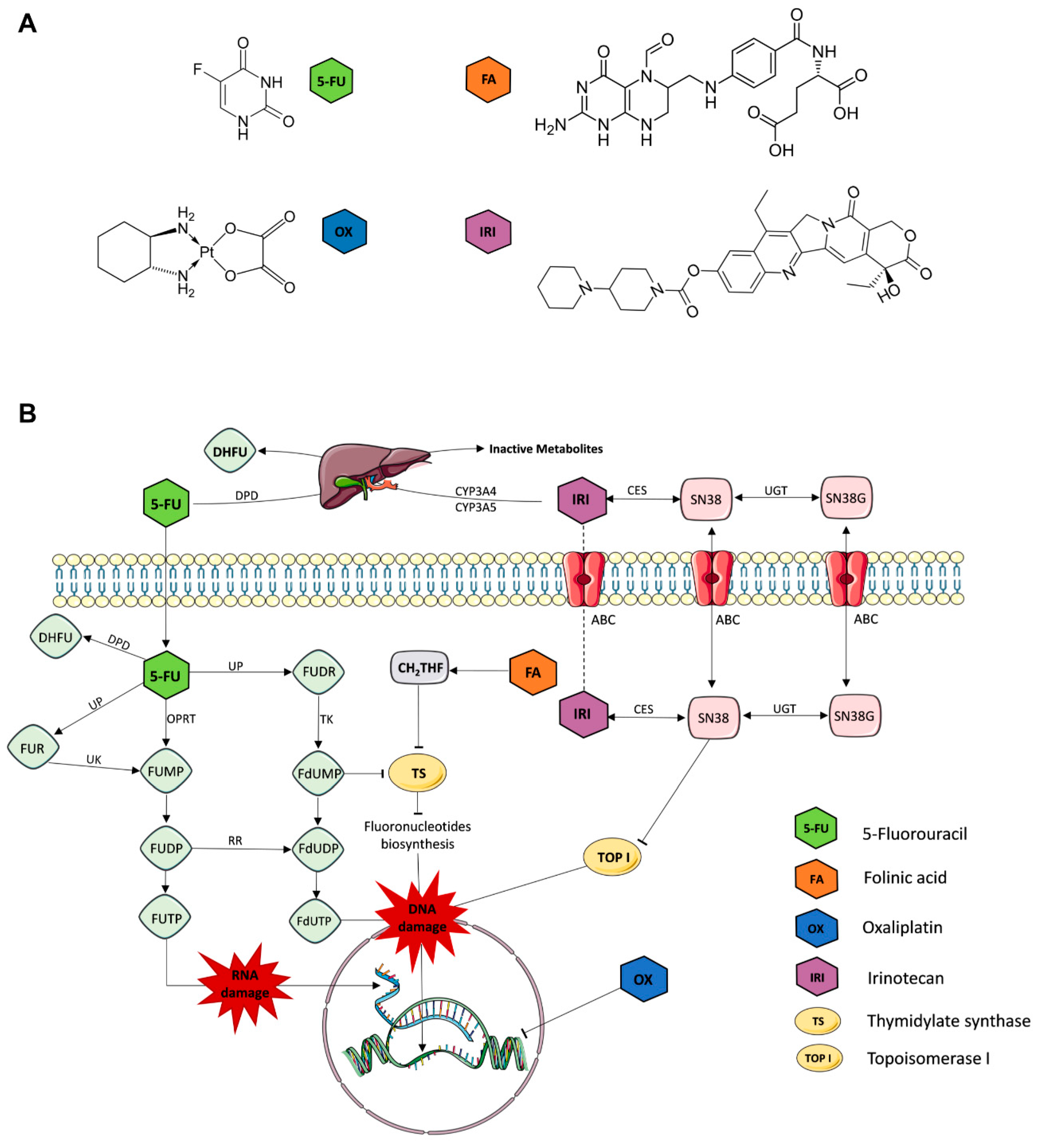
| Cell Line | Duke’s Type | Cell Status | MSI/CIN | CIMP | Mutations/Deregulations |
| DLD1 | C | undifferentiated | MSI | CIMP + | APC l1417fs, R2166, KRAS G13D, PIK3CA E545K; D549N, TP53 S241F |
| HCT116 | A | undifferentiated | MSI | CIMP + | KRAS G13D, PIK3CA H1047R |
| SW620 | C | undifferentiated | MSS; CIN pos46 | CIMP − | APC Q1338, KRAS G12V, TP53 R273H; P309S |
| LS174T | B | colon-like | MSI | CIMP − | KRAS G12D, PIK3CA H1047R, BRAF p.D211Gc |
| Drug | Chemical Formula | IUPAC Chemical Nomenclature | Abb. | ||
| folinic acid (leucovorin) | C20H23N7O7 | (2S)-2-[[4-[(2-amino-5-formyl-4-oxo-3,6,7,8-tetrahydropteridin-6-yl)methylamino]benzoyl]amino]pentanedioic acid | FA | ||
| 5-fluorouracil | C4H3FN2O2 | 5-fluoro-1H-pyrimidine-2,4-dione | FU | ||
| oxaliplatin | C8H12N2O4Pt | [(1R,2R)-2-azanidylcyclohexyl]azanide;oxalate;platinum(4+) | OX | ||
| irinotecan | C33H38N4O6 | [(19S)-10,19-diethyl-19-hydroxy-14,18-dioxo-17-oxa-3,13-diazapentacyclo[1 1.8.0.02,11.04,9.015,20]henicosa-1(21),2,4(9),5,7,10,15(20)-heptaen-7-yl] 4-piperidin-1-ylpiperidine-1-carboxylate | IRI | ||
| 7-Ethyl-10-hydroxycamptothecin * | C22H20N2O5 | (19S)-10,19-diethyl-7,19-dihydroxy-17-oxa-3,13-diazapentacyclo[11.8.0.02,11.04,9.015,20]henicosa-1(21),2,4(9),5,7,10,15(20)-heptaene-14,18-dione | SN | ||
| Drug | DLD1 | HCT116 | SW620 | LS174T |
|---|---|---|---|---|
| FA [µM] 5-FU [µM] | 0.5 2.0 | 0.5 2.0 | 0.25 6.0 | 0.5 6.0 |
| ATP levels [% CTRL] ±SD | 83.2 2.1 | 83.4 3.8 | 91.5 4.5 | 86.6 4.9 |
| CI FF | 1.65 | 0.97 | 0.62 | 0.82 |
| Drug | CUD | DLD1 ODC | HCT116 ODC | SW620 ODC | LS174T ODC |
|---|---|---|---|---|---|
| FA [µM] | 0.5 | 0.5 | 0.5 | 0.25 | 0.5 |
| 5-FU [µM] | 10 | 2 | 2 | 6 | 6 |
| SN [µM] | 0.1 | 0.02 | 0.003 | 0.004 | 0.004 |
| OX [µM] | 0.6 | 0.5 | 0.2 | 0.3 | 0.2 |
| ATP levels [% CTRL] ±SD | - | 48 4.9 | 35 0.7 | 37 8.6 | 45 2.2 |
| CI FF/OX/SN | - | 1.61 | 2.60 | 3.45 | 2.47 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zoetemelk, M.; Ramzy, G.M.; Rausch, M.; Nowak-Sliwinska, P. Drug-Drug Interactions of Irinotecan, 5-Fluorouracil, Folinic Acid and Oxaliplatin and Its Activity in Colorectal Carcinoma Treatment. Molecules 2020, 25, 2614. https://doi.org/10.3390/molecules25112614
Zoetemelk M, Ramzy GM, Rausch M, Nowak-Sliwinska P. Drug-Drug Interactions of Irinotecan, 5-Fluorouracil, Folinic Acid and Oxaliplatin and Its Activity in Colorectal Carcinoma Treatment. Molecules. 2020; 25(11):2614. https://doi.org/10.3390/molecules25112614
Chicago/Turabian StyleZoetemelk, Marloes, George M. Ramzy, Magdalena Rausch, and Patrycja Nowak-Sliwinska. 2020. "Drug-Drug Interactions of Irinotecan, 5-Fluorouracil, Folinic Acid and Oxaliplatin and Its Activity in Colorectal Carcinoma Treatment" Molecules 25, no. 11: 2614. https://doi.org/10.3390/molecules25112614
APA StyleZoetemelk, M., Ramzy, G. M., Rausch, M., & Nowak-Sliwinska, P. (2020). Drug-Drug Interactions of Irinotecan, 5-Fluorouracil, Folinic Acid and Oxaliplatin and Its Activity in Colorectal Carcinoma Treatment. Molecules, 25(11), 2614. https://doi.org/10.3390/molecules25112614






