Supramolecular Electrochemical Sensor for Dopamine Detection Based on Self-Assembled Mixed Surfactants on Gold Nanoparticles Deposited Graphene Oxide
Abstract
1. Introduction
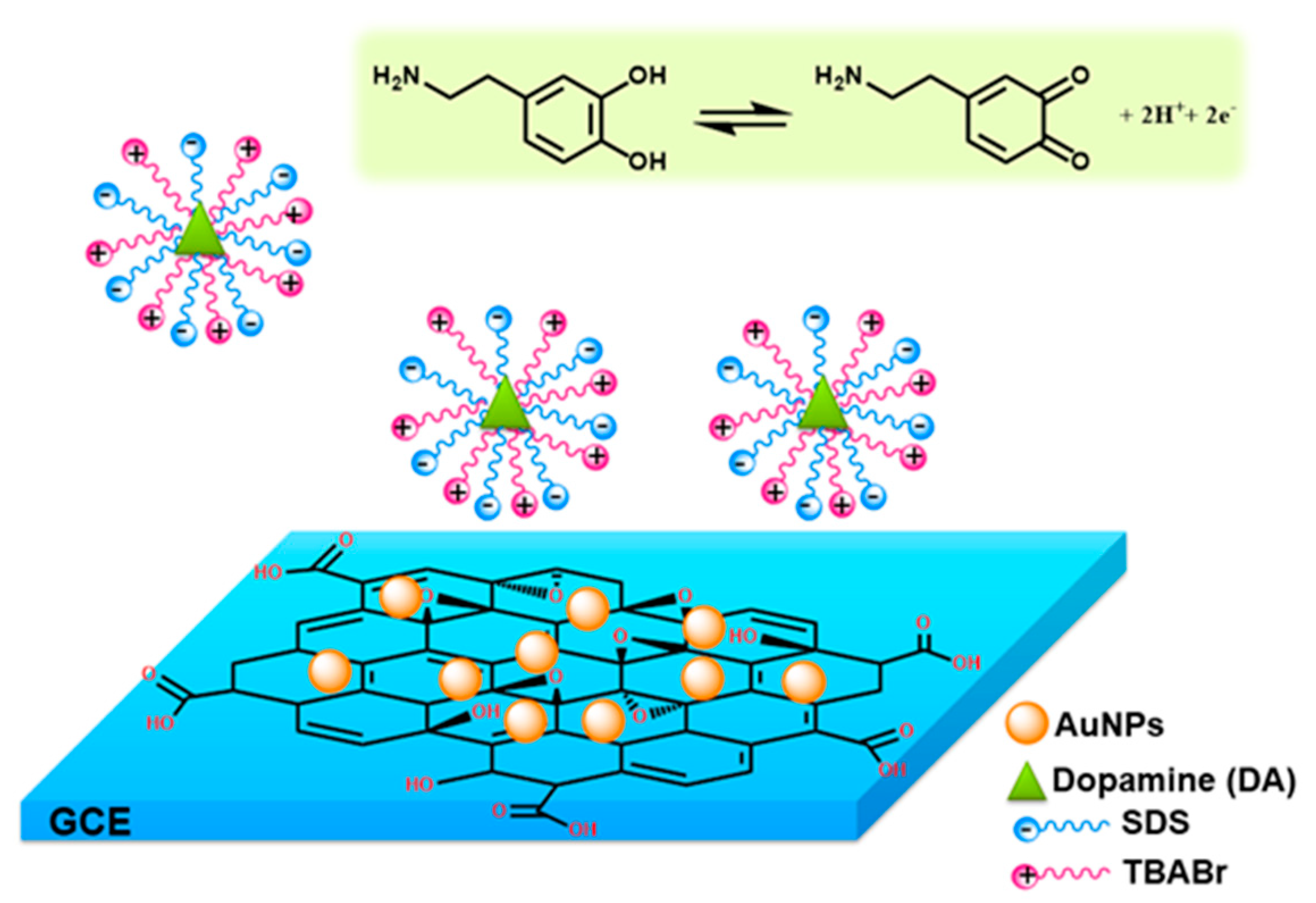
2. Results and Discussion
2.1. Morphological Characterization of the AuNPs/GO/GCE
2.2. Electrochemical Characterization of the Modified Electrodes
2.3. Electrochemical Behavior of DA at Modified Electrodes in the Presence of Supramolecular Assemblies of Mixed Surfactants
2.4. Effect of pH
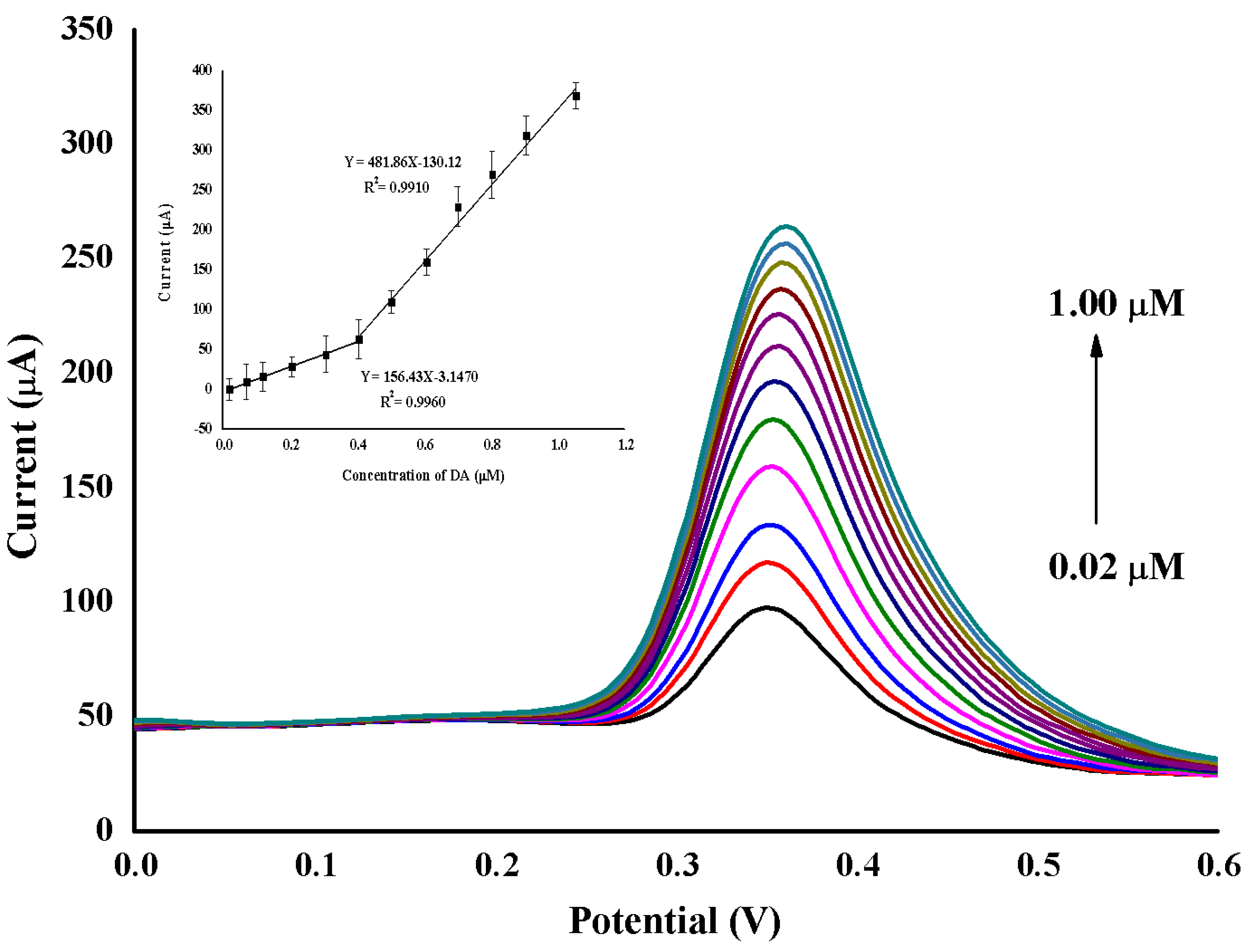
2.5. Analytical Performance
2.6. Interferences, Stability, and Reproducibility of the Supramolecular Electrochemical Sensor
3. Experimental Section
3.1. Material and Methods
3.2. Preparation of the AuNPs/GO/GCE
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ferancová, A.; Korgová, E.; Buzinkaiová, T.; Kutner, W.; Štěpánek, I.; Labuda, J. Electrochemical sensors using screen-printed carbon electrode assemblies modified with the β-cyclodextrin or carboxymethylated β-cyclodextrin polymer films for determination of tricyclic antidepressive drugs. Anal. Chim. Acta 2001, 447, 47–54. [Google Scholar]
- Liu, A.; Honma, I.; Zhou, H. Amperometric biosensor based on tyrosinase-conjugated polysacchride hybrid film: Selective determination of nanomolar neurotransmitters metabolite of 3,4-dihydroxyphenylacetic acid (DOPAC) in biological fluid. Biosens. Bioelectron. 2005, 21, 809–816. [Google Scholar] [PubMed]
- Amiri, M.; Dadfarnia, S.; Haji Shabani, A.M.; Sadjadi, S. Non-enzymatic sensing of dopamine by localized surface plasmon resonance using carbon dots-functionalized gold nanoparticles. J. Pharm. Biomed. Anal. 2019, 172, 223–229. [Google Scholar] [CrossRef]
- Wei, X.; Zhang, Z.; Wang, Z. A simple dopamine detection method based on fluorescence analysis and dopamine polymerization. Microchem. J. 2019, 145, 55–58. [Google Scholar] [CrossRef]
- Liu, C.; Gomez, F.A.; Miao, Y.; Cui, P.; Lee, W. A colorimetric assay system for dopamine using microfluidic paper-based analytical devices. Talanta 2019, 194, 171–176. [Google Scholar]
- Nikolajsen, R.P.; Hansen, Å.M. Analytical methods for determining urinary catecholamines in healthy subjects. Anal. Chim. Acta 2001, 449, 1–15. [Google Scholar]
- Gottås, A.; Ripel, Å.; Boix, F.; Vindenes, V.; Mørland, J.; Øiestad, E.L. Determination of dopamine concentrations in brain extracellular fluid using microdialysis with short sampling intervals, analyzed by ultra high performance liquid chromatography tandem mass spectrometry. J. Pharmacol. Toxicol. Methods 2015, 74, 75–79. [Google Scholar] [CrossRef]
- Jiao, J.; Zuo, J.; Pang, H.; Tan, L.; Chen, T.; Ma, H. A dopamine electrochemical sensor based on Pd-Pt alloy nanoparticles decorated polyoxometalate and multiwalled carbon nanotubes. J. Electroanal. Chem. 2018, 827, 103–111. [Google Scholar] [CrossRef]
- Yang, Y.; Li, M.; Zhu, Z. A novel electrochemical sensor based on carbon nanotubes array for selective detection of dopamine or uric acid. Talanta 2019, 201, 295–300. [Google Scholar] [CrossRef]
- Eom, G.; Oh, C.; Moon, J.; Kim, H.; Kim, M.K.; Kim, K.; Seo, J.-W.; Kang, T.; Lee, H.J. Highly sensitive and selective detection of dopamine using overoxidized polypyrrole/sodium dodecyl sulfate-modified carbon nanotube electrodes. J. Electroanal. Chem. 2019, 848, 113295. [Google Scholar]
- Riches, P.L.; Wright, A.F.; Ralston, S.H. Recent insights into the pathogenesis of hyperuricaemia and gout. Hum. Mol. Genet. 2009, 18, R177–R184. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Du, J.; Wang, H.; Zou, C.; Jiang, F.; Yang, P.; Du, Y. A facile electrochemical sensor based on reduced graphene oxide and Au nanoplates modified glassy carbon electrode for simultaneous detection of ascorbic acid, dopamine and uric acid. Sens. Actuators B Chem. 2014, 204, 302–309. [Google Scholar] [CrossRef]
- Qi, S.; Zhao, B.; Tang, H.; Jiang, X. Determination of ascorbic acid, dopamine, and uric acid by a novel electrochemical sensor based on pristine graphene. Electrochim. Acta 2015, 161, 395–402. [Google Scholar] [CrossRef]
- Zhu, Q.; Bao, J.; Huo, D.; Yang, M.; Wu, H.; Hou, C.; Fa, H. 3DGH-Fc based electrochemical sensor for the simultaneous determination of ascorbic acid, dopamine and uric acid. J. Electroanal. Chem. 2017, 799, 459–467. [Google Scholar]
- Palanisamy, S.; Thirumalraj, B.; Chen, S.-M.; Ali, M.A.; Al-Hemaid, F.M.A. Palladium nanoparticles decorated on activated fullerene modified screen printed carbon electrode for enhanced electrochemical sensing of dopamine. J. Colloid Interface Sci. 2015, 448, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Mukdasai, S.; Langsi, V.; Pravda, M.; Srijaranai, S.; Glennon, J.D. A highly sensitive electrochemical determination of norepinephrine using l-cysteine self-assembled monolayers over gold nanoparticles/multi-walled carbon nanotubes electrode in the presence of sodium dodecyl sulfate. Sens. Actuators B Chem. 2016, 236, 126–135. [Google Scholar]
- He, W.; Ding, Y.; Zhang, W.; Ji, L.; Zhang, X.; Yang, F. A highly sensitive sensor for simultaneous determination of ascorbic acid, dopamine and uric acid based on ultra-small Ni nanoparticles. J. Electroanal. Chem. 2016, 775, 205–211. [Google Scholar]
- Jin, J.; Mei, H.; Wu, H.; Wang, S.; Xia, Q.; Ding, Y. Selective detection of dopamine based on Cu2O@Pt core-shell nanoparticles modified electrode in the presence of ascorbic acid and uric acid. J. Alloys Compd. 2016, 689, 174–181. [Google Scholar] [CrossRef]
- Xu, G.; Li, B.; Cui, X.T.; Ling, L.; Luo, X. Electrodeposited conducting polymer PEDOT doped with pure carbon nanotubes for the detection of dopamine in the presence of ascorbic acid. Sens. Actuators B Chem. 2013, 188, 405–410. [Google Scholar]
- Wang, W.; Xu, G.; Cui, X.T.; Sheng, G.; Luo, X. Enhanced catalytic and dopamine sensing properties of electrochemically reduced conducting polymer nanocomposite doped with pure graphene oxide. Biosens. Bioelectron. 2014, 58, 153–156. [Google Scholar] [CrossRef]
- Ojani, R.; Raoof, J.-B.; Maleki, A.A.; Safshekan, S. Simultaneous and sensitive detection of dopamine and uric acid using a poly(L-methionine)/gold nanoparticle-modified glassy carbon electrode. Chin. J. Catal. 2014, 35, 423–429. [Google Scholar]
- Li, Y.; Song, H.; Zhang, L.; Zuo, P.; Ye, B.; Yao, J.; Chen, W. Supportless electrochemical sensor based on molecularly imprinted polymer modified nanoporous microrod for determination of dopamine at trace level. Biosens. Bioelectron. 2016, 78, 308–314. [Google Scholar] [PubMed]
- Niu, X.; Yang, W.; Guo, H.; Ren, J.; Gao, J. Highly sensitive and selective dopamine biosensor based on 3,4,9,10-perylene tetracarboxylic acid functionalized graphene sheets/multi-wall carbon nanotubes/ionic liquid composite film modified electrode. Biosens. Bioelectron. 2013, 41, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Ku, S.; Palanisamy, S.; Chen, S.-M. Highly selective dopamine electrochemical sensor based on electrochemically pretreated graphite and nafion composite modified screen printed carbon electrode. J. Colloid Interface Sci. 2013, 411, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Huang, G.; Li, J.; Wang, Z.; Xu, X. Graphene oxide-Ag/poly-l-lysine modified glassy carbon electrode as an electrochemical sensor for the determination of dopamine in the presence of ascorbic acid. J. Electroanal. Chem. 2015, 759, 113–121. [Google Scholar]
- Wang, C.; Li, J.; Shi, K.; Wang, Q.; Zhao, X.; Xiong, Z.; Wang, Y. Graphene coated by polydopamine/multi-walled carbon nanotubes modified electrode for highly selective detection of dopamine and uric acid in the presence of ascorbic acid. J. Electroanal. Chem. 2016, 770, 56–61. [Google Scholar]
- Zhou, M.; Zhai, Y.; Dong, S. Electrochemical Sensing and Biosensing Platform Based on Chemically Reduced Graphene Oxide. Anal. Chem. 2009, 81, 5603–5613. [Google Scholar]
- Wu, C.; Sun, D.; Li, Q.; Wu, K. Electrochemical sensor for toxic ractopamine and clenbuterol based on the enhancement effect of graphene oxide. Sens. Actuators B Chem. 2012, 168, 178–184. [Google Scholar] [CrossRef]
- Goh, B.-M.; Wang, Y.; Reddy, M.-V.; Ding, Y.-L.; Lu, L.; Bunker, C.; Loh, K.-P. Filling the voids of graphene foam with graphene “Eggshell” for improved lithium-ion storage. ACS Appl. Mater. Interfaces 2014, 6, 9835–9841. [Google Scholar] [CrossRef]
- Petnikota, S.; Rotte, N.-K.; Reddy, M.-V.; Srikanth, V.-V.; Chowdari, B.-V. MgO-decorated few-layered graphene as an anode for Li-ion batteries. ACS Appl. Mater. Interfaces 2015, 7, 2301–2309. [Google Scholar] [CrossRef]
- Atta, N.F.; El-Ads, E.H.; Ahmed, Y.M.; Galal, A. Determination of some neurotransmitters at cyclodextrin/ionic liquid crystal/graphene composite electrode. Electrochim. Acta 2016, 199, 319–331. [Google Scholar] [CrossRef]
- Zhang, S.J.; Kang, K.; Niu, L.M.; Kang, W.J. Electroanalysis of neurotransmitters via 3D gold nanoparticles and a graphene composite coupled with a microdialysis device. J. Electroanal. Chem. 2019, 834, 249–257. [Google Scholar] [CrossRef]
- Adumitrăchioaie, A.; Tertiș, M.; Suciu, M.; Graur, F.; Cristea, C. A novel immunosensing platform for serotonin detection in complex real samples based on graphene oxide and chitosan. Electrochim. Acta 2019, 311, 50–61. [Google Scholar] [CrossRef]
- Palanisamy, S.; Velusamy, V.; Ramaraj, S.; Chen, S.-W.; Yang, T.C.K.; Balu, S.; Banks, C.E. Facile synthesis of cellulose microfibers supported palladium nanospindles on graphene oxide for selective detection of dopamine in pharmaceutical and biological samples. Mater. Sci. Eng. 2019, 98, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Niyogi, S.; Bekyarova, E.; Itkis, M.E.; McWilliams, J.L.; Hamon, M.A.; Haddon, R.C. Solution Properties of Graphite and Graphene. J. Am. Chem. Soc. 2006, 128, 7720–7721. [Google Scholar] [CrossRef] [PubMed]
- Babaei, A.; Taheri, A.R. Nafion/Ni(OH)2 nanoparticles-carbon nanotube composite modified glassy carbon electrode as a sensor for simultaneous determination of dopamine and serotonin in the presence of ascorbic acid. Sens. Actuators B Chem. 2013, 176, 543–551. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Arashpour, B.; Rezaei, B.; Allafchian, A.R. Voltammetric behavior of dopamine at a glassy carbon electrode modified with NiFe2O4 magnetic nanoparticles decorated with multiwall carbon nanotubes. Mater. Sci. Eng. C 2014, 39, 78–85. [Google Scholar] [CrossRef]
- Vinoth, V.; Wu, J.J.; Asiri, A.M.; Anandan, S. Simultaneous detection of dopamine and ascorbic acid using silicate network interlinked gold nanoparticles and multi-walled carbon nanotubes. Sens. Actuators B Chem. 2015, 210, 731–741. [Google Scholar] [CrossRef]
- Yang, S.; Yin, Y.; Li, G.; Yang, R.; Li, J.; Qu, L. Immobilization of gold nanoparticles on multi-wall carbon nanotubes as an enhanced material for selective voltammetric determination of dopamine. Sens. Actuators B Chem. 2013, 178, 217–221. [Google Scholar] [CrossRef]
- Zhu, W.; Chen, T.; Ma, X.; Ma, H.; Chen, S. Highly sensitive and selective detection of dopamine based on hollow gold nanoparticles-graphene nanocomposite modified electrode. Colloids Surf. B 2013, 111, 321–326. [Google Scholar] [CrossRef]
- Jia, D.; Dai, J.; Yuan, H.; Lei, L.; Xiao, D. Selective detection of dopamine in the presence of uric acid using a gold nanoparticles-poly(luminol) hybrid film and multi-walled carbon nanotubes with incorporated β-cyclodextrin modified glassy carbon electrode. Talanta 2011, 85, 2344–2351. [Google Scholar] [CrossRef]
- Khudaish, E.A.; Al-Nofli, F.; Rather, J.A.; Al-Hinaai, M.; Laxman, K.; Kyaw, H.H.; Al-Harthy, S. Sensitive and selective dopamine sensor based on novel conjugated polymer decorated with gold nanoparticles. J. Electroanal. Chem. 2016, 761, 80–88. [Google Scholar] [CrossRef]
- Wu, X.; Radovic, L.R. Catalytic oxidation of carbon/carbon composite materials in the presence of potassium and calcium acetates. Carbon 2005, 43, 333–344. [Google Scholar] [CrossRef]
- Román-Martínez, M.C.; Cazorla-Amorós, D.; Linares-Solano, A.; De Lecea, C.S.-M.; Yamashita, H.; Anpo, M. Metal-support interaction in Pt/C catalysts. Influence of the support surface chemistry and the metal precursor. Carbon 1995, 33, 3–13. [Google Scholar]
- Nascentes, C. Cloud point formation based on mixed micelles in the presence of electrolytes for cobalt extraction and preconcentration. Talanta 2003, 61, 759–768. [Google Scholar] [CrossRef]
- Ezoddin, M.; Shemirani, F.; Khani, R. Application of mixed-micelle cloud point extraction for speciation analysis of chromium in water samples by electrothermal atomic absorption spectrometry. Desalination 2010, 262, 183–187. [Google Scholar] [CrossRef]
- Li, J.; Kuang, D.; Feng, Y.; Zhang, F.; Xu, Z.; Liu, M. A graphene oxide-based electrochemical sensor for sensitive determination of 4-nitrophenol. J. Hazard. Mater. 2012, 201–202, 250–259. [Google Scholar] [CrossRef]
- Mukdasai, S.; Moore, E.; Glennon, J.D.; He, X.; Nesterenko, E.P.; Nesterenko, P.N.; Srijaranai, S. Comparison of electrochemical property between multiwalled carbon nanotubes and porous graphitized carbon monolith modified glassy carbon electrode for the simultaneous determination of ascorbic acid and uric acid. J. Electroanal. Chem. 2014, 731, 53–59. [Google Scholar] [CrossRef]
- Godajdar, B.M.; Ansari, B. Preparation of novel magnetic dicationic ionic liquid polymeric phase transfer catalyst and their application in nucleophilic substitution reactions of benzyl halides in water. J. Mol. Liq. 2015, 202, 34–39. [Google Scholar] [CrossRef]
- Kukusamude, C.; Quirino, J.P.; Srijaranai, S. A coacervative extraction based on single-chain and double-chain cationic surfactants. J. Chromatogr. A 2016, 1472, 10–15. [Google Scholar] [CrossRef]
- Mata, J.; Varade, D.; Ghosh, G.; Bahadur, P. Effect of tetrabutylammonium bromide on the micelles of sodium dodecyl sulfate. Colloids Surf. A Physicochem. Eng. Asp. 2004, 245, 69–73. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, T.; Ryu, J.H.; Yoo, Y.J. Iron oxide/carbon black (Fe2O3/CB) composite electrode for the detection of reduced nicotinamide cofactors using an amperometric method under a low overpotential. Biosens. Bioelectron. 2010, 25, 1160–1165. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Li, M.; Li, N.; Gu, Z.; Duan, X. Electrocatalytic oxidation of norepinephrine at a reduced C60-[dimethyl-(β-cyclodextrin)]2 and Nafion chemically modified electrode. Electrochim. Acta 2002, 47, 2673–2678. [Google Scholar] [CrossRef]
- Gao, L.-L.; Sun, W.-J.; Yin, X.-M.; Bu, R.; Gao, E.-Q. Graphite paste electrodes modified with a sulfo-functionalized metal-organic framework (type MIL-101) for voltammetric sensing of dopamine. Microchim. Acta 2019, 186, 762. [Google Scholar]
- Messaound, N.B.; Ghica, M.E.; Dridi, C.; Ali, M.B.; Brett, C.M.A. Electrochemical sensor based on multiwalled carbon nanotube andgold nanoparticle modified electrode for the sensitive detection of bisphenol A. Sens. Actuators B Chem. 2017, 253, 513–522. [Google Scholar] [CrossRef]
- Chen, X.; Chen, J.; Dong, H.; Yu, Q.; Zhang, S.; Chen, H. Sensitive detection of dopamine using a platinum microelectrode modified by reduced graphene oxide and gold nanoparticles. J. Electroanal. Chem. 2019, 848, 113244. [Google Scholar]
- Do, P.T.; Do, P.Q.; Nguyen, H.B.; Nguyen, V.C.; Tran, D.L.; Le, T.H.; Tran, Q.H. A highly sensitive electrode modified with graphene, gold nanoparticles, and molecularly imprinted over-oxidized polypyrrole for electrochemical determination of dopamine. J. Mol. Liq. 2014, 198, 307–312. [Google Scholar] [CrossRef]
- Wang, J.; Yang, B.; Zhong, J.; Yan, B.; Zhang, K.; Zhai, C.; Yang, P. Dopamine and uric acid electrochemical sensor based on a glassy carbon electrode modified with cubic Pd and reduced graphene oxide nanocomposite. J. Colloid Interface Sci. 2017, 497, 172–180. [Google Scholar] [CrossRef]
- Ejaz, A.; Joo, Y.; Jeon, S. Fabrication of 1,4-bis(aminomethyl)benzene and cobalt hydroxide @ graphene oxide for selective detection of dopamine in the presence of ascorbic acid and serotonin. Sens. Actuators B Chem. 2017, 240, 297–307. [Google Scholar]
- Goyal, R.N.; Gupta, V.K.; Oyama, M.; Bachheti, N. Gold nanoparticles modified indium tin oxide electrode for the simultaneous determination of dopamine and serotonin: Application in pharmaceutical formulations and biological fluids. Talanta 2007, 72, 976–983. [Google Scholar]
- Nuengmatcha, P.; Mahachai, R.; Chanthai, S. Removal of Hg(II) from Aqueous Solution Using Graphene Oxide as Highly Potential Adsorbent. Asian J. Chem. 2014, 26, S85–S88. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
Sample Availability: Not available. |






| Method | Linear Range (µM) | Correlation Coefficient (R2) | Detection Limit (µM) | References |
|---|---|---|---|---|
| TBABr/SDS⋅⋅⋅AuNPs/GO/GCE | 0.02–1.00 | 0.9960 and 0.9910 | 0.010 | Present work |
| AuNPs/rGO/Pt wire | 0.05–3.0 | 0.9980 | 0.016 | [56] |
| AuNPs/Gr/OPPy-MIP/GCE | 0.5–8.0 | 0.9900 | 0.10 | [57] |
| Cubic Pd/RGO/GCE | 0.45–421 | 0.9968 | 0.18 | [58] |
| Co(OH)2/BAMB/GO | 3–20 and 25–100 | 0.9905 0.9944 | 0.40 | [59] |
| MWCNTs/EDAS/AuNPs/GCE | 0.5–50 | 0.9968 | 0.08 | [37] |
| AuNPs/MWCNTs/GCE | 0.06–8 | 0.9984 | 0.04 | [39] |
| AuNPs/PTAP/GCE | 0.15–1.5 | 0.9925 | 0.017 | [42] |
| Analyte | Human Serum Sample 1 (n = 3) | Human Serum Sample 2 (n = 3) | |||
|---|---|---|---|---|---|
| Spiked (µM) | Found (µM) | Recovery (%) | Found (µM) | Recovery (%) | |
| DA | - | nd * | - | nd * | - |
| 0.10 | 0.097 | 97.26 ± 8.55 | 0.104 | 104.21 ± 6.01 | |
| 0.20 | 0.207 | 103.66 ± 3.41 | 0.199 | 99.50 ± 1.33 | |
| 0.30 | 0.303 | 101.19 ± 3.68 | 0.302 | 100.73 ± 2.64 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uppachai, P.; Srijaranai, S.; Poosittisak, S.; Md Isa, I.; Mukdasai, S. Supramolecular Electrochemical Sensor for Dopamine Detection Based on Self-Assembled Mixed Surfactants on Gold Nanoparticles Deposited Graphene Oxide. Molecules 2020, 25, 2528. https://doi.org/10.3390/molecules25112528
Uppachai P, Srijaranai S, Poosittisak S, Md Isa I, Mukdasai S. Supramolecular Electrochemical Sensor for Dopamine Detection Based on Self-Assembled Mixed Surfactants on Gold Nanoparticles Deposited Graphene Oxide. Molecules. 2020; 25(11):2528. https://doi.org/10.3390/molecules25112528
Chicago/Turabian StyleUppachai, Pikaned, Supalax Srijaranai, Suta Poosittisak, Illyas Md Isa, and Siriboon Mukdasai. 2020. "Supramolecular Electrochemical Sensor for Dopamine Detection Based on Self-Assembled Mixed Surfactants on Gold Nanoparticles Deposited Graphene Oxide" Molecules 25, no. 11: 2528. https://doi.org/10.3390/molecules25112528
APA StyleUppachai, P., Srijaranai, S., Poosittisak, S., Md Isa, I., & Mukdasai, S. (2020). Supramolecular Electrochemical Sensor for Dopamine Detection Based on Self-Assembled Mixed Surfactants on Gold Nanoparticles Deposited Graphene Oxide. Molecules, 25(11), 2528. https://doi.org/10.3390/molecules25112528






