New Methyl Threonolactones and Pyroglutamates of Spilanthes acmella (L.) L. and Their Bone Formation Activities
Abstract
1. Introduction
2. Results and Discussion
2.1. Identification of Compounds
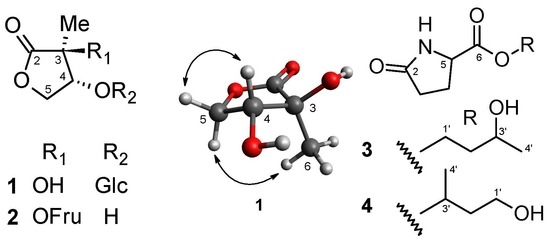
2.1.1. Structure of 2-C-methyl-d-threono-1,4-lactone-3-O-β-d-glucopyranoside (1)
2.1.2. Structure of 2-C-methyl-d-threono-1,4-lactone-3-O-α-d-fructofuranoside (2)
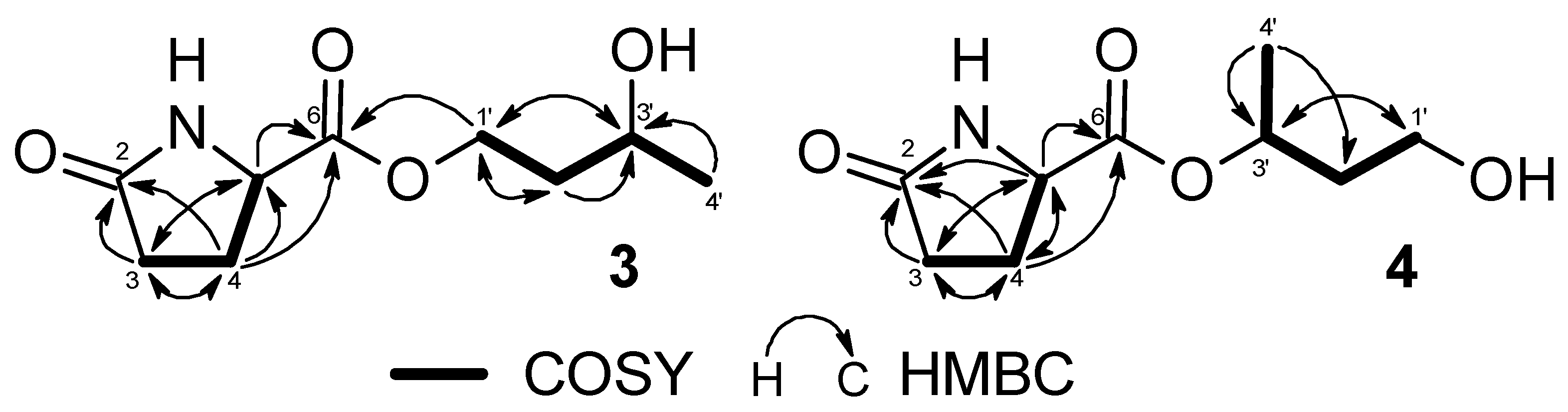
2.1.3. Structure of 1,3-butanediol-1-pyroglutamate (3)
2.1.4. Structure of 1,3-butanediol 3-pyroglutamate (4)
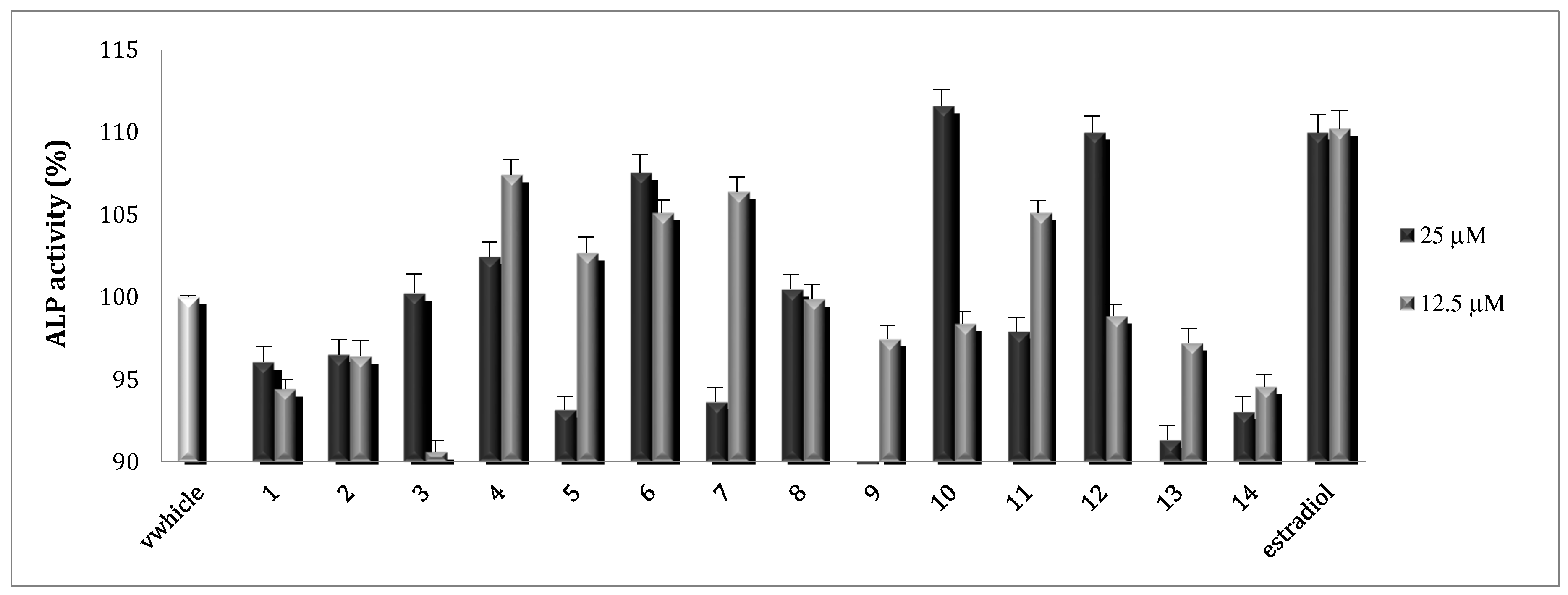
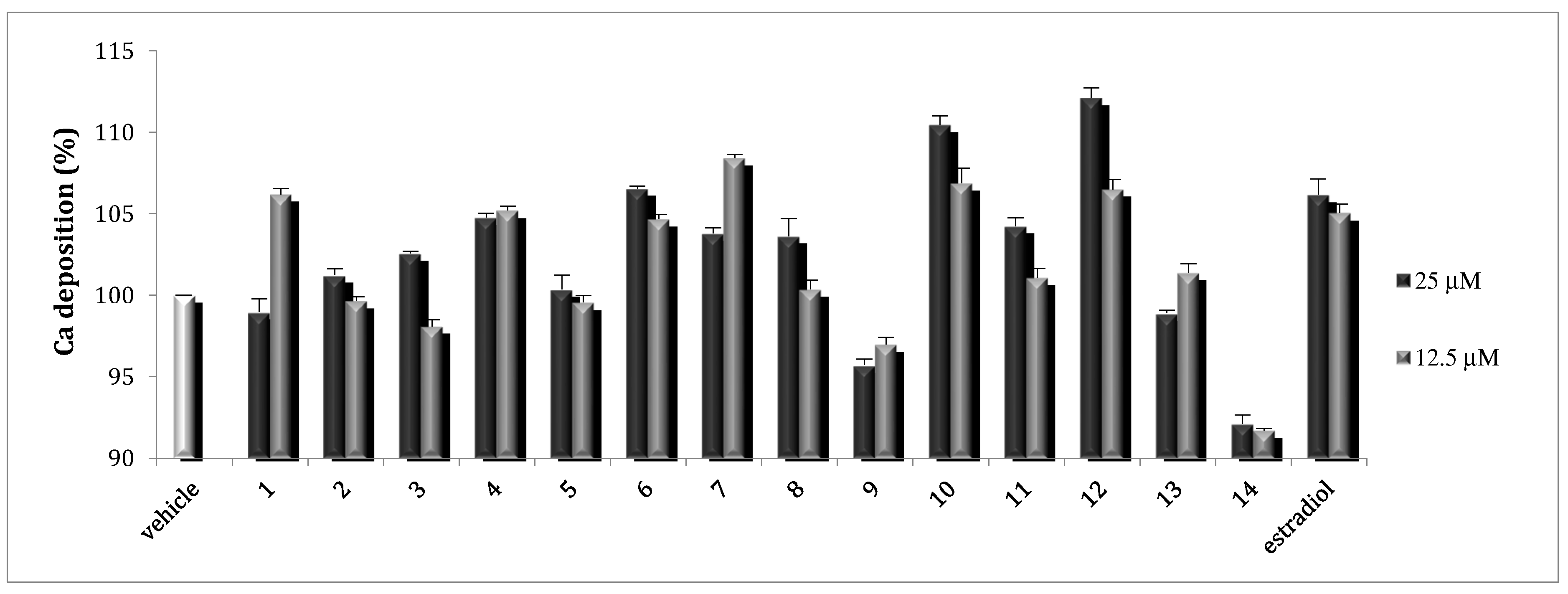
2.2. Osteoblast Activity
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.3.1. 2-C-methyl-d-threono-1,4-lactone-4-O-β-d-glucopyranoside (1)
3.3.2. 2-C-methyl-d-threono-1,4-lactone-3-O-α-d-fructofuranoside (2)
3.3.3. 1,3-butanediol-1-pyroglutamate (3)
3.3.4. 1,3-butanediol-3-pyroglutamate (4)
3.3.5. Acid Hydrolysis for Identification of Sugar Moiety of 1 and 2
3.4. Osteoblast Activities
3.4.1. Cell Culture
3.4.2. Alkaline Phosphatase (ALP) Activity
3.4.3. Mineralization of MC3T3-E1
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vandenbroucke, A.; Luyten, F.P.; Flamaing, J.; Gielen, E. Pharmacological treatment of osteoporosis in the oldest old. Clin. Interv. Aging 2017, 12, 1065–1077. [Google Scholar] [CrossRef]
- Sahu, J.; Jain, K.; Jain, B.; Sahu, R.K. A review on phytopharmacology and micropropagation of Spilanthes acmella. Pharmacologyonline 2011, 2, 1105–1110. [Google Scholar]
- Tiwari, K.L.; Jadhav, S.K.; Joshi, V. An updated review on medicinal herb genus Spilanthes. Chin. J. Integr. Med. 2011, 9, 1170–1178. [Google Scholar] [CrossRef]
- Sharma, V.; Boonen, J.; Chauhan, N.S.; Thakur, M.; Spiegeleer, B.D.; Dixit, V.K. Spilanthes acmella ethanolic flower extract: LC-MS alkylamide profiling and its effects on sexual behavior in male rats. Phytomedicine 2011, 18, 1161–1169. [Google Scholar] [CrossRef] [PubMed]
- Wongsawatkul, O.; Prachayasittikul, S.; Chartchalerm, I.N.A.; Satayavivad, J.; Ruchirawat, S.; Prachayasittikul, V. Vasorelaxant and antioxidant activities of Spilanthes acmella Murr. Int. J. Mol. Sci. 2008, 9, 2724–2744. [Google Scholar] [CrossRef] [PubMed]
- Ramsewak, R.S.; Erickson, A.J.; Nair, M.G. Bioactive N-isobutylamides from the flower buds of Spilanthes acmella. Phytochemistry 1999, 51, 729–732. [Google Scholar] [CrossRef]
- Storey, C.; Salem, J.I. Lay use of Amazonian plants for the treatment of tuberculosis. Acta Amaz. 1997, 27, 175–182. [Google Scholar] [CrossRef]
- Hossan, S.; Agarwala, B.; Sarwar, S.; Karim, M.; Jahan, R.; Rahmatullah, M. Traditional use of medicinal plants in Bangladesh to treat urinary tract infections and sexually transmitted diseases. Ethnobot. Res. Appl. 2010, 8, 61–74. [Google Scholar] [CrossRef]
- Arora, S.; Vijay, S.; Kumar, D. Phytochemical and antimicrobial studies on the leaves of Spilanthes acmella. J. Chem. Pharm. Res. 2011, 3, 145–150. [Google Scholar]
- Jondiko, I.J.O. A mosquito larvicide in Spilanthes mauritiana. Phytochemistry 1986, 25, 2289–2290. [Google Scholar] [CrossRef]
- Kadir, H.A.; Zakaria, M.B.; Kechil, A.A.; Azirun, M.S. Toxicity and electrophysiological effects of Spilanthes acmella Murr. extracts on Periplaneta americana L. Pest. Manag. Sci 1989, 25, 329–335. [Google Scholar] [CrossRef]
- Prachayasittikul, S.; Suphapong, S.; Worachartcheewan, A.; Lawung, R.; Ruchirawat, S.; Prachayasittikul, V. Bioactive metabolites from Spilanthes acmella Murr. Molecules 2009, 14, 850–867. [Google Scholar] [CrossRef] [PubMed]
- Narayana, K.R.; Reddy, M.S.; Chaluvadi, M.R.; Krishna, D.R. Bioflavonoids classification, pharmacological, biochemical effects and therapeutic potential. Indian J. Pharmacol. 2001, 33, 2–16. [Google Scholar]
- Laswati, H.; Subadi, I.; Widyowati, R.; Agil, M.; Pangkahila, J.A. Spilanthes acmella and physical exercise increased testosterone levels and osteoblast cells in glucocorticoid-induced osteoporosis male mice. Bali Med. J. 2015, 4, 76–81. [Google Scholar] [CrossRef]
- Widyowati, R. Alkaline phosphatase activity of Graptophyllum pictum and Spilanthes acmella fractions against MC3T3-E1 cells as marker of osteoblast differentiation cells. Int. J. Pharm. Pharm. Sci. 2011, 3, 34–37. [Google Scholar]
- Uriburu, M.L.; Gil, R.R.; Sosa, V.E.; Fuente, J.R. Caffeoyl esters of threonic acid and its lactone from Viguiera pazensis. J. Arg. Chem. Soc. 2008, 96, 55–61. [Google Scholar]
- Kitajima, J.; Ishikawa, T.; Tanaka, T.; Ida, Y. Water-soluble constituents of fennel IX. Glucides and nucleosides. Chem. Pharm. Bull. 1999, 47, 988–992. [Google Scholar] [CrossRef]
- Barraclough, P.; Dieterich, P.; Spray, C.A.; Young, D.W. Two separate and distinct syntheses of stereospecifically deuterated samples of (2S)-proline. Org. Biomol. Chem. 2006, 4, 1483–1491. [Google Scholar] [CrossRef]
- Otsuka, H.; Takeda, Y.; Yamasaki, K.; Takeda, Y. Structural elucidation of Dendranthemosides A and B: Two new β-ionone glucosides from Dendranthema shiwogiku. Planta Med. 1992, 58, 373–375. [Google Scholar] [CrossRef]
- Marino, S.D.; Borbone, N.; Zollo, F.; Lanaro, A.; Meglio, P.D.; Lorizzi, M. Megastigmane and phenolic components from Laurus nobilis L. leaves and their inhibitory effects on nitric oxide production. J. Agric. Food Chem. 2004, 52, 7525–7531. [Google Scholar] [CrossRef]
- Miyase, T.; Ueno, A.; Takizawa, N.; Kobayashi, H.; Karasawa, H. Studies on the glycosides of Epimedium grandiflorum MORR. Var. thunbergianum (MIQ.) NAKAI. I. Chem. Pharm. Bull. 1987, 35, 1109–1117. [Google Scholar] [CrossRef]
- Rosa, S.; Giulio, A.; Tommonaro, G. Aliphatic and aromatic glycosides from the cell cultures of Lycopersicon esculentum. Phytochemistry 1996, 42, 1031–1034. [Google Scholar] [CrossRef]
- Zhou, H.; Qin, M.; Hong, J.; Ni, Y.; Wu, G. Chemical constituents of Viola yedoensis. Chin. J. Nat. Med. 2009, 7, 290–292. [Google Scholar] [CrossRef]
- Chen, H.; Geng, C.; Cao, T.; Zhang, X.; Ma, Y.; Huang, X.; Chen, J. Chemical structure of capsicuoside A from fruits of Capsium annum. China J. Chin. Mater. Med. 2013, 38, 1934–1937. [Google Scholar]
- Tanaka, T.; Nakashima, T.; Ueda, T.; Tomii, K.; Kouno, I. Facile discrimination of aldose enantiomers by reversed-phase HPLC. Chem. Pharm. Bull. 2007, 55, 899–901. [Google Scholar] [CrossRef]
- Kasai, R.; Suzuno, M.; Asakawa, J.; Tanaka, O. Carbon-13 chemical shifts of isoprenoid-β-d-glucopyranosides and -β-d-mannopyranosides. Stereochemical influences of aglycone alcohols. Tetrahedron. Lett. 1977, 18, 175–178. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, D.; Zhao, Y.; Gao, H.; Hu, Y.; Hu, J. Fructose-derived carbohydrates from Alisma orientalis. Nat. Prod. Res. 2009, 23, 1013–1020. [Google Scholar] [CrossRef]
- Stein, G.S.; Lian, J.B. Molecular mechanisms mediating developmental and hormone-regulated expression of genes in osteoblasts: An integrated relationship of cell growth and differentiation. In Cellular and Molecular Biology of Bone; Noda, M., Ed.; Academic Press: Tokyo, Japan, 1993; pp. 47–95. [Google Scholar]
- Backer, C.A.; Bakhuizen van den Brink Jr, R.C. Flora of Java (Spermatophytes only); Noordhoff: Groningen, The Netherlands, 1965; p. 408. [Google Scholar]
- Heyne, K. Tumbuhan Berguna Indonesia III; Badan Litbang Departemen Kehutanan: Jakarta, Indonesia, 1987; p. 1456. [Google Scholar]
- Cuong, N.X.; Tuan, T.A.; Kiem, P.V.; Minh, C.V.; Choi, E.M.; Kim, Y.H. New cembranoid diterpenes from the Vietnamese soft coral Sarcophyton mililatensis stimulate osteoblastic differentiation in MC3T3-E1 cells. Chem. Pharm. Bull. 2008, 56, 988–992. [Google Scholar] [CrossRef]
- Hamazaki, T.; Suzuki, N.; Widyowati, R.; Miyahara, T.; Kadota, S.; Ochiai, H.; Hamazaki, K. The depressive effects of 5,8,11-eicosatrienoic acid (20:3n-9) on osteoblasts. Lipids 2009, 44, 97–102. [Google Scholar] [CrossRef]
- Widyowati, R.; Tezuka, Y.; Miyahara, T.; Awale, S.; Kadota, S. Alkaline phosphatase (ALP) enhancing iridoid glucosides from the Indonesian medicinal plant Barleria lupulina. Nat. Prod. Commun. 2010, 5, 1711–1716. [Google Scholar] [CrossRef]
- Yan, X.T.; Lee, S.H.; Li, W.; Sun, Y.N.; Yang, S.Y.; Jang, H.D.; Kim, Y.H. Evaluation of the antioxidant and anti-osteoporosis activities of chemical constituents of the fruits of Prunus mume. Food Chem. 2014, 156, 408–415. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1–14 are available from the authors. |
 1H–1H COSY and
1H–1H COSY and  HMBC correlations; (b) NOESY correlations.
HMBC correlations; (b) NOESY correlations.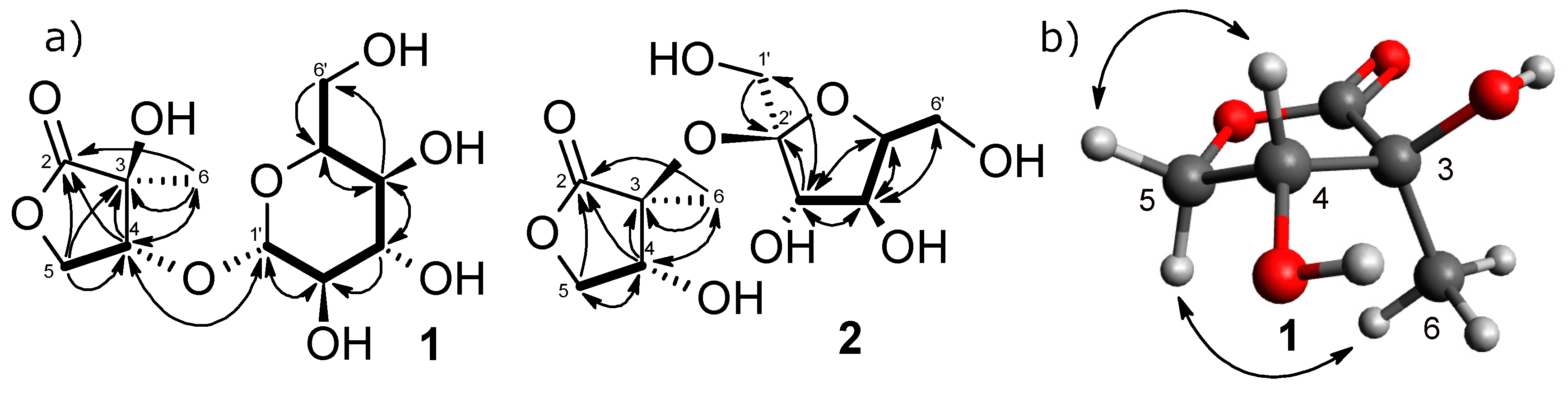
| Position | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| 3 | - | - | 2.31 ddd (17.1, 9.9, 5.5) 2.37 ddd (17.1, 9.4, 7.3) | 2.32 ddd (16.9, 9.7, 5.6) 2.37 ddd (16.9, 9.4, 7.1) |
| 4 | 4.43 t-like (6.5) | 4.17 dd (5.5, 4.4) | 2.16 m 2.48 m | 2.17 dddd (13.0, 9.4, 5.6, 4.7) 2.49 dddd (13.0, 9.7, 9.2, 7.1) |
| 5 | 4.10 dd (9.5, 6.4, α) 4.52 dd (9.5, 6.5, β) | 3.97 dd (9.4, 4.4, α) 4.48 dd (9.4, 5.5, β) | 4.29 dd (9.1, 4.4) | 4.24 dd (9.2, 4.7) |
| 6 | 1.41 s (3H) | 1.35 s (3H) | - | - |
| 1′ | 4.37 d (7.7) | 3.63 d (12.1) 3.71 d (12.1) | 4.27 m (2H) | 3.65 dt-like (10.6, 6.3) 3.68 dt-like (10.6, 6.7) |
| 2′ | 3.238 dd (9.2, 7.7) | - | 1.75 m 1.79 m | 1.62 dtd (13.8, 6.7, 4.8) 1.67 ddt (13.8, 7.7, 6.3) |
| 3′ | 3.36 t-like (9.1) | 4.04 d (4.2) | 3.86 dqd (8.2, 6.3, 4.4) | 3.89 dqd (7.7, 6.3, 4.8) |
| 4′ | 3.237 dd (9.8, 9.1) | 3.89 dd (6.4, 4.2) | 1.20 d (3H, 6.3) | 1.18 d (3H,6.3) |
| 5′ | 3.34 ddd (9.8, 7.4, 2.5) | 3.84 ddd (6.4, 4.9, 3.1) | ||
| 6′ | 3.60 dd (11.6, 7.4) 3.91 dd (11.6, 2.5) | 3.64 dd (11.9, 4.9) 3.78 dd (11.9, 3.1) |
| Position | 1 | 2 | 3 | 4 | 1a |
|---|---|---|---|---|---|
| 2 | 179.4 | 179.3 | 181.2 | 181.3 | 180.2 |
| 3 | 75.7 (±0) * | 75.9 | 30.4 | 30.6 | 75.7 |
| 4 | 83.4 (+7.4) * | 75.6 | 26.0 | 26.3 | 76.0 |
| 5 | 69.6 (−3.4) * | 72.9 | 57.3 | 57.4 | 73.0 |
| 6 | 19.2 | 17.6 | 174.2 | 176.2 | 17.7 |
| 1’ | 104.0 | 60.6 | 64.0 | 60.5 | |
| 2’ | 74.9 | 109.2 | 38.8 | 42.6 | |
| 3’ | 78.2 | 82.6 | 65.5 | 66.3 | |
| 4’ | 72.2 | 79.0 | 23.9 | 23.9 | |
| 5’ | 78.1 | 84.7 | |||
| 6’ | 63.2 | 62.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Widyowati, R.; Sulistyowaty, M.I.; Uyen, N.H.; Sugimoto, S.; Yamano, Y.; Otsuka, H.; Matsunami, K. New Methyl Threonolactones and Pyroglutamates of Spilanthes acmella (L.) L. and Their Bone Formation Activities. Molecules 2020, 25, 2500. https://doi.org/10.3390/molecules25112500
Widyowati R, Sulistyowaty MI, Uyen NH, Sugimoto S, Yamano Y, Otsuka H, Matsunami K. New Methyl Threonolactones and Pyroglutamates of Spilanthes acmella (L.) L. and Their Bone Formation Activities. Molecules. 2020; 25(11):2500. https://doi.org/10.3390/molecules25112500
Chicago/Turabian StyleWidyowati, Retno, Melanny Ika Sulistyowaty, Nguyen Hoang Uyen, Sachiko Sugimoto, Yoshi Yamano, Hideaki Otsuka, and Katsuyoshi Matsunami. 2020. "New Methyl Threonolactones and Pyroglutamates of Spilanthes acmella (L.) L. and Their Bone Formation Activities" Molecules 25, no. 11: 2500. https://doi.org/10.3390/molecules25112500
APA StyleWidyowati, R., Sulistyowaty, M. I., Uyen, N. H., Sugimoto, S., Yamano, Y., Otsuka, H., & Matsunami, K. (2020). New Methyl Threonolactones and Pyroglutamates of Spilanthes acmella (L.) L. and Their Bone Formation Activities. Molecules, 25(11), 2500. https://doi.org/10.3390/molecules25112500