Characteristics of New Peptides GQLGEHGGAGMG, GEHGGAGMGGGQFQPV, EQGFLPGPEESGR, RLARAGLAQ, YGNPVGGVGH, and GNPVGGVGHGTTGT as Inhibitors of Enzymes Involved in Metabolic Syndrome and Antimicrobial Potential
Abstract
1. Introduction
2. Results
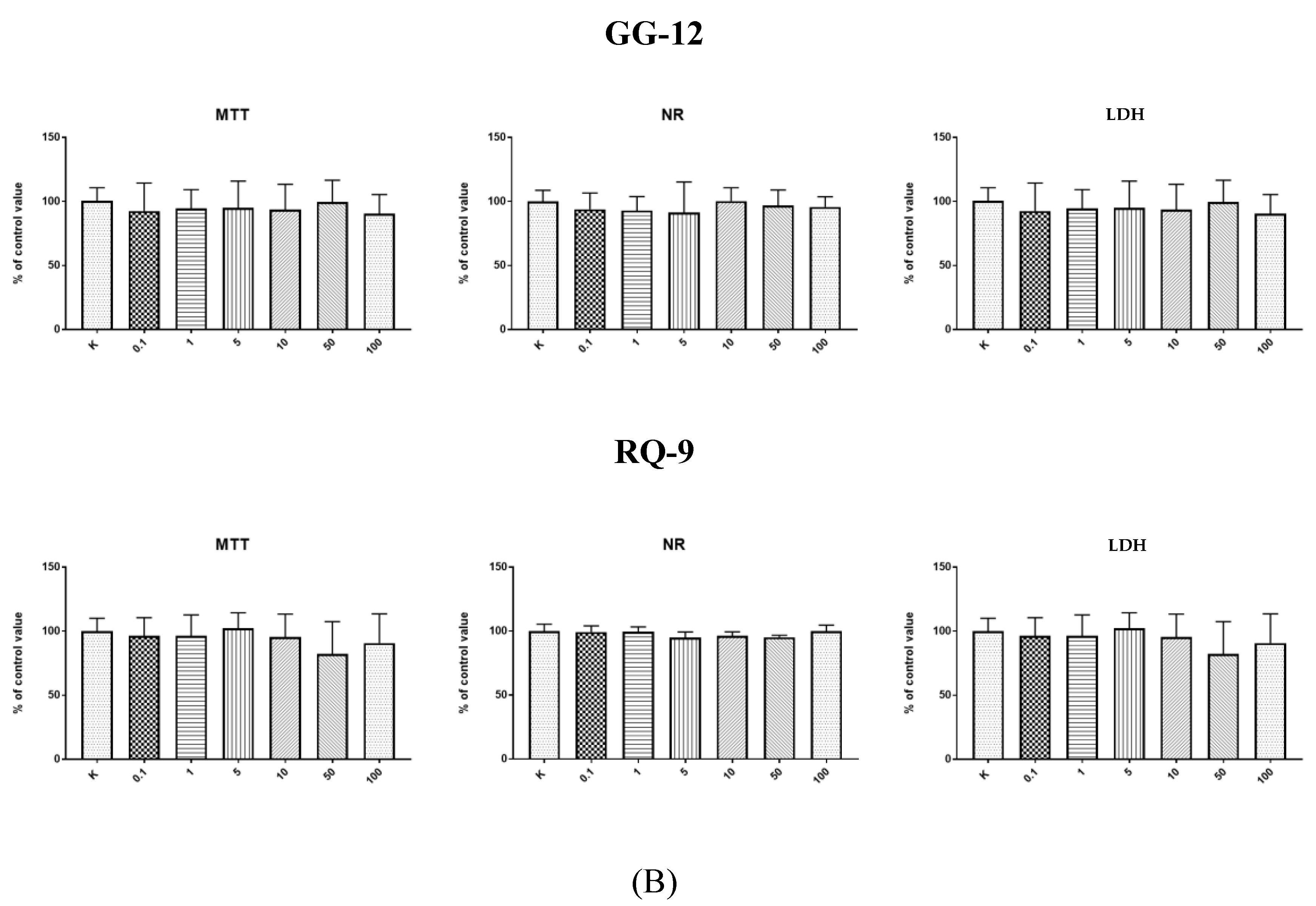
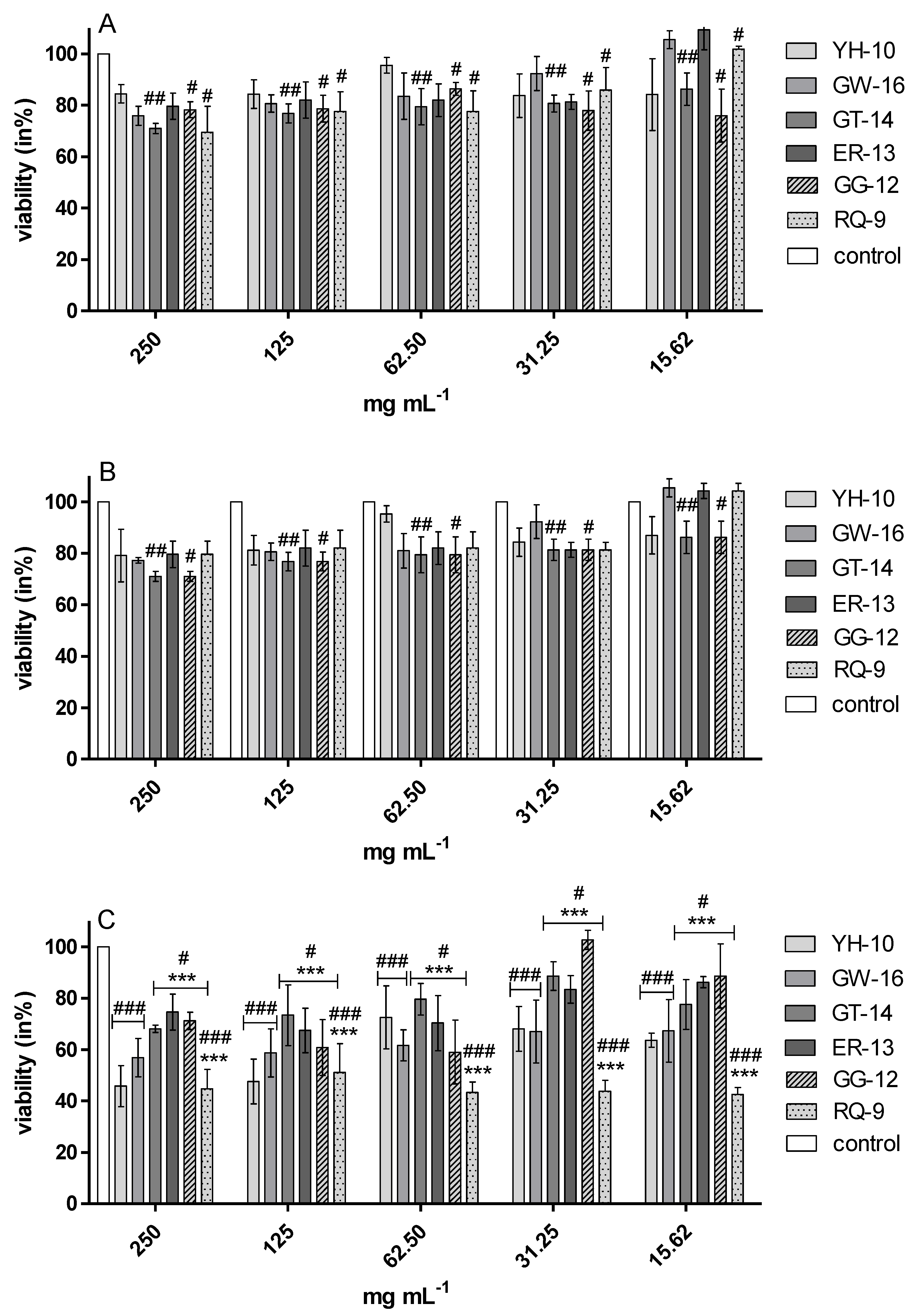
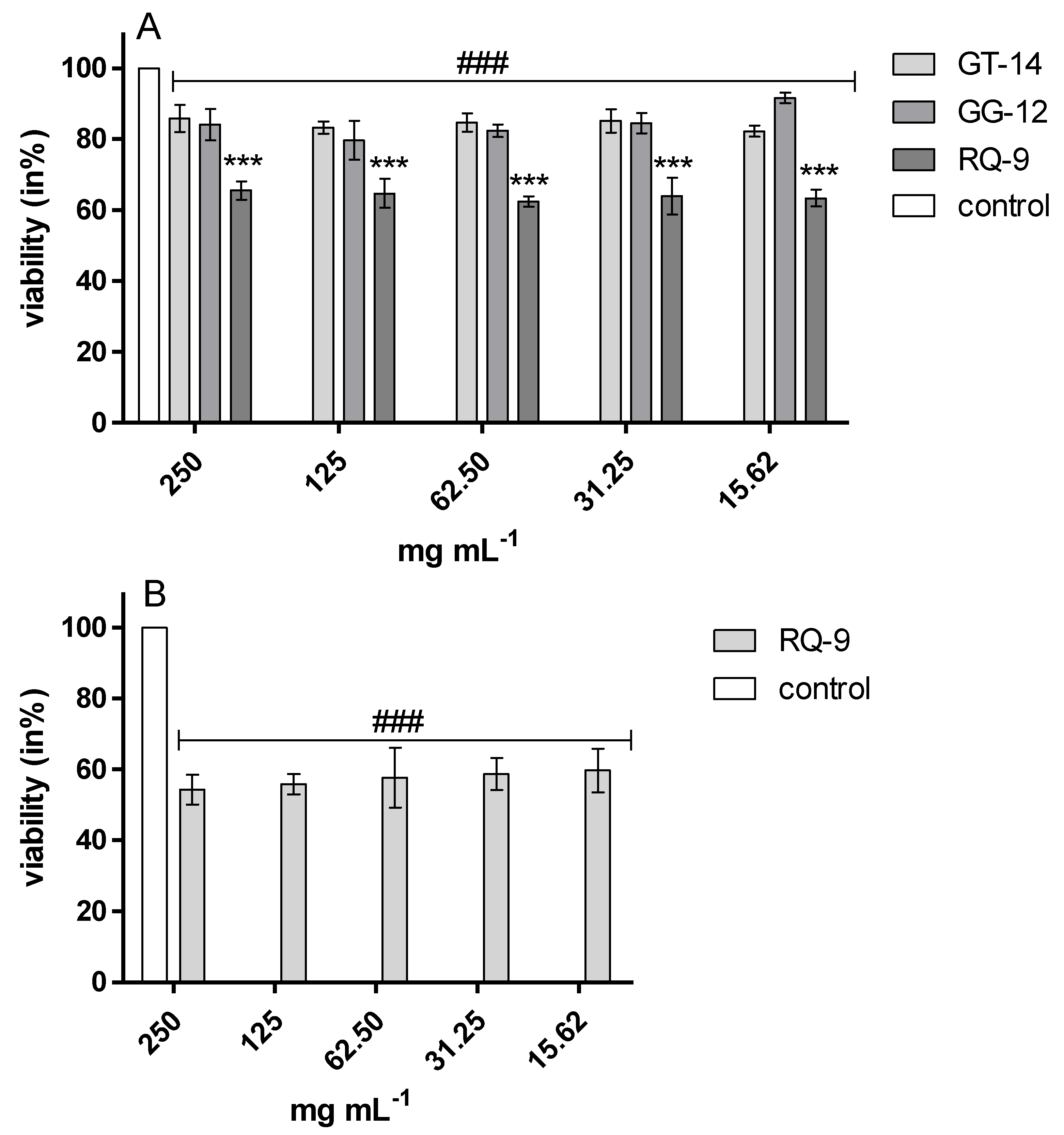
2.1. Cytotoxic Effect-Endothelial Cells
2.2. Physicochemical Parameters
2.3. Effect on Enzyme Activity
2.4. Antibacterial Properties of Peptides
3. Discussion
4. Materials and Methods
4.1. Analysis of the Physicochemical Characteristics of Synthetic Peptides
4.2. Determination of Cytotoxic Activity of Synthetic Peptides
4.2.1. MTT Test
4.2.2. NR Test
4.2.3. LDH Test
4.3. Peptides as an Enzyme Inhibitory
4.3.1. ACE Inhibitor Activity
4.3.2. α-amylase Inhibitory Activity
4.3.3. α-Glucosidase Inhibitory Activity
4.3.4. Pancreatic Lipase Inhibitory Activity
4.3.5. Lipoxidase (LOX) Inhibitory Activity
4.3.6. COX-1 and COX-2 Inhibitory Activities
4.4. Kinetics Parameters of Enzymes Inhibitors
4.5. Antimicrobial Activity
4.5.1. Determination of Minimum Inhibitory Concentration (MIC)
4.5.2. Estimation of Biotoxicity of Synthetic Peptides Using Resazurin Reduction Assay
4.6. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dekker, I.P.; Marijnissen, R.M.; Giltay, E.J.; Van Der Mast, R.C.; Oude, R.C.; Rhebergen, D.; Rius, N. The role of metabolic syndrome in late-life depression over 6 years: The NESDO study. J. Affect. Disord. 2019, 257, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Badely, M.; Sepandi, M.; Samadi, M.; Parastouei, K. The effect of whey protein on the components of metabolic syndrome in overweight and obese individuals; a systematic review and meta- analysis. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 3121–3131. [Google Scholar] [CrossRef] [PubMed]
- Akif, M.; Schwager, S.L.; Anthony, C.S.; Czarny, B.; Beau, F.; Dive, V.; Sturrock, E.D.; Acharya, K.R. Novel mechanism of inhibition of human angiotensin-I-converting enzyme (ACE) by a highly specific phosphinic tripeptide. Biochem. J. 2011, 59, 53–59. [Google Scholar] [CrossRef]
- Villadóniga, C.; María, A.; Cantera, B. New ACE-inhibitory peptides derived from α -lactalbumin produced by hydrolysis with Bromelia antiacantha peptidases. Biocatal. Agric. Biotechnol. 2019, 20, 101258. [Google Scholar] [CrossRef]
- Mamilla, R.K.; Mishra, V.K. Effect of germination on antioxidant and ACE inhibitory activities of legumes. LWT-Food Sci. Technol. 2017, 75, 51–58. [Google Scholar] [CrossRef]
- Khueychai, S.; Jangpromma, N.; Choowongkomon, K. A novel ACE inhibitory peptide derived from alkaline hydrolysis of ostrich (Struthio camelus) egg white ovalbumin. Process Biochem. 2018, 73, 235–245. [Google Scholar] [CrossRef]
- Lee, S.Y.; Hur, S.J. Purification of novel angiotensin converting enzyme inhibitory peptides from beef myo fi brillar proteins and analysis of their effect in spontaneously hypertensive rat model. Biomed. Pharmacother. 2019, 116, 109046. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, L.; Sun, Q.; Song, G.; Huang, J. Extraction, identification and structure-activity relationship of antioxidant peptides from sesame (Sesamum indicum L.) protein hydrolysate. Food Res. Int. 2018, 116, 707–7016. [Google Scholar] [CrossRef]
- Joy, O.; Liu, L.; Zhang, S.; Lu, J.; Pang, X.; Lv, J. α-Glucosidase and ACE dual inhibitory protein hydrolysates and peptide fractions of sprouted quinoa yoghurt beverages inoculated with Lactobacillus casei. Food Chem. 2019, 299, 124985. [Google Scholar]
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Publ. Gr. 2017, 14, 88–98. [Google Scholar] [CrossRef]
- Ganesan, M.S.; Raja, K.K.; Narasimhan, K.; Murugesan, S. Design, synthesis, α-amylase inhibition and in silico docking study of novel quinoline bearing proline derivatives. J. Mol. Struct. 2020, 1208, 127873. [Google Scholar] [CrossRef]
- Ramadhan, A.H.; Nawas, T.; Zhang, X.; Pembe, M.; Xia, W.; Xu, Y. Purification and identification of a novel antidiabetic peptide from Chinese giant salamander (Andrias davidianus) protein hydrolysate against α -amylase and α -glucosidase. Int. J. Food Prop. 2018, 2912, 1–13. [Google Scholar] [CrossRef]
- Wang, J.; Wu, T.; Fang, L.; Liu, C.; Liu, X.; Li, H.; Shi, J.; Li, M.; Min, W. Anti-diabetic ef ect by walnut (Juglans mandshurica Maxim.)-derived peptide LPLLR through inhibiting αglucosidase and α-amylase, and alleviating insulin resistance of hepatic HepG2 cells. J. Funct. Foods 2020, 69, 103944. [Google Scholar] [CrossRef]
- Su, H.; Ruan, Y.; Li, Y.; Chen, J.; Yin, Z.; Zhang, Q. In vitro and in vivo inhibitory activity of taxifolin on three digestive enzymes. Int. J. Biol. Macromol. 2020, 150, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Stefanucci, A.; Dimmito, M.P.; Dall’Acqua, S.; Luisi, G.; Mirzaie, S.; Novellino, E.; Mollica, A. Discovery of novel amide tripeptides as pancreatic lipase inhibitors by virtual screening. New J. Chem. 2019, 43, 3208–3217. [Google Scholar] [CrossRef]
- Lunder, M.; Bratkovi, T.; Kreft, S.; Štrukelj, B. Peptide inhibitor of pancreatic lipase selected by phage display using different elution strategies. J. Lipid Res. 2017, 21, 1–18. [Google Scholar] [CrossRef]
- Martinez-Villaluenga, C.; Rupasinghe, S.G.; Schuler, M.A.; De, E.G. Peptides from purified soybean b -conglycinin inhibit fatty acid synthase by interaction with the thioesterase catalytic domain. FEBS J. 2010, 277, 1481–1493. [Google Scholar] [CrossRef]
- Khan, S.A.; Ali, A.; Khan, S.A.; Zahran, S.A.; Damanhouri, G.; Azhar, E.; Qadri, I. Unraveling the complex relationship triad between lipids, obesity, and Inflammation. Mediators Inflamm. 2014, 2014, 1–16. [Google Scholar] [CrossRef]
- Montoya-Rodríguez, A.; González, E.; Mejía, D. Pure peptides from amaranth (Amaranthus hypochondriacus) proteins inhibit LOX-1 receptor and cellular markers associated with atherosclerosis development in vitro. FRIN 2015, 77, 204–214. [Google Scholar] [CrossRef]
- Karaś, M.; Jakubczyk, A.; Szymanowska, U.; Krystyna, J.; Lewicki, S.; Złotek, U. Different temperature treatments of millet grains affect the biological activity of protein hydrolyzates. Nutrients 2019, 11, 550. [Google Scholar] [CrossRef]
- Jakubczyk, A.; Szymanowska, U.; Karaś, M.; Złotek, U. Potential anti-inflammatory and lipase inhibitory peptides generated by in vitro gastrointestinal hydrolysis of heat treated millet grains gastrointestinal hydrolysis of heat treated millet grains. CyTA-J. Food 2019, 17, 324–333. [Google Scholar] [CrossRef]
- Lammi, C.; Aiello, G.; Boschin, G.; Arnoldi, A. Multifunctional peptides for the prevention of cardiovascular disease: A new concept in the area of bioactive food-derived peptides. J. Funct. Foods 2019, 55, 135–145. [Google Scholar] [CrossRef]
- Fillería, S.F.G.; Tironi, V.A. Prevention of in vitro oxidation of low density lipoproteins (LDL) by amaranth peptides released by gastrointestinal digestion. J. Funct. Foods 2017, 34, 197–206. [Google Scholar] [CrossRef]
- Vargas, G.; Azarbal, J.; Mbbs, R.T. A Comparative Review of Established Diets for Prevention of Cardiovascular Disease and Newer Dietary Strategies. Curr. Probl. Cardiol. 2020, 100582. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, N.; Barbera, A.; Dominguez, M.; Torres, A.; Hernandez, M.; Henadez, I.; Gil, R.; Ancizar, J.; Garay, H.; Reyes, O.; et al. Therapeutic effect of an altered peptide ligand derived from heat-shock protein 60 by suppressing of inflammatory cytokines secretion in two animal models of rheumatoid arthritis. Autoimmunity 2012, 45, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Conconi, M.T.; Ghezzo, F.; Dettin, M.; Urbani, L.; Grandi, C.; Guidolin, D.; Nico, B.; Di Bello, C.; Paolo, P. Effects on in vitro and in vivo angiogenesis induced by small peptides carrying adhesion sequences. Pept. Sci. 2010, 16, 349–357. [Google Scholar] [CrossRef]
- Bizouarne, N.; Denis, V.; Legrand, A.; Monsigny, M.; Kieda, C. Original article A SV-40 immortalized murine endothelial cell line from peripheral lymph node high endothelium expresses a new -L-fucose binding protein. Biol. Cell 1993, 79, 209–218. [Google Scholar] [CrossRef]
- Bizouarne, N.; Mitterrand, M.; Monsigny, M.; Kieda, C.; De Biochimie, L.; Moldculaire, C.D.B. Characterization of membrane sugar-specific receptors in cultured high endothelial cells from mouse peripheral lymph nodes. Biol. Cell 1993, 79, 27–35. [Google Scholar] [CrossRef]
- Wang, B.; Li, B. Effect of molecular weight on the transepithelial transport and peptidase degradation of casein-derived peptides by using Caco-2 cell model. Food Chem. 2017, 218, 1–8. [Google Scholar] [CrossRef]
- Lu, R.L.Z.; Chen, Y.S.S. Molecular Design, Structural Analysis and Antifungal Activity of Derivatives of Peptide CGA-N46. Interdiscip. Sci. Comput. Life Sci. 2016, 8, 319–326. [Google Scholar]
- Enany, S. Structural and functional analysis of hypothetical and conserved proteins of Clostridium tetani. J. Infect. Public Health 2014, 7, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, M. In vitro and in silico studies of novel synthetic ACE-inhibitory peptides derived from Saccharomyces cerevisiae protein hydrolysate. Bioorg. Chem. 2019, 87, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Sakulnarmrat, K.; Konczak, I. Composition of native Australian herbs polyphenolic-rich fractions and in vitro inhibitory activities against key enzymes relevant to metabolic syndrome. Food Chem. 2012, 134, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Atlas, S.A. The Renin-Angiotensin Aldosterone System: Pathophysiological Role and Pharmacologic Inhibition. J. Manag. Care Pharm. 2007, 13, 9–20. [Google Scholar] [CrossRef]
- Kharazmi-khorassani, J.; Asoodeh, A.; Tanzadehpanah, H. Bioorganic Chemistry Antioxidant and angiotensin-converting enzyme (ACE) inhibitory activity of thymosin alpha-1 (Thα1) peptide. Bioorg. Chem. 2019, 87, 743–752. [Google Scholar] [CrossRef]
- Tanzadehpanah, H.; Asoodeh, A.; Reza, M.; Chamani, J. Identi fi cation of a novel angiotensin-I converting enzyme inhibitory peptide from ostrich egg white and studying its interactions with the enzyme. Innov. Food Sci. Emerg. Technol. 2013, 18, 212–219. [Google Scholar] [CrossRef]
- Yaba, E.; Rafik, A.; Mostafa, B. α 67-106 of bovine hemoglobin: A new family of antimicrobial and angiotensin I-converting enzyme inhibitory peptides. Eur. Food Res. Technol. 2011, 232, 637–646. [Google Scholar]
- Kim, S.; Ngo, D.; Vo, T. Marine Fish-Derived Bioactive Peptides as Potential Antihypertensive Agents. In Advances in Food and Nutrition Research; Academic Press: Lincoln, NE, USA, 2012; Volume 65, pp. 249–260. [Google Scholar]
- Salampessy, J.; Reddy, N.; Phillips, M.; Kailasapathy, K. Isolation and characterization of nutraceutically potential ACE-Inhibitory peptides from leatherjacket (Meuchenia sp.) protein hydrolysates. LWT-Food Sci. Technol. 2017, 80, 430–436. [Google Scholar] [CrossRef]
- Toopcham, T.; Mes, J.J.; Wichers, H.J.; Roytrakul, S.; Yongsawatdigul, J. Bioavailability of angiotensin I-converting enzyme (ACE) inhibitory peptides derived from Virgibacillus halodenitrificans SK1-3-7 proteinases hydrolyzed tilapia muscle proteins. Food Chem. 2017, 220, 190–197. [Google Scholar] [CrossRef]
- Raghavan, S.; Kristinsson, H.G. ACE-inhibitory activity of tilapia protein hydrolysates. Food Chem. 2009, 117, 582–588. [Google Scholar] [CrossRef]
- Mirzaei, M.; Mirdamadi, S.; Safavi, M. Structural analysis of ACE-inhibitory peptide (VL-9) derived from Kluyveromyces marxianus protein hydrolysate. J. Mol. Struct. 2020, 1213, 128199. [Google Scholar] [CrossRef]
- Grijnhagen-riska, C. Competitive inhibitor binding assay (CIBA) of captopril and other ACE inhibitors. Clin. Chim. Acta 1987, 162, 53–60. [Google Scholar] [CrossRef]
- Lordan, S.; Smyth, T.J.; Soler-vila, A.; Stanton, C.; Ross, R.P. The a-amylase and a-glucosidase inhibitory effects of Irish seaweed extracts. Food Chem. 2013, 141, 2170–2176. [Google Scholar] [CrossRef]
- Jiang, M.; Yan, H.; He, R.; Ma, Y. Purification and a molecular docking study of α-glucosidase-inhibitory peptides from a soybean protein hydrolysate with ultrasonic pretreatment. Eur. Food Res. Technol. 2018, 244, 1995–2005. [Google Scholar] [CrossRef]
- Ktari, N.; Ben, R.; Salem, S.; Bkhairia, I.; Ben, S.; Nasri, R.; Ben, R.; Nasri, M. Functional properties and biological activities of peptides from zebra blenny protein hydrolysates fractionated using ultra filtration. Food Biosci. 2020, 34, 100539. [Google Scholar] [CrossRef]
- Fu, C.; Yang, X.; Lai, S.; Liu, C. Structure, antioxidant and α -amylase inhibitory activities of longan pericarp proanthocyanidins. J. Funct. Foods 2015, 14, 23–32. [Google Scholar] [CrossRef]
- Fu, C.; Jiang, Y.; Guo, J.; Su, Z. Natural Products with Anti-obesity E ff ects and Di ff erent Mechanisms of Action. J. Agric. Food Chem. 2016, 64, 9571–9585. [Google Scholar] [CrossRef]
- Man, B.; Cheung, Y.; Cheung, T.T.; Samaranayake, N.R. Therapeutic Advances in Drug Safety Safety of antiobesity drugs. Ther. Adv. Drug Saf. 2013, 1–11. [Google Scholar]
- Peters, G.; Bywater, R. Influence of a Lipid Interface on Protein Dynamics in a Fungal Lipase. Biophys. J. 2001, 81, 3052–3065. [Google Scholar] [CrossRef][Green Version]
- Jakubczyk, A.; Baraniak, B. Angiotensin I converting enzyme inhibitory peptides obtained after in vitro hydrolysis of pea (Pisum sativum var. Bajka) globulins. Biomed Res. Int. 2014, 2014, 438459. [Google Scholar] [CrossRef] [PubMed]
- López-Expósito, I.; Pellegrini, A.; Amigo, L.R.I. Synergistic Effect Between Different Milk-Derived Peptides and Proteins. J. Dairy Sci. 2008, 91, 2184–2189. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, D.; Kordowska-wiater, M.; Karaś, M.; Zięba, E. Release kinetics and antimicrobial properties of the potassium sorbate-loaded edible films made from pullulan, gelatin and their blends. Food Hydrocoll. 2019, 101, 105539. [Google Scholar] [CrossRef]
- Bintsis, T. Foodborne pathogens. AIMS Microbiol. 2017, 3, 529–563. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Tang, J. Candida albicans infection and intestinal immunity. Microbiol. Res. 2017, 198, 27–35. [Google Scholar] [CrossRef]
- Lum, K.Y.; Tay, S.T.; Le, C.F.; Lee, V.S.; Sabri, N.H.; Velayuthan, R.D.; Hassan, H.; Sekaran, S.D. Activity of Novel Synthetic Peptides against Candida albicans. Sci. Rep. 2015, 5, 1–12. [Google Scholar] [CrossRef]
- Satana, D.; Genc, G.E.; Erturan, Z. The antifungal susceptibilities of oral Candida spp isolates from HIV-infected patients. African J. Microbiol. Res. 2010, 4, 466–470. [Google Scholar]
- Salas, C.E.; Badillo-corona, J.A.; Ramírez-sotelo, G.; Oliver-salvador, C. Biologically Active and Antimicrobial Peptides from Plants. Biomed Res. Int. 2015, 2015, 11. [Google Scholar] [CrossRef]
- Lei, J.; Sun, L.; Huang, S.; Zhu, C.; Li, P.; He, J.; Mackey, V.; Coy, D.H. The antimicrobial peptides and their potential clinical applications. Am. J. Transl. Res. 2019, 11, 3919–3931. [Google Scholar]
- Amadou, I.; Le, G.; Amza, T.; Sun, J.; Shi, Y. Puri fi cation and characterization of foxtail millet-derived peptides with antioxidant and antimicrobial activities. Food Res. Int. 2013, 51, 422–428. [Google Scholar] [CrossRef]
- Sousa, J.C.; Berto, R.F.; Gois, A.; Hono, E.R.; Konno, K.; Richardson, M.; Rocha, M.F.G.; Camargo, A.C.M.; Pimenta, D.C.; Cardi, B.A.; et al. Leptoglycin: A new Glycine/Leucine-rich antimicrobial peptide isolated from the skin secretion of the South American frog Leptodactylus pentadactylus (Leptodactylidae). Toxicon 2009, 54, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Osaka, I.; Hefty, P.S. Simple Resazurin-Based Microplate Assay for Measuring Chlamydia. Antimicrob. Agents Chemother. 2013, 57, 2838–2840. [Google Scholar] [CrossRef] [PubMed]
- Cusimano, M.G.; Spinello, A.; Barone, G.; Schillaci, D.; Cascioferro, S.; Magistrato, A.; Parrino, B.; Arizza, V.; Vitale, M. A Synthetic Derivative of Antimicrobial Peptide Holothuroidin 2 from Mediterranean Sea Cucumber (Holothuria tubulosa) in the Control of Listeria monocytogenes. Mar. Drug 2019, 17, 159. [Google Scholar] [CrossRef] [PubMed]
- Rokicki, D.; Zdanowski, R.; Lewicki, S.; Leśniak, M.; Suska, M.; Wojdat, E.; Skopińska-Rózewska, E.; Skopiński, P. Inhibition of proliferation, migration and invasiveness of endothelial murine cells culture induced by resveratrol. Cent. Eur. J. Immunol. 2014, 39, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Świeca, M.; Baraniak, B.; Gawlik-Dziki, U. In vitro digestibility and starch content, predicted glycemic index and potential in vitro antidiabetic effect of lentil sprouts obtained by different germination techniques. Food Chem. 2013, 138, 1414–1420. [Google Scholar] [CrossRef]
- Jakubczyk, A.; Świeca, M.; Gawlik-Dziki, U.; Dziki, D. Nutritional potential and inhibitory activity of bread fortified with green coffee beans against enzymes involved in metabolic syndrome pathogenesis. LWT-Food Sci. Technol. 2018, 95, 78–84. [Google Scholar] [CrossRef]
- Jakubczyk, A.; Karaś, M.; Złotek, U.; Szymanowska, U.; Baraniak, B.; Bochnak, J. Peptides obtained from fermented faba bean seeds (Vicia faba) as potential inhibitors of an enzyme involved in the pathogenesis of metabolic syndrome. LWT-Food Sci. Technol. 2019, 105, 306–313. [Google Scholar] [CrossRef]
- Szymanowska, U.; Jakubczyk, A.; Baraniak, B.; Kur, A. Characterisation of lipoxygenase from pea seeds (Pisum sativum var. Telephone L.). Food Chem. 2009, 116, 906–910. [Google Scholar] [CrossRef]
Sample Availability: Samples of the peptides are available from the authors. |

| Peptide Sequence (Abbreviation) | Molecular Weight g/mol | Net Charge | Theoretical pI | Instability Index | Aliphatic Index | GRAVY Index | Water Solubility |
|---|---|---|---|---|---|---|---|
| YGNPVGGVGH (YH-10) | 956.03 | +0.1 | 6.74 | 3.76 (stable) | 58.00 | −0.280 | poor |
| GEHGGAGMGGGQFQPV (GV-16) | 1485.59 | −0.9 | 5.24 | 30.66 (stable) | 24.38 | −0.463 | poor |
| GNPVGGVGHGTTGT (GT-14) | 1210.27 | +0.1 | 6.74 | −13.99 (stable) | 41.43 | −0.314 | poor |
| EQGFLPGPEESGR (ER-13) | 1402.48 | −2.0 | 4.25 | 92.12 (unstable) | 30.00 | −1.315 | good |
| GQLGEHGGAGMG (GG-12) | 1070.14 | −0.9 | 5.24 | −8.83 (stable) | 40.83 | −0.425 | poor |
| RLARAGLAQ (RQ-9) | 955.12 | +2.0 | 12.00 | 8.89 (stable) | 120.00 | 0.011 | good |
| Peptide (Abbreviation) | IC50 (µg/mL) | |||
|---|---|---|---|---|
| ACE | α-Amylase | α-Glucosidase | Lipase | |
| YGNPVGGVGH (YH-10) | 498.79 ± 3.66 a | 76.75 ± 7.9 a | nd | 102.25 ± 1.40 ab |
| GEHGGAGMGGGQFQPV (GV-16) | 728.30 ± 4.01 b | 66.22 ± 18.86 a | nd | 62.32 ± 4.44 ab |
| GNPVGGVGHGTTGT (GT-14) | 525.63 ± 3.08 c | 60.53 ± 2.35 a | nd | 104.21 ± 4.23 b |
| EQGFLPGPEESGR (ER-13) | 641.16 ± 2.18 d | 71.65 ± 10.94 a | nd | 76.81 ± 18.33 ab |
| GQLGEHGGAGMG (GG-12) | 561.60 ± 3.15 e | 56.72 ± 8.67 a | nd | 60.62 ± 17.20 a |
| RLARAGLAQ (RQ-9) | nd | 66.74 ± 5.91 a | nd | 97.31 ± 28.57 ab |
| Peptide (Abbreviation) | IC50 (µg/mL) | ||
|---|---|---|---|
| LOX | COX-1 | COX-2 | |
| YGNPVGGVGH (YH-10) | 121.66 ± 2.16 a | 16.61 ± 1.13 a | 15.53 ± 1.78 a |
| GEHGGAGMGGGQFQPV (GV-16) | 101.69 ± 2.72 b | 11.88 ± 0.79 b | 44.81 ± 2.03 b |
| GNPVGGVGHGTTGT (GT-14) | 185.71 ± 6.11 c | 14.84 ± 1.71 a | 4.43 ± 0.87 c |
| EQGFLPGPEESGR (ER-13) | 84.35 ± 4.62 d | 6.71 ± 0.47 c | 4.31 ± 0.99 c |
| GQLGEHGGAGMG (GG-12) | 140.26 ± 2.75 e | 7.61 ± 0.77 c | 16.85 ± 2.53 a |
| RLARAGLAQ (RQ-9) | 196.09 ± 3.43 c | 0.31 ± 0.01 d | 4.77 ± 1.01 c |
| ACE | |||||||
|---|---|---|---|---|---|---|---|
| Reaction without Inhibitor | YH-10 | GV-16 | GT-14 | ER-13 | GG-12 | RQ-9 | |
| Km (mM) | 0.56 | 1.29 | 3.29 | 2.99 | 1.86 | 2.21 | na |
| Vmax | 0.008 | 0.006 | 0.008 | 0.008 | 0.008 | 0.008 | na |
| Type of Inhibition | - | uncompetitive | competitive | competitive | competitive | competitive | na |
| α-amylase | |||||||
| Km (mM) | 121.02 | 117.71 | 121.02 | 60.05 | 58.61 | 36.45 | 17.51 |
| Vmax | 1.04 | 0.86 | 0.73 | 0.42 | 0.56 | 0.45 | 0.30 |
| Type of Inhibition | - | uncompetitive | noncompetitive | uncompetitive | uncompetitive | uncompetitive | uncompetitive |
| Pancreatic Lipase | |||||||
| Km (mM) | 306.40 | 306.40 | 94.92 | 100.77 | 306.40 | 145.30 | 47.33 |
| Vmax | 2.04 | 0.68 | 0.35 | 0.34 | 2.01 | 0.70 | 0.06 |
| Type of Inhibition | - | noncompetitive | uncompetitive | uncompetitive | noncompetitive | uncompetitive | uncompetitive |
| LOX | |||||||
|---|---|---|---|---|---|---|---|
| Reaction without Inhibitor | YH-10 | GV-16 | GT-14 | ER-13 | GG-12 | RQ-9 | |
| Km (mM) | 1.41 | 1.41 | 1.41 | 1.41 | 1.41 | 4.16 | 5.08 |
| Vmax | 0.49 | 0.34 | 0.25 | 0.28 | 0.32 | 0.49 | 0.49 |
| Type of Inhibition | - | noncompetitive | noncompetitive | noncompetitive | noncompetitive | competitive | competitive |
| COX-1 | |||||||
| Km (mM) | 0.65 | 0.53 | 0.30 | 0.59 | 0.41 | 0.55 | 0.49 |
| Vmax | 0.42 | 0.32 | 0.23 | 0.34 | 0.27 | 0.32 | 0.31 |
| Type of Inhibition | - | uncompetitive | uncompetitive | uncompetitive | uncompetitive | uncompetitive | uncompetitive |
| COX-2 | |||||||
| Km (mM) | 0.12 | 0.07 | 0.09 | 0.11 | 0.12 | 0.07 | 0.08 |
| Vmax | 0.28 | 0.14 | 0.15 | 0.15 | 0.15 | 0.13 | 0.14 |
| Type of Inhibition | - | uncompetitive | uncompetitive | uncompetitive | noncompeptitive | uncompetitive | uncompetitive |
| Peptide Sequence (Abbreviation) | E. coli ATCC 25922 | S. aureus ATCC 29737 | S. enterica ATCC 4931 | L. monocytogenes ATCC BAA-2660 | C. albicans ATCC 90028 |
|---|---|---|---|---|---|
| MIC (mg/mL) | |||||
| YGNPVGGVGH (YH-10) | nd | nd | nd | 125 | nd |
| GEHGGAGMGGGQFQPV (GV-16) | nd | nd | nd | 62.50 | nd |
| GNPVGGVGHGTTGT (GT-14) | 250 | 250 | nd | 250 | nd |
| EQGFLPGPEESGR (ER-13) | nd | nd | nd | 125 | nd |
| GQLGEHGGAGMG (GG-12) | nd | 250 | nd | 250 | nd |
| RLARAGLAQ (RQ-9) | 250 | nd | 15.62 | 15.62 | 15.62 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Złotek, U.; Jakubczyk, A.; Rybczyńska-Tkaczyk, K.; Ćwiek, P.; Baraniak, B.; Lewicki, S. Characteristics of New Peptides GQLGEHGGAGMG, GEHGGAGMGGGQFQPV, EQGFLPGPEESGR, RLARAGLAQ, YGNPVGGVGH, and GNPVGGVGHGTTGT as Inhibitors of Enzymes Involved in Metabolic Syndrome and Antimicrobial Potential. Molecules 2020, 25, 2492. https://doi.org/10.3390/molecules25112492
Złotek U, Jakubczyk A, Rybczyńska-Tkaczyk K, Ćwiek P, Baraniak B, Lewicki S. Characteristics of New Peptides GQLGEHGGAGMG, GEHGGAGMGGGQFQPV, EQGFLPGPEESGR, RLARAGLAQ, YGNPVGGVGH, and GNPVGGVGHGTTGT as Inhibitors of Enzymes Involved in Metabolic Syndrome and Antimicrobial Potential. Molecules. 2020; 25(11):2492. https://doi.org/10.3390/molecules25112492
Chicago/Turabian StyleZłotek, Urszula, Anna Jakubczyk, Kamila Rybczyńska-Tkaczyk, Paula Ćwiek, Barbara Baraniak, and Sławomir Lewicki. 2020. "Characteristics of New Peptides GQLGEHGGAGMG, GEHGGAGMGGGQFQPV, EQGFLPGPEESGR, RLARAGLAQ, YGNPVGGVGH, and GNPVGGVGHGTTGT as Inhibitors of Enzymes Involved in Metabolic Syndrome and Antimicrobial Potential" Molecules 25, no. 11: 2492. https://doi.org/10.3390/molecules25112492
APA StyleZłotek, U., Jakubczyk, A., Rybczyńska-Tkaczyk, K., Ćwiek, P., Baraniak, B., & Lewicki, S. (2020). Characteristics of New Peptides GQLGEHGGAGMG, GEHGGAGMGGGQFQPV, EQGFLPGPEESGR, RLARAGLAQ, YGNPVGGVGH, and GNPVGGVGHGTTGT as Inhibitors of Enzymes Involved in Metabolic Syndrome and Antimicrobial Potential. Molecules, 25(11), 2492. https://doi.org/10.3390/molecules25112492






