Characterization of Ionic Liquid Lignins Isolated from Spruce Wood with 1-Butyl-3-methylimidazolium Acetate and Methyl Sulfate and Their Binary Mixtures with DMSO
Abstract
1. Introduction
2. Results and Discussion
2.1. Molecular Weight Distributions of Ionic Liquid Lignins
2.2. Functional and Elemental Compositions of Ionic Liquid Lignins
2.3. 2D NMR Spectra and Structural Characterization of Ionic Liquid Lignins
2.4. High-Resolution Mass Spectrometry of Lignin Preparations
3. Materials and Methods
3.1. Reagents and Materials
3.2. Lignin Preparations
3.3. Analytical Procedures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Faßbach, T.A.; Kirchmann, R.; Behr, A.; Vorholt, A.J. Recycling of homogeneous catalysts in reactive ionic liquid–solvent-free aminofunctionalizations of alkenes. Green Chem. 2017, 19, 5243–5249. [Google Scholar] [CrossRef]
- Konnerth, H.; Prechtl, M.H.G. Selective Hydrogenation of N-Heterocyclic Compounds using Ru Nanocatalysts in Ionic Liquids. Green Chem. 2017, 19, 2762–2767. [Google Scholar] [CrossRef]
- Bisht, M.; Mondal, D.; Pereira, M.M.; Freire, M.G.; Venkatesu, P.; Coutinho, J.A.P. Long-term protein packaging in cholinium-based ionic liquids: Improved catalytic activity and enhanced stability of cytochrome c against multiple stresses. Green Chem. 2017, 19, 4900–4911. [Google Scholar] [CrossRef] [PubMed]
- Kilpeläinen, I.; Xie, H.; King, A.; Granstrom, M.; Heikkinen, S.; Argyropoulos, D.S. Dissolution of Wood in Ionic Liquids. J. Agric. Food Chem. 2007, 55, 9142–9148. [Google Scholar] [CrossRef]
- Zhao, H.; Baker, G.A.; Wagle, D.V.; Ravula, S.; Zhang, Q. Tuning task-specific ionic liquids for the extractive desulfurization of liquid fuel. ACS Sustain. Chem. Eng. 2016, 4, 4771–4780. [Google Scholar] [CrossRef]
- Fox, E.T.; Weaver, J.E.F.; Henderson, W.A. Tuning Binary Ionic Liquid Mixtures: Linking Alkyl Chain Length to Phase Behavior and Ionic Conductivity. J. Phys. Chem. C 2012, 116, 5270–5274. [Google Scholar] [CrossRef]
- Shu, H.; Xu, Y. Tuning the strength of cation coordination interactions of dual functional ionic liquids for improving CO2 capture performance. Int. J. Greenh. Gas Control 2020, 94, 11807–11814. [Google Scholar] [CrossRef]
- Ladesov, A.V.; Kosyakov, D.S.; Bogolitsyn, K.G.; Gorbova, N.S. Solvatochromic polarity parameters for binary mixtures of 1-butyl-3-methylimidazolium acetate with water, methanol, and dimethylsulfoxide. Russ. J. Phys. Chem. A 2015, 89, 1814–1820. [Google Scholar] [CrossRef]
- Lan, W.; Liu, C.-F.; Sun, R.-C. Fractionation of Bagasse into Cellulose, Hemicelluloses, and Lignin with Ionic Liquid Treatment Followed by Alkaline Extraction. J. Agric. Food Chem. 2011, 59, 8691–8701. [Google Scholar] [CrossRef]
- Liu, C.; Li, Y.; Hou, Y. Behavior of oxygen-containing groups in grass lignin during dissolution in basic ionic liquids. Cellulose 2019, 26, 737–749. [Google Scholar] [CrossRef]
- Achinivu, E.C. Protic ionic liquids for lignin extraction—A lignin characterization study. Int. J. Mol. Sci. 2018, 19, 482. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, L.; Zhang, Z.; Wellard, R.M.; Bartley, J.P.; O’Hara, I.M.; Doherty, W.O.S. Characterisation of lignins isolated from sugarcane bagasse pretreated with acidified ethylene glycol and ionic liquids. Biomass Bioenergy 2014, 70, 498–512. [Google Scholar] [CrossRef]
- Wen, J.-L.; Sun, S.-L.; Xue, B.-L.; Sun, R.-C. Quantitative Structures and Thermal Properties of Birch Lignins after Ionic Liquid Pretreatment. J. Agric. Food Chem. 2013, 61, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Pinkert, A.; Goeke, D.F.; Marsh, K.N.; Pang, S. Extracting wood lignin without dissolving or degrading cellulose: Investigations on the use of food additive-derived ionic liquids. Green Chem. 2011, 13, 3124–3136. [Google Scholar] [CrossRef]
- Brandt, A.; Chen, L.; van Dongen, B.E.; Welton, T.; Hallett, J.P. Structural changes in lignins isolated using an acidic ionic liquid water mixture. Green Chem. 2015, 17, 5019–5034. [Google Scholar] [CrossRef]
- Qu, Y.; Luo, H.; Li, H.; Xu, J. Comparison on structural modification of industrial lignin by wet ball milling and ionic liquid pretreatment. Biotechnol. Rep. 2015, 6, 1–7. [Google Scholar] [CrossRef]
- Sowmiah, S.; Srinivasadesikan, V.; Tseng, M.-C.; Chu, Y.-H. On the Chemical Stabilities of Ionic Liquids. Molecules 2009, 14, 3780. [Google Scholar] [CrossRef]
- Chiarotto, I.; Feroci, M.; Inesi, A. First direct evidence of N-heterocyclic carbene in BMIm acetate ionic liquids. An electrochemical and chemical study on the role of temperature. New J. Chem. 2017, 41, 7840–7843. [Google Scholar] [CrossRef]
- Ladesov, A.V.; Belesov, A.V.; Kuznetsova, M.V.; Pochtovalova, A.S.; Malkov, A.V.; Shestakov, S.L.; Kosyakov, D.S. Fractionation of Wood with Binary Solvent 1-Butyl-3-methylimidazolium Acetate + Dimethyl Sulfoxide. Russ. J. Appl. Chem. 2018, 91, 663–670. [Google Scholar] [CrossRef]
- Brandt, A.; Hallett, J.P.; Leak, D.J.; Murphy, R.J.; Welton, T. The effect of the ionic liquid anion in the pretreatment of pine wood chips. Green Chem. 2010, 12, 672–679. [Google Scholar] [CrossRef]
- Wen, J.-L.; Sun, S.-L.; Xue, B.-L.; Sun, R.-C. Recent Advances in Characterization of Lignin Polymer by Solution-State Nuclear Magnetic Resonance (NMR) Methodology. Materials 2013, 6, 359–391. [Google Scholar] [CrossRef] [PubMed]
- Kosyakov, D.S.; Ul’yanovskii, N.V.; Anikeenko, E.A.; Gorbova, N.S. Negative ion mode atmospheric pressure ionization methods in lignin mass spectrometry: A comparative study. Rapid Commun. Mass Spectrom. 2016, 30, 2099–2108. [Google Scholar] [CrossRef] [PubMed]
- Pikovskoi, I.I.; Kosyakov, D.S.; Shavrina, I.S.; Ul’yanovskii, N.V. Study of Nettle (Urtica dióica) Lignin by Atmospheric Pressure Photoionization Orbitrap Mass Spectrometry. J. Anal. Chem. 2019, 74, 1412–1420. [Google Scholar] [CrossRef]
- Pikovskoi, I.I.; Kosyakov, D.S.; Faleva, A.V.; Shavrina, I.S.; Kozhevnikov, A.Y.; Ul’yanovskii, N.V. Study of the Carex Lignin by high-resolution mass spectrometry and nuclear magnetic resonance spectroscopy. Russ. Chem. Bull. 2020, in press. [Google Scholar]
- Van Krevelen, D. Graphical statistical method for the study of structure and reaction processes of coal. Fuel 1950, 29, 269–284. [Google Scholar]
- Pepper, J.M.; Baylis, P.E.; Adler, E. The isolation and properties of lignin obtained by the acidolysis of spruce and aspen woods in dioxane-water. Can. J. Chem. 1959, 37, 1241–1245. [Google Scholar] [CrossRef]
- Granata, A.; Argyropoulos, D.S. 2-Chloro-4,4,5,5-tetramethyl-1,3,2-dioxaphospholane, a reagent for the accurate determination of the uncondensed and condensed phenolic moieties in lignins. J. Agric. Food Chem. 1995, 43, 1538–1544. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
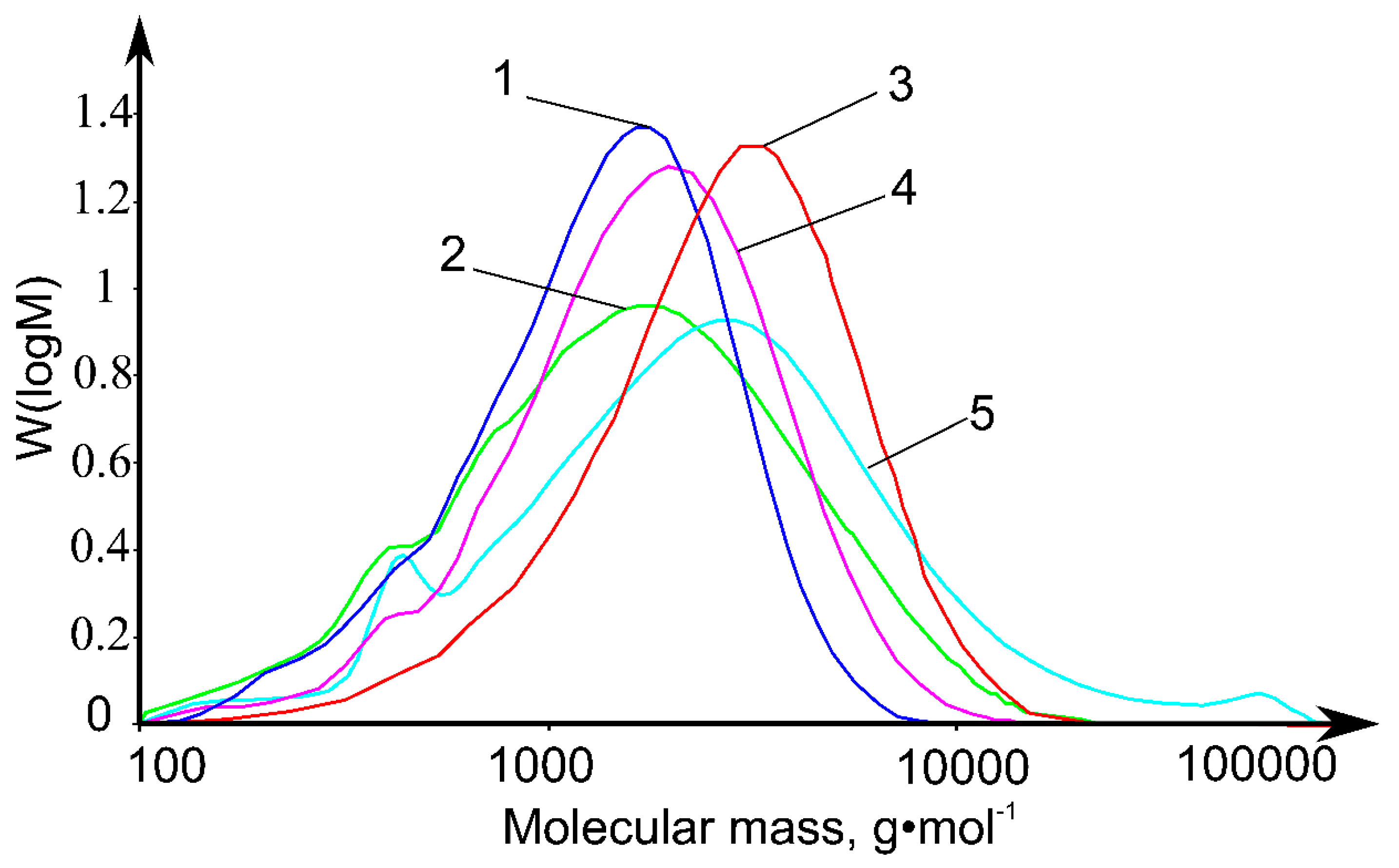


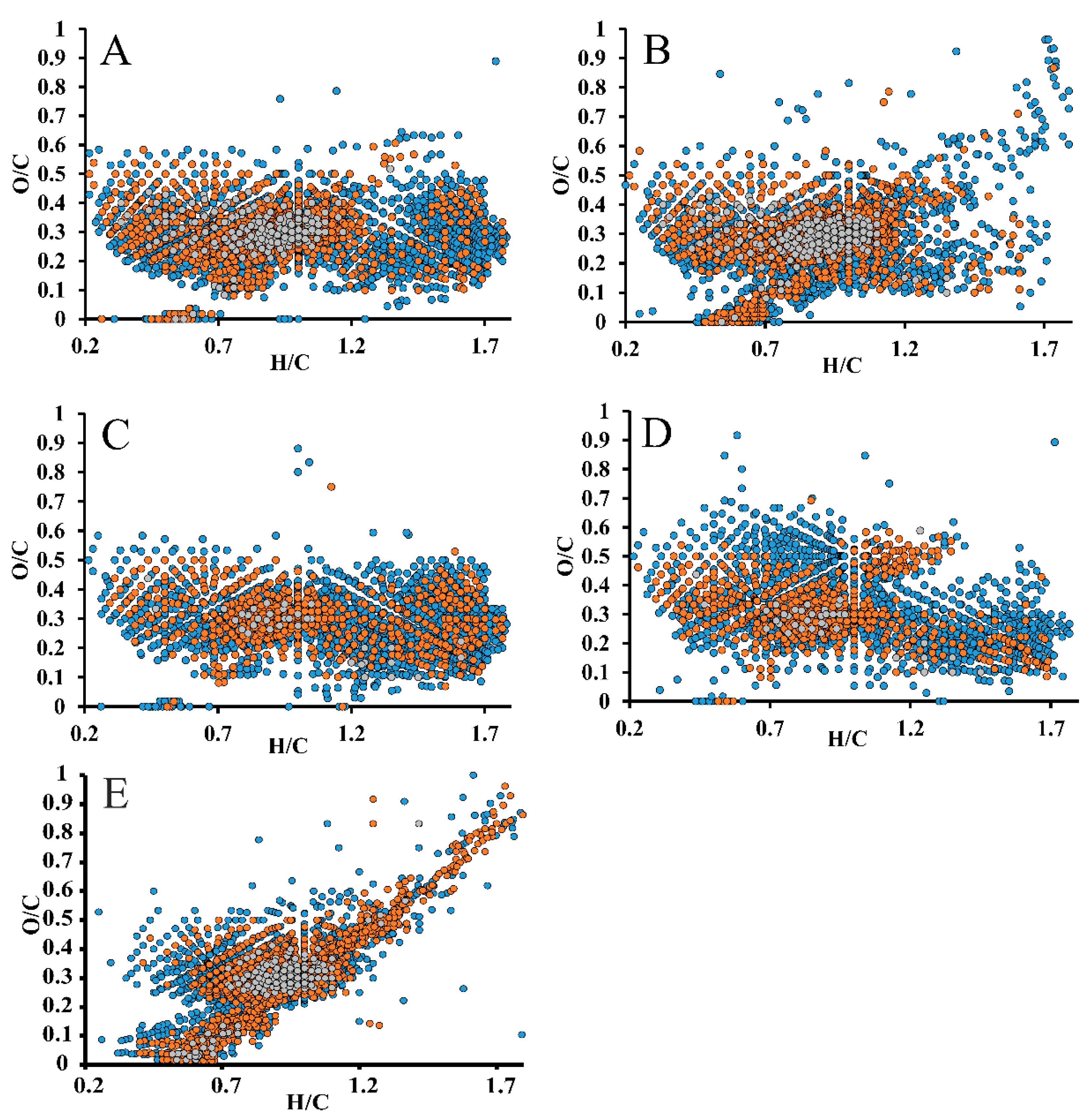
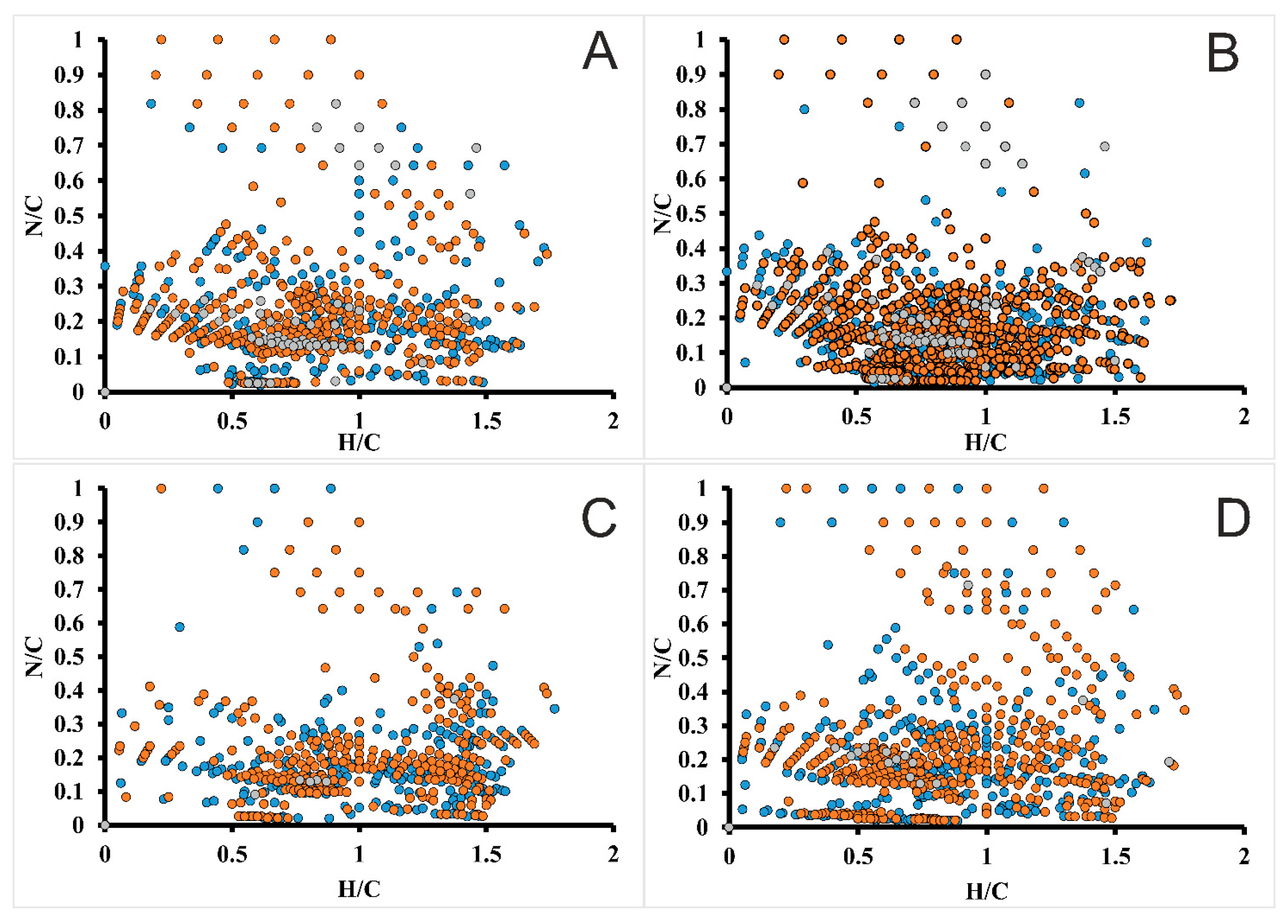
| Sample | Mw, g·mol−1 | Mn, g·mol−1 | PDI (Mw/Mn) |
|---|---|---|---|
| IL lignin ([bmim]MeSO4) | 3.4 | 1.9 | 1.8 |
| IL lignin ([bmim]MeSO4 + DMSO) | 2.1 | 1.2 | 1.8 |
| IL lignin ([bmim]OAc) | 1.6 | 0.98 | 1.7 |
| IL lignin ([bmim]Oac + DMSO) | 2.1 | 0.98 | 2.1 |
| DL | 4.6 | 1.8 | 2.5 |
| Sample | Functional Groups and Their Content | ||||||
|---|---|---|---|---|---|---|---|
| as a % of Sample Weight | per 100 Aromatic Units | ||||||
| OHtotal | OHphen. | OHaliph. | COOH | OCH3 | Acetyl | C=Oald. | |
| IL lignin ([bmim]MeSO4) | 1.5 | 1.2 | 0.3 | 0.3 | 78.4 | 0 | 4.3 |
| IL lignin ([bmim]MeSO4 + DMSO) | 5.3 | 2.0 | 3.3 | 0.5 | 72.9 | 16.7 | 0 |
| IL lignin ([bmim]OAc) | 3.7 | 1.8 | 1.9 | 0.9 | 69.8 | 0 | 6.9 |
| IL lignin ([bmim]OAc + DMSO) | 4.6 | 2.1 | 2.6 | 0.3 | 69.3 | 15.3 | 0 |
| DL | 9.1 | 3.3 | 5.8 | 0.1 | 96.1 | 6.1 | 10.4 |
| Sample | Elemental Composition, % | ||||
|---|---|---|---|---|---|
| C | H | N | S | O | |
| IL lignin ([bmim]MeSO4) | 62.7 | 6.9 | 2.4 | 2.9 | 25.1 |
| IL lignin ([bmim]MeSO4 + DMSO) | 68.5 | 9.6 | 1.3 | 0.0 | 20.6 |
| IL lignin ([bmim]OAc) | 65.4 | 7.5 | 0.9 | 3.8 | 22.4 |
| IL lignin ([bmim]OAc + DMSO) | 66.6 | 8.9 | 4.5 | 0.0 | 20.0 |
| DL | 62.6 | 7.3 | 0.0 | 0.0 | 30.1 |
| Sample | β-O-4 | α-O-4/β-5 | α-O-γ(γ-O-α)/β-β |
|---|---|---|---|
| [bmim]MeSO4 | 6.0 | 4.95 | 2.39 |
| [bmim]OAc | 20.8 | 4.68 | 3.38 |
| [bmim]MeSO4-DMSO | 15.1 | 6.28 | 2.10 |
| [bmim]OAc-DMSO | 15.0 | 4.25 | 2.18 |
| DLS | 21.4 | 7.37 | 3.53 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belesov, A.V.; Ladesov, A.V.; Pikovskoi, I.I.; Faleva, A.V.; Kosyakov, D.S. Characterization of Ionic Liquid Lignins Isolated from Spruce Wood with 1-Butyl-3-methylimidazolium Acetate and Methyl Sulfate and Their Binary Mixtures with DMSO. Molecules 2020, 25, 2479. https://doi.org/10.3390/molecules25112479
Belesov AV, Ladesov AV, Pikovskoi II, Faleva AV, Kosyakov DS. Characterization of Ionic Liquid Lignins Isolated from Spruce Wood with 1-Butyl-3-methylimidazolium Acetate and Methyl Sulfate and Their Binary Mixtures with DMSO. Molecules. 2020; 25(11):2479. https://doi.org/10.3390/molecules25112479
Chicago/Turabian StyleBelesov, Artyom V., Anton V. Ladesov, Ilya I. Pikovskoi, Anna V. Faleva, and Dmitry S. Kosyakov. 2020. "Characterization of Ionic Liquid Lignins Isolated from Spruce Wood with 1-Butyl-3-methylimidazolium Acetate and Methyl Sulfate and Their Binary Mixtures with DMSO" Molecules 25, no. 11: 2479. https://doi.org/10.3390/molecules25112479
APA StyleBelesov, A. V., Ladesov, A. V., Pikovskoi, I. I., Faleva, A. V., & Kosyakov, D. S. (2020). Characterization of Ionic Liquid Lignins Isolated from Spruce Wood with 1-Butyl-3-methylimidazolium Acetate and Methyl Sulfate and Their Binary Mixtures with DMSO. Molecules, 25(11), 2479. https://doi.org/10.3390/molecules25112479






