Supercritical Fluid Applications in the Design of Novel Antimicrobial Materials
Abstract
1. Introduction
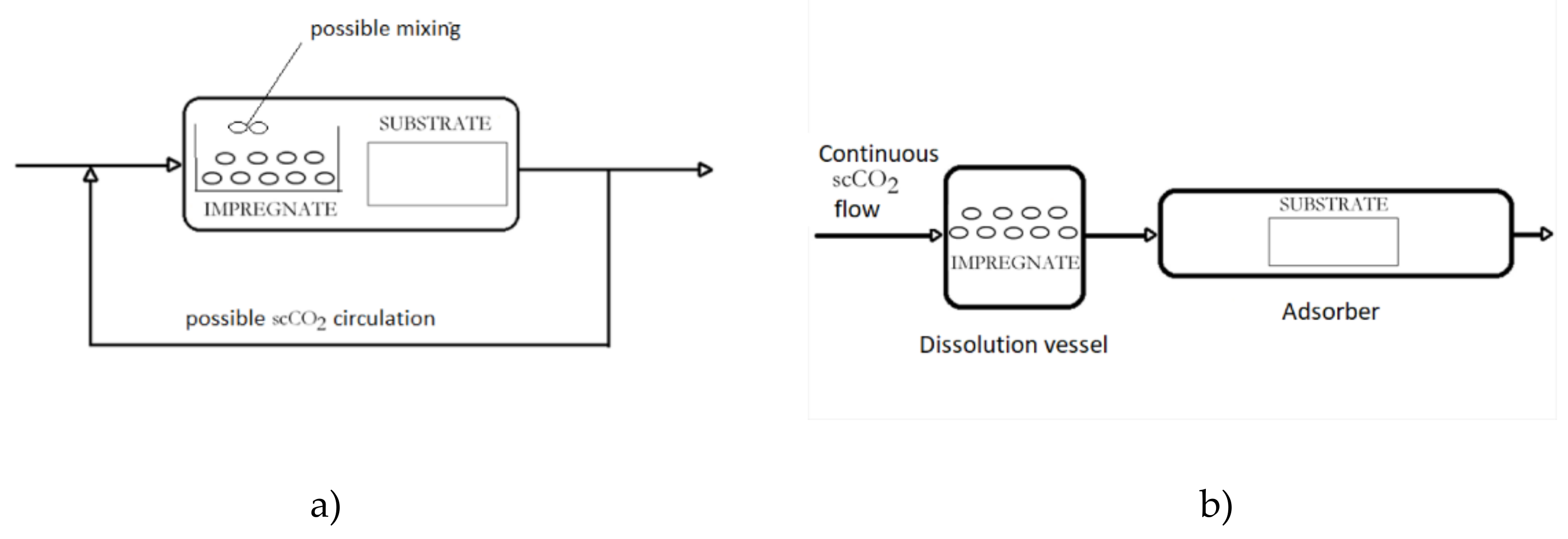
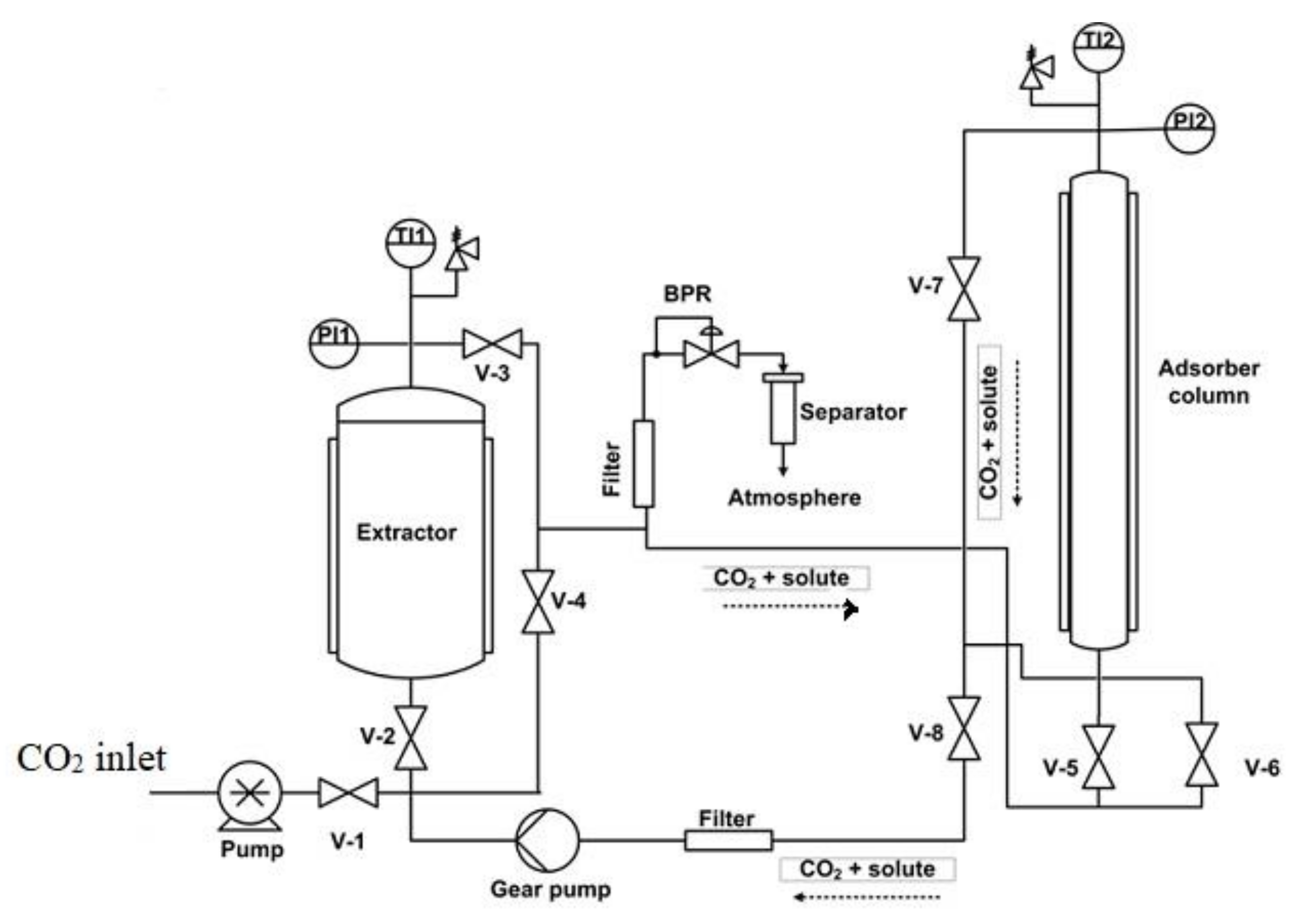
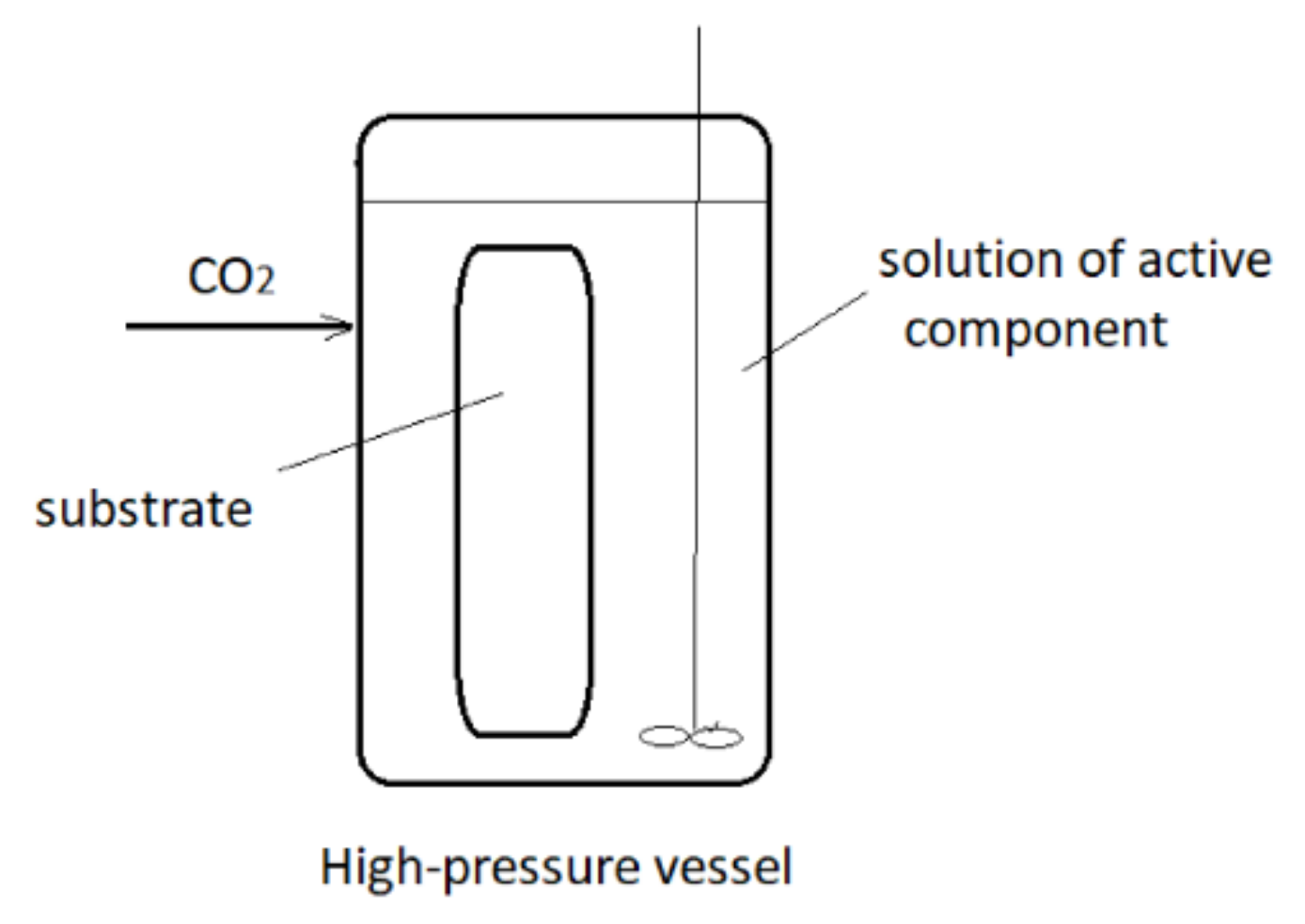
2. Supercritical Solvent Impregnation (SSI)
2.1. Impregnation of Textiles and Fibers
2.2. Impregnation of Polymeric Forms Other than Textiles and Fibers
3. Supercritical Assisted Impregnation (SAI) and High-Pressure Assisted Impregnation (HPAI)
4. Supercritical Solvent Impregnation or Supercritical Assisted Impregnation Coupled with Polymerization in scCO2
5. Supercritical Foaming
6. Supercritical Drying of Metal-Carrying Gels
7. Other Methodologies Applied to the Development of Antibacterial Materials
8. Discussion
Funding
Conflicts of Interest
References
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Antibiotic Resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/antibiotic-resistance (accessed on 4 March 2020).
- Centers for Disease Control and Prevention. Antibiotic/Antimicrobial Resistance (AR/AMR). Available online: https://www.cdc.gov/drugresistance/biggest-threats.html (accessed on 4 March 2020).
- Antibiotic-Resistant Infections Threaten Modern Medicine. Available online: https://www.cdc.gov/drugresistance/pdf/threats-report/Threat-Modern-Medicine-508.pdf (accessed on 4 March 2020).
- World Health Organization Regional Office for Europe. Moving Towards a Multisectorial Approach to Tackling Antimicrobial Resistance. Available online: http://www.euro.who.int/en/countries/france/news/news/2020/3/moving-towards-a-multisectoral-approach-to-tackling-antimicrobial-resistance (accessed on 20 March 2020).
- Kiellow, A.W.; Henriksen, O. Supercritical wood impregnation. J. Supercrit. Fluid. 2009, 50, 297–304. [Google Scholar] [CrossRef][Green Version]
- Fernandes, J.; Kjellow, A.W.; Henriksen, O. Modeling and optimization of the supercritical wood impregnation process-Focus on pressure and temperature. J. Supercrit. Fluid. 2012, 66, 307–314. [Google Scholar] [CrossRef]
- Cookson, Q.L. Treatment of eucalypt heartwood using supercritical carbon dioxide as a Carrier. In Proceedings of the 27th Products Research Conference, CSIRO Forestry and Forest Products, Clayton, Australia, 12–13 November 2001. [Google Scholar]
- Iversen, S.B.; Larsen, T.; Henriksen, O.; Felsvang, K. The world’s first commercial supercritical wood treatement plant. In The International Society for the Advancement of Supercritical Fluids, Proceedings of the 6th International Symposium on Supercritical Fluids, Versailles, France, 28−30 April 2003; Institute National Polytechnique de Lorraine: Vandoeuvre-lès-Nancy, France, 2003. [Google Scholar]
- Van der Kraan, M.; Cid, M.V.F.; Woerlee, G.F.; Veugelers, W.J.T.; Witkamp, G.J. Dyeing of natural and synthetic textiles in supercritical carbon dioxide with disperse reactive dyes. J. Supercrit. Fluid. 2007, 40, 470–476. [Google Scholar] [CrossRef]
- Banchero, M. Supercritical fluid dyeing of synthetic and natural textiles—A review. Color. Technol. 2012, 129, 2–17. [Google Scholar] [CrossRef]
- Dyecoo. Available online: http//www.dyecoo.com/ (accessed on 20 March 2020).
- Nascimento, G.G.F.; Locatelli, J.; Freitas, P.C.; Silva, G.L. Antibacterial activity of plant extracts and phytochemicals on antibiotic-resistant bacteria. Braz. J. Microbiol. 2000, 31, 247–256. [Google Scholar] [CrossRef]
- Cheesman, M.J.; Ilanko, A.; Blonk, B.; Cock, I.E. Developing New Antimicrobial Therapies: Are Synergistic Combinations of Plant Extracts/Compounds with Conventional Antibiotics the Solution? Pharmacogn. Rev. 2017, 11, 57–72. [Google Scholar]
- Lewis, K.; Ausubel, F.M. Prospects for plant-derived antibacterials. Nat. Biotechnol. 2006, 24, 1504–1507. [Google Scholar] [CrossRef]
- Mišić, D. The applicability of supercritical extracts in clinical treatment of bacterial infections in humans and animals. In Supercritical CO2 Extraction and its Applications; Rój, E., Ed.; Polish Foundations of the Opportunities Industrialization Centers “OIC Poland”: Lublin, Poland, 2014; pp. 23–34. [Google Scholar]
- Cosentino, S.; Tuberoso, C.I.; Pisano, B.; Satta, M.; Mascia, V.; Arzedi, E.; Palmas, F. In vitro antimicrobial activity and chemical composition of Sardinian thymus essential oils. Lett. Appl. Microbiol. 1999, 29, 130–135. [Google Scholar] [CrossRef]
- Nostro, A.; Blanco, A.R.M.; Cannatelli, A.; Enea, V.; Flamini, G.; Morelli, I.; Roccaro, A.S.; Alonzo, V. Susceptibility of methicillin-resistant staphylococci to oregano essential oil, carvacrol and thymol. FEMS Microbiol. Lett. 2004, 230, 191–195. [Google Scholar] [CrossRef]
- U.S. Food & Drug Administration. CFR-Code of Federal Regulations Title 21. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=172.515&SearchTerm=thymol (accessed on 25 March 2020).
- Milovanovic, S.; Stamenic, M.; Markovic, D.; Ivanovic, J.; Zizovic, I. Supercritical impregnation of cellulose acetate with thymol. J. Supercrit. Fluid. 2015, 97, 107–115. [Google Scholar] [CrossRef]
- Kramer, A.; Schwebke, I.; Kampf, G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect. Dis. 2006, 6, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Milovanovic, S.; Stamenic, M.; Markovic, D.; Radetic, M.; Zizovic, I. Solubility of thymol in supercritical carbon dioxide and its impregnation on cotton gauze. J. Supercrit. Fluid. 2013, 84, 173–181. [Google Scholar] [CrossRef]
- Milovanovic, S.; Radetic, M.; Misic, D.; Asanin, J.; Leontijevic, V.; Ivanovic, J.; Zizovic, I. High pressure modified cotton in wound dressing applications. In Cotton Fibers: Characteristics, Uses and Performance; Gordon, S., Abidi, N., Eds.; Nova Science Publishers: New York, NY, USA, 2017; pp. 117–205. [Google Scholar]
- Nithyakalyani, D.; Ramachandran, T.; Rajendran, R.; Mahalakshmi, M. Assessment of antibacterial activity of herbal finished surface modified polypropylene non-woven fabric against bacterial pathogens of wound. J. Appl. Polym. Sci. 2013, 129, 672–681. [Google Scholar] [CrossRef]
- Markovic, D.; Milovanovic, S.; Radetic, M.; Jokic, B.; Zizovic, I. Impregnation of corona modified polypropylene non-woven material with thymol in supercritical carbon dioxide for antimicrobial application. J. Supercrit. Fluid. 2015, 101, 215–221. [Google Scholar] [CrossRef]
- Marković, D.; Milovanović, S.; De Clerck, K.; Zizovic, I.; Stojanović, D.; Radetić, M. Development of material with strong antimicrobial activity by high pressure CO2 impregnation of polyamide nanofibers with thymol. J. CO2 Util. 2018, 26, 19–27. [Google Scholar] [CrossRef]
- Sanchez-Sanchez, J.; Fernández-Ponce, M.T.; Casas, L.; Mantell, C.; Martínez de la Ossa, E.J. Impregnation of mango leaf extract into a polyester textile using supercritical carbon dioxide. J. Supercrit. Fluid. 2017, 128, 208–217. [Google Scholar] [CrossRef]
- Fanovich, M.A.; Ivanovic, J.; Misic, D.; Alvarez, M.V.; Jaeger, P.; Zizovic, I.; Eggers, R. Development of polycaprolactone scaffold with antibacterial activity by the integrated supercritical extraction and impregnation process. J. Supercrit. Fluid. 2013, 78, 42–53. [Google Scholar] [CrossRef]
- Ivanovic, J.; Milovanovic, S.; Stamenic, M.; Fanovich, M.A.; Jaeger, P.; Zizovic, I. Application of an Integrated Supercritical Extraction and Impregnation Process for Incorporation of Thyme Extracts into Different Carriers. In Handbook of Supercritical Fluids; Osborne, J., Ed.; Nova Science Publishers: New York, NY, USA, 2014; pp. 257–281. [Google Scholar]
- Zizovic, I.; Jaeger, P.T.; Pietsch, A. Production of materials with antimicrobial properties by a combined supercritical extraction and impregnation process. In Proceedings of the International Symposium on Supercritical Fluids, Antibes-Juan-les-Pins, Valence, France, 22–25 April 2018. [Google Scholar]
- Zizovic, I.; Ivanovic, J.; Milovanovic, S.; Stamenic, M. Impregnations using supercritical carbon dioxide. In Supercritical CO2 Extraction and its Applications; Rój, E., Ed.; Polish Foundations of the Opportunities Industrialization Centers ”OIC Poland”: Lublin, Poland, 2014; pp. 23–34. [Google Scholar]
- Zizovic, I.; Ivanovic, J.; Milovanovic, S.; Adamovic, T. Application of supercritical fluids in development of materials with antibacterial properties. In Supercritical Fluid Applications; Roj, E., Ed.; New Chemical Syntheses Institute: Pulawy, Poland, 2016; pp. 45–60. [Google Scholar]
- Maksimovic, S.; Tadic, V.; Radmanovic, T.; Milovanovic, S.; Stankovic, M.; Zizovic, I. Utilization of the integrated process of supercritical extraction and impregnation for incorporation of Helichrysum italicum extract into corn starch xerogel. Chem. Ind. Chem. Eng. Q. 2018, 24, 191–200. [Google Scholar] [CrossRef]
- Fanovich, M.A.; Ivanovic, J.; Zizovic, I.; Misic, D.; Jaeger, P. Functionalization of polycaprolactone/hydroxyapatite scaffolds with Usnea lethariiformis extract by using supercritical CO2. Mater. Sci. Eng. C 2016, 58, 204–212. [Google Scholar] [CrossRef]
- Rój, E.; Tadić, V.M.; Mišić, D.; Zizovic, I.; Arsić, I.; Dobrzyńska-Inger, A.; Kostrzewa, D. Supercritical carbon dioxide hops extracts with antimicrobial properties. Open Chem. 2015, 13, 1157–1171. [Google Scholar] [CrossRef]
- Gittard, S.D.; Hojo, D.; Hyde, G.K.; Scarel, G.; Narayan, R.J.; Parsons, G.N. Antifungal textiles formed using silver deposition in supercritical carbon dioxide. J. Mater. Eng. Perform. 2010, 19, 368–373. [Google Scholar] [CrossRef]
- Chen, Y.; Zhong, X.; Zhang, Q. Synthesis of CO2-Philic Polysiloxane with N-Halamine Side Groups for Biocidal Coating on Cotton. Ind. Eng. Chem. Res. 2012, 51, 9260–9265. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Q.; Han, Q.; Mi, Y.; Sun, S.; Feng, C.; Xiao, H.; Yu, P.; Yang, C. Synthesis of polysiloxane with 5,5-dimethylhydantoin-based N-halamine pendants for biocidal functionalization of polyethylene by supercritical impregnation. J. Appl. Polym. Sci. 2017, 134, 44721–44729. [Google Scholar]
- Chen, Y.; Yu, P.; Feng, C.; Wang, Y.; Han, Q.; Zhang, Q. Synthesis of polysiloxane with quaternized N-halamine moieties for antibacterial coating of polypropylene via supercritical impregnation technique. Appl. Surf. Sci. 2017, 419, 683–691. [Google Scholar] [CrossRef]
- Chen, Y.; He, Q.; Ren, G.; Feng, C.; Li, N.; Yu, H.; Han, Q. Preparation of biocidal 4-ethyl-4-(hydroxymethyl)oxazolidin-2-one-based N-halamine polysiloxane for impregnation of polypropylene in supercritical CO2. J. Appl. Polym. Sci. 2018, 135, 46624–46631. [Google Scholar] [CrossRef]
- Borkow, G.; Gabbay, J. Biocidal textiles can help fight nosocomial infections. Med. Hypotheses 2008, 70, 990–994. [Google Scholar] [CrossRef]
- Bayraktar, Z.; Kiran, E. Miscibility, Phase Separation, and Volumetric Properties in Solutions of Poly(dimethylsiloxane) in Supercritical Carbon Dioxide. J. Appl. Polym. Sci. 2000, 75, 1397–1403. [Google Scholar] [CrossRef]
- O’Neill, M.L.; Cao, Q.; Fang, M.; Johnston, K.P. Solubility of Homopolymers and Copolymers in Carbon Dioxide. Ind. Eng. Chem. Res. 1998, 37, 3067–3079. [Google Scholar] [CrossRef]
- Elmaaty, A.T.; Ma, J.; El-Taweel, F.; El-Aziz, E.A.; Okubayashi, S. Facile Bifunctional Dyeing of Polyester under Supercritical Carbon Dioxide Medium with New Antibacterial Hydrazono Propanenitrile Dyes. Ind. Eng. Chem. Res. 2014, 53, 15566–15570. [Google Scholar] [CrossRef]
- Elmaaty, A.T.; El-Aziz, A.E.; Ma, J.; El-Taawel, F.; Okubayashi, S. Eco-Friendly Disperse Dyeing and Functional Finishing of Nylon 6 Using Supercritical Carbon Dioxide. Fibers 2015, 3, 309–322. [Google Scholar] [CrossRef]
- Ma, J.; Elmaaty, A.T.; Okubayashi, S. Effect of Supercritical Carbon Dioxide on Dyeability and Physical Properties of Ultra-High-Molecular-Weight Polyethylene Fiber. AUTEX Res. J. 2019, 19, 228–235. [Google Scholar] [CrossRef]
- Milovanovic, S.; Markovic, D.; Aksentijevic, K.; Stojanovic, D.B.; Ivanovic, J.; Zizovic, I. Application of cellulose acetate for controlled release of thymol. Carbohyd. Polym. 2016, 147, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Milovanovic, S.; Adamovic, T.; Aksentijevic, K.; Misic, D.; Ivanovic, J.; Zizovic, I. Cellulose Acetate Based Material with Antibacterial Properties Created by Supercritical Solvent Impregnation. Int. J. Polym. Sci. 2017, 2017, 1–9. [Google Scholar] [CrossRef]
- Zizovic, I.; Senerovic, L.; Moric, I.; Adamovic, T.; Jovanovic, M.; Kalagasidis Krusic, M.; Misic, D.; Stojanovic, D.; Milovanovic, S. Utilization of supercritical carbon dioxide in fabrication of cellulose acetate films with anti-biofilm effects against Pseudomonas aeruginosa and Staphylococcus aureus. J. Supercrit. Fluid. 2018, 140, 11–20. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S.F. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef]
- Oppenheimer-Shaanan, Y.; Steinberg, N.; Kolodkin-Gal, I. Small molecules are natural triggers for the disassembly of biofilms. Trends Microbiol. 2013, 21, 594–601. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- World Health Organization. Prioritization of Pathogens to Guide Discovery, Research and Development of New Antibiotics for Drug-Resistant Bacterial Infections, Including Tuberculosis; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Adamovic, T.; Milovanovic, S.; Markovic, D.; Zizovic, I. Impregnation of cellulose acetate films with carvacrol using supercritical carbon dioxide. Tehnika 2018, 73, 19–25. [Google Scholar] [CrossRef]
- Varona, S.; Rodríguez-Rojo, S.; Martín, A.; José Cocero, M.; Duarte, C.M.M. Supercritical impregnation of lavandin (Lavandula hybrida) essential oil in modified starch. J. Supercrit. Fluid. 2011, 58, 313–319. [Google Scholar] [CrossRef]
- Senerović, L.; Morić, I.; Zizovic, I. SSI in the antibacterial mats design. Unpublished work. 2020. [Google Scholar]
- Darpentigny, C.; Marcoux, P.R.; Menneteau, M.; Michel, B.; Ricoul, F.; Jean, B.; Bras, J.; Nonglaton, G. Antimicrobial cellulose nanofibril porous materials obtained by supercritical impregnation of thymol. ACS Appl. Bio Mater. 2020. [Google Scholar] [CrossRef]
- Terzić, I.; Ivanović, J.; Žižović, I.; Lučić Škorić, M.; Milosavljević, N.; Milašinović, N.; Kalagasidis Krušić, M. A novel chitosan gels: Supercritical CO2 drying and impregnation with thymol. Polym. Eng. Sci. 2018, 58, 2192–2199. [Google Scholar] [CrossRef]
- Dias, A.M.A.; Braga, M.E.M.; Seabra, I.J.; Ferreira, P.; Gil, M.H.; de Sousa, H.C. Development of natural-based wound dressings impregnated with bioactive compounds and using supercritical carbon dioxide. Int. J. Pharmaceut. 2011, 408, 9–19. [Google Scholar] [CrossRef]
- Tsutsumi, C.; Fukukawa, N.; Sakafuji, J.; Oro, K.; Hata, K.; Nakayama, Y.; Shiono, T. Impregnation of Poly(L-lactide-ran-cyclic carbonate) Copolymers with Useful Compounds with Supercritical Carbon Dioxide. J. Appl. Polym. Sci. 2011, 121, 1431–1441. [Google Scholar] [CrossRef]
- Lim, L.T.; Auras, R.; Rubino, M. Processing technologies for poly(lactic acid). Prog. Polym. Sci. 2008, 33, 820–852. [Google Scholar] [CrossRef]
- Yu, J.P.; Guan, Y.X.; Yao, S.J.; Zhu, Z.Q. Preparation of Roxithromycin-Loaded Poly(l-lactic Acid) Films with Supercritical Solution Impregnation. Ind. Eng. Chem. Res. 2011, 50, 13813–13818. [Google Scholar] [CrossRef]
- Torres, A.; Ilabaca, E.; Rojas, A.; Rodríguez, F.; Galotto, M.J.; Guarda, A.; Villegas, C.; Romero, J. Effect of processing conditions on the physical, chemical and transport properties of polylactic acid films containing thymol incorporated by supercritical impregnation. Eur. Polym. J. 2017, 89, 195–210. [Google Scholar] [CrossRef]
- Villegas, C.; Torres, A.; Rios, M.; Rojas, A.; Romero, J.; de Dicastillo, C.L.; Valenzuela, X.; Galotto, M.J.; Guarda, A. Supercritical impregnation of cinnamaldehyde into polylactic acid as a route to develop antibacterial food packaging materials. Food Res. Int. 2017, 99, 650–659. [Google Scholar] [CrossRef]
- Villegas, C.; Arrieta, M.P.; Rojas, A.; Torres, A.; Faba, S.; Toledo, M.J.; Gutierrez, M.A.; Zavalla, E.; Romero, J.; Galotto, M.J.; et al. PLA/organoclay bionanocomposites impregnated with thymol and cinnamaldehyde by supercritical impregnation for active and sustainable food packaging, Compos. Part B—Eng. 2019, 176. [Google Scholar] [CrossRef]
- Milovanovic, S.; Hollermann, G.; Errenst, C.; Pajnik, J.; Frerich, S.; Kroll, S.; Rezwan, K.; Ivanovic, J. Supercritical CO2 impregnation of PLA/PCL films with natural substances for bacterial growth control in food packaging. Food Res. Int. 2018, 107, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.; Romero, J.; Macan, A.; Guarda, A.; Galotto, M.J. Near critical and supercritical impregnation and kinetic release of thymol in LLDPE films used for food packaging. J. Supercrit. Fluid. 2014, 85, 41–48. [Google Scholar] [CrossRef]
- Goni, M.L.; Ganan, N.A.; Strumia, M.C.; Martinia, R.E. Eugenol-loaded LLDPE films with antioxidant activity by supercriticalcarbon dioxide impregnation. J. Supercrit. Fluid. 2016, 111, 28–35. [Google Scholar] [CrossRef]
- Medeiros, G.R.; Ferreira, S.R.S.; Carciofi, B.A.M. High pressure carbon dioxide for impregnation of clove essential oil in LLDPE films. Innov. Food Sci. Emerg. 2017, 41, 206–215. [Google Scholar] [CrossRef]
- Rojas, A.; Torres, A.; Martínez, F.; Salazar, L.; Villegas, C.; Galotto, M.J.; Guarda, A.; Romero, J. Assessment of kinetic release of thymol from LDPE nanocomposites by supercritical impregnation: Effect of depressurization rate and nanoclay content. Eur. Polym. J. 2017, 93, 294–306. [Google Scholar] [CrossRef]
- Rojas, A.; Torres, A.; Añazco, A.; Villegas, C.; Galotto, M.J.; Guarda, A.; Romero, J. Effect of pressure and time on scCO2-assisted incorporation of thymol into LDPE-based nanocomposites for active food packaging. J. CO2 Util. 2018, 26, 434–444. [Google Scholar] [CrossRef]
- Bierhalz, A.C.K.; da Silva, M.A.; de Sousa, H.C.; Braga, M.E.M.; Kieckbusch, T.G. Influence of natamycin loading methods on the physical characteristics of alginate active films. J. Supercrit. Fluid. 2013, 76, 74–82. [Google Scholar] [CrossRef]
- De Souza, A.C.; Dias, A.M.A.; Sousa, H.C.; Tadini, C.C. Impregnation of cinnamaldehyde into cassava starch biocompositefilms using supercritical fluid technology for the development of foodactive packaging. Carbohyd. Polym. 2014, 102, 830–837. [Google Scholar] [CrossRef]
- Milovanovic, S.; Jankovic-Castvan, I.; Ivanovic, J.; Zizovic, I. Effect of Starch Xero- and Aerogels Preparation on the Supercritical CO2 Impregnation of Thymol. Starch—Starke 2015, 67, 174–182. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, P.; Ren, G.; Zhang, Q.; Han, Q.; Teng, H. Interpenetration of Polyethylene Terephthalate with Biocidal Quaternary Ammonium /N-Chloramine Polysiloxane in Supercritical CO2. Ind. Eng. Chem. Res. 2017, 56, 9560–9568. [Google Scholar] [CrossRef]
- Xu, W.Z.; Yang, L.; Charpentier, P.A. Preparation of Antibacterial Softwood via Chemical Attachment of Quaternary Ammonium Compounds Using Supercritical CO2. ACS Sustain. Chem. Eng. 2016, 4, 1551–1561. [Google Scholar] [CrossRef]
- Renner, M.; Weidner, E.; Brandin, G. High-pressure carbon dioxide tanning. Chem. Eng. Res. Des. 2009, 87, 987–996. [Google Scholar] [CrossRef]
- Mölders, N.; Renner, M.; Errenst, C.; Weidner, E. Incorporation of antibacterial active additives inside polycarbonatesurfaces by using compressed carbon dioxide as transport aid. J. Supercrit. Fluid. 2018, 132, 83–90. [Google Scholar] [CrossRef]
- Niu, A.; Han, Y.; Wu, J.; Yu, N.; Xu, Q. Synthesis of One-Dimensional Carbon Nanomaterials Wrapped by Silver Nanoparticles and Their Antibacterial Behavior. J. Phys. Chem. C 2010, 114, 12728–12735. [Google Scholar] [CrossRef]
- Haldorai, Y.; Kim, B.K.; Jo, Y.L.; Shim, J.J. Ag@graphene oxide nanocomposite as an efficient visible-light plasmonic photocatalyst for the degradation of organic pollutants: A facile green synthetic approach. Mater. Chem. Phys. 2014, 143, 1452–1461. [Google Scholar] [CrossRef]
- Karthäuser, J. Method of Producing an Interpenetrating Polymer Network (IPN), the IPN and Use Thereof. U.S. Patent No. US7687585 B2, 30 March 2010. [Google Scholar]
- Steffensen, S.L.; Vestergaard, M.H.; Groenning, M.; Alm, M.; Franzyk, H.; Nielsen, H.M. Sustained prevention of biofilm formation on a novel silicone matrix suitable for medical devices. Eur. J. Pharm. Biopharm. 2015, 94, 305–311. [Google Scholar] [CrossRef]
- Steffensen, S.L.; Vestergaard, M.H.; Moller, E.H.; Groenning, M.; Alm, M.; Franzyk, H.; Nielsen, H.M. Soft hydrogels interpenetrating silicone-a polymer network for drug-releasing medical devices. J. Biomed. Mater. Res. B Appl. Biomater. 2015, 104, 402–410. [Google Scholar] [CrossRef]
- Stenger, M.; Klein, K.; Grønnemose, R.B.; Klitgaard, J.K.; Kolmos, H.J.; Lindholt, J.S.; Alm, M.; Thomsen, P.; Andersen, T.E. Co-release of dicloxacillin and thioridazine from catheter material containing an interpenetrating polymer network for inhibiting device-associated Staphylococcus aureus infection. J. Control. Release 2016, 241, 125–134. [Google Scholar] [CrossRef]
- Maki, D.G.; Tambyah, P.A. Engineering out the risk for infection with urinary catheters. Emerg. Infect. Dis. 2001, 7, 342–347. [Google Scholar] [CrossRef]
- Schumm, K.; Lam, T.B.L. Types of urethral catheters for management of shortterm voiding problems in hospitalized adults: A short version cochrane review. Neurourol. Urodyn. 2008, 27, 110–121. [Google Scholar] [CrossRef]
- Raad, I.; Hanna, H.; Maki, D. Intravascular catheter-related infections: Advances in diagnosis, prevention, and management. Lancet Infect. Dis. 2007, 7, 645–657. [Google Scholar] [CrossRef]
- Dimick, J.B.; Pelz, R.K.; Consunji, R.; Swoboda, S.M.; Hendrix, C.W.; Lipsett, P.A. Increased resource use associated with catheter-related bloodstream infection in the surgical intensive care unit. Arch. Surg. 2001, 136, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Correia, V.G.; Bonifacio, V.D.B.; Raje, V.P.; Casimiro, T.; Moutinho, G.; da Silva, C.L.; Pinho, M.G.; Aguiar-Ricardo, A. Oxazoline-Based Antimicrobial Oligomers: Synthesis by CROP Using Supercritical CO2. Macromol. Biosci. 2011, 11, 1128–1137. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A. Green solvents for sustainable organic synthesis: State of the art. Green Chem. 2005, 7, 267–278. [Google Scholar] [CrossRef]
- Correia, V.G.; Ferraria, A.M.; Pinho, M.G.; Aguiar-Ricardo, A. Antimicrobial Contact-Active Oligo(2-oxazoline)s-Grafted Surfaces for Fast Water Disinfection at the Point-of-Use. Biomacromolecules 2015, 16, 3904–3915. [Google Scholar] [CrossRef]
- Brogden, K.A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005, 3, 238–250. [Google Scholar] [CrossRef]
- Zasloff, M. Antimicrobial Peptides, Innate Immunity, and the Normally Sterile Urinary Tract. J. Am. Soc. Nephrol. 2007, 18, 2810–2816. [Google Scholar] [CrossRef]
- Ivanovic, J.; Knauer, S.; Fanovich, A.; Milovanovic, S.; Stamenic, M.; Jaeger, P.; Zizovic, I.; Eggers, R. Supercritical CO2 sorption kinetics and thymol impregnation of PCL and PCL-HA. J. Supercrit. Fluid. 2016, 107, 486–498. [Google Scholar] [CrossRef]
- García-González, C.A.; Barros, J.; Rey-Rico, A.; Redondo, P.; Gómez-Amoza, J.L.; Concheiro, A.; Alvarez-Lorenzo, C.; Monteiro, F.J. Antimicrobial Properties and Osteogenicity of Vancomycin-Loaded Synthetic Scaffolds Obtained by Supercritical Foaming. ACS Appl. Mater. Interfaces 2018, 10, 3349–3360. [Google Scholar] [CrossRef]
- Milovanovic, S.; Markovic, D.; Mrakovic, A.; Kuska, R.; Zizovic, I.; Frerich, S.; Ivanovica, J. Supercritical CO2-assisted production of PLA and PLGA foams for controlled thymol release. Mater. Sci. Eng. C 2019, 99, 394–404. [Google Scholar] [CrossRef]
- Cai, J.; Kimura, S.; Wada, M.; Kuga, S. Nanoporous Cellulose as Metal Nanoparticles Support. Biomacromolecules 2009, 10, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Rao, C.N.R.; Muller, A.; Cheetham, A.K. The Chemistry of Nanomaterials: Synthesis, Properties and Applications; Wiley-VCH Verlag Gmbh & Co. KGaA: Weinheim, Germany, 2004. [Google Scholar]
- Raman, S.P.; Keil, C.; Dieringer, P.; Hübner, C.; Bueno, A.; Gurikov, P.; Nissen, J.; Holtkamp, M.; Karst, U.; Haase, H.; et al. Alginate aerogels carrying calcium, zinc and silver cations for woundcare: Fabrication and metal detection. J. Supercrit. Fluid. 2019, 153, 104545. [Google Scholar] [CrossRef]
- Nešić, A.; Gordić, M.; Davidović, S.; Radovanović, Ž.; Nedeljković, J.; Smirnova, I.; Gurikov, P. Pectin-based nanocomposite aerogels for potential insulated food packaging application. Carbohydr. Polym. 2018, 195, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Tewari, P.H.; Hunt, A.J.; Lofftus, K.D. Ambient-temperature supercritical drying of transparent silica aerogels. Mater. Lett. 1985, 3, 363–367. [Google Scholar] [CrossRef]
- García-González, C.A.; Alnaief, M.; Smirnova, I. Polysaccharide-based aerogels—Promising biodegradable carriers for drug delivery systems. Carbohydr. Polym. 2011, 86, 1425–1438. [Google Scholar] [CrossRef]
- Subrahmanyam, R.; Gurikov, P.; Meissner, I.; Smirnova, I. Preparation of biopolymer aerogels using green solvents. J. Vis. Exp. 2016, 113, e54116. [Google Scholar] [CrossRef]
- Li, H.; Zhong, J.; Zhu, H.; Yang, Y.; Ding, M.; Luo, L.; Huo, Y.; Li, H. Hybrid Cu2O/TiO2 Nanocomposites with Enhanced Photocatalytic Antibacterial Activity toward Acinetobacter baumannii. ACS Appl. Bio Mater. 2019, 2, 4892–4903. [Google Scholar] [CrossRef]
- McConnell, M.J.; Actis, L.; Pachon, J. Acinetobacter baumannii: Human infections, factors contributing to pathogenesis and animal models. Fem. Microbiol. Rev. 2013, 37, 130–155. [Google Scholar] [CrossRef]
- Sui, R.H.; Charpentier, P. Synthesis of metal oxide nanostructures by direct sol-gel chemistry in supercritical fluids. Chem. Rev. 2012, 112, 3057–3082. [Google Scholar] [CrossRef]
- Sahraneshin, A.; Takami, S.; Hojo, D.; Minami, K.; Arita, T.; Adschiri, T. Synthesis of shape-controlled and organic-hybridized hafnium oxide nanoparticles under sub- and supercritical hydrothermal conditions. J. Supercrit. Fluid. 2012, 62, 190–196. [Google Scholar] [CrossRef]
- Bhartia, B.; Puniredd, S.R.; Jayaraman, S.; Gandhimathi, C.; Sharma, M.; Kuo, Y.C.; Chen, C.H.; Reddy, V.J.; Troadec, C.; Srinivasan, M.P. Highly Stable Bonding of Thiol Monolayers to Hydrogen-Terminated Si via Supercritical Carbon Dioxide: Toward a Super Hydrophobic and Bioresistant Surface. ACS Appl. Mater. Interfaces 2016, 8, 24933–24945. [Google Scholar] [CrossRef] [PubMed]
- Katayama, S.; Zhao, L.; Yonezawa, S.; Iwai, Y. Modification of the surface of cotton with supercritical carbon dioxide and water to support nanoparticles. J. Supercrit. Fluid. 2012, 61, 199–205. [Google Scholar] [CrossRef]
- Cuadra, I.A.; Martínez-Casado, F.J.; Cheda, J.A.R.; Redondo, M.I.; Pando, C.; Cabañas, A. Production and Characterization of a New Copper(II) Propanoate-Isonicotinamide Adduct Obtained via Slow Evaporation and using Supercritical CO2 as an Antisolvent. Cryst. Growth Des. 2019, 19, 620–629. [Google Scholar] [CrossRef]
- Imbuluzqueta, E.; Elizondo, E.; Gamazo, C.; Moreno-Calvo, E.; Veciana, J.; Ventosa, N.; Blanco-Prieto, M.J. Novel bioactive hydrophobic gentamicin carriers for the treatment of intracellular bacterial infections. Acta Biomater. 2011, 7, 1599–1608. [Google Scholar] [CrossRef] [PubMed]
- Saelo, S.; Assatarakul, K.; Sane, A.; Suppakul, P. Fabrication of Novel Bioactive Cellulose-Based Films Derived from Caffeic Acid Phenethyl Ester-Loaded Nanoparticles via a Rapid Expansion Process: RESOLV. J. Agric. Food Chem. 2016, 64, 6694–6707. [Google Scholar] [CrossRef] [PubMed]
- Varona, S.; Rodríguez Rojo, S.; Martín, Á.; Cocero, M.J.; Serra, A.T.; Crespo, T.; Duarte, C.M.M. Antimicrobial activity of lavandin essential oil formulations against three pathogenic food-borne bacteria. Ind. Crop. Prod. 2013, 42, 243–250. [Google Scholar] [CrossRef]
- Varona, S.; Kareth, S.; Martín, Á.; Cocero, M.J. Formulation of lavandin essential oil with biopolymers by PGSS for application as biocide in ecological agriculture. J. Supercrit. Fluid. 2010, 54, 369–377. [Google Scholar] [CrossRef]
- Santo, I.E.; Campardelli, R.; Albuquerque, E.C.; de Melo, S.V.; Della Porta, G.; Reverchon, E. Liposomes preparation using a supercritical fluid assisted continuous process. Chem. Eng. J. 2014, 249, 153–159. [Google Scholar] [CrossRef]
- Trucillo, P.; Campardelli, R.; Scognamiglio, M.; Reverchon, E. Control of liposomes diameter at micrometric and nanometric level using a supercritical assisted technique. J. CO2 Util. 2019, 31, 119–127. [Google Scholar] [CrossRef]
- Trucillo, P.; Ferrari, P.F.; Campardelli, R.; Reverchon, E.; Perego, P. A Supercritical Assisted Process for the Production of Amoxicillin Loaded Liposomes for Anti-microbial Applications. J. Supercrit. Fluid. 2020. [Google Scholar] [CrossRef]
- Campardelli, R.; Trucillo, P.; Reverchon, E. Supercritical assisted process for the efficient production of liposomes containing antibiotics for ocular delivery. J. CO2 Util. 2018, 25, 235–241. [Google Scholar] [CrossRef]
- Trucillo, P.; Cardea, S.; Baldino, L.; Reverchon, E. Production of liposomes loaded alginate aerogels using two supercritical CO2 assisted techniques. J. CO2 Util. 2020, 39, 101161. [Google Scholar] [CrossRef]
- Weidner, E. Impregnation via Supercritical Fluids—Principles and Applications. In Proceedings of the 10th International Symposium on Supercritical Fluids (ISSF 2012), San Francisco, CA, USA, 13–16 May 2012. [Google Scholar]
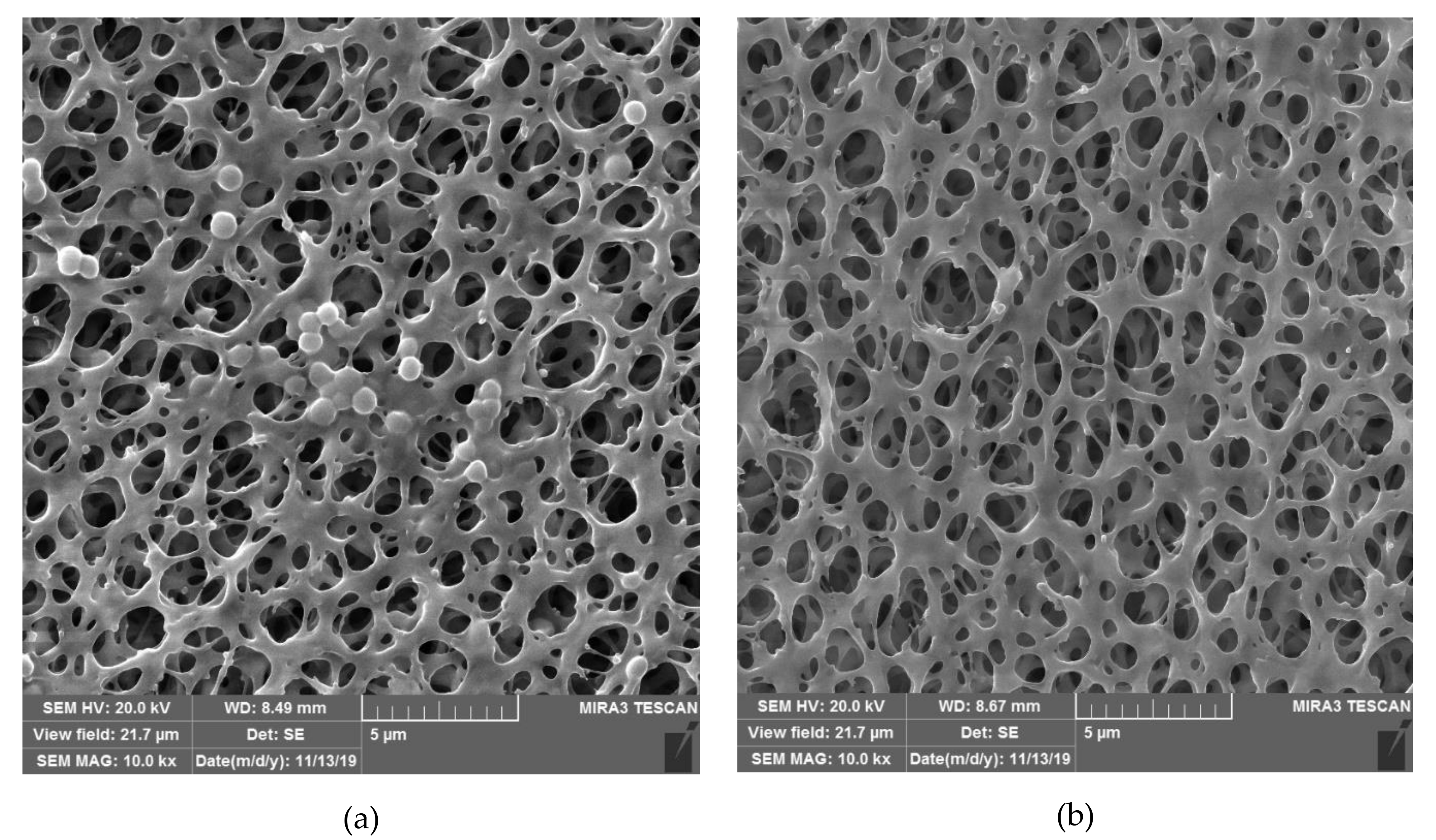
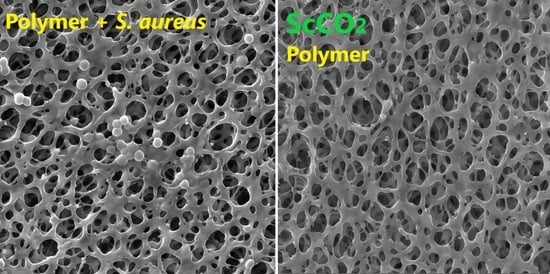
| Active Substance | Technique and Main Process Parameters | Solid Material | Loading (Result) | Microorganism | Reference |
|---|---|---|---|---|---|
| Thymol | SSI, 35 °C, 15.5 MPa, 1–24 h | Cotton fibers | 1.74–19.6% | E. coli, S. aureus, B. subtilis, E. faecalis, C. albicans | [22] |
| Carvacrol | SSI, 50 °C, 10–30 MPa, 1–24 h | Cotton fibers | 4–14.4% | E. coli, S. aureus | [23] |
| Thymol | SSI, 35 °C, 15.5 MPa, 4 h | Polypropylene fibers | 0.5–11.2% | E. coli, S. aureus, C. albicans | [25] |
| Thymol | SSI, 35 °C, 10 and 20 MPa, 0.5–4 h Near-critical, 25 °C, 7 MPa, 0.5–4 h | Polyamide nanofibers | 22.6–59.2% 6.51–33.8% | E. coli, S. aureus, C. albicans | [26] |
| Mango leaf extract | SSI, 35 and 55 °C, 40 and 50 MPa, 22 h Methanol cosolvent | Polyester fibers | 1.1–2.8% polyphenols | E. coli | [27] |
| Thyme extract | SFE-SSI, 35 °C, 15 MPa, batch 5 h | Cotton fibers Cellulose acetate Polypropylene fibers PCL Chitosan | 7.18% 1.44% 4.78% 9.04% 0.96% | [29] | |
| Usnea barbata extract Curry plant Lemon balm | SFE-SSI, 40 °C, 30 MPa, batch 5 h | LDPE Polypropilene fibers Cotton fibers | 3.05% 3.99% 2.24% | [30] | |
| Hop extract | SFE-SSI, 35 °C, 15 MPa, batch 5 h SFE-SSI, 50 °C, 29 MPa, batch 5 h | PCL Polypropylene fibers Starch xerogel | 6.04% 4.36% 2.58% | [31] | |
| Thyme extract Thymol Thymol Thymol | SFE-SSI, 110 °C, 30 MPa, 2 h batch + 2 h flow SSI, 35–110 °C, 10 and 30 MPa, 2–4 h SSI, 35 °C, 7.5 MPa, 2 h SSI, 35 °C, 15 and 30 MPa, 2 h | PLA PLA PLGA Starch | 1.2% 4.9–6.6% 3.0% 14.7–31.9% | [32] | |
| Ag(hepta), Ag(cod)(hfac) | SSI, 40 °C, 21 MPa, 10–15 h Reduction in H2 + scCO2 | Cotton fabric | Silver coating | C. albicans | [36] |
| N-halamine polysiloxane | SSI, 50 °C, 25 MPa, 3 h | Cotton fibers | 60 nm coating | E. coli, S. aureus | [37] |
| N-halamine polysiloxane | SSI, 50 °C, 28 MPa, overnight | Polyethylene fibers | 73 nm coating | E. coli, S. aureus | [38] |
| N-halamine polysiloxane | SSI, 50 °C, 28 MPa, overnight | Polypropylene fibers | Coating | E. coli, S. aureus | [39,40] |
| Hydrazono propanenitrile dyes | SSI, 120 °C, 15 MPa, 1–3 h Methanol cosolvent | Polyester fabric | Dyeing | E. coli, S. aureus | [44] |
| Hydrazono propanenitrile dyes | SSI, 80–120 °C, 5–15 MPa, 1–3 h | Polyamide fabric | Dyeing | E. coli, S. aureus, P. aeruginosa, B. subtilis | [45] |
| Hydrazono propanenitrile dyes | SSI, 120 °C, 20 MPa, 1–3 h With or without decalin cosolvent | UHMW polyethylene fiber | Dyeing | E. coli, S. aureus, B. cereus | [46] |
| Thymol | SSI, 35 °C, 10 and 20 MPa, 2–45 h | Cellulose acetate | 5–72% | S. aureus, C. albicans | [20] |
| Thymol | SSI, 35 °C, 10 MPa, 2–32 h | Cellulose acetate | 5–66% | S. Typhimurium, S. Enteritidis, L. monocytogenes, L. ivanovii, L. innocua, Corynebacterium, R. equi, B. anthracis, B. cereus, B. subtilis, S. pneumoniae, S. pyogenes, S. aureus, MRSA, K. pneumoniae, P. aeruginosa, E. coli, Acinetobacter, P. mirabilis | [47] |
| Carvacrol | SSI, 50 °C, 10–30 MPa, 2–18 h | Celulose acetate | 5–60% | MRSA, E.coli, Acinetobacter, B. anthracis, B. cereus, B. subtilis, Corynebacterium, K. pneumoniae, L. ivanovii, L. monocytogenes, R. equi, S. Enteritidis, S. pyogenes, S. pneumoniae | [48] |
| Thymol | SSI, 35 °C, 15.5 MPa, 0.5–16 h | Cellulose acetate | 8–64% | S. aureus, MRSA, P. aeruginosa | [49] |
| Carvacrol | SSI, 50 °C, 21 MPa, 0.5 and 2 h | Cellulose acetate | 2.5–31.4% | [54] | |
| Thymol | SSI, 40 °C, 10 MPa, 1 h | Cellulose nanofibril mats | 4.1–8.3% | E. coli, S. epidermidis, C. albicans | [57] |
| Thymol | SSI, 35 °C, 10 MPa, 2–6 h | Chitosan-itaconic acid-methacrylic acid | 1.0–4.6% | [58] | |
| Thymol | SSI, 40 °C, 20 MPa, 3 h Near-critical, 30 °C, 10 MPa, 3 h | N-carboxybutylchitosan Agarose | 0.8–2.5% | [59] | |
| d-limonene | SSI, 40 °C, 20 MPa, 3 h | Poly(L-lactide-ran-cyclic carbonate) | 0.15–5.3% | [60] | |
| Roxithromycin | SSI, 40–70 °C, 8–30 MPa, 0.5–4 h | PLA | 0.5–10.5% | [62] | |
| Thymol | SSI, 40 °C, 9 and 12 MPa, 3 h | PLA | 13.5–20.5% | [63] | |
| Cinnamaldehyde | SSI, 40 °C, 9 and 12 MPa, 3 h | PLA | 8–13% | E. coli, S. aureus | [64] |
| Thymol Cinnamaldehyde | SSI, 40 °C, 12 MPa, 3 h | PLA+nanoclay | 17% 11% | E. coli, S. aureus | [65] |
| Thymol Thyme extract | SSI, 40 °C, 10 MPa, 1–15 h SFE-SSI, 40 °C, 10 MPa, 2–6 h | PLA/PCL | 8–35.8% 4.3–5% | E. coli, B. subtilis | [66] |
| Usnea lethariiformis extract | SFE-SSI, 40 °C/30 MPa SFE; 35 °C/15 MPa SSI; 2 h flow + 1 h circ. | PCL | 0.2–2.8% | MRSA, L. innocua | [28] |
| Usnea lethariiformis extract | SFE-SSI, 40 °C/30 MPa SFE; 35 °C/17 MPa SSI; 2 h flow + 1 h circ. | PCL+hydrohyapatite | 1.7–5.9% | MRSA | [34] |
| Thymol | SSI and near-critical, 40 °C, 7–12 MPa, 4 h | LLDPE | 1.5–3.8% | [67] | |
| Eugenol | SSI, 40 °C, 10–15 MPa, 4 h | LLDPE | 1–6% | [68] | |
| Clove bud essential oil | SSI, 25–45 °C, 15 and 25 MPa, 4 h | LLDPE | 1–4% | [69] | |
| Thymol | SSI, 40 °C, 12 MPa, 1 h | LDPE+nanoclay | 0.36–1.19% | [70] | |
| Thymol | SSI, 40 °C, 9–12 MPa, 0.5–5 h | LDPE+nanoclay | 0.82–1.62% | S. aureus, E. coli | [71] |
| Natamycin | SSI, 40 °C, 20 MPa, 2.5–14 h with or without ethanol cosolvent | Alginate | 0.3–1.6% | [72] | |
| Cinnamaldehyde | SSI, 35 °C, 15 and 2 MPa, 3 h | Starch | 0.1–0.25% | [73] | |
| Thymol | SSI, 35 °C, 15.5, 24 h | Starch | 1.15–4.02% | [74] | |
| Curry plant extract | SFE-SSI, 40 °C, 35 MPa, 5 h | Starch | 1.26% | [33] | |
| Lavandin essential oil | SSI, 40–50 °C, 10–12 MPa, 2 h | n-octenyl succinate modified starch | 2.5–15% | [55] | |
| Quaternary ammonium/N-chloramine polysiloxane | SSI, 50 °C, 28 MPa, overnight | PET | 70 nm coating | S. aureus, E. coli | [75] |
| Quaternary ammonium compounds | SSI and chemical reaction, 100 °C, 41.4 MPa, 20 h Hexamethylene diisocyanate as a linker | Softwood | E. coli | [76] | |
| Silver nitrate | HPAI, 20 °C, 12 MPa, 10 min SAI, 40 and 80 °C, 12 MPa, 10 min Ethanol solution of AgNO3 | Polycarbonate | 2.4 mg/kg 23.4 mg/kg | E. coli | [78] |
| Silver NPs (AgNO3 precursor) | SAI, 65 °C, 12 MPa, 3 h Ethanol solution, glucose as a reducer | Carbon nanomaterials | E.coli | [79] | |
| Silver NPs (AgNO3 precursor) | SAI, 65 °C, 12 MPa, 3 h Ethanol solution, glucose as a reducer | Graphene oxide | E. coli, S. aureus, L. anguillarum | [80] | |
| Ciprofloxacin loaded in IPN material | SSI or SAI + Polymerization SSI/SAI, 40 °C, 20–25 MPa, 20 min–16 h Polymerization, 75 °C, 30–36 MPa, 3 h | IPN material based on silicone elastomer and PHEMA | 13–38% PHEMA | S. aureus | [82] |
| Ciprofloxacin loaded in IPN material | SSI + polymerization SSI, 40 °C, 20 MPa, 16 h | IPN material based on PDMS and PHEMA | 25% PHEMA | S. aureus | [83] |
| Polymerization, 75 °C, 30 MPa, 3 h | |||||
| Dicloxacillin Dicloxacillin and thioridazine | SSI + polymerization SSI, 40 °C, 20–25 MPa, 16 h Polymerization, 75 °C, 30 MPa, 3 h | IPN material based on silicone elastomer and PHEMA | 25.29–41.68% PHEMA | S. aureus, MRSA | [84] |
| 2-oxazoline-based oligomers | SSI + polymerization SSI, 40 °C, 18 MPa, 24 h Polymerization, 65 °C, 18 MPa, 20 h Reaction with tertiary amine, 40 °C, 18 MPa, 20 h | Chitosan | E.coli, S. aureus | [91] | |
| Vancomycin | Foaming from solid dispersion | PCL and chitosan | 1–5% | E. coli, S. aureus | [95] |
| 40 °C, 14 MPa, 1 h | |||||
| Thymol | SSI+ foaming in one step, 35 and 40 °C, 10–30 MPa, 2 h | PCL PCL+hydroxyapatite | 12–18% | [94] | |
| Thymol | SSI + foaming in one step, 25–50 °C, 7.5–15 MPa, 2–24 h | PLA PLGA | 0.92–6.62% | [96] | |
| Silver, gold and platinum NPs | Sc drying of metal-carrying gels Two steps: 5.3 MPa, 4 °C for 6 h, and 10 MPa, 40 °C for 0.5 h | Cellulose | Aerogel containing metal particles | [97] | |
| Ca-Zn Ca-Zn-Ag | High-pressure gelation, 50 MPa, 24 h, room temperature; Sc drying of metal-carrying gel at 50 °C, 12 MPa, 2 h, 20 g/min CO2 flowrate | Calcium-alginate | Aerogel containing metal particles | [99] | |
| TiO2 NPs | Sc drying of metal-carrying gel at 50–60 °C, 11–13 MPa, 5 h, 0.2 kg/h CO2 flowrate | Pectin | Aerogel containing NPs | E. coli | [100] |
| Cu2O and TiO2 | Sc solvothermal process in ethanol as supercrit. fl., 243 °C, 6.4 MPa, 70 min | Cu2O-TiO2 nanocomposites | A. baumannii, P. aeruginosa, E. coli, S. aureus | [104] | |
| Alkylthiols | Sc CO2 grafting at 100 °C and 10 MPa, 120 min | Oxide-free silicon | Deposited monolayer | [108] | |
| TiO2 NPs | Physical treatment of fibers, 40 °C, 20 MPa, 60 min, fast decompression 0.80 MPa/min−1 | Cotton fibers | NPs modified cotton | [109] | |
| Isonicotinamide and copper(II) propanoate | Antisolvent precipitation (SAS)–ethanol soluton, 40 °C, 10 MPa, 1 mL/min | Ligand crystals produced | [110] | ||
| Gentamicin | Antisolvent precipitation from acetone solution at 10 MPa, 25 °C | GEN-AOT complex | Micronized solid | E. coli | [111] |
| Caffeic acid phenethyl ester | RESOLV–ethanol solution at 17.3 MPa and 50 °C; nozzle at 80 °C | NPs produced | P. aeruginosa, C. albicans, L. monocytogenes | [112] | |
| Lavandin essential oil | PGSS drying, 104–130 °C, 6–10 MPa PGSS, 70 °C, 6–8.5 MPa | Soybean lecithin, n-octenyl succinic anhydride modified starch, PCL | Oil encapsulated in polymer | E. coli, S. aureus, B. Baccereus | [113] |
| Lavandin essential oil | PGSS, 76–84 °C, 5.4–8.5 MPa PGSS drying, 108–127 °C, 9–12.4 MPa | PEG n-octenyl succinic anhydride modified starch | Oil encapsulated in polymer | [114] | |
| Vancomycin | SuperLip, 40 °C, 10 MPa | Phospholipids | Liposomes | [116] | |
| Amoxicillin | SuperLip, 40 °C, 10 MPa | Phospholipids | Liposomes | E. coli | [117] |
| Ampicillin Ofloxacin | SuperLip, 40 °C, 10 MPa | Phospholipids | Liposomes | [118] | |
| Amoxicillin | SuperLip, 40 °C, 10 MPa Sc Drying, 35 °C, 20 MPa, 6 h, 1 kg/h scCO2 flowrate | Phospholipids Alginate | Liposomes entrapped in aerogel | [119] |
| High-Pressure Methodology | Reference |
|---|---|
| Supercritical Solvent Impregnation (SSI) | [20,22,23,25,26,27,28,29,30,31,32,33,34,36,37,38,39,40,44,45,46,47,48,49,54,55,57,58,59,62,63,64,65,66,67,68,69,70,71,72,73,74,75,94,96] |
| Supercritical Assisted Impregnation (SAI) High-pressure Assisted Impregnation (HPAI) | [78,79,80] [78] |
| SSI/SAI + polymerization | [82,83,84,91] |
| SSI/SAI + chemical reaction other than polymerization | [76,91,108] |
| Supercritical foaming | [28,34,94,95,96] |
| Supercritical drying | [97,99,100,119] |
| Supercritical solvothermal process 1 | [104] |
| Antisolvent techniques | [110,111] |
| RESOLV | [112] |
| PGSS | [113,114] |
| Physical surface modification | [109] |
| Liposome formation (SuperLip) | [116,117,118,119] |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zizovic, I. Supercritical Fluid Applications in the Design of Novel Antimicrobial Materials. Molecules 2020, 25, 2491. https://doi.org/10.3390/molecules25112491
Zizovic I. Supercritical Fluid Applications in the Design of Novel Antimicrobial Materials. Molecules. 2020; 25(11):2491. https://doi.org/10.3390/molecules25112491
Chicago/Turabian StyleZizovic, Irena. 2020. "Supercritical Fluid Applications in the Design of Novel Antimicrobial Materials" Molecules 25, no. 11: 2491. https://doi.org/10.3390/molecules25112491
APA StyleZizovic, I. (2020). Supercritical Fluid Applications in the Design of Novel Antimicrobial Materials. Molecules, 25(11), 2491. https://doi.org/10.3390/molecules25112491