Rice-Associated Rhizobacteria as a Source of Secondary Metabolites against Burkholderia glumae
Abstract
1. Introduction
2. Results and Discussion
2.1. In Vitro Antagonistic Activity of Isolated Rhizobacteria
2.2. Activities that Promote Plant Growth and Promising Strain Identification
2.2.1. In Vitro Rice Seed Germination and Seedling Growth
2.2.2. In Vitro Inorganic Phosphate Solubilization
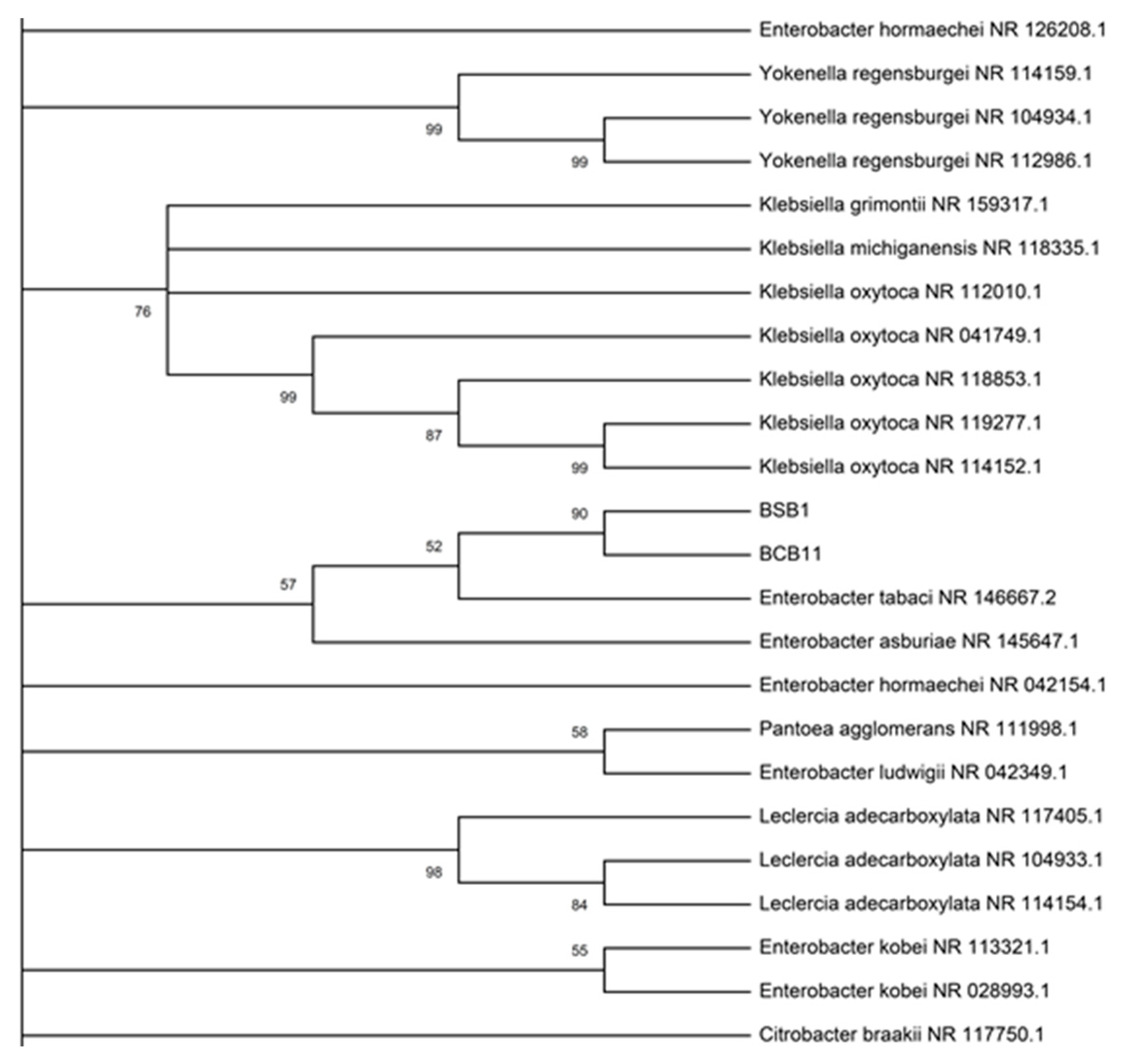
2.2.3. Identification of Promising Strains
2.3. EtOAc Extract Antibacterial Activity and Metabolomics Profiles
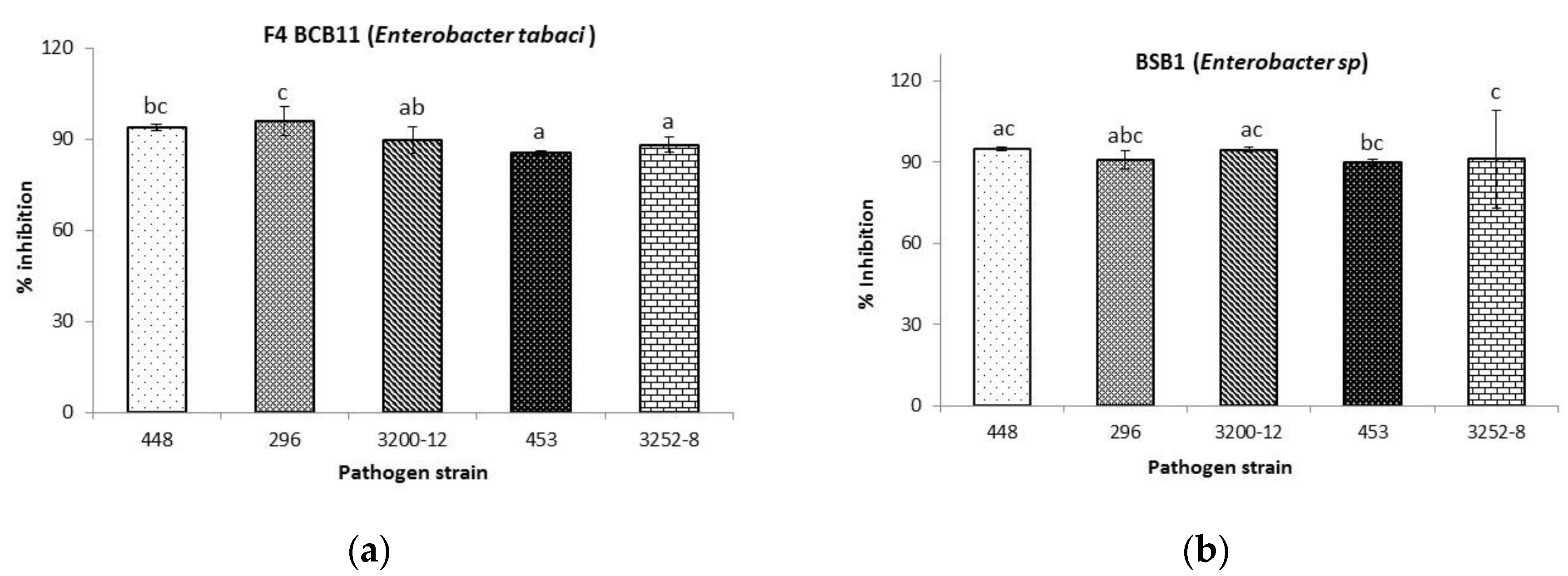
2.3.1. Antibacterial Activity toward Burkholderia glumae
2.3.2. Metabolomic Profiles in Rice-Associated Rhizobacteria with Antagonistic Activity
Metabolomics Profiles in BCB11 EtOAc Extract
Metabolomics Profile in BSB1 EtOAc Extract
Antibacterial Activity of the Main Compound Found in EtOAC Extracts, 3-Phenylpropanoic Acid, against B. glumae
3. Materials and Methods
3.1. Materials
3.2. B. glumae Strains
3.3. Sample Collection and Rhizospheric Bacteria Isolation in Rice
3.4. Identification of Promising Strains
3.5. Antagonistic In Vitro Activity Toward B. glumae Strains
3.6. Fermentation and Metabolite Extraction
3.7. Antibacterial EtOAc Extract Activity toward B. glumae
3.8. In Vitro Plant-Growth-Promoting Activity
3.8.1. Seed Germination and Seedling Growth
3.8.2. Phosphate Solubilization Assessment
3.9. Metabolites Extraction and Metabolomic Analysis
3.9.1. Active Fractions Chromatographic Separations
3.9.2. Fourier Transform Infrared (FT-IR)
3.9.3. Nuclear Magnetic Resonance (NMR)
3.9.4. Gas Chromatography–Mass Spectrometry (GC–MS)
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- McBratney, A.; Field, D.J.; Koch, A. The dimensions of soil security. Geoderma 2014, 213, 203–213. [Google Scholar] [CrossRef]
- Mohanty, T.R.; Maity, S.K.; Roul, P.K.; Sahoo, K.C. Studies on yield, economics and energetics of rice (oryza sativa l.) in relation to crop establishment methods and nutrient management practices. Int. J. Bio Resour. Stress Manag. 2014, 5, 495–501. [Google Scholar] [CrossRef]
- Seck, P.A.; Aliou, D.; Samarendu, M.; Wopereis, M.C.S. Crops that feed the world 7: Rice. Food Secur. 2012, 4, 7–24. [Google Scholar] [CrossRef]
- Karki, H.S.; Bishnu, K.S.; Jae, W.H.; Donald, E.G.; Barphagha, I.K.; Milton, C.R.; Rebecca, A.M.; Beom, S.K.; Jong, H.H. Diversities in virulence, antifungal activity, pigmentation and dna fingerprint among strains of burkholderia glumae. PLoS ONE 2012, 7, e45376. [Google Scholar] [CrossRef]
- Mizobuchi, R.; Sato, H.; Fukuoka, S.; Tsushima, S.; Imbe, T.; Yano, M. Identification of qrbs1, a qtl involved in resistance to bacterial seedling rot in rice. Theor. Appl. Genet. 2013, 126, 2417–2425. [Google Scholar] [CrossRef]
- Cui, Z.-Q.; Zhu, B.; Xie, G.-L.; Li, B.; Huang, S.-W. Research status and prospect of burkholderia glumae, the pathogen causing bacterial panicle blight. Rice Sci. 2016, 23, 111–118. [Google Scholar]
- Magbanua, Z.V.; Mark, A.; Teresia, B.; Hsu, C.-Y.; Showmaker, K.C.; Philippe, C.; Peng, D.; Peterson, D.G.; Lu, S. Transcriptomic dissection of the rice—Burkholderia glumae interaction. BMC Genom. 2014, 15, 755. [Google Scholar] [CrossRef]
- García-Cristobal, J.; García-Villaraco, A.; Ramos, B.; Gutierrez-Mañero, J.; Lucas, J.A. Priming of pathogenesis related-proteins and enzymes related to oxidative stress by plant growth promoting rhizobacteria on rice plants upon abiotic and biotic stress challenge. J. Plant Physiol. 2015, 188, 72–79. [Google Scholar] [CrossRef]
- Ham, J.H.; Melanson, R.A.; Rush, M.C. Burkholderia glumae: Next major pathogen of rice? Mol. Plant Pathol. 2011, 12, 329–339. [Google Scholar] [CrossRef]
- Shrestha, B.K.; Hari, S.K.; Donald, E.G.; Nootjarin, J.K.; Jong, H.H. Biological control activities of rice-associated bacillus sp. strains against sheath blight and bacterial panicle blight of rice. PLoS ONE 2016, 11, e0146764. [Google Scholar] [CrossRef]
- Mwajita, M.R.; Hunja, M.; Akio, T.; Esther, M.K. Evaluation of rhizosphere, rhizoplane and phyllosphere bacteria and fungi isolated from rice in kenya for plant growth promoters. SpringerPlus 2013, 2, 606. [Google Scholar] [CrossRef]
- Finkel, O.M.; Gabriel, C.; Sur, H.P.; Isai, S.G.; Jeffery, L.D. Understanding and exploiting plant beneficial microbes. Curr. Opin. Plant Biol. 2017, 38, 155–163. [Google Scholar] [CrossRef]
- Mauchline, T.H.; Jacob, G.M. Life in earth-the root microbiome to the rescue? Curr. Opin. Microbiol. 2017, 37, 23–28. [Google Scholar] [CrossRef]
- Tkacz, A.; Philip, P. Role of root microbiota in plant productivity. J. Exp. Bot. 2015, 66, 2167–2175. [Google Scholar] [CrossRef]
- Kumar, H.; Dubey, R.C.; Maheshwari, D.K. Seed-Coating fenugreek with burkholderia rhizobacteria enhances yield in field trials and can combat fusarium wilt. Rhizosphere 2017, 3, 92–99. [Google Scholar] [CrossRef]
- Pinstrup-Andersen, P. Food security: Definition and measurement. Food Secur. 2009, 1, 5–7. [Google Scholar] [CrossRef]
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Lawrence, H.; David, L.; Muir, J.F.; Jules, P.; Sherman, R.; Thomas, S.M.; Camilla, T. Food security: The challenge of feeding 9 billion people. Science 2010, 327, 812–818. [Google Scholar] [CrossRef]
- Barbara, D.C.; Pieter, T.; Christine, V.; Cammue, B.P.A.; Kemal, K. What lies beneath: Belowground defense strategies in plants. Trends Plant Sci. 2015, 20, 91–101. [Google Scholar]
- Chamam, A.; Florence, W.-D.; Gilles, C.; Cédric, B.; Claire, P.-C. Differential responses of oryza sativa secondary metabolism to biotic interactions with cooperative, commensal and phytopathogenic bacteria. Planta 2015, 242, 1439–1452. [Google Scholar] [CrossRef]
- Zhang, R.; Jorge, M.V.; Shen, Q.R. The unseen rhizosphere root–soil–microbe interactions for crop production. Curr. Opin. Microbiol. 2017, 37, 8–14. [Google Scholar] [CrossRef]
- Grobelak, A.; Napora, A.; Kacprzak, M. Using plant growth-promoting rhizobacteria (pgpr) to improve plant growth. Ecol. Eng. 2015, 84, 22–28. [Google Scholar] [CrossRef]
- Mariutto, M.; Marc, O. Chapter two-molecular patterns of rhizobacteria involved in plant immunity elicitation. In Advances in Botanical Research; Harsh, B., Janine, S., Eds.; Academic Press: London, UK, 2015; pp. 21–56. [Google Scholar]
- Osman, J.R.; Gustavo, F.; Michael, S.D. Bacterial diversity of the rhizosphere and nearby surface soil of rice (oryza sativa) growing in the camargue (france). Rhizosphere 2017, 3, 112–122. [Google Scholar] [CrossRef]
- Hossain, M.T.; Ajmal, K.; Eu, J.C.; Rashid, M.H.-O.; Young, R.C. Biological control of rice bakanae by an endophytic bacillus oryzicola yc7007. Plant Pathol. J. 2016, 32, 228–241. [Google Scholar] [CrossRef]
- Suárez-Moreno, Z.R.; Vinchira-Villarraga, D.M.; Vergara-Morales, D.I.; Castellanos, L.; Ramos, F.A.; Guarnaccia, C.; Degrassi, G.; Venturi, V.; Moreno-Sarmiento, N. Plant-Growth promotion and biocontrol properties of three streptomyces spp. isolates to control bacterial rice pathogens. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef]
- Mahmoud, S.Y.; Ziedan, E.-S.H.; Farrag, E.S.; Kalafalla, R.S.; Ali, A.M. Antifungal activity of pyocyanin produced by pseudomonas aeruginosa against fusarium oxysporum schlech a root-rot phytopathogenic fungi. IJPRIF 2016, 9, 43–50. [Google Scholar]
- Leonid, C.; Brandis, A.; Ismailov, Z.; Chet, I. Pyrrolnitrin production by an enterobacter agglomerans strain with a broad spectrum of antagonistic activity towards fungal and bacterial phytopathogens. Curr. Microbiol. 1996, 32, 208–212. [Google Scholar]
- Hans-Peter, F.; Krastel, P.; Müller, J.; Gebhardt, K.; Zeeck, A. Enterobactin: The characteristic catecholate siderophore of enterobacteriaceae is produced by streptomyces species. FEMS Microbiol. Lett. 2001, 196, 147–151. [Google Scholar]
- Kumar, V.; Narula, N. Solubilization of inorganic phosphates and growth emergence of wheat as affected by azotobacter chroococcum mutants. Biol. Fertil. Soils 1999, 28, 301–305. [Google Scholar] [CrossRef]
- Verma, J.P.; Yadav, J.; Tiwari, K.N.; Lavakush, S.V. Impact of plant growth promoting rhizobacteria on crop production. Int. J. Agric. Res. 2010, 5, 954–983. [Google Scholar] [CrossRef]
- Mathurot, C.; Lumyong, S. Screening and optimization of indole-3-acetic acid production and phosphate solubilization from rhizobacteria aimed at improving plant growth. Curr. Microbiol. 2011, 62, 173–181. [Google Scholar]
- Zhao, J.; Zhang, X.Y.; Wang, Z.Y.; Cao, B.; Deng, S.J.; Bi, M.C.; Zhang, Y. Enhanced biodegradation of atrazine by arthrobacter sp. dns10 during co-culture with a phosphorus solubilizing bacteria: Enterobacter sp. p1. Ecotoxicol. Environ. Saf. 2019, 172, 159–166. [Google Scholar]
- Duan, Y.-Q.; Zhou, X.-K.; Li, D.-Y.; Li, Q.-Q.; Dang, L.-Z.; Zhang, Y.-G.; Qiu, L.-H.; Nimaichand, S.; Li, W.-J. Enterobacter tabaci sp. nov., a novel member of the genus enterobacter isolated from a tobacco stem. Antonie Van Leeuwenhoek 2015, 108, 1161–1169. [Google Scholar] [CrossRef]
- Lagos, L.; Maruyama, F.; Nannipieri, P.; Mora, M.L.; Ogram, A.; Jorquera, M.A. Current overview on the study of bacteria in the rhizosphere by modern molecular techniques: A mini review. J. Soil Sci. Plant Nutr. 2015, 15, 504–523. [Google Scholar]
- Wang, L.; Qiu, P.; Long, X.-F.; Zhang, S.; Zeng, Z.-G.; Tian, Y.-Q. Comparative analysis of chemical constituents, antimicrobial and antioxidant activities of ethylacetate extracts of polygonum cuspidatum and its endophytic actinomycete, streptomyces sp. A0916. Chin. J. Nat. Med. 2016, 14, 117–123. [Google Scholar] [CrossRef]
- Zhu, Y.-J.; Zhou, H.-T.; Hu, Y.H.; Tang, J.-Y.; Su, M.-X.; Guo, Y.-J.; Chen, Q.-X.; Liu, B. Antityrosinase and antimicrobial activities of 2-phenylethanol, 2-phenylacetaldehyde and 2-phenylacetic acid. Food Chem. 2011, 124, 298–302. [Google Scholar] [CrossRef]
- Narayana, K.J.; Prabhakar, P.; Vijayalakshmi, M.; Venkateswarlu, Y.; Krishna, P.S. Biological activity of phenylpropionic acid isolated from a terrestrial streptomycetes. Pol. J. Microbiol. 2007, 56, 191. [Google Scholar]
- Guzman, J.D. Natural cinnamic acids, synthetic derivatives and hybrids with antimicrobial activity. Molecules 2014, 19, 19292–19349. [Google Scholar] [CrossRef]
- Balasubramanian, N.; Belsare, D.; Pharande, D.; Mourya, V.; Dhake, A. Esters, amides and substituted derivatives of cinnamic acid: Synthesis, antimicrobial activity and qsar investigations. Eur. J. Med. Chem. 2004, 39, 827–834. [Google Scholar]
- Rodrigo, R.; Cajas-Madriaga, D.; Martínez, M.; Martín, A.S.; Pérez, C.; Fajardo, V.; Becerra, J. Biological activity of macromycetes isolated from chilean subantarctic ecosystems. J. Chil. Chem. Soc. 2013, 58, 2016–2019. [Google Scholar]
- Chen, H.; Fujita, M.; Feng, Q.H.; Clardy, J.; Fink, G.R. Tyrosol is a quorum-sensing molecule in <em>candida albicans. Proc. Natl. Acad. Sci. USA 2004, 101, 5048–5052. [Google Scholar] [CrossRef]
- Concepción, R.; Medina, E.; Vargas, J.; Brenes, M.; Castro, A.D. In vitro activity of olive oil polyphenols against helicobacter pylori. J. Agric. Food Chem. 2007, 55, 680–686. [Google Scholar]
- Antonio, C.M.; Abriouel, H.; López, R.L.; Omar, N.B.; Valdivia, E.; Gálvez, A. enhanced bactericidal activity of enterocin as-48 in combination with essential oils, natural bioactive compounds and chemical preservatives against listeria monocytogenes in ready-to-eat salad. Food Chem. Toxicol. 2009, 47, 2216–2223. [Google Scholar]
- Huberman, L.; Gollop, N.; Mumcuoglu, K.Y.; Breuer, E.; Bhusare, S.R.; Shai, Y.; Galun, R. Antibacterial substances of low molecular weight isolated from the blowfly, lucilia sericata. Med Vet. Entomol. 2007, 21, 127–131. [Google Scholar] [CrossRef]
- Carolina, C.; Moreno-Arribas, M.V.; Martín-Álvarez, P.J.; Bills, G.; Vicente, M.F.; Basilio, A.; Rivas, C.L.; Requena, T.; Rodríguez, J.M.; Bartolomé, B. Antimicrobial activity of phenolic acids against commensal, probiotic and pathogenic bacteria. Res. Microbiol. 2010, 161, 372–382. [Google Scholar]
- Maris, A.J.A.V.; Konings, W.N.; Dijken, J.P.V.; Pronk, J.T. Microbial export of lactic and 3-hydroxypropanoic acid: Implications for industrial fermentation processes. Metab. Eng. 2004, 6, 245–255. [Google Scholar] [CrossRef]
- Tamblyn, K.C.; Conner, D.E. Bactericidal activity of organic acids against salmonella typhimurium attached to broiler chicken skint. J. Food Prot. 1997, 60, 629–633. [Google Scholar] [CrossRef]
- Alessandro, P.; Petrini, M. Tryptophol and derivatives: Natural occurrence and applications to the synthesis of bioactive compounds. Nat. Prod. Rep. 2019, 36, 490–530. [Google Scholar]
- Oswaldo, G.-L.; Trigos, Á.; Fernández, F.J.; Yañez-Morales, M.D.J.; Saucedo-Castañeda, G. Tyrosol and tryptophol produced by ceratocystis adiposa. World J. Microbiol. Biotechnol. 2007, 23, 1473–1477. [Google Scholar]
- Hala Yassin, E.-K.; El-Sheekh, M.M. Induction of the synthesis of bioactive compounds of the marine alga tetraselmis tetrathele (west) butcher grown under salinity stress. Egypt. J. Aquat. Res. 2016, 42, 385–391. [Google Scholar]
- Xu, L.-L.; Han, T.; Wu, J.-Z.; Zhang, Q.-Y.; Zhang, H.; Huang, B.-K.; Rahman, K.; Qin, L.-P. Comparative research of chemical constituents, antifungal and antitumor properties of ether extracts of panax ginseng and its endophytic fungus. Phytomedicine 2009, 16, 609–616. [Google Scholar] [CrossRef]
- Jutta, L.-M. Auxin conjugates: Their role for plant development and in the evolution of land plants. J. Exp. Bot. 2011, 62, 1757–1773. [Google Scholar]
- Patten, C.L.; Bernard, R.G. Role of pseudomonas putida indoleacetic acid in development of the host plant root system. Appl. Environ. Microbiol. 2002, 68, 3795–3801. [Google Scholar] [CrossRef] [PubMed]
- Adriana, B.; Ferrara, L.; Pezzo, M.D.; Mele, G.; Sorbo, S.; Bassi, P.; Montesano, D. Antibacterial and antioxidant activities of ethanol extract from paullinia cupana mart. J. Ethnopharmacol. 2005, 102, 32–36. [Google Scholar]
- Maddox, C.E.; Laur, L.M.; Li, T. Antibacterial activity of phenolic compounds against the phytopathogen xylella fastidiosa. Curr. Microbiol. 2009, 60, 53. [Google Scholar] [CrossRef] [PubMed]
- Eun-Ju, L.; Jung, H.-J.; Park, E.-H.; Kang, H.-J. Anti-angiogenic, anti-inflammatory and anti-nociceptive activity of 4-hydroxybenzyl alcohol. J. Pharm. Pharmacol. 2007, 59, 1235–1240. [Google Scholar]
- Elodie, D.; Petrault-Laprais, M.; Maurois, P.; Pages, N.; Bac, P.; Bordet, R.; Vamecq, J. Experimental stroke protection induced by 4-hydroxybenzyl alcohol is cancelled by bacitracin. Neurosci. Res. 2009, 64, 137–142. [Google Scholar]
- Yu, S.-S.; Zhao, J.; Zheng, W.-P.; Zhao, Y. Neuroprotective effect of 4-hydroxybenzyl alcohol against transient focal cerebral ischemia via anti-apoptosis in rats. Brain Res. 2010, 1308, 167–175. [Google Scholar] [CrossRef]
- Vikas, S.; Kumar, P.; Pathak, D. Biological importance of the indole nucleus in recent years: A comprehensive review. J. Heterocycl. Chem. 2010, 47, 491–502. [Google Scholar]
- Cortés-Zavaleta, O.; López-Malo, A.; Hernández-Mendoza, A.; García, H.S. Antifungal activity of lactobacilli and its relationship with 3-phenyllactic acid production. Int. J. Food Microbiol. 2014, 173, 30–35. [Google Scholar] [CrossRef]
- Hwang, B.K.; Lim, S.W.; Kim, B.S.; Lee, J.Y.; Moon, S.S. Isolation and in vivo and in vitro antifungal activity of phenylacetic acid and sodium phenylacetate from streptomyces humidus. Appl. Environ. Microbiol. 2001, 67, 3739–3745. [Google Scholar] [CrossRef]
- Prema, P.; Smila, D.; Palavesam, A.; Immanuel, G. Production and characterization of an antifungal compound (3-phenyllactic acid) produced by lactobacillus plantarum strain. Food Bioprocess Technol. 2010, 3, 379–386. [Google Scholar] [CrossRef]
- Lee, J.-H.; Wendisch, V.F. Biotechnological production of aromatic compounds of the extended shikimate pathway from renewable biomass. J. Biotechnol. 2017, 257, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Averesch, N.J.H.; Krömer, J.O. Metabolic engineering of the shikimate pathway for production of aromatics and derived compounds—present and future strain construction strategies. Front. Bioeng. Biotechnol. 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Audrey, W.; Chavez, V.; Huang, R.S.P.; Wahed, A.; Actor, J.K.; Dasgupta, A. Chapter 4-media for the clinical microbiology laboratory. In Microbiology and Molecular Diagnosis in Pathology; Wanger, A., Chavez, V., Huang, R.S.P., Wahed, A., Actor, J.K., Dasgupta, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 51–60. [Google Scholar]
- Guillermo, G. Production of aromatic compounds in bacteria. Curr. Opin. Biotechnol. 2009, 20, 651–658. [Google Scholar]
- Dias, F.M.S.; Gomez, J.G.C.; Silva, L.F. Exploring the microbial production of aromatic fine chemicals to overcome the barriers of traditional methods. Adv. Appl. Sci. Res. 2017, 8, 94–109. [Google Scholar]
- Harris, D.M.M.; Berrué, F.; Kerr, R.; Patten, C.L. Metabolomic analysis of indolepyruvate decarboxylase pathway derivatives in the rhizobacterium enterobacter cloacae. Rhizosphere 2018, 6, 98–111. [Google Scholar] [CrossRef]
- Chen, W.P.; Kuo, T.T. A simple and rapid method for the preparation of gram-negative bacterial genomic DNA. Nucleic Acids Res. 1993, 21, 2260. [Google Scholar] [CrossRef]
- Kumar, S.; Glen, S.; Li, M.; Knyaz, C.; Tamura, K. Mega X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard—Tenth Edition; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |
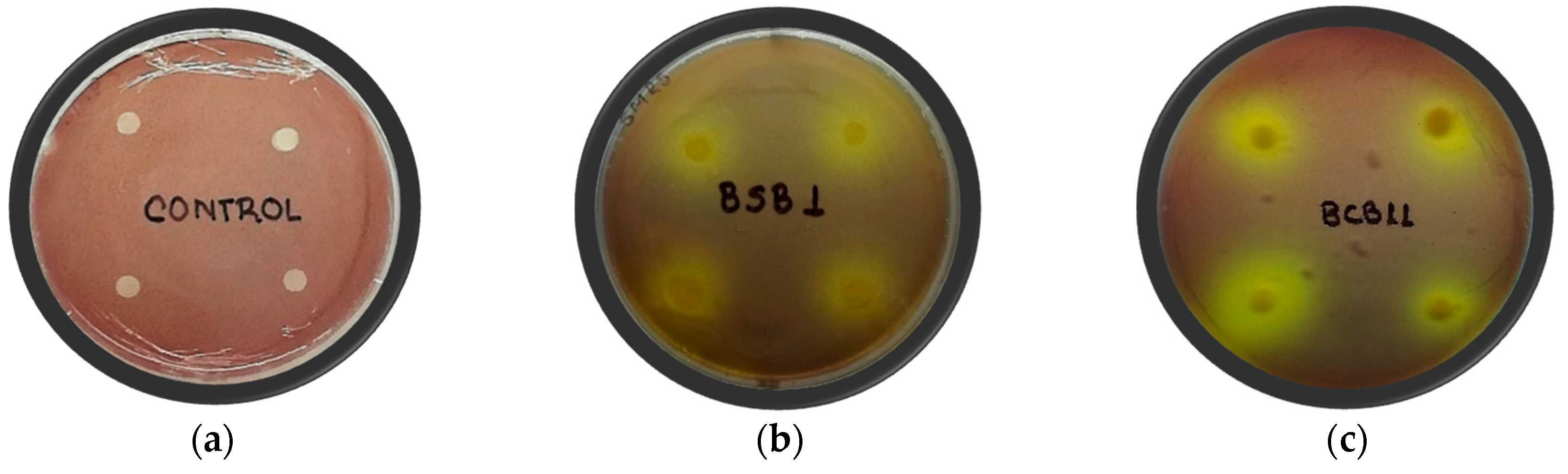

| Rhizobacteria | Burkholderia glumae Strains | ||||
|---|---|---|---|---|---|
| 296 | 448 | 453 | 3200-12 | 3252-8 | |
| Halozone (mm) | |||||
| BCB11 | 9.1 ± 1.6 c | N.D | N.D | N.D | 7.6 ± 1.2 b |
| BSB1 | 6.7 ± 0.8 b | 5.7 ± 0.9 b | N.D | N.D | 6.8 ± 1.2 b |
| Control | 22.1 ± 1.9 a | 17.3 ± 0.7 a | 27.3 ± 2.0 | 32.9 ± 3.8 | 21.0 ± 1.9 a |
| Bacteria | Germination % | Shoot Length (cm) | Root (cm) | Seedling Weight Dry (mg) | Shoot/Root Ratio (s/r) |
|---|---|---|---|---|---|
| “FEDEARROZ 67” | |||||
| BCB11 | 76.67 ± 4.71 a | 6.55 ± 0.97 a | 4.49 ± 0.84 a | 10.69 ± 0.30 a | 1.46 ± 0.33 a |
| BSB1 | 90.00 ± 8.16 b | 5.02 ± 0.76 b | 4.40 ± 0.68 a | 12.02 ± 0.32 b | 1.14 ± 0.26 a |
| Control | 70.48 ± 8.19 a | 6.35 ± 0.92 a | 4.26 ± 0.72 a | 11.19 ± 0.78 a,b | 1.49 ± 0.26 a |
| “FEDEARROZ 2000” | |||||
| BCB11 | 96.67 ± 4.71 a | 6.46 ± 0.90 a | 6.90 ± 1.00 b | 14.94 ± 2.73 a | 0.93 ± 0.18 a |
| BSB1 | 96.67 ± 4.71 a | 7.42 ± 0.93 b | 7.71 ± 1.12 a | 14.69 ± 2.15 a | 0.96 ± 0.18 a |
| Control | 96.67 ± 4.71 a | 6.96 ± 1.03 a,b | 7.95 ± 1.19 a | 15.95 ± 1.70 a | 0.87 ± 0.19 a |
| Compound | Retention Time (min) | BCB11 | BSB1 |
|---|---|---|---|
| 1 | 3.408 | Lactic Acid | Lactic Acid |
| 2 | 5.498 | Benzoic acid | Benzoic acid |
| 3 | 6.108 | Benzeneacetic acid | Benzeneacetic acid |
| 4 | 6.270 | Butanedioic acid | Butanedioic acid |
| 5 | 6.396 | - | Catechol |
| 6 | 6.643 | Uracil | Uracil |
| 7 | 6.770 | - | 2,5-dihydroxy-3,6-dihydro-3,6-dimethylpyrazine |
| 8 | 6.815 | Nonanoic acid | Nonanoic acid |
| 9 | 7.455 | 2,4-Dihydroxy-5-methyl-pyrimidine | - |
| 10 | 7.630 | 3-phenylpropanoic acid | 3-phenylpropanoic acid |
| 11 | 7.742 | beta-Alanine | beta-Alanine |
| 12 | 7.926 | - | Indole |
| 13 | 8.017 | Decanoic acid | Decanoic acid |
| 14 | 8.650 | 4-Hydroxybenzyl alcohol | 4-Hydroxybenzyl alcohol |
| 15 | 8.715 | N-(2-phenylethyl)-acetamide | N-(2-phenylethyl)-acetamide |
| 16 | 8.863 | N-Acetylphenylethylamine | - |
| 17 | 9.114 | Cinnamic acid | - |
| 18 | 9.449 | Tyrosol | Tyrosol |
| 19 | 9.642 | N-Phenethylpropionamide | - |
| 20 | 10.223 | 4-Hydroxybenzeneacetic acid | 4-Hydroxybenzeneacetic acid |
| 21 | 11.570 | Phloretic acid | Phloretic acid |
| 22 | 11.665 | - | Benzyl benzoate |
| 23 | 12.408 | Myristic acid | Myristic acid |
| 24 | 13.024 | Tryptophol | Tryptophol |
| 25 | 13.326 | - | 8-Phenyloctanoic acid |
| 26 | 13.711 | 3-Indolacetic acid | 3-Indolacetic acid |
| 27 | 14.346 | Palmitic acid | Palmitic acid |
| 28 | 14.730 | (Z)-octadec-9-enenitrile | (Z)-octadec-9-enenitrile |
| 29 | 14.839 | 3-Indolepropionic acid | - |
| 30 | 15.228 | 5-Hydroxytryptophol | 5-Hydroxytryptophol |
| 31 | 15.919 | Oleic Acid | Oleic Acid |
| 32 | 16.129 | Stearic acid | Stearic acid |
| 33 | 18.216 | (Z)-Docos-9-enenitrile | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peñaloza Atuesta, G.C.; Murillo Arango, W.; Eras, J.; Oliveros, D.F.; Méndez Arteaga, J.J. Rice-Associated Rhizobacteria as a Source of Secondary Metabolites against Burkholderia glumae. Molecules 2020, 25, 2567. https://doi.org/10.3390/molecules25112567
Peñaloza Atuesta GC, Murillo Arango W, Eras J, Oliveros DF, Méndez Arteaga JJ. Rice-Associated Rhizobacteria as a Source of Secondary Metabolites against Burkholderia glumae. Molecules. 2020; 25(11):2567. https://doi.org/10.3390/molecules25112567
Chicago/Turabian StylePeñaloza Atuesta, Giann Carlos, Walter Murillo Arango, Jordi Eras, Diego Fernándo Oliveros, and Jonh Jairo Méndez Arteaga. 2020. "Rice-Associated Rhizobacteria as a Source of Secondary Metabolites against Burkholderia glumae" Molecules 25, no. 11: 2567. https://doi.org/10.3390/molecules25112567
APA StylePeñaloza Atuesta, G. C., Murillo Arango, W., Eras, J., Oliveros, D. F., & Méndez Arteaga, J. J. (2020). Rice-Associated Rhizobacteria as a Source of Secondary Metabolites against Burkholderia glumae. Molecules, 25(11), 2567. https://doi.org/10.3390/molecules25112567





