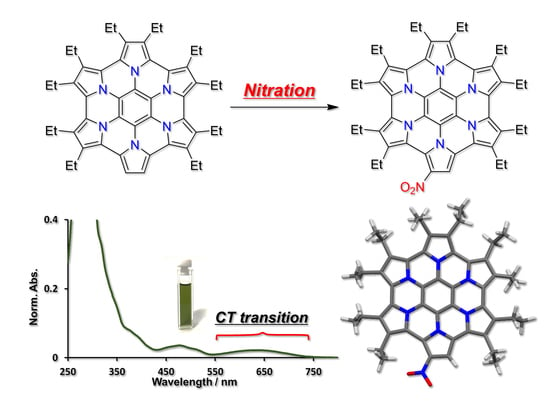
Synthesis and Properties of NitroHPHAC: The First Example of Substitution Reaction on HPHAC
Abstract
1. Introduction
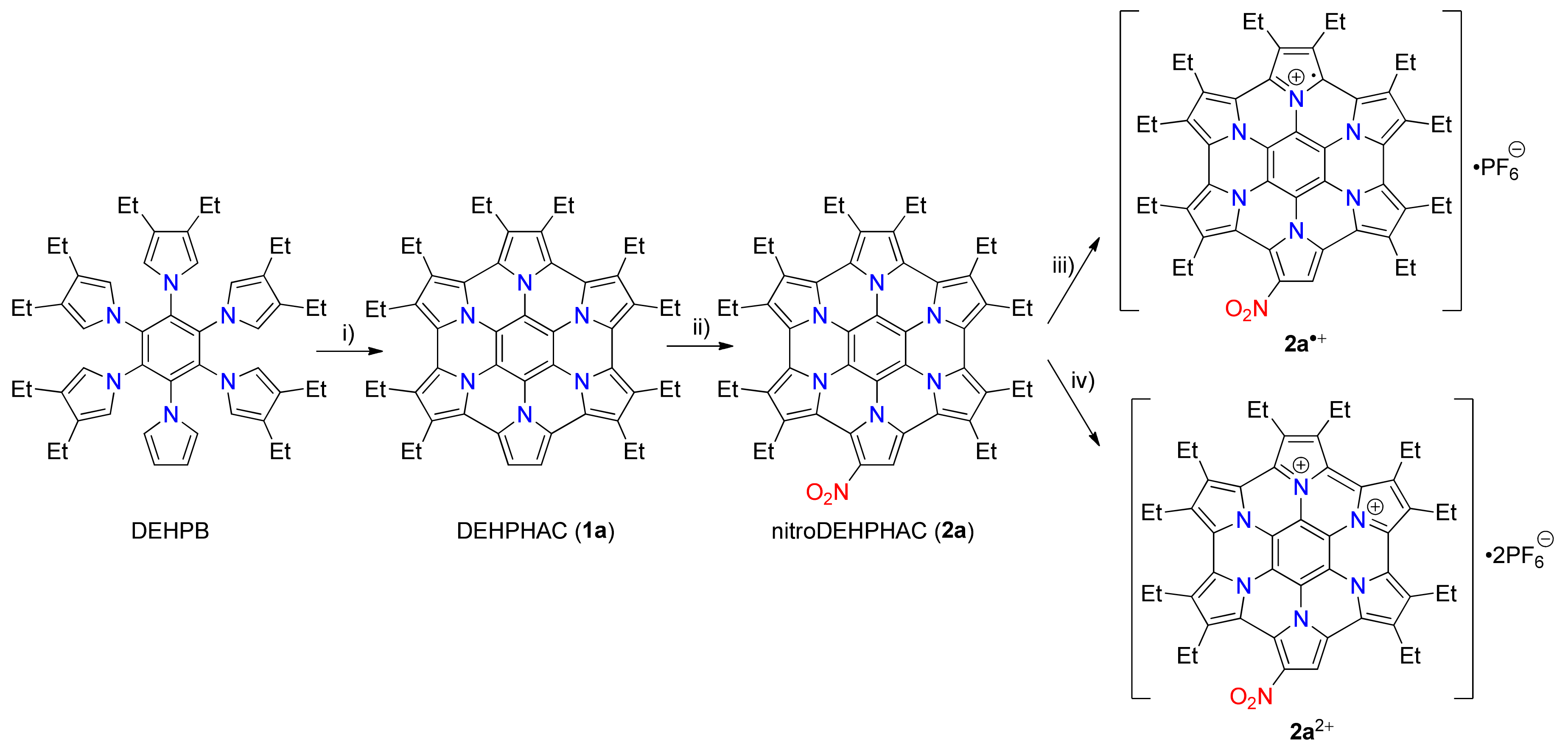
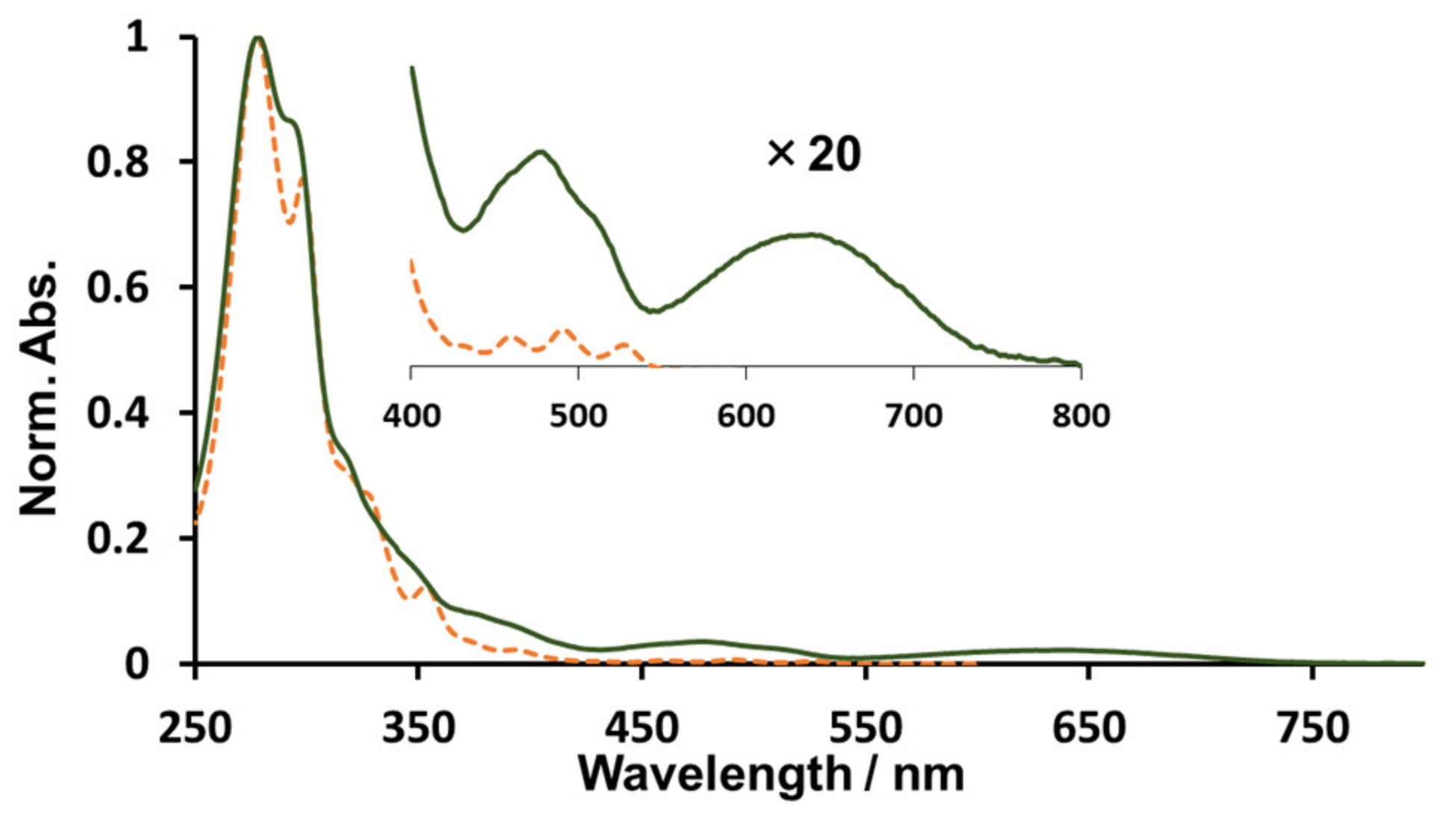
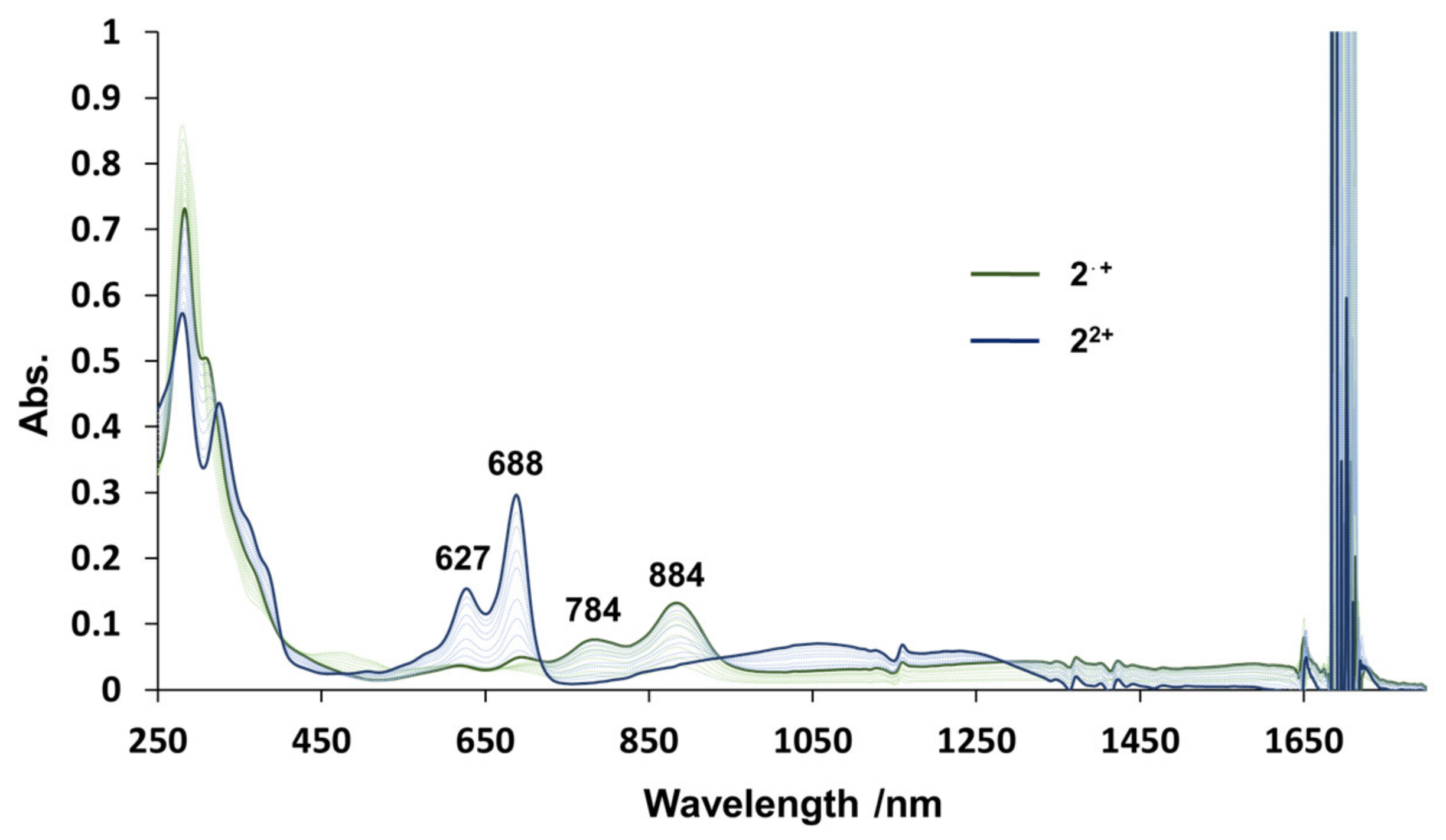
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. Synthesis of N-[2,3,4,5,6-Pentakis(3, 4-Dimethylpyrrolyl)Phenyl]Pyrrole (DEHPB)
4.2. Synthesis of 1,2,3,4,5,6,7,8,9,10-Decaethylhexapyrrolo [2 ,1,5-bc:2′,1′,5′-ef:2′′,1′′,5′′-hi:2′′′,1′′′,5′′′-kl:2′′′′,1′′′′,5′′′′-no:2′′′′′,1′′′′′,5′′′′′-qr][2a,4a,6a,8a,10a,12a]Hexaazacoronene (DEHPHAC 1a)
4.3. Synthesis of nitroDEHPHAC 2a
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yan, G.; Yang, M. Recent advances in the synthesis of aromatic nitro compounds. Org. Biomol. Chem. 2013, 11, 2554–2566. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Hiroto, S.; Lee, S.; Son, M.; Hisaki, I.; Yoshida, T.; Kim, D.; Kobayashi, N.; Shinokubo, H. Synthesis of Highly Twisted and Fully π-Conjugated Porphyrinic Oligomers. J. Am. Chem. Soc. 2015, 137, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Akita, M.; Hiroto, S.; Shinokubo, H. Oxidative Annulation of β-Aminoporphyrins into Pyrazine-Fused Diporphyrins. Angew. Chem. Int. Ed. 2012, 51, 2894–2897. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, H.; Wachi, N.; Hiroto, S.; Shinokubo, H. Oxidation of 2-Amino-Substituted BODIPYs Providing Pyrazine-Fused BODIPY Trimers. Chem. Commun. 2014, 50, 2715–2717. [Google Scholar] [CrossRef] [PubMed]
- Goto, K.; Yamaguchi, R.; Hiroto, S.; Ueno, H.; Kawai, T.; Shinokubo, H. Intermolecular Oxidative Annulation of 2-Aminoanthracenes to Diazaacenes and Aza [7] helicenes. Angew. Chem. Int. Ed. 2012, 51, 10333–10336. [Google Scholar] [CrossRef]
- Kosugi, Y.; Itoho, K.; Okazaki, H.; Yanai, T. Unexpected Formation of Dibenzo [a,h] phenazine in the Reaction Between 1-Naphthyliminodimagnesium Dibromide and 1-Nitronaphthalene. J. Org. Chem. 1995, 60, 5690–5692. [Google Scholar] [CrossRef]
- Ueta, K.; Tanaka, T.; Osuka, A. Synthesis and Characterizations of meso-Nitrocorroles. Chem. Lett. 2018, 47, 916–919. [Google Scholar] [CrossRef]
- Smith, K.M.; Barnett, G.H.; Evans, B.; Martynenko, Z. Novel meso-Substitution Reactions of Metalloporphyrins. J. Am. Chem. Soc. 1979, 101, 5953–5961. [Google Scholar] [CrossRef]
- Saltsman, I.; Mahammed, A.; Goldberg, I.; Tkachenko, E.; Botoshansky, M.; Gross, Z. Selective Substitution of Corroles: Nitration, Hydroformylation, and Chlorosulfonation. J. Am. Chem. Soc. 2002, 124, 7411–7420. [Google Scholar] [CrossRef]
- Stefanelli, M.; Mastroianni, M.; Nardis, S.; Licoccia, S.; Fronczek, F.R.; Smith, K.M.; Zhu, W.; Ou, Z.; Kadish, K.M.; Paolesse, R. Functionalization of Corroles: The Nitration Reaction. Inorg. Chem. 2007, 46, 10791–10799. [Google Scholar] [CrossRef]
- Nishiyama, A.; Tanaka, Y.; Mori, S.; Furuta, H.; Shimizu, S. Oxidative Nitration Reaction of Antiaromatic 5,15-Dioxaporphyrin. J. Porphyr. Phthalocyanines 2020, 24, 355–361. [Google Scholar] [CrossRef]
- Mikus, A.; Rosa, M.; Ostrowski, S. Isomers of β,β-Dinitro-5,10,15,20-Tetraphenylporphyrin Derivatives: Valuable Starting Materials for Further Transformations. Molecules 2019, 24, 838. [Google Scholar] [CrossRef]
- Sun, B.; Ou, Z.; Yang, S.; Meng, D.; Lu, G.; Fang, Y.; Kadish, K.M. Synthesis and Electrochemistry of β-Pyrrole Nitro-Substituted Cobalt(ii) Porphyrins. The Effect of the NO2 Group on Redox Potentials, the Electron Transfer Mechanism and Catalytic Reduction of Molecular Oxygen in Acidic Media. Dalton Trans. 2014, 43, 10809–10815. [Google Scholar] [CrossRef]
- Yadav, P.; Kumar, R.; Saxena, A.; Butcher, R.J.; Sankar, M. β-Trisubstituted “Push–Pull” Porphyrins—Synthesis and Structural, Photophysical, and Electrochemical Redox Properties. Eur. J. Inorg. Chem. 2017, 2017, 3269–3274. [Google Scholar] [CrossRef]
- Takase, M.; Enkelmann, V.; Sebastiani, D.; Baumgarten, M.; Müllen, K. Annularly fused hexapyrrolohexaazacoronenes: An extended π-system with multiple interior nitrogen atoms displays stable oxidation states. Angew. Chem. Int. Ed. 2007, 46, 5524–5527. [Google Scholar] [CrossRef] [PubMed]
- Oki, K.; Takase, M.; Mori, S.; Shiotari, A.; Sugimoto, Y.; Ohara, K.; Okujima, T.; Uno, H. Synthesis, structures, and properties of core-expanded azacoronene analogue: A twisted π-system with two n-doped heptagons. J. Am. Chem. Soc. 2018, 140, 10430–10434. [Google Scholar] [CrossRef] [PubMed]
- Navakouski, M.; Zhylitskaya, H.; Chmielewski, P.J.; Żyła-Karwowska, M.; Stępień, M. Electrophilic aromatic coupling of hexapyrrolylbenzenes. A mechanistic analysis. J. Org. Chem. 2020, 85, 187–194. [Google Scholar] [CrossRef]
- Takase, M.; Narita, T.; Fujita, W.; Asano, M.S.; Nishinaga, T.; Benten, H.; Yoza, K.; Müllen, K. Pyrrole-fused azacoronene family: The influence of replacement with dialkoxybenzenes on the optical and electronic properties in neutral and oxidized states. J. Am. Chem. Soc. 2013, 135, 8031–8040. [Google Scholar] [CrossRef]
- Gońka, E.; Chmielewski, P.J.; Lis, T.; Stępień, M. Expanded hexapyrrolohexaazacoronenes. Near-infrared absorbing chromophores with interrupted peripheral conjugation. J. Am. Chem. Soc. 2014, 136, 16399–16410. [Google Scholar] [CrossRef]
- Żyła, M.; Gońka, E.; Chmielewski, P.J.; Cybińska, J.; Stępień, M. Synthesis of a Peripherally Conjugated 5-6-7 Nanographene. Chem. Sci. 2016, 7, 286–294. [Google Scholar] [CrossRef]
- Żyła-Karwowska, M.; Zhylitskaya, H.; Cybińska, J.; Lis, T.; Chmielewski, P.J.; Stępień, M. An electron-deficient azacoronene obtained by radial π-extension. Angew. Chem. Int. Ed. 2016, 55, 14658–14662. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, Y.; Takase, M.; Okujima, T.; Mori, S.; Uno, H. Synthesis and Redox Properties of Pyrrole- and Azulene-Fused Azacoronene. Org. Lett. 2019, 21, 1900–1903. [Google Scholar] [CrossRef] [PubMed]
- Uno, H.; Ishiwata, M.; Muramatsu, K.; Takase, M.; Mori, S.; Okujima, T. Oxidation Behavior of 1,3-Dihydrothieno [3,4-a] DEHPHAC. Bull. Chem. Soc. Jpn. 2019, 92, 973–981. [Google Scholar] [CrossRef]
- Navakouski, M.; Zhylitskaya, H.; Chmielewski, P.J.; Lis, T.; Cybińska, J.; Stępień, M. Stereocontrolled synthesis of chiral heteroaromatic propellers with small optical bandgaps. Angew. Chem. Int. Ed. 2019, 131, 4983–4987. [Google Scholar] [CrossRef]
- Oki, K.; Takase, M.; Mori, S.; Uno, H. Synthesis and isolation of antiaromatic expanded azacoronene via intramolecular vilsmeier-type reaction. J. Am. Chem. Soc. 2019, 141, 16255–16259. [Google Scholar] [CrossRef] [PubMed]
- Moshniaha, L.; Żyła-Karwowska, M.; Chmielewski, P.J.; Lis, T.; Cybinska, J.; Gońka, E.; Oschwald, J.; Drewello, T.; Medina Rivero, S.; Casado, J.; et al. Aromatic Nanosandwich Obtained by σ-Dimerization of a Nanographenoid π-Radical. J. Am. Chem. Soc. 2020, 142, 3626–3635. [Google Scholar] [CrossRef]
- Tokárová, Z.; Balogh, R.; Tisovský, P.; Hrnčariková, K.; Végh, D. Direct nucleophilic substitution of polyfluorobenzenes with pyrrole and 2,5-dimethylpyrrole. J. Fluor. Chem. 2017, 204, 59–64. [Google Scholar] [CrossRef]
- Ooi, S.; Yoneda, T.; Tanaka, T.; Osuka, A. meso-Free Corroles: Syntheses, Structures, Properties, and Chemical Reactivities. Chem. Eur. J. 2015, 21, 7772–7779. [Google Scholar] [CrossRef]
- Osuka, A.; Shimidzu, H. meso, meso-Linked Porphyrin Arrays. Angew. Chem. Int. Ed. Engl. 1997, 36, 135–137. [Google Scholar] [CrossRef]
- Boese, R.; Biäser, D.; Nussbaumer, M.; Krygowski, T.M. Low temperature crystal and molecular structure of nitrobenzene. Struct. Chem. 1992, 3, 363–368. [Google Scholar] [CrossRef]
- Kertesz, M. Pancake Bonding: An Unusual Pi-Stacking Interaction. Chem. Eur. J. 2019, 25, 400–416. [Google Scholar] [CrossRef]
- Tateno, M.; Takase, M.; Iyoda, M.; Komatsu, K.; Nishinaga, T. Steric Control in the π-Dimerization of Oligothiophene Radical Cations Annelated with Bicyclo [2.2.2] octane Units. Chem. Eur. J. 2013, 19, 5457–5467. [Google Scholar] [CrossRef] [PubMed]
- Morita, Y.; Suzuki, S.; Fukui, K.; Nakagawa, S.; Kitagawa, H.; Kishida, H.; Okamoto, H.; Naito, A.; Sekine, A.; Ohashi, Y.; et al. Thermochromism in an organic crystal based on the coexistence of σ- and π-dimers. Nat. Mater. 2008, 7, 48–51. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, H.; Hiroto, S.; Shinokubo, H. Reversible σ-Bond Formation in Bowl-Shaped π-Radical Cations: The Effects of Curved and Planar Structures. J. Am. Chem. Soc. 2018, 140, 4649–4655. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds DEHPB, 1a, and 2a are available from the authors. |




| Bond | Neutral | Radical Cation | Dication |
|---|---|---|---|
| Cα-Cα Cβ-Cβ Cα-Cβ | 1.482(3) 1.435(3) 1.391(5) | 1.463(8) 1.418(4) 1.407(7) | 1.442(6) 1.396(5) 1.429(5) |
| Cα-N | 1.394(4) | 1.392(6) | 1.389(7) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sasaki, Y.; Takase, M.; Mori, S.; Uno, H. Synthesis and Properties of NitroHPHAC: The First Example of Substitution Reaction on HPHAC. Molecules 2020, 25, 2486. https://doi.org/10.3390/molecules25112486
Sasaki Y, Takase M, Mori S, Uno H. Synthesis and Properties of NitroHPHAC: The First Example of Substitution Reaction on HPHAC. Molecules. 2020; 25(11):2486. https://doi.org/10.3390/molecules25112486
Chicago/Turabian StyleSasaki, Yoshiki, Masayoshi Takase, Shigeki Mori, and Hidemitsu Uno. 2020. "Synthesis and Properties of NitroHPHAC: The First Example of Substitution Reaction on HPHAC" Molecules 25, no. 11: 2486. https://doi.org/10.3390/molecules25112486
APA StyleSasaki, Y., Takase, M., Mori, S., & Uno, H. (2020). Synthesis and Properties of NitroHPHAC: The First Example of Substitution Reaction on HPHAC. Molecules, 25(11), 2486. https://doi.org/10.3390/molecules25112486