Non-Functionalized Fullerenes and Endofullerenes in Aqueous Dispersions as Superoxide Scavengers
Abstract
1. Introduction
2. Results
2.1. Preparation, Purification, and Characterization of Aqueous Fullerene Dispersions
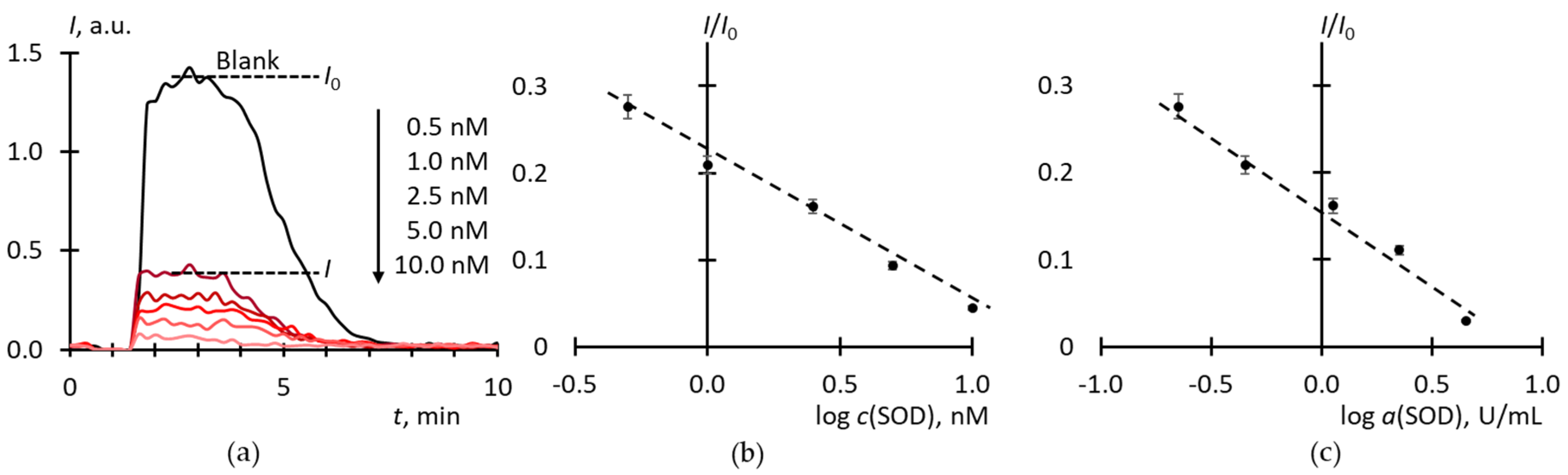
2.2. Superoxide Scavenging by SOD
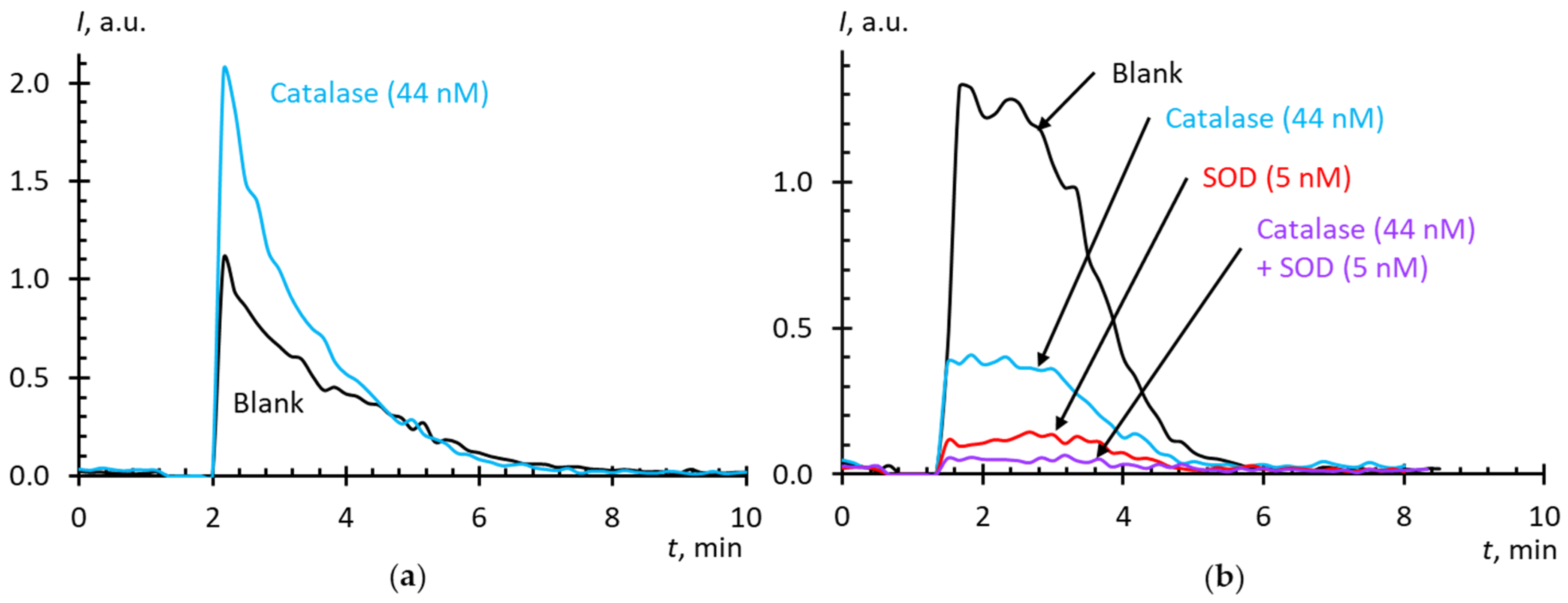
2.3. Blank Experiments
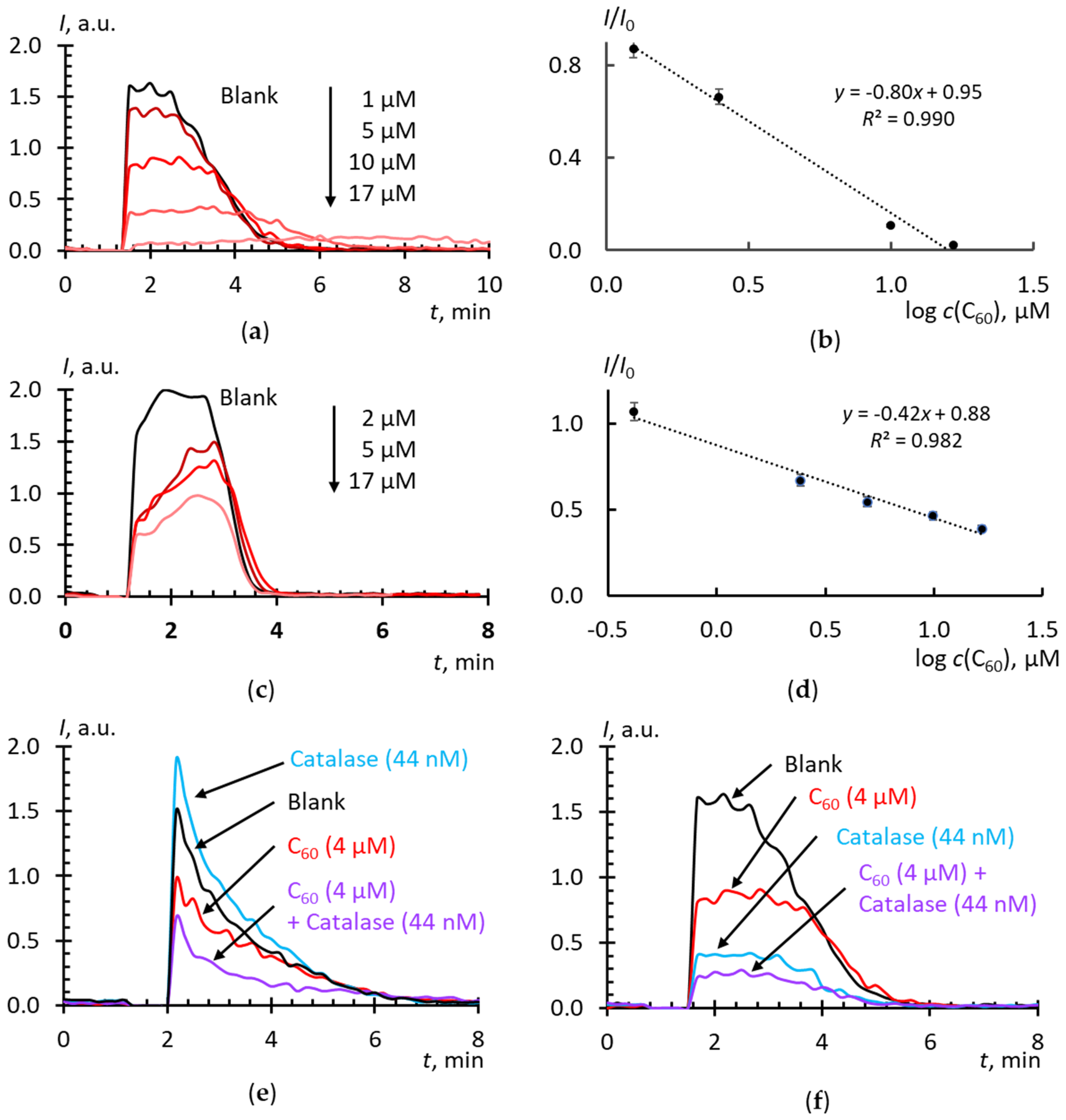
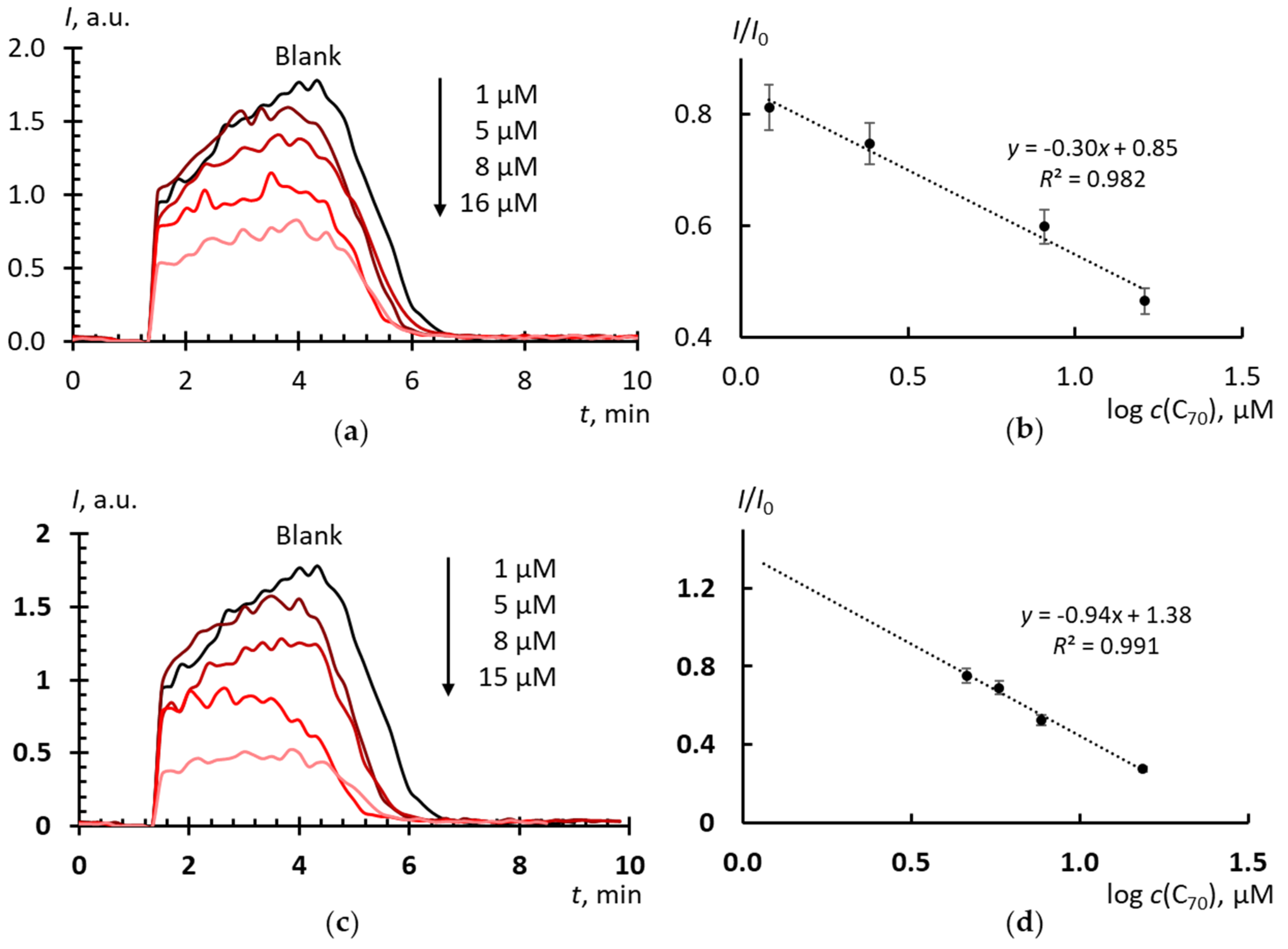
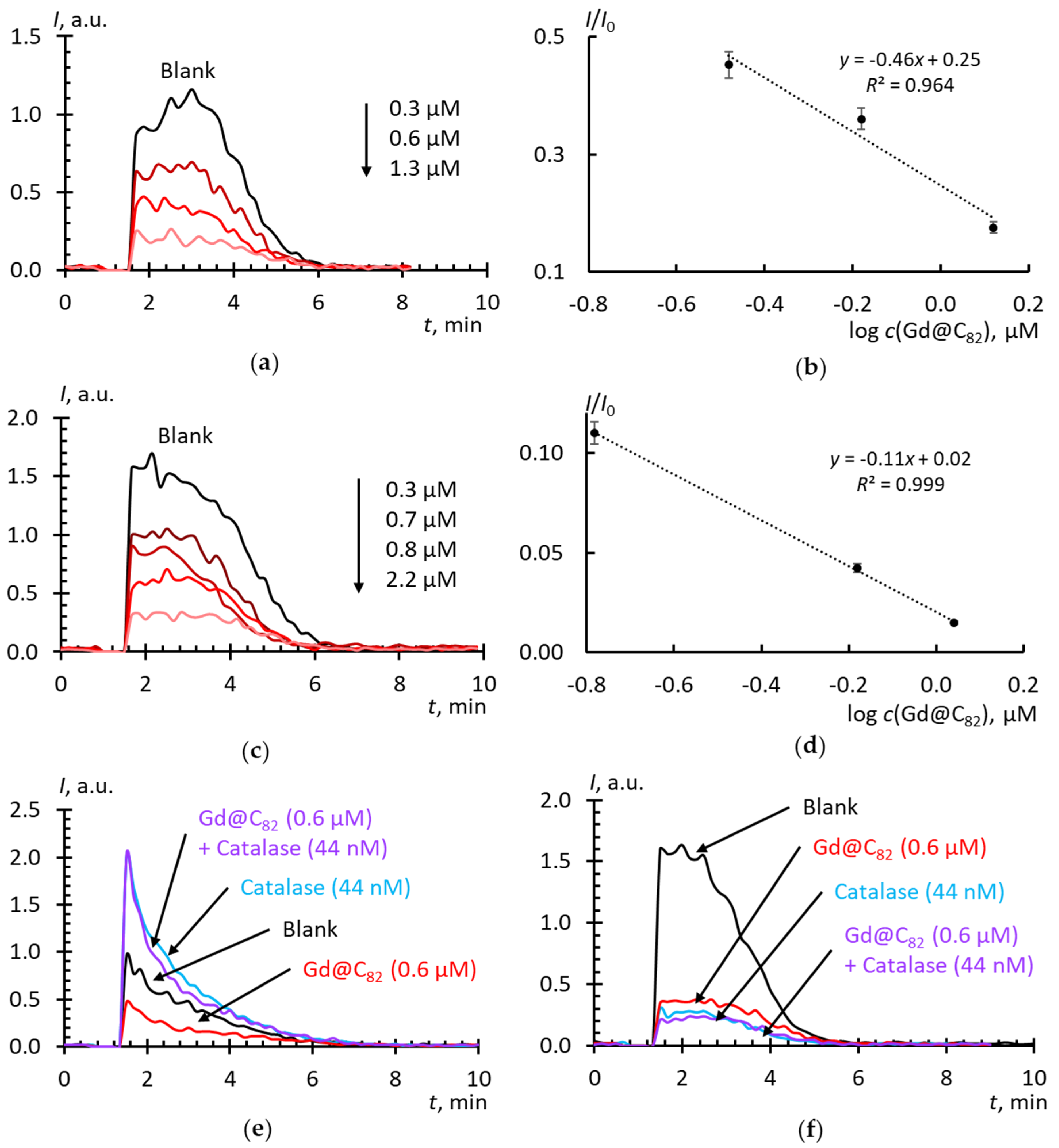
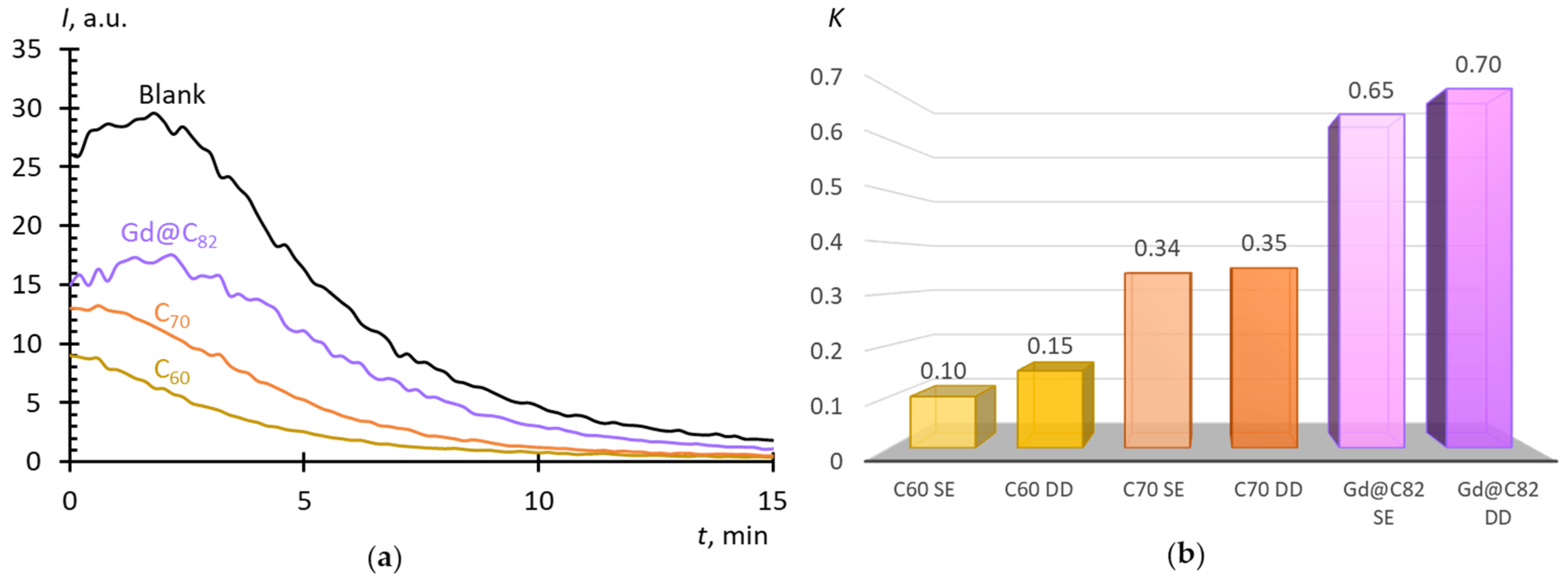
2.4. Superoxide Scavenging by Fullerenes in Aqueous Dispersions
2.5. Superoxide Scavenging Potential of Aqueous Fullerene Dispersions in Cells
3. Discussion
- The AFDs prepared by solvent replacement have a slightly higher antioxidant activity than those prepared by direct dispersion.
- With both methods of AFD preparation, fullerenes can be arranged in the row Gd@C82 > C60 > C70 with respect to the ability to scavenge SAR; and C60 and C70 differ in the mechanism of interaction with SAR from SOD, which allows them to be rather considered superoxide scavengers, in contrast to Gd@C82, which, presumably, is a SOD mimic.
- With respect to the intracellular SAR, the activity of fullerenes decreases in the row C60 > C70> Gd@C82.
3.1. Preparation Procedure
3.2. Chemiluminescence System Based on Xanthine/Xanthine Oxidase
3.3. Activity Mechanisms
3.4. Intracellular SAR-Scavenging Activity
4. Materials and Methods
4.1. Chemicals
4.2. Sample Preparation and Characterization
4.2.1. Preparation of Aqueous Fullerene Dispersions by Direct Ultrasound Probe Sonication
4.2.2. Preparation of Aqueous Fullerene Dispersions by Solvent-Replacement Ultrasound Probe Sonication
4.2.3. Characterization
4.3. Chemiluminescence Experiments
4.3.1. Chemiluminescence Analysis of Superoxide Scavenging Potential
4.3.2. NADH-Stimulated Lucigenin-Enhanced Chemiluminescence of Human Fibroblasts
4.4. Cluster Parameters in Aqueous Fullerene Dispersions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- Sies, H. Oxidative Stress; Elsevier Science: London, UK, 2013. [Google Scholar]
- Ray, P.D.; Huang, B.W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 1–21. [Google Scholar] [CrossRef] [PubMed]
- McCord, J.M.; Edeas, M.A. SOD, oxidative stress and human pathologies: A brief history and a future vision. Biomed. Pharmacother. 2005, 59, 139–142. [Google Scholar] [CrossRef]
- Sheng, Y.; Abreu, I.A.; Cabelli, D.E.; Maroney, M.J.; Miller, A.F.; Teixeira, M.; Valentine, J.S. Superoxide dismutases and superoxide reductases. Chem Rev. 2014, 114, 3854–3918. [Google Scholar] [CrossRef]
- Salvemini, D.; Muscoli, C.; Riley, D.P.; Cuzzocrea, S. Superoxide dismutase mimetics. Pulm. Pharmacol. Ther. 2002, 15, 439–447. [Google Scholar] [CrossRef]
- Batinic-Haberle, I.; Tovmasyan, A.; Roberts, E.R.; Vujaskovic, Z.; Leong, K.W.; Spasojevic, I. SOD therapeutics: Latest insights into their structure-activity relationships and impact on the cellular redox-based signaling pathways. Antioxid. Redox Signal. 2014, 20, 2372–2415. [Google Scholar] [CrossRef]
- Bonetta, R. Potential therapeutic applications of MnSODs and SOD-mimetics. Chemistry 2018, 24, 5032–5041. [Google Scholar] [CrossRef]
- Wei, H.; Wang, E. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes. Chem. Soc. Rev. 2013, 42, 6060–6093. [Google Scholar] [CrossRef]
- Madkour, L.H. Antioxidants, Therapeutic Options, and Regulation of the Immune Responses. In Nanoparticles Induce Oxidative and Endoplasmic Reticulum Stresses; Springer International Publishing: Cham, Switzerland, 2020; pp. 631–665. [Google Scholar]
- Wu, J.; Wang, X.; Wang, Q.; Lou, Z.; Li, S.; Zhu, Y.; Qin, L.; Wei, H. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes (II). Chem. Soc. Rev. 2019, 48, 1004–1076. [Google Scholar] [CrossRef]
- Yan, W.; Seifermann, S.M.; Pierrat, P.; Brase, S. Synthesis of highly functionalized C60 fullerene derivatives and their applications in material and life sciences. Org. Biomol. Chem. 2015, 13, 25–54. [Google Scholar] [CrossRef] [PubMed]
- Dugan, L.L.; Turetsky, D.M.; Du, C.; Lobner, D.; Wheeler, M.; Almli, C.R.; Shen, C.K.; Luh, T.Y.; Choi, D.W.; Lin, T.S. Carboxyfullerenes as neuroprotective agents. Proc. Natl. Acad. Sci. USA 1997, 94, 9434–9439. [Google Scholar] [CrossRef] [PubMed]
- Yan, X. Nanozymology: Connecting Biology and Nanotechnology. Springer: Singapore, 2020. [Google Scholar]
- Li, R.; Zhen, M.; Guan, M.; Chen, D.; Zhang, G.; Ge, J.; Gong, P.; Wang, C.; Shu, C. A novel glucose colorimetric sensor based on intrinsic peroxidase-like activity of C60-carboxyfullerenes. Biosens Bioelectron 2013, 47, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.S.; Hardt, J.I.; Quick, K.L.; Kim-Han, J.S.; Erlanger, B.F.; Huang, T.T.; Epstein, C.J.; Dugan, L.L. A biologically effective fullerene (C60) derivative with superoxide dismutase mimetic properties. Free Radic. Biol. Med. 2004, 37, 1191–1202. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, G.D.; Roursgaard, M.; Jensen, K.A.; Poulsen, S.S.; Larsen, S.T. In vivo biology and toxicology of fullerenes and their derivatives. Basic Clin. Pharmacol. Toxicol. 2008, 103, 197–208. [Google Scholar] [CrossRef]
- Aschberger, K.; Johnston, H.J.; Stone, V.; Aitken, R.J.; Tran, C.L.; Hankin, S.M.; Peters, S.A.; Christensen, F.M. Review of fullerene toxicity and exposure--appraisal of a human health risk assessment, based on open literature. Regul. Toxicol. Pharmacol. Rtp 2010, 58, 455–473. [Google Scholar] [CrossRef]
- Gharbi, N.; Pressac, M.; Hadchouel, M.; Szwarc, H.; Wilson, S.R.; Moussa, F. [60]fullerene is a powerful antioxidant in vivo with no acute or subacute toxicity. Nano. Lett. 2005, 5, 2578–2585. [Google Scholar] [CrossRef]
- Yin, J.J.; Lao, F.; Fu, P.P.; Wamer, W.G.; Zhao, Y.; Wang, P.C.; Qiu, Y.; Sun, B.; Xing, G.; Dong, J.; et al. The scavenging of reactive oxygen species and the potential for cell protection by functionalized fullerene materials. Biomaterials 2009, 30, 611–621. [Google Scholar] [CrossRef]
- Hudhomme, P.; O’Brien, P.; De La Puente, F.L.; Kroto, H.; Hiroshi, I.; Nierengarten, J.F.; Craighead, H.; Guldi, D.; Paolucci, F.; Burley, G. Fullerenes: Principles and Applications; Royal Society of Chemistry: Cambridge, UK, 2011. [Google Scholar]
- Miyazawa, K.; Ochiai, Y.; Tachibana, M.; Kizuka, T.; Nakamura, S. Fullerene Nanowhiskers; Jenny Stanford Publishing: Singapore, 2019. [Google Scholar]
- Jorio, A. Bioengineering Applications of Carbon Nanostructures; Springer International Publishing: Cham, Switzerland, 2015. [Google Scholar]
- Lin, A.M.; Fang, S.F.; Lin, S.Z.; Chou, C.K.; Luh, T.Y.; Ho, L.T. Local carboxyfullerene protects cortical infarction in rat brain. Neurosci. Res. 2002, 43, 317–321. [Google Scholar] [CrossRef]
- Hayyan, M.; Hashim, M.A.; AlNashef, I.M. Superoxide ion: Generation and chemical implications. Chem. Rev. 2016, 116, 3029–3085. [Google Scholar] [CrossRef]
- Braun, K.; Dunsch, L.; Pipkorn, R.; Bock, M.; Baeuerle, T.; Yang, S.; Waldeck, W.; Wiessler, M. Gain of a 500-fold sensitivity on an intravital MR contrast agent based on an endohedral gadolinium-cluster-fullerene-conjugate: A new chance in cancer diagnostics. Int. J. Med. Sci. 2010, 7, 136–146. [Google Scholar] [CrossRef][Green Version]
- Shinohara, H.; Tagmatarchis, N.; Kroto, H. Endohedral Metallofullerenes: Fullerenes with Metal Inside; Wiley: Hoboken, NJ, USA, 2015. [Google Scholar]
- Chen, C.; Xing, G.; Wang, J.; Zhao, Y.; Li, B.; Tang, J.; Jia, G.; Wang, T.; Sun, J.; Xing, L.; et al. Multihydroxylated [Gd@C82(OH)22]n nanoparticles: Antineoplastic activity of high efficiency and low toxicity. Nano Lett. 2005, 5, 2050–2057. [Google Scholar] [CrossRef]
- Ko, W.B.; Heo, J.Y.; Nam, J.H.; Lee, K.B. Synthesis of a water-soluble fullerene [C60] under ultrasonication. Ultrasonics 2004, 41, 727–730. [Google Scholar] [CrossRef] [PubMed]
- Mikheev, I.V.; Khimich, E.S.; Rebrikova, A.T.; Volkov, D.S.; Proskurnin, M.A.; Korobov, M.V. Quasi-equilibrium distribution of pristine fullerenes C60 and C70 in a water–toluene system. Carbon 2017, 111, 191–197. [Google Scholar] [CrossRef]
- Mikheev, I.V.; Kareev, I.E.; Bubnov, V.P.; Volkov, D.S.; Korobov, M.V.; Proskurnin, M.A. Aqueous dispersions of unmodified Y@C82 (C2v) endohedral metallofullerene. Chemistry Select. 2017, 2, 8936–8940. [Google Scholar] [CrossRef]
- Sadana, A.; Ahuja, S. Bioseparations of Proteins: Unfolding/Folding and Validations; Elsevier Science: San Diego, CA, USA, 1997. [Google Scholar]
- Taurozzi, J.S.; Hackley, V.A.; Wiesner, M. Preparation of nanoparticle dispersions from powdered material using ultrasonic disruption. Nist Spec. Publ. 2012, 1200, 1200–1202. [Google Scholar]
- Storch, J.; Ferber, E. Detergent-amplified chemiluminescence of lucigenin for determination of superoxide anion production by NADPH oxidase and xanthine oxidase. Anal. Biochem 1988, 169, 262–267. [Google Scholar] [CrossRef]
- Qi, J.; Sun, L.Q.; Qian, S.Y.; Yu, B.Y. A novel multi-hyphenated analytical method to simultaneously determine xanthine oxidase inhibitors and superoxide anion scavengers in natural products. Anal. Chim Acta 2017, 984, 124–133. [Google Scholar] [CrossRef]
- Radi, R.; Tan, S.; Prodanov, E.; Evans, R.A.; Parks, D.A. Inhibition of xanthine oxidase by uric acid and its influence on superoxide radical production. Biochim Biophys Acta 1992, 1122, 178–182. [Google Scholar] [CrossRef]
- Lynch, R.E.; Fridovich, I. Autoinactivation of xanthine oxidase: The role of superoxide radical and hydrogen peroxide. Biochim Biophys Acta 1979, 571, 195–200. [Google Scholar] [CrossRef]
- Link, E.M.; Riley, P.A. Role of hydrogen peroxide in the cytotoxicity of the xanthine/xanthine oxidase system. Biochem J. 1988, 249, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Lacy, F.; Gough, D.A.; Schmid-Schonbein, G.W. Role of xanthine oxidase in hydrogen peroxide production. Free Radic. Biol. Med. 1998, 25, 720–727. [Google Scholar] [CrossRef]
- Radi, R.A.; Rubbo, H.; Prodanov, E. Comparison of the effects of superoxide dismutase and cytochrome c on luminol chemiluminescence produced by xanthine oxidase-catalyzed reactions. Biochim. Biophys. Acta 1989, 994, 89–93. [Google Scholar] [CrossRef]
- Bedouhene, S.; Moulti-Mati, F.; Hurtado-Nedelec, M.; Dang, P.M.; El-Benna, J. Luminol-amplified chemiluminescence detects mainly superoxide anion produced by human neutrophils. Am. J. Blood Res. 2017, 7, 41–48. [Google Scholar]
- Baker, M.A.; Krutskikh, A.; Curry, B.J.; Hetherington, L.; Aitken, R.J. Identification of cytochrome-b5 reductase as the enzyme responsible for NADH-dependent lucigenin chemiluminescence in human spermatozoa. Biol. Reprod. 2005, 73, 334–342. [Google Scholar] [CrossRef]
- Schepetkin, I.A. Lucigenin as a substrate of microsomal NAD(P)H-oxidoreductases. Biochemistry. Biokhimiia 1999, 64, 25–32. [Google Scholar] [PubMed]
- Yang, S.; Mulet, X.; Gengenbach, T.; Waddington, L.; Seeber, A.; Zhen, M.; Wang, C.; Muir, B.W.; Such, G.K.; Hao, X. Limitations with solvent exchange methods for synthesis of colloidal fullerenes. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2017, 514, 21–31. [Google Scholar] [CrossRef]
- Brant, J.A.; Labille, J.; Bottero, J.Y.; Wiesner, M.R. Characterizing the impact of preparation method on fullerene cluster structure and chemistry. Langmuir 2006, 22, 3878–3885. [Google Scholar] [CrossRef]
- Nath, S.; Pal, H.; Sapre, A.V. Effect of solvent polarity on the aggregation of fullerenes: A comparison between C60 and C70. Chem. Phys. Lett. 2002, 360, 422–428. [Google Scholar] [CrossRef]
- Colmenares, J.C.; Chatel, G. Sonochemistry: From Basic Principles to Innovative Applications; Springer International Publishing: Cham, Switzerland, 2017. [Google Scholar]
- Mikheev, I.V.; Pirogova, M.O.; Bolotnik, T.A.; Volkov, D.S.; Korobov, M.V.; Proskurnin, M.A. Optimization of the solvent-exchange process for high-yield synthesis of aqueous fullerene dispersions. Nanosyst. Phys. Chem. Math. 2018, 1, 41–45. [Google Scholar] [CrossRef]
- Kluge, H.; Broz, J.; Eder, K. Effect of benzoic acid on growth performance, nutrient digestibility, nitrogen balance, gastrointestinal microflora and parameters of microbial metabolism in piglets. J. Anim. Physiol. Anim. Nutr. (Berl.) 2006, 90, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.; Packer, L.; Hänninen, O. Handbook of Oxidants and Antioxidants in Exercise; Elsevier Science: Amsterdam, Netherlands, 2000. [Google Scholar]
- Deguchi, S.; Alargova, R.G.; Tsujii, K. stable dispersions of fullerenes, C60 and C70, in water. Preparation and characterization. Langmuir 2001, 17, 6013–6017. [Google Scholar] [CrossRef]
- Avdeev, M.V.; Khokhryakov, A.A.; Tropin, T.V.; Andrievsky, G.V.; Klochkov, V.K.; Derevyanchenko, L.I.; Rosta, L.; Garamus, V.M.; Priezzhev, V.B.; Korobov, M.V.; et al. Structural features of molecular-colloidal solutions of C60 fullerenes in water by small-angle neutron scattering. Langmuir 2004, 20, 4363–4368. [Google Scholar] [CrossRef]
- Mikheev, I.V.; Usoltseva, L.O.; Ivshukov, D.A.; Volkov, D.S.; Korobov, M.V.; Proskurnin, M.A. Approach to the assessment of size-dependent thermal properties of disperse solutions: Time-resolved photothermal lensing of aqueous pristine fullerenes C60 and C70. J. Phys. Chem. C 2016, 120, 28270–28287. [Google Scholar] [CrossRef]
- Nagano, T.; Fridovich, I. Does the aerobic xanthine oxidase reaction generate singlet oxygen? Photochem. Photobiol. 1985, 41, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, C.; Fridovich, I. A mechanism for the production of ethylene from methional. The generation of the hydroxyl radical by xanthine oxidase. J. Biol. Chem. 1970, 245, 4641–4646. [Google Scholar]
- Bannister, J.V.; Bannister, W.H.; Hill, H.A.; Thornalley, P.J. Enhanced production of hydroxyl radicals by the xanthine-xanthine oxidase reaction in the presence of lactoferrin. Biochim Biophys Acta 1982, 715, 116–120. [Google Scholar] [CrossRef]
- Kuppusamy, P.; Zweier, J.L. Characterization of free radical generation by xanthine oxidase. Evidence for hydroxyl radical generation. J. Biol. Chem. 1989, 264, 9880–9884. [Google Scholar]
- Mori, H.; Arai, T.; Mori, K.; Tsutsui, H.; Makino, K. Use of M4PO and oxygen-17 in the study on hydroxyl radical generation in the hypoxanthine-xanthine oxidase reaction. Biochem. Mol. Biol. Int. 1994, 32, 523–529. [Google Scholar]
- Britigan, B.E.; Pou, S.; Rosen, G.M.; Lilleg, D.M.; Buettner, G.R. Hydroxyl radical is not a product of the reaction of xanthine oxidase and xanthine. The confounding problem of adventitious iron bound to xanthine oxidase. J. Biol. Chem. 1990, 265, 17533–17538. [Google Scholar]
- Pichorner, H.; Jessner, G.; Ebermann, R. tBOOH acts as a suicide substrate for catalase. Arch. Biochem Biophys 1993, 300, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Kono, Y.; Fridovich, I. Superoxide radical inhibits catalase. J. Biol. Chem. 1982, 257, 5751–5754. [Google Scholar]
- Miura, T.; Sakurai, K.; Ogiso, T. Enhanced lipid peroxidation of erythrocyte membranes and phosphatidylcholine liposomes induced by a xanthine oxidase system in the presence of catalase. Chem Pharm. Bull. (Tokyo) 1984, 32, 3227–3234. [Google Scholar] [CrossRef] [PubMed]
- Artemchik, V.D.; Kurchenko, V.P.; Metelitsa, D.I. Peroxidase activity of catalase with respect to aromatic amines. Biokhimiia 1985, 50, 826–832. [Google Scholar] [PubMed]
- Goyal, M.M.; Basak, A. Hydroxyl radical generation theory: A possible explanation of unexplained actions of mammalian catalase. Int. J. Biochem. Mol. Biol. 2012, 3, 282–289. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Goyal, M.M. Hydroxyl radical generation by mammalian catalase: A few experimental evidences. Biochem. Pharmacol. Open Access 2012, 1, 1000103. [Google Scholar] [CrossRef]
- Wilhelm, J.; Vilim, V. Variables in xanthine oxidase-initiated luminol chemiluminescence: Implications for chemiluminescence measurements in biological systems. Anal. Biochem. 1986, 158, 201–210. [Google Scholar] [CrossRef]
- Rost, M.; Karge, E.; Klinger, W. What do we measure with luminol-, lucigenin- and penicillin-amplified chemiluminescence? 1. Investigations with hydrogen peroxide and sodium hypochlorite. J. Biolumin Chemilumin 1998, 13, 355–363. [Google Scholar] [CrossRef]
- Riehl, T.E.; Malehorn, C.L.; Hinze, W.L. Characterisation and evaluation of the use of membrane mimetic agents to amplify chemiluminescence from the lucigenin-hydrogen peroxide reaction system. Analyst 1986, 111, 931–939. [Google Scholar] [CrossRef]
- Krusic, P.J.; Wasserman, E.; Keizer, P.N.; Morton, J.R.; Preston, K.F. Radical reactions of C60. Science 1991, 254, 1183–1185. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, J.; Ma, H.; Zhen, M.; Guo, J.; Wang, L.; Jiang, L.; Shu, C.; Wang, C. Biocompatible [60]/[70] fullerenols: Potent defense against oxidative injury induced by reduplicative chemotherapy. Acs Appl. Mater. Interfaces 2017, 9, 35539–35547. [Google Scholar] [CrossRef] [PubMed]
- Popov, A.A. Endohedral Fullerenes: Electron. Transfer and Spin; Springer International Publishing: Cham, Switzerland, 2017. [Google Scholar]
- Kimura, S.; Jellinck, P.H. Rat intestinal peroxidase: Inhibition by endogenous xanthine and xanthine oxidase. Arch. Biochem Biophys 1985, 241, 141–148. [Google Scholar] [CrossRef]
- Matsuoka, M.; Jin, J. Application of electrochemiluminescence for the evaluation of the antioxidant capacity of some phenolic compounds against superoxide anion radicals. Anal. Sci 2015, 31, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Bensasson, R.V.; Brettreich, M.; Frederiksen, J.; Gottinger, H.; Hirsch, A.; Land, E.J.; Leach, S.; McGarvey, D.J.; Schonberger, H. Reactions of e(-)(aq), CO(2)(*)(-), HO(*), O(2)(*)(-) and O(2)((1)delta(g)) with a dendro[60]fullerene and C(60)[C(COOH)(2)](n) (n = 2-6). Free Radic. Biol. Med. 2000, 29, 26–33. [Google Scholar] [CrossRef]
- Okuda, K.; Hirota, T.; Hirobe, M.; Nagano, T.; Mochizuki, M.; Mashino, T. Synthesis of various water-soluble G60 derivatives and their superoxide-quenching activity. Fuller. Sci. Technol. 2000, 8, 127–142. [Google Scholar] [CrossRef]
- Witte, P.; Beuerle, F.; Hartnagel, U.; Lebovitz, R.; Savouchkina, A.; Sali, S.; Guldi, D.; Chronakis, N.; Hirsch, A. Water solubility, antioxidant activity and cytochrome C binding of four families of exohedral adducts of C60 and C70. Org. Biomol. Chem. 2007, 5, 3599–3613. [Google Scholar] [CrossRef]
- Ali, S.S.; Hardt, J.I.; Dugan, L.L. SOD activity of carboxyfullerenes predicts their neuroprotective efficacy: A structure-activity study. Nanomedicine 2008, 4, 283–294. [Google Scholar] [CrossRef]
- Liu, G.F.; Filipovic, M.; Ivanovic-Burmazovic, I.; Beuerle, F.; Witte, P.; Hirsch, A. High catalytic activity of dendritic C60 monoadducts in metal-free superoxide dismutation. Angew. Chem. 2008, 47, 3991–3994. [Google Scholar] [CrossRef]
- Jalilov, A.S.; Zhang, C.; Samuel, E.L.; Sikkema, W.K.; Wu, G.; Berka, V.; Kent, T.A.; Tsai, A.L.; Tour, J.M. Mechanistic study of the conversion of superoxide to oxygen and hydrogen peroxide in carbon nanoparticles. Acs Appl. Mater. Interfaces 2016, 8, 15086–15092. [Google Scholar] [CrossRef]
- Wu, G.; Berka, V.; Derry, P.J.; Mendoza, K.; Kakadiaris, E.; Roy, T.; Kent, T.A.; Tour, J.M.; Tsai, A.L. Critical comparison of the superoxide dismutase-like activity of carbon antioxidant nanozymes by direct superoxide consumption kinetic measurements. Acs Nano 2019, 13, 11203–11213. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-S.; Conceicao, J.; Jin, C.; Smalley, R.E. Threshold photodetachment of cold C−60. Chem. Phys. Lett. 1991, 182, 5–11. [Google Scholar] [CrossRef]
- Boltalina, O.V.; Ioffe, I.N.; Sorokin, I.D.; Sidorov, L.N. Electron affinity of some endohedral lanthanide fullerenes. J. Phys. Chem. A 1997, 101, 9561–9563. [Google Scholar] [CrossRef]
- Ptasinska, S.; Echt, O.; Denifl, S.; Stano, M.; Sulzer, P.; Zappa, F.; Stamatovic, A.; Scheier, P.; Mark, T.D. Electron attachment to higher fullerenes and to Sc3N@C80. J. Phys. Chem. A 2006, 110, 8451–8456. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Kikuchi, K.; Oguri, F.; Nakao, Y.; Suzuki, S.; Achiba, Y.; Yamamoto, K.; Funasaka, H.; Takahashi, T. Electrochemical properties of fullerenolanthanides. Tetrahedron 1996, 52, 4973–4982. [Google Scholar] [CrossRef]
- Haufler, R.E.; Conceicao, J.; Chibante, L.P.F.; Chai, Y.; Byrne, N.E.; Flanagan, S.; Haley, M.M.; O’Brien, S.C.; Pan, C. Efficient production of C60 (buckminsterfullerene), C60H36, and the solvated buckide ion. J. Phys. Chem. 1990, 94, 8634–8636. [Google Scholar] [CrossRef]
- Boudon, C.; Gisselbrecht, J.-P.; Gross, M.; Herrmann, A.; Rüttimann, M.; Crassous, J.; Cardullo, F.; Echegoyen, L.; Diederich, F. Redox characteristics of covalent derivatives of the higher fullerenes C70, C76, and C78. J. Am. Chem. Soc. 1998, 120, 7860–7868. [Google Scholar] [CrossRef]
- Popov, A.A.; Yang, S.; Dunsch, L. Endohedral fullerenes. Chem Rev. 2013, 113, 5989–6113. [Google Scholar] [CrossRef]
- Shinohara, H. Endohedral metallofullerenes. Rep. Prog. Phys. 2000, 63, 843–892. [Google Scholar] [CrossRef]
- Senapati, L.; Schrier, J.; Whaley, K.B. Electronic transport, structure, and energetics of endohedral Gd@C82 metallofullerenes. Nano Lett. 2004, 4, 2073–2078. [Google Scholar] [CrossRef]
- Spurlin, T.A.; Gewirth, A.A. Effect of C60 on solid supported lipid bilayers. Nano Lett. 2007, 7, 531–535. [Google Scholar] [CrossRef]
- Ikeda, A.; Matsumoto, M.; Akiyama, M.; Kikuchi, J.; Ogawa, T.; Takeya, T. Direct and short-time uptake of [70]fullerene into the cell membrane using an exchange reaction from a [70]fullerene-gamma-cyclodextrin complex and the resulting photodynamic activity. Chem. Commun. (Camb.) 2009, 12, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.C.; Moghadam, B.Y.; Westerhoff, P.; Posner, J.D. Distribution of fullerene nanomaterials between water and model biological membranes. Langmuir 2011, 27, 11899–11905. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Zhao, L.; Kang, S.G.; Pan, J.; Song, Y.; Zhang, M.; Xing, G.; Wang, F.; Li, J.; Zhou, R.; et al. Impacts of fullerene derivatives on regulating the structure and assembly of collagen molecules. Nanoscale 2013, 5, 7341–7348. [Google Scholar] [CrossRef]
- Bubnov, V.P.; Laukhina, E.E.; Kareev, I.E.; Koltover, V.K.; Prokhorova, T.G.; Yagubskii, E.B.; Kozmin, Y.P. Endohedral metallofullerenes: A convenient gram-scale preparation. Chem. Mater. 2002, 14, 1004–1008. [Google Scholar] [CrossRef]
- Lakshmi, D.; Whitcombe, M.J.; Davis, F.; Sharma, P.S.; Prasad, B.B. Electrochemical detection of uric acid in mixed and clinical samples: A review. Electroanalysis 2011, 23, 305–320. [Google Scholar] [CrossRef]
- Goel, A.; Howard, J.B.; Vander Sande, J.B. Size analysis of single fullerene molecules by electron microscopy. Carbon 2004, 42, 1907–1915. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Dresselhaus, G.; Eklund, P.C. Science of Fullerenes and Carbon Nanotubes: Their Properties and Applications; Elsevier Science: San Diego, CA, USA, 1996. [Google Scholar]
- Rao, C.N.R.; Govindaraj, A.; O’Brien, P.; Kroto, H.; Craighead, H. Nanotubes and Nanowires; Royal Society of Chemistry: Cambridge, UK, 2015. [Google Scholar]
Sample Availability: Samples of the aqueous dispersions of fullerenes and endofullerene compounds are available from the authors. |

| Fullerene | Preparation Technique | c;, mM | Particle Size after a 0.22 μm Filter, nm | ζ-Potential, mV | ć × 10–13, Clusters/L | ć, pM | nc × 10–6 | nc,surf × 10–4 | η,% | ć, act, μM |
|---|---|---|---|---|---|---|---|---|---|---|
| C60 | Direct sonication | 0.0831 | 110 ± 5 | –28.4 ± 0.2 | 1.40 | 23.2 | 3.6 | 5.7 | 1.60 | 1.3 |
| C60 | Solvent-replacement sonication | 0.0901 | 100 ± 3 | –29.0 ± 0.3 | 2.01 | 33.5 | 2.7 | 4.7 | 1.76 | 1.6 |
| C70 | Direct sonication | 0.0811 | 113 ± 2 | −29.5 ± 0.3 | 2.30 | 38.2 | 2.1 | 4.0 | 1.90 | 1.5 |
| C70 | Solvent-replacement sonication | 0.0771 | 111 ± 3 | –30.9 ± 0.3 | 2.32 | 38.5 | 2.0 | 3.9 | 1.94 | 1.5 |
| Gd@C82 | Direct sonication of solid Gd@C82-enriched sample | 0.0222 | 95 ± 5 | –32.3 ± 0.3 | 6.07 | 100.1 | 0.20 | 0.88 | 4.07 | 0.89 |
| Gd@C82 | Solvent-replacement sonication of toluene HPLC-grade Gd@C82 solution | 0.0112 | 90 ± 2 | –25.2 ± 0.3 | 3.43 | 59.9 | 0.18 | 0.79 | 4.29 | 0.45 |
| Sample | Calibration Functions I/I0 vs.c (or a for SOD) | Calibration Functions I/I0 vs. Active Cluster Concentration (ć, act) | Concentration of Semi-Suppression of Reference (Blank) CL (c1/2) | Rel. to SOD Efficiency, × 106 |
|---|---|---|---|---|
| SOD | I/I0 = (–0.18 ± 0.01) × a(U/mL) + (0.16 ± 0.03), r = 0.9820 | — | 0.03 ± 0.005 nM | — |
| AFD C60 (direct dispergation) | I/I0 = (–0.80 ± 0.09) × c (µM) + (0.96 ± 0.06), r = 0.9980 | I/I0 = (–0.60 ± 0.06) × c (µM) – (0.36 ± 0.06), r = 0.9952 | 4.0 ± 0.1 µM | 7.5 |
| AFD C60 (solvent replacement) | I/I0 = (–0.42 ± 0.03) × c (µM) + (0.87 ± 0.5), r = 0.9960 | I/I0 = (–0.27 ± 0.03) × c (µM) + (0.70 ± 0.22), r = 0.9771 | 2.0 ± 0.4 µM | 15 |
| AFD C70 (direct dispergation) | I/I0 = (–0.30 ± 0.02) × c (µM) + (0.85 ± 0.09), r = 0.9860 | I/I0 = (–0.30 ± 0.02) × c (µM) + (0.8 ± 0.0), r = 0.9913 | 14.5 ± 2.1 µM | 2 |
| AFD C70 (solvent replacement) | I/I0 = (–0.94 ± 0.08) × c (µM) + (1.34 ± 0.3), r = 0.9980 | I/I0 = (–0.94 ± 0.08) × c (µM) + (0.23 ± 0.07), r = 0.9913 | 9.0 ± 0.7 µM | 3 |
| Gd@C82 (direct dispergation) | I/I0 = (–0.46 ± 0.3) × c (µM) + (0.25 ± 0.05), r = 0.9640 | I/I0 = (–0.46 ± 0.3) × c (µM) + (0.25 ± 0.05), r = 0.9818 | 0.28 ± 0.05 µM | 100 |
| Gd@C82 (solvent replacement) | I/I0 = (–0.12 ± 0.01) × c (µM) + (0.02 ± 0.002), r = 0.9980 | /I0 = (–0.12 ± 0.02) × c (µM) + (0.13 ± 0.04), r = 0.9998 | 0.07 ± 0.005 µM | 400 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
V. Mikheev, I.; M. Sozarukova, M.; V. Proskurnina, E.; E. Kareev, I.; A. Proskurnin, M. Non-Functionalized Fullerenes and Endofullerenes in Aqueous Dispersions as Superoxide Scavengers. Molecules 2020, 25, 2506. https://doi.org/10.3390/molecules25112506
V. Mikheev I, M. Sozarukova M, V. Proskurnina E, E. Kareev I, A. Proskurnin M. Non-Functionalized Fullerenes and Endofullerenes in Aqueous Dispersions as Superoxide Scavengers. Molecules. 2020; 25(11):2506. https://doi.org/10.3390/molecules25112506
Chicago/Turabian StyleV. Mikheev, Ivan, Madina M. Sozarukova, Elena V. Proskurnina, Ivan E. Kareev, and Mikhail A. Proskurnin. 2020. "Non-Functionalized Fullerenes and Endofullerenes in Aqueous Dispersions as Superoxide Scavengers" Molecules 25, no. 11: 2506. https://doi.org/10.3390/molecules25112506
APA StyleV. Mikheev, I., M. Sozarukova, M., V. Proskurnina, E., E. Kareev, I., & A. Proskurnin, M. (2020). Non-Functionalized Fullerenes and Endofullerenes in Aqueous Dispersions as Superoxide Scavengers. Molecules, 25(11), 2506. https://doi.org/10.3390/molecules25112506






