The Effect of Bioactive Glass-Enhanced Orthodontic Bonding Resins on Prevention of Demineralization: A Systematic Review
Abstract
:1. Introduction
2. Results
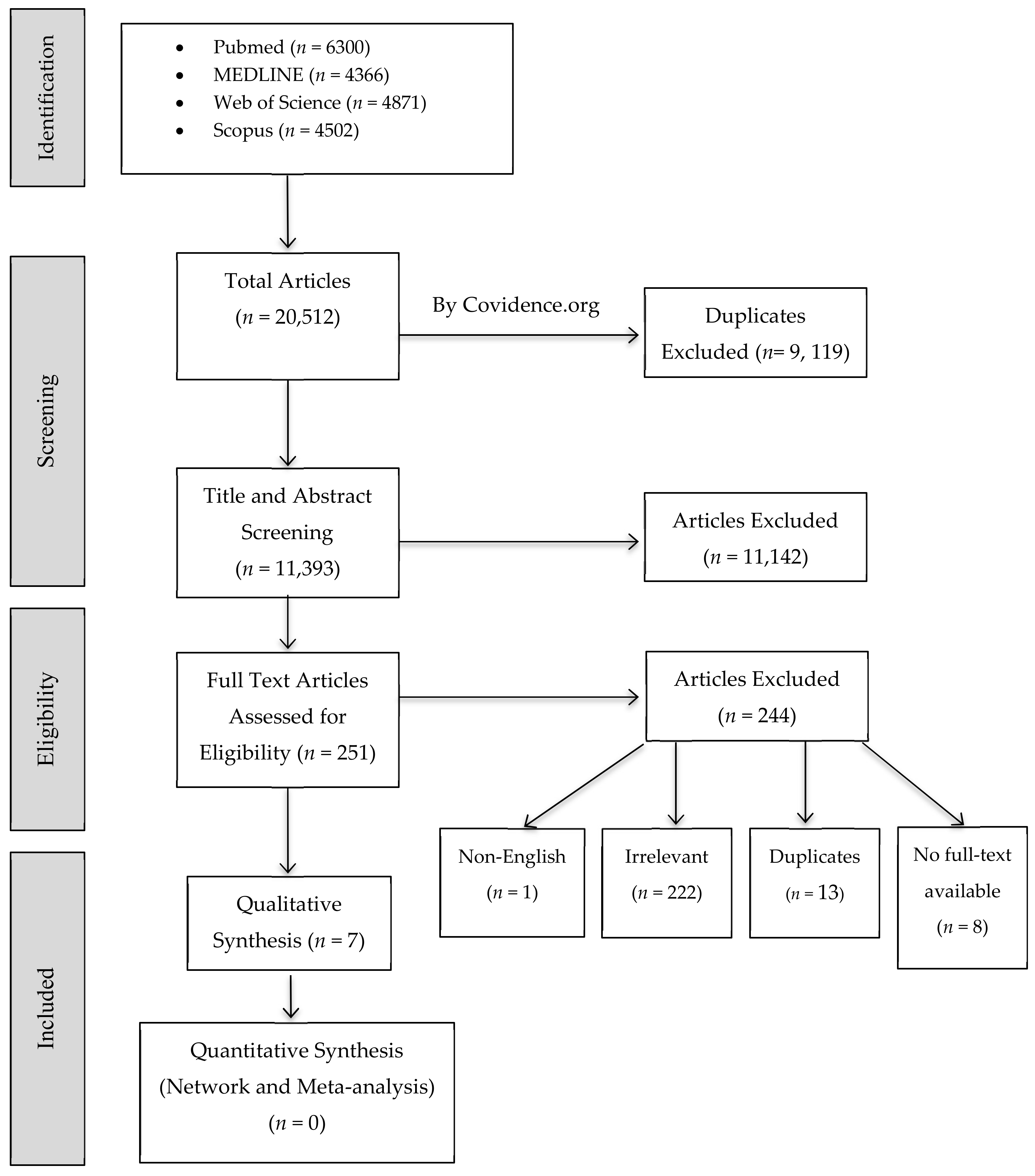
2.1. Study Selection
2.2. Risk of Bias Appraisal
2.3. Study Characteristics
2.3.1. BAG and Included Studies Characteristics
2.3.2. Protocols
2.3.3. Methodologies and Assessment Techniques
2.3.4. Summary of Findings
3. Discussion
4. Materials and Methods
4.1. Research Question
4.2. Definitions
4.3. Search Strategy
4.4. Inclusion and Exclusion Criteria
4.5. Studies Screening and Selection
4.6. Data Extraction
4.7. Quality Assessment
4.8. Assessment of Heterogeneity
4.9. Data Synthesis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fejerskov, O.; Nyvad, B.; Kidd, E.A.M. Dental Caries: The Disease and Its Clinical Management; Fejerskov, O., Nyvad, B., Kidd, E.A.M., Eds.; Blackwell Munksgaard: Oxford, UK, 2003; pp. 71–99. [Google Scholar]
- Chambers, C.; Stewart, S.; Su, B.; Sandy, J.; Ireland, A. Prevention and treatment of demineralisation during fixed appliance therapy: A review of current methods and future applications. Br. Dent. J. 2013, 215, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Sundararaj, D.; Venkatachalapathy, S.; Tandon, A.; Pereira, A. Critical evaluation of incidence and prevalence of white spot lesions during fixed orthodontic appliance treatment: A meta-analysis. J. Int. Soc. Prev. Communi. Dent. 2015, 5, 433–439. [Google Scholar] [CrossRef] [Green Version]
- Khalaf, K. Factors Affecting the Formation, Severity and Location of White Spot Lesions during Orthodontic Treatment with Fixed Appliances. J. Oral Maxillofac. Res. 2014, 5, e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomes, S.C.; Varela, C.C.; da Veiga, S.L.; Rösing, C.K.; Oppermann, R.V. Periodontal conditions in subjects following orthodontic therapy. A preliminary study. Eur. J. Orthodontics 2007, 29, 477–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Featherstone, J.D.B. The science and practice of caries prevention. J. Am. Dent. Assoc. 2000, 131, 887–899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatterjee, R.; Kleinberg, I. Effect of orthodontic band placement on the chemical composition of human incisor tooth plaque. Arch. Oral Biol. 1979, 24, 97–100. [Google Scholar] [CrossRef]
- KnÖSel, M.; Bojes, M.; Jung, K.; Ziebolz, D.; Renger, S. Increased susceptibility for white spot lesions by surplus orthodontic etching exceeding bracket base area. Orthod. Fr. 2015, 86, 233–244. [Google Scholar] [CrossRef]
- Khoroushi, M.; Kachuie, M. Prevention and Treatment of White Spot Lesions in Orthodontic Patients. Contemp Clin. Dent. 2017, 8, 11–19. [Google Scholar] [CrossRef]
- Nam, H.-J.; Kim, Y.-M.; Kwon, Y.H.; Yoo, K.-H.; Yoon, S.-Y.; Kim, I.-R.; Park, B.-S.; Son, W.-S.; Lee, S.-M.; Kim, Y.-I. Fluorinated Bioactive Glass Nanoparticles: Enamel Demineralization Prevention and Antibacterial Effect of Orthodontic Bonding Resin. Materials 2019, 12, 1813. [Google Scholar] [CrossRef] [Green Version]
- Fallahinejad Ghajari, M.; Eslamian, L.; Naji Rad, A.; Morovati, S.P. Efficacy of Glass Ionomer Cements for Prevention of White Spot Lesions During Orthodontic Banding: A Randomized Clinical Trial. J. Dent. 2015, 12, 913–920. [Google Scholar]
- Pithon, M.M.; Dos Santos, M.J.; Andrade, C.S.; Leao Filho, J.C.; Braz, A.K.; de Araujo, R.E.; Tanaka, O.M.; Fidalgo, T.K.; Dos Santos, A.M.; Maia, L.C. Effectiveness of varnish with CPP-ACP in prevention of caries lesions around orthodontic brackets: An OCT evaluation. Eur. J. Orthod. 2015, 37, 177–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.M.; Kim, I.R.; Park, B.S.; Lee, D.A.-O.; Ko, C.C.; Son, W.S.; Kim, Y.I. Remineralization Property of an Orthodontic Primer Containing a Bioactive Glass with Silver and Zinc. Materials 2017, 10, 1253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernando, D.; Attik, N.; Pradelle-Plasse, N.; Jackson, P.; Grosgogeat, B.; Colon, P. Bioactive glass for dentin remineralization: A systematic review. Mater. Sci. Eng. C. Mater. Biol. Appl. 2017, 76, 1369–1377. [Google Scholar] [CrossRef] [PubMed]
- Huang, J. Biology and Engineering of Stem Cell Niches 2017, 1st ed.; Academic Press: Boston, MA, USA, 2017. [Google Scholar]
- Terzopoulou, Z.A.-O.; Baciu, D.; Gounari, E.A.-O.; Steriotis, T.A.-O.; Charalambopoulou, G.A.-O.; Tzetzis, D.A.-O.; Bikiaris, D.A.-O. Composite Membranes of Poly(epsilon-caprolactone) with Bisphosphonate-Loaded Bioactive Glasses for Potential Bone Tissue Engineering Applications. Molecules 2019, 24, 3067. [Google Scholar] [CrossRef] [Green Version]
- Hench, L.L. The story of Bioglass. J. Mater. Sci. Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef]
- Vichery, C.; Nedelec, J.M. Bioactive Glass Nanoparticles: From Synthesis to Materials Design for Biomedical Applications. Materials 2016, 9, 288. [Google Scholar] [CrossRef] [Green Version]
- Filho, O.P.; La Torre, G.P.; Hench, L.L. Effect of crystallization on apatite-layer formation of bioactive glass 45S5. J. Biomed. Mater. Res. 1996, 30, 509–514. [Google Scholar] [CrossRef]
- Wilson, J.; Pigott, G.H.; Schoen, F.J.; Hench, L.L. Toxicology and biocompatibility of bioglasses. J. Biomed. Mater. Res. 1981, 15, 805–817. [Google Scholar] [CrossRef]
- Skallevold, H.E.; Rokaya, D.; Khurshid, Z.; Zafar, M.S. Bioactive Glass Applications in Dentistry. Int. J. Mol. Sci. 2019, 20, 5960. [Google Scholar] [CrossRef] [Green Version]
- Kokubo, T. Bioactive glass ceramics: Properties and applications. Biomaterials 1991, 12, 155–163. [Google Scholar] [CrossRef]
- Kokubo, T.; Kushitani, H.; Sakka, S.; Kitsugi, T.; Yamamuro, T. Solutions able to reproduce in vivo surface-structure changes in bioactive glass-ceramic A-W. J. Biomed. Mater. Res. 1990, 24, 721–734. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, H.R.; Gaddam, A.A.-O.; Rebelo, A.A.-O.; Brazete, D.; Stan, G.E.; Ferreira, J.A.-O. Bioactive Glasses and Glass-Ceramics for Healthcare Applications in Bone Regeneration and Tissue Engineering. Materials 2018, 11, 2530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Begum, S.; Johnson, W.E.; Worthington, T.; Martin, R.A. The influence of pH and fluid dynamics on the antibacterial efficacy of 45S5 Bioglass. Biomed. Mater. 2016, 11, 015006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drago, L.; Toscano, M.; Bottagisio, M. Recent Evidence on Bioactive Glass Antimicrobial and Antibiofilm Activity: A Mini-Review. Materials 2018, 11, 326. [Google Scholar] [CrossRef] [Green Version]
- Allan, I.; Newman, H.; Wilson, M. Antibacterial activity of particulate Bioglass® against supra- and subgingival bacteria. Biomaterials 2001, 22, 1683–1687. [Google Scholar] [CrossRef]
- Kim, Y.-M.; Kim, D.-H.; Song, C.W.; Yoon, S.-Y.; Kimd, S.-Y.; Nad, H.S.; Chung, J.; Kim, Y.-I.; Kwon, Y.H. Antibacterial and remineralization effects of orthodontic bonding agents containing bioactive glass. Korean J. Orthod. 2018, 48, 163–171. [Google Scholar] [CrossRef]
- Lee, S.A.-O.; Yoo, K.H.; Yoon, S.Y.; Kim, I.A.-O.; Park, B.S.; Son, W.S.; Ko, C.C.; Son, S.A.; Kim, Y.I. Enamel Anti-Demineralization Effect of Orthodontic Adhesive Containing Bioactive Glass and Graphene Oxide: An In-Vitro Study. Materials 2018, 11, 1728. [Google Scholar] [CrossRef] [Green Version]
- Song, H.-K.; Yoo, K.-H.; Yoon, S.-Y.; Chung, J.; Son, W.-S.; Lee, S.-M.; Kim, Y.-I. In Vitro Effect of Gallium-Doped Bioactive Glass on Enamel Anti-Demineralization and Bond Strength of Orthodontic Resins. Appl. Sci. 2019, 9, 4918. [Google Scholar] [CrossRef] [Green Version]
- John, Ł.; Janeta, M.; Szafert, S. Synthesis of cubic spherosilicates for self-assembled organic–inorganic biohybrids based on functionalized methacrylates. New J. Chem. 2018, 42, 39–47. [Google Scholar] [CrossRef] [Green Version]
- Owens, G.J.; Singh, R.K.; Foroutan, F.; Alqaysi, M.; Han, C.-M.; Mahapatra, C.; Kim, H.-W.; Knowles, J.C. Sol–gel based materials for biomedical applications. Prog. Mater. Sci. 2016, 77, 1–79. [Google Scholar] [CrossRef] [Green Version]
- Chlanda, A.; Oberbek, P.; Heljak, M.; Kijeńska-Gawrońska, E.; Bolek, T.; Gloc, M.; John, Ł.; Janeta, M.; Woźniak, M.J. Fabrication, multi-scale characterization and in-vitro evaluation of porous hybrid bioactive glass polymer-coated scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2019, 94, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Marović, D.; Šariri, K.; Demoli, N.; Ristić, M.; Hiller, K.-A.; Škrtić, D.; Rosentritt, M.; Schmalz, G.; Tarle, Z. Remineralizing amorphous calcium phosphate based composite resins: The influence of inert fillers on monomer conversion, polymerization shrinkage, and microhardness. Croat. Med. J. 2016, 57, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Jurczyk, M. Bionanomaterials for Dental Applications; Jurczyk, M., Ed.; CRC Press: Boca Raton, FL, USA, 2012; Volume 39, p. 422. [Google Scholar]
- Minick, G.T.; Oesterle, L.J.; Newman, S.M.; Shellhart, W.C. Bracket bond strengths of new adhesive systems. Am. J. Orthod. Dentofacial. Orthoped. 2009, 135, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Marovic, D.; Tarle, Z.; Hiller, K.A.; Muller, R.; Rosentritt, M.; Skrtic, D.; Schmalz, G. Reinforcement of experimental composite materials based on amorphous calcium phosphate with inert fillers. Dent. Mater. 2014, 30, 1052–1060. [Google Scholar] [CrossRef]
- Taha, A.A.; Patel, M.P.; Hill, R.G.; Fleming, P.S. The effect of bioactive glasses on enamel remineralization: A systematic review. J. Dent. 2017, 67, 9–17. [Google Scholar] [CrossRef]
- Kohda, N.; Iijima, M.; Kawaguchi, K.; Toshima, H.; Muguruma, T.; Endo, K.; Mizoguchi, I. Inhibition of enamel demineralization and bond-strength properties of bioactive glass containing 4-META/MMA-TBB-based resin adhesive. Eur. J. Oral Sci. 2015, 123. [Google Scholar] [CrossRef]
- Manfred, L.; Covell, D.A.; Crowe, J.J.; Tufekci, E.; Mitchell, J.C. A novel biomimetic orthodontic bonding agent helps prevent white spot lesions adjacent to brackets. Angle. Orthod. 2013, 83, 97–103. [Google Scholar] [CrossRef] [Green Version]
- Firzok, H.; Zahid, S.; Asad, S.; Manzoor, F.; Khan, A.S.; Shah, A.T. Sol-gel derived fluoridated and non-fluoridated bioactive glass ceramics-based dental adhesives: Compositional effect on re-mineralization around orthodontic brackets. J. Non-Cryst. Solids 2019, 521, 119469. [Google Scholar] [CrossRef]
- Shirazi, M.; Tamadon, M.; Izadi, M. Effect of addition of bioactive glass to resin modified glass ionomer cement on enamel demineralization under orthodontic brackets. J. Clin. Exp. Dent. 2019, 11. [Google Scholar] [CrossRef]
- Kantoor, P.; Srivastava, N.; Rana, V.; Adlakha, V.K. Alterations in the mechanical properties of the extracted human teeth to be used as biological restorations on storing them in different storage media: Anin vitrostudy. Dent. Traumatol. 2015, 31, 308–313. [Google Scholar] [CrossRef]
- ten Cate, J.M. Models and Role Models. Caries Res. 2015, 49, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Tsichlaki, A.; Chin, S.Y.; Pandis, N.; Fleming, P.S. How long does treatment with fixed orthodontic appliances last? A systematic review. Am. J. Orthodontics Dentofacial Orthopedics 2016, 149, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Faggion, C.M., Jr. Guidelines for Reporting Pre-clinical In Vitro Studies on Dental Materials. J. Evidence-Based Dental Practice 2012, 12, 182–189. [Google Scholar] [CrossRef]
- Khoroushi, M.; Mousavinasab, S.M.; Keshani, F.; Hashemi, S. Effect of resin-modified glass ionomer containing bioactive glass on the flexural strength and morphology of demineralized dentin. Oper. Dent. 2013, 38, E1–E10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.; Qi, X.; Boey, F.; Zhang, H. Graphene-based composites. Chem. Soc. Rev. 2012, 41, 666–686. [Google Scholar] [CrossRef] [PubMed]
- Percival, S.L.; Bowler, P.G.; Russell, D. Bacterial resistance to silver in wound care. J. Hospital Infect. 2005, 60, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Zhang, Y.; He, G.-X.; Katagori, N.; Chen, H. A comparison of conventional methods for the quantification of bacterial cells after exposure to metal oxide nanoparticles. BMC Microbiol. 2014, 14, 222. [Google Scholar] [CrossRef] [Green Version]
- Brown, M.L.; Davis, H.B.; Tufekci, E.; Crowe, J.J.; Covell, D.A.; Mitchell, J.C. Ion release from a novel orthodontic resin bonding agent for the reduction and/or prevention of white spot lesions. An in vitro study. Angle Orthodontist 2011, 81, 1014–1020. [Google Scholar] [CrossRef]
- Bayne, S.C. Correlation of clinical performance with ‘in vitro tests’ of restorative dental materials that use polymer-based matrices. Dent. Mater. 2011, 28, 52–71. [Google Scholar] [CrossRef]
- Wiegand, A.; Attin, T. Design of Erosion/Abrasion Studies – Insights and Rational Concepts. Caries. Res. 2011, 45, 53–59. [Google Scholar] [CrossRef] [Green Version]
- DeLong, R.; Douglas, W.H. An artificial oral environment for testing dental materials. IEEE. Trans. Biomed. Eng. 1991, 38, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Featherstone, J.D.B.; Glena, R.; Shariati, M.; Shields, C.P. Dependence of in vitro Demineralization of Apatite and Remineralization of Dental Enamel on Fluoride Concentration. J. Dent. Res. 1990, 69, 620–625. [Google Scholar] [CrossRef] [PubMed]
- West, N.X.; Davies, M.; Amaechi, B.T. In vitro and in situ Erosion Models for Evaluating Tooth Substance Loss. Caries Res. 2011, 45, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Meurman, J.H.; Rytömaa, I.; Kari, K.; Laakso, T.; Murtomaa, H. Salivary pH and Glucose after Consuming Various Beverages, Including Sugar-Containing Drinks. Caries Res. 1987, 21, 353–359. [Google Scholar] [CrossRef]
- Meurman, J.H.; ten Cate, J.M. Pathogenesis and modifying factors of dental erosion. Eur. J. Oral. Sci. 1996, 104, 199–206. [Google Scholar] [CrossRef]
- Hara, A.T.; Ando, M.; González-Cabezas, C.; Cury, J.A.; Serra, M.C.; Zero, D.T. Protective Effect of the Dental Pellicle against Erosive Challenges in situ. J. Dent. Res. 2006, 85, 612–616. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Prisma Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 4, 1–9. [Google Scholar]
- Abou Neel, E.A.; Aljabo, A.; Strange, A.; Ibrahim, S.; Coathup, M.; Young, A.M.; Bozec, L.; Mudera, V. Demineralization-remineralization dynamics in teeth and bone. Int. J. Nanomedicine 2016, 11, 4743–4763. [Google Scholar] [CrossRef]
- Rosa, W.L.; Piva, E.; Silva, A.F. Bond strength of universal adhesives: A systematic review and meta-analysis. J. Dent. 2015, 43. [Google Scholar] [CrossRef]
- Sarkis-Onofre, R.; Skupien, J.A.; Cenci, M.S.; Moraes, R.R.; Pereira-Cenci, T. The role of resin cement on bond strength of glass-fiber posts luted into root canals: A systematic review and meta-analysis of in vitro studies. Oper. Dent. 2014, 39, 31–44. [Google Scholar] [CrossRef]
| Sampling Bias | Assessment Bias | Reporting Bias | Risk of Bias | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Study | Caries-Free Teeth | Sample Size Calculation | Sample Preparation | Sample Randomization | Presence of Control Group | Blinding | Definitive Values | Quantitative Analysis | |
| Manfreda et al., 2013 [40] |  |  |  |  |  |  |  |  | Medium |
| Kohda et al., 2015 [39] |  |  |  |  |  |  |  |  | Medium |
| Kim et al., 2018 [28] |  |  |  |  |  |  |  |  | High |
| Lee et al., 2018 [29] |  |  |  |  |  |  |  |  | Medium |
| Firzoka et al., 2019 [41] |  |  |  |  |  |  |  |  | Medium |
| Shirazi et al., 2019 [42] |  |  |  |  |  |  |  |  | Low |
| Song et al., 2019 [30] |  |  |  |  |  |  |  |  | Medium |
 Yes
Yes  No
No  Not Applicable Parameter.
Not Applicable Parameter.| Study | Group Sample Size | Teeth Type | Teeth Storage Media | Sample Preparation | BAG Composition | BAG Synthesis Method | BAG Particle Size | BAG Ratio/ Weight | Control Groups | Tested Groups |
|---|---|---|---|---|---|---|---|---|---|---|
| Manfreda et al., 2013 [40] | 10 | Human, non-carious third molars | 0.5% Chloramine-T solution at 4 °C | Cleansing with non-fluoridated pumice and water using a prophylaxis cup | 62BAG 65BAG 81BAG 85BAG | Sol-gel | 62BAG: 75 m2/g 65BAG:144 m2/g 81BAG:320 m2/g 85BAG:268 m2/g | 62BAG: 58:100 65BAG: 49:100 81BAG: 37:100 85BAG: 33:100 (BAG: Monomer) | TXT | 62BAG-Bond 65BAG-Bond 81BAG-Bond 85BAG-Bond |
| Kohda et al., 2015 [39] | 10 | Human, non-carious, upper premolars | Cleansing with non-fluoridated pumice and water using a prophylaxis cup | 45.0% SiO2 + 24.5% Na2O + 24.5% CaO + 6.0% P2O5 | Melting and grinding | 100 µm | 0, 10, 20, 30, 40 or 50% | PMMA powder containing 0% BAG + 4-META/MMA + TBB | PMMA powder containing 10, 20, 40 or 50% BAG + 4-META/MMA + TBB | |
| Kim et al., 2018 [28] | Not Clear | Human premolar | Silver- and Zinc-doped BAGs: A0 A1 A1Z5 Z5 | Sol-gel | 10 or 15% | CF TXT | CF + 10% A0 CF + 10% A1 CF + 10% A1Z5 CF + 15% A1Z5 CF + 10% Z5 | |||
| Lee et al., 2018 [29] | 9 | Human, non-carious, upper premolars | Cleansing with non-fluoridated pumice and water using a prophylaxis cup | BAG@GO | Sol-gel | 1, 3 or 5% | LV | LV + 1, 3 or 5% BAG@GO | ||
| Firzoka et al., 2019 [41] | 30 | Human, non-carious, premolars | Normal Saline at 4 °C | Disinfecting with 0.5% Chloramine-T solution for 24 h | F-BGC-1 BGC-1 F-BGC-2 BGC-2 | Sol-gel | F-BGC-1: 69.89 m2/g BGC-1: 71.08 m2/g F-BGC-2: 65.45 m2/g BGC-2: 65.34 m2/g | 5% | TXT | TXT + F-BGC-1 TXT + BGC-1 TXT + F-BGC-2 TXT + BGC-2 |
| Shirazi et al., 2019 [42] | 20 | Human, non-carious, premolars | 0.1% Thymol solution for one week then stored in distilled water at 6 °C |
| 30% | TXT Fuji II LC | Fuji II LC + 30% BAG | |||
| Song et al., 2019 [30] | 10 | Human, non-carious, premolars |
| Gallium-Doped BAG; GaMBN | Modified sol-gel | 404.09 m2/g | 1, 3 or 5% | CF | CF + 1, 3, or 5% GaMBN |
| Study | Sample Preparation | pH Cycling Protocol | Outcome Measurement Method | |||
|---|---|---|---|---|---|---|
| Demineralization | Remineralization | Days of Cycle Repetition | Notes | |||
| Manfreda et al., 2013 [40] |
| For 6 h, teeth were immersed in 40 mL demineralization solution consisting of 2.0 mM Ca, 2.0 mM PO4 and 0.075 mM CH3COOH at pH 4.4. | For 18 h, teeth were immersed in 40 mL of remineralization solution consisting of 1.5 mM Ca, 0.9 mM PO4, 0.1 5 M KCl and 20 mM C2H6AsNaO2 at pH 7. | 14 |
| Knoop Microhardness Testing: Hardness was measured using Knoop indenter. |
| Kohda et al., 2015 [39] |
| For 4 h, teeth were immersed in 2 mL demineralization solution consisting of 2 mM CaCl2 and 2 mM NaH2PO4 with 50 mM CH3COOH) at pH 4.55. | For 20 h, teeth were immersed in 2 mL remineralization solution consisting of 2 mM CaCl2 and 2 mM NaH2PO4 with 0.1 M of NaOH at pH 6.8. | 14 | Nano-indentation Testing: Hardness was measured using Berkovich indenter. | |
| Kim et al., 2018 [28] |
| For 6 h, teeth were immersed in a demineralization solution consisting of 2.0 mM Ca(NO3)2·4H2O, 2.0 mM KH2PO4 and 75.0 mM CH3COOH at pH of 4.4. | For 18 h, teeth were immersed in a remineralization solution consisting of 20.2 mM C2H12AsNaO5, 1.5 mM, Ca(NO3)2·4H2O, 0.9 mM KH2PO4 and 130 mM CaCl2 at pH 6.8. | 14 |
| Micro-CT Scanning: Intensity histograms were used to measure the lesion depth, remineralization zone width and mineral loss. |
| Lee et al., 2018 [29] |
| For 6 h, teeth were immersed in a demineralization solution (Biosesang, Seoul, Korea) | For 18 h, teeth were immersed in an anti-demineralization solution (Biosesang, Seoul, Korea) | 14 |
| Micro-CT Scanning: Brightness histograms were used to measure the anti-demineralization length. |
| Firzoka et al., 2019 [41] |
| For 6 h, teeth were immersed in 4 mL demineralization solution consisting of CaCl2, Na3PO4, CH3COOH, KOH and thymol crystals at pH 4.4. | For 18 h, teeth were immersed in 4 mL remineralization solution consisting of CaCl2, Na3PO4, KCl and thymol crystals at pH 7. | 14 |
| |
| Shirazi et al., 2019 [42] |
| For 6 h, teeth were immersed in 10 mL demineralization solution consisting of 2.2 mM CaCl2, 50 M CH3COOH and 2.2 mM KH2PO4, 35.78 mL of 1 M C6H8O7, 14.22 mL of 1 M C2H3NaO2, 0.0022 M KH2PO4 and 0.0022 M CaCl2 at pH 4.3. | For 18 h, teeth were immersed in 10 mL remineralization solution consisting of 1.5 mM CaCl2, 150 mM KCl and 0.9 mM KH2PO4 at pH 7. | 21 | Fresh solutions were used each week. | Polarized Light Microscopy: Depth of the lesion was measured. |
| Song et al., 2019 [30] |
| For 6 h, teeth were immersed in 500 mL demineralization solution consisting of 2 mM Ca(NO3).4H2O, 2 mM KH2PO4 and 75 mM CH3COOH at pH 4.4. | For 18 h, teeth were immersed in 500 mL remineralization solution consisting of CH3COOH.4H2O, 0.9 mM KH2PO4, 130 mM KCl and 20.2 mM NaC2H6AsO2.3H2O at pH 7. | 14 |
| Micro-CT Scanning: Brightness histograms were used to measure the anti-demineralization length. |
| Assessment Tool | Study | Intervention (Mean ± SD) | Control (Mean ± SD) | Summary of Results |
|---|---|---|---|---|
| Micro-computed Tomography | Kim et al., 2018 [28] | Lesion Depth: CF + 10% A0: (0.17 ± 0.018 µm) CF + 10% A1: (0.095 ± 0.014 µm) CF + 10% A1Z5: (0.091 ± 0.017 µm) CF + 10% Z5 (0.073 ± 0.011 µm) Mineral Loss: CF + 10% A0: (198.95 ± 33.42) CF + 10% A1: (219.04 ± 63.73) CF + 10% A1Z5: (183.15 ± 48.2) CF + 10% Z5 (113.95 ± 21.09) Remineralization Zone Width: CF + 10% A0: (0.292 ± 0.088 µm) CF + 10% A1: (0.257 ± 0.058 µm) CF + 10% A1Z5: (0.236 ± 0.56 µm) CF + 10% Z5 (0.345 ± 0.024 µm) | Lesion Depth: CF: (0.093 ± 0.025 µm) * TXT: (0.099 ± 0.022 µm) * Mineral Loss: CF: (219.08 ± 64) * TXT: (172.83 ± 43.79) * Remineralization Zone Width: CF: (0.143 ± 0.02 µm) * TXT: (0.048 ± 0.026 µm) * |
|
| Lee et al., 2018 [29] | LV + 1% BAG@GO: (132.4 ± 49 µm) LV + 3% BAG@GO: (228.7 ± 135.3 µm) LV + 5% BAG@GO: (218.4 ± 57 µm) | LV (1.3 ± 0.2 µm) * |
| |
| Song et al., 2019 [30] | CF + 1% GaMBN: (477.5 ± 260.5 µm) CF + 3% GaMBN: (728.4 ± 266.8 µm) CF + 5% GaMBN: (970.3 ± 370.9 µm) | CF (53.7 ± 22.2 µm) ** |
| |
| Hardness Testing | Manfreda et al., 2013 [40] ᵻ | − | − | All the BAG containing orthodontic bonding agents (BAG-Bonds) outperformed the commercial control in regard to enamel hardness surrounding the brackets. |
| Kohda et al., 2015 [39] | Values were provided in a supplemental document. | Values were provided in a supplemental document. | All the BAG containing 4META/MMA-TBB-based resins outperformed the commercial control in regard to enamel hardness surrounding the brackets. | |
| Polarized Light Microscopy | Shirazi et al., 2019 [42] | Fuji II LC + 30% BAG (73.8 ± 22.29 µm) | TXT (182.98 ± 20.69 µm) * Fuji II LC (118.08 ± 29.42 µm) * | BAG containing RMGIC showed higher ability to prevent demineralization by a significant reduction in demineralization depth under orthodontic brackets in comparison to the commercial controls. |
| Fourier Transform Infrared and Spectroscopy Scanning Electron | Firzok et al., 2019 [41]ᵻ | − | − |
|
| Antibacterial Effect | ||||
|---|---|---|---|---|
| Assessment Tool | Study | Intervention (Mean ± SD) | Control (Mean ± SD) | Summary of Results |
| Optical Density | Kim et al., 2018 [28] | CF + 10% A0: (0.22 OD at 620 nm) CF + 10% A1: (0.3 OD at 620 nm) CF + 10% A1Z5: (0.22 at OD 620 nm) CF + 15% A1Z5: (0.29 at OD 620 nm) CF + 10% Z5 (0.28 at OD 620 nm) | CF: (0.38 OD at 620 nm) * TXT: (0.35 OD at 620 nm) * | All interventional resins showed significantly lower absorbance values than control resins. |
| Lee et al., 2018 [29] | In 24 h, | In 24 h, |
| |
| LV + 1% BAG@GO: (2.1 ± 0.2% at 620 nm) LV + 3% BAG@GO: (3 ± 2.6% at 620 nm) LV + 5% BAG@GO: (4.2 ± 2.8% at 620 nm) | LV (67.2 ± 14.5% at 620 nm) * | |||
| In 48 h, | In 48 h, | |||
| LV + 1% BAG@GO: (0.6 ± 0.2% at 620 nm) LV + 3% BAG@GO: (0.6 ± 0.1% at 620 nm) LV + 5% BAG@GO: (0.5 ± 0.1% at 620 nm) | LV (62 ± 9.8% at 620 nm) * | |||
| Song et al., 2019 [30] ᵻ | − | − |
| |
| Ion Release | ||||||
|---|---|---|---|---|---|---|
| Assessment Tool | Study | Intervention (Mean ± SD) | Control (Mean ± SD) | Summary of Results | ||
| Ion Release | Kim et al., 2018 [28]ᵻ | − | − |
| ||
| Kohda et al., 2015 [39]ᵻ | − | − |
| |||
| Song et al., 2019 [30] | In 1 day | In 7 days | In 14 days | Barely released |
| |
| CF + 1% GaMBN | CF + 1% GaMBN | CF + 1% GaMBN | ||||
| Ca: (3.8 ± 0.1) ppm * | Ca: (7.2 ± 0.2) ppm * | Ca: (7.1 ± 0.1) ppm * | ||||
| P: (0.4 ± 0) ppm * | P: (1.2 ± 0) ppm * | P: (0.8 ± 0) ppm * | ||||
| Ga: (0.2 ± 0) ppm * | Ga: (1.5 ± 0) ppm * | Ga: (2.1 ± 0.1) ppm * | ||||
| CF + 3% GaMBN | CF + 3% GaMBN | CF + 3% GaMBN | ||||
| Ca: (6.4 ± 0.1) ppm * | Ca: (17.8 ± 0.8) ppm * | Ca: (16.6 ± 0.1) ppm * | ||||
| P: (1 ± 0) ppm * | P: (3.3 ± 0.1) ppm * | P: (3.4 ± 0.1) ppm * | ||||
| Ga: (0.6 ± 0) ppm * | Ga: (4.8 ± 0.3) ppm * | Ga: (5.7 ± 0.5) ppm * | ||||
| CF + 5% GaMBN | CF + 5% GaMBN | CF + 5% GaMBN | ||||
| Ca: (6.3 ± 0.2) ppm * | Ca: (19.5 ± 0.3) ppm * | Ca: (27.2 ± 0.8) ppm * | ||||
| P: (1.3 ± 0.1) ppm * | P: (4.5 ± 0.1) ppm * | P: (6.4 ± 0.1) ppm * | ||||
| Ga: (0.5 ± 0) ppm * | Ga: (6.7 ± 0.3) ppm * | Ga: (6.7 ± 0.1) ppm * | ||||
| Acid Neutralization | ||||
|---|---|---|---|---|
| Assessment Tool | Study | Intervention (Mean ± SD) | Control (Mean ± SD) | Summary of Results |
| pH Change | Kohda et al., 2015 [39] ᵻ | − | − |
|
| Search | Terms |
|---|---|
| 1 | (“Resins, Synthetic”[Mesh] OR “Dental Bonding”[Mesh]) OR “Dental Materials”[Mesh]) |
| 2 | (“bioactive glass”[tw] OR “bioglass”[tw] OR “bioceramic”[tw]) |
| 3 | (“Tooth Demineralization”[Mesh] OR “Tooth Remineralization”[Mesh]) |
| 4 | (“Remineralization”[tw] OR “Remineralisation”[tw], OR “Demineralization”[tw], OR “Demineralisation”[tw]) |
| 5 | #1 OR #2 |
| 6 | #3 OR #4 |
| 7 | #5 AND #6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alamri, A.; Salloot, Z.; Alshaia, A.; Ibrahim, M.S. The Effect of Bioactive Glass-Enhanced Orthodontic Bonding Resins on Prevention of Demineralization: A Systematic Review. Molecules 2020, 25, 2495. https://doi.org/10.3390/molecules25112495
Alamri A, Salloot Z, Alshaia A, Ibrahim MS. The Effect of Bioactive Glass-Enhanced Orthodontic Bonding Resins on Prevention of Demineralization: A Systematic Review. Molecules. 2020; 25(11):2495. https://doi.org/10.3390/molecules25112495
Chicago/Turabian StyleAlamri, Abdulaziz, Zainah Salloot, Alaa Alshaia, and Maria Salem Ibrahim. 2020. "The Effect of Bioactive Glass-Enhanced Orthodontic Bonding Resins on Prevention of Demineralization: A Systematic Review" Molecules 25, no. 11: 2495. https://doi.org/10.3390/molecules25112495





