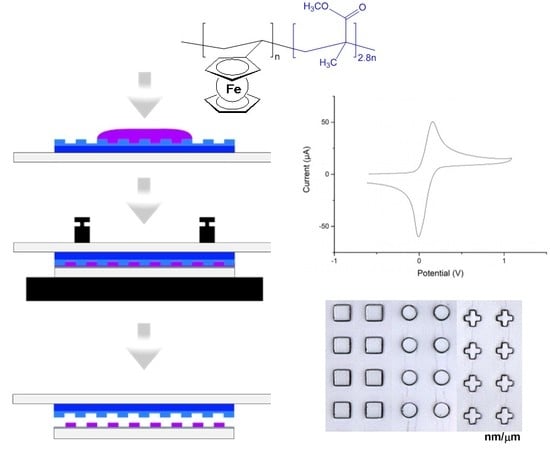
3D Micro/Nanopatterning of a Vinylferrocene Copolymer
Abstract
1. Introduction
2. Results and Discussion
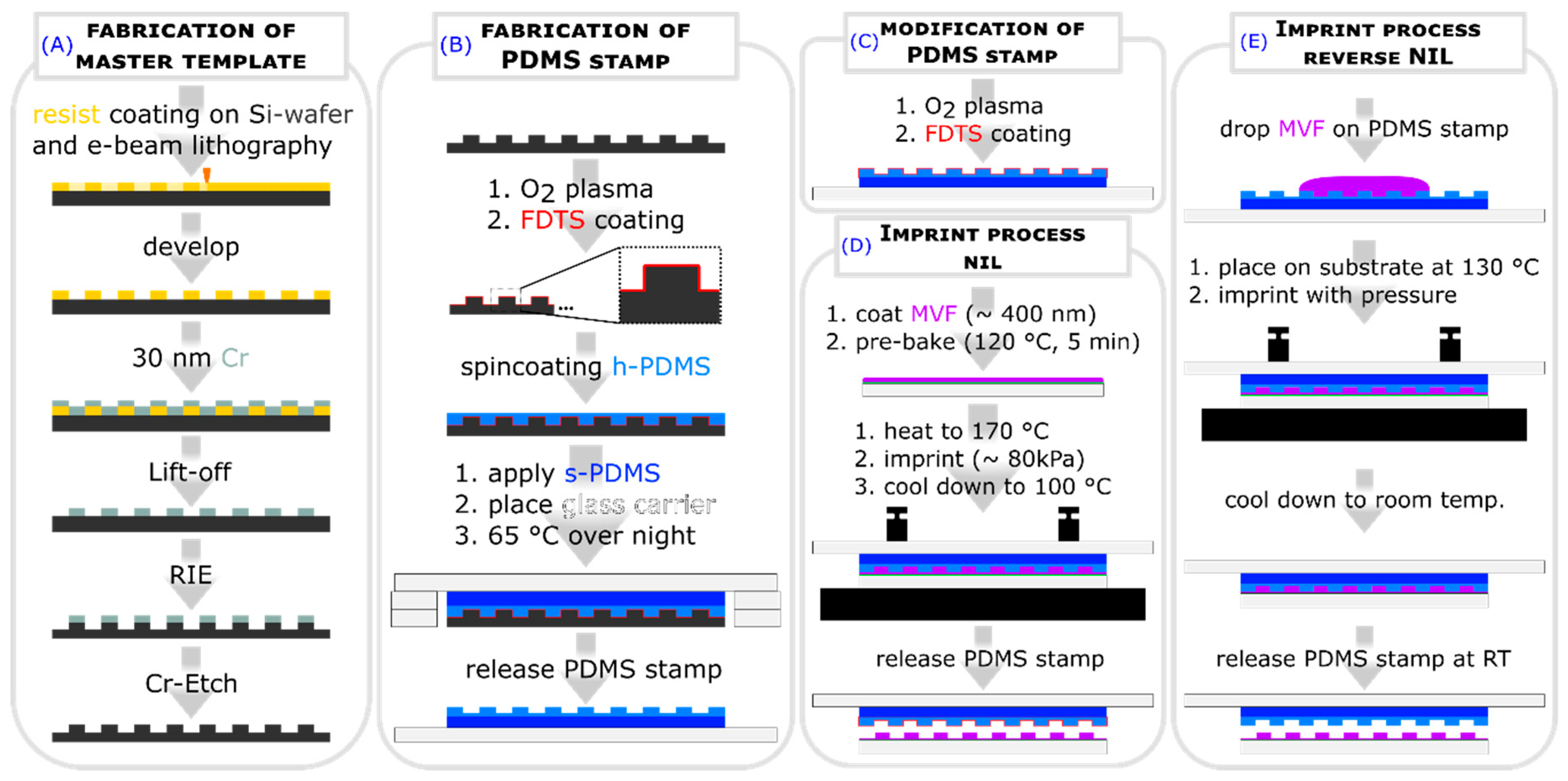
2.1. Fabrication of Master Template
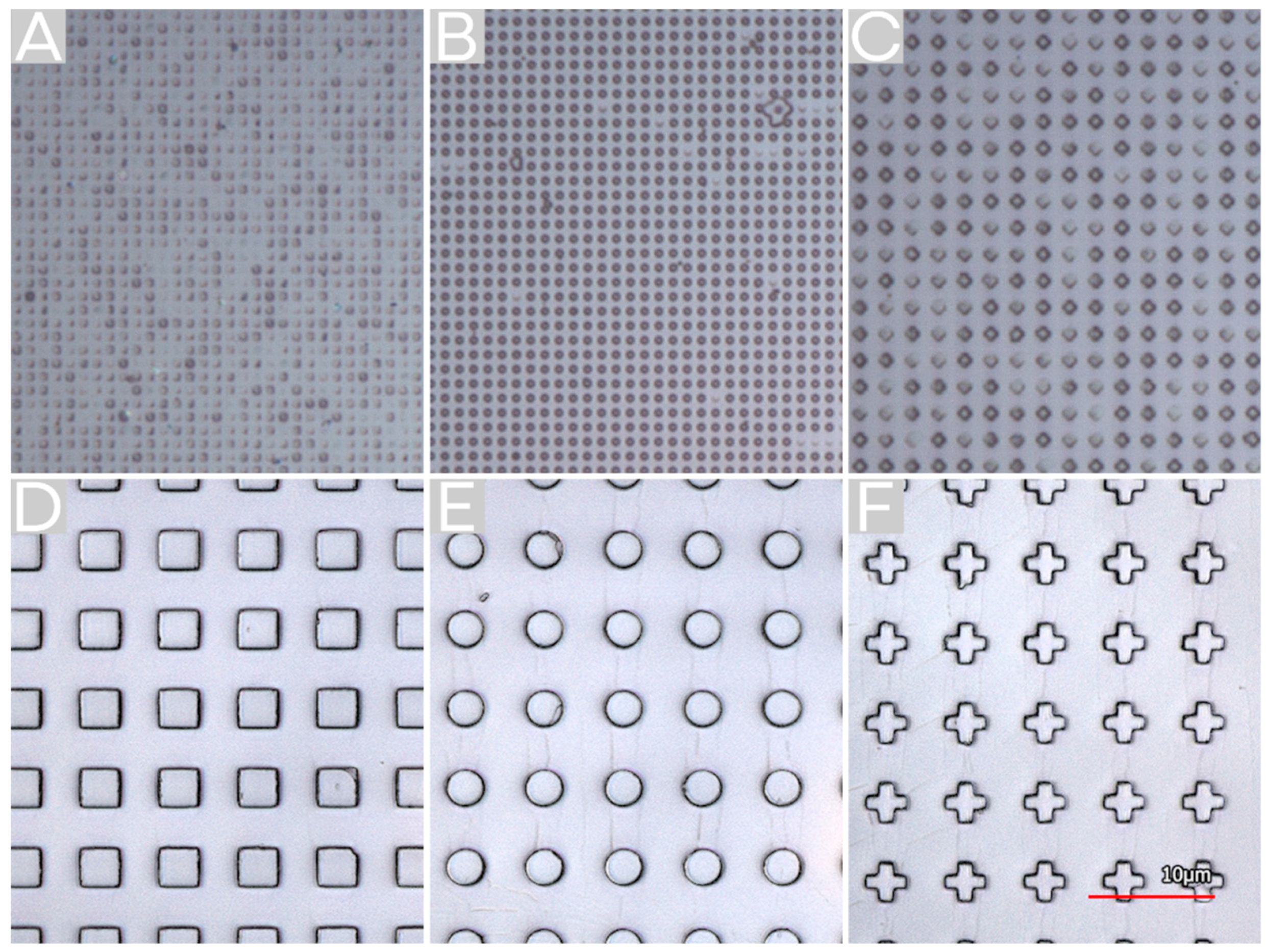
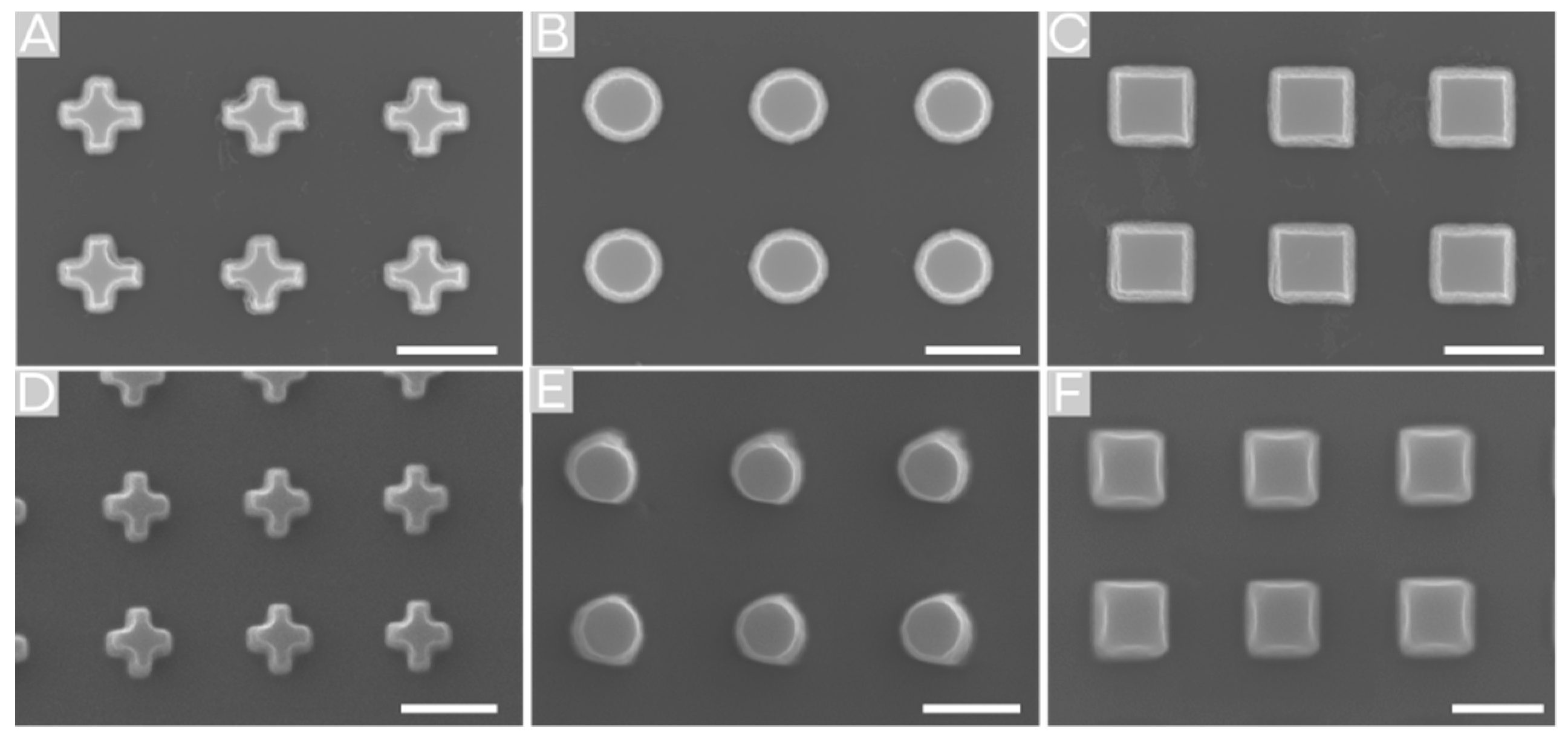
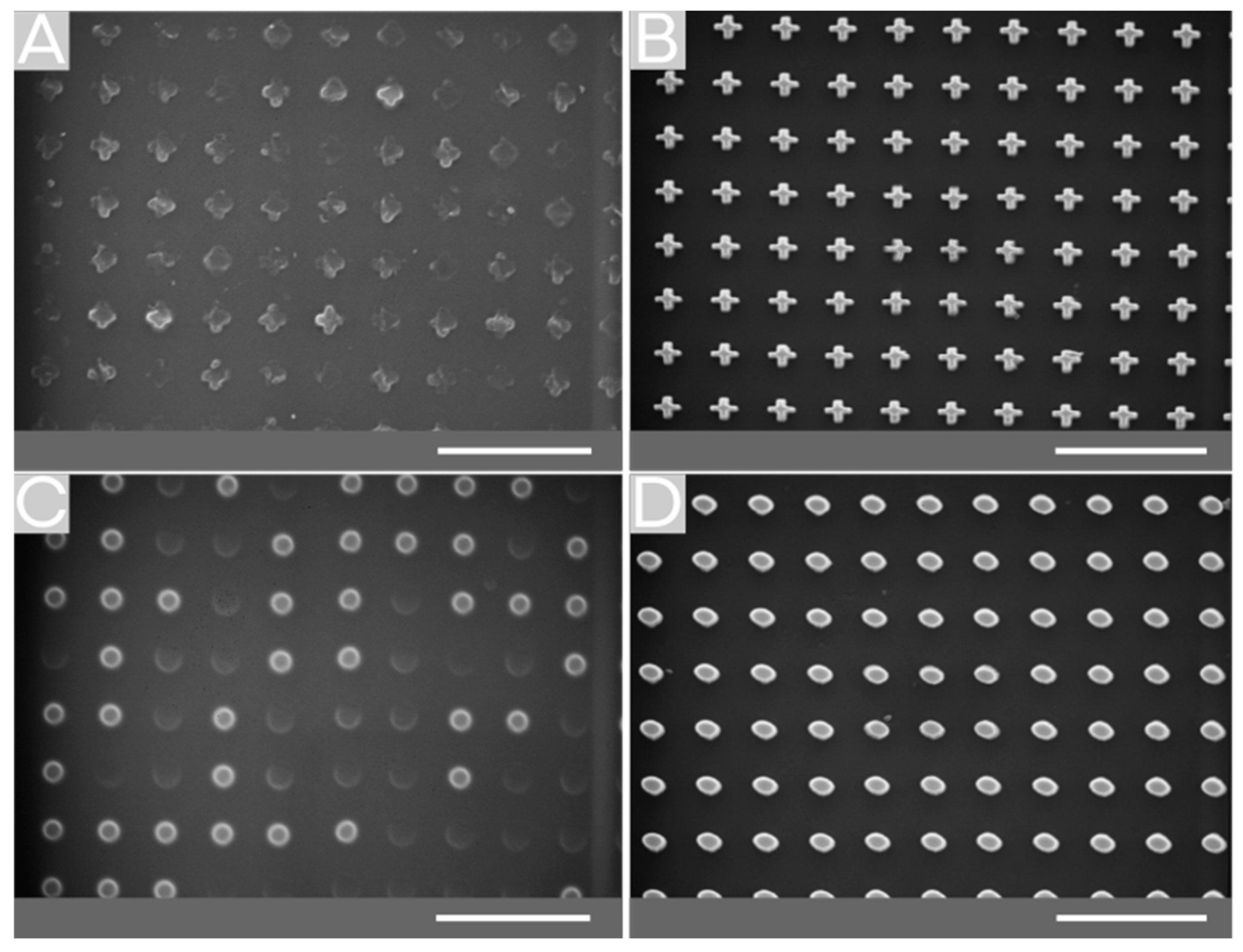
2.2. Surface Structuring by Thermal NIL
2.3. Surface Structuring by Reverse NIL
2.4. Comparison of Fabricating MVF Structured Surfaces via Thermal NIL vs. Reverse NIL
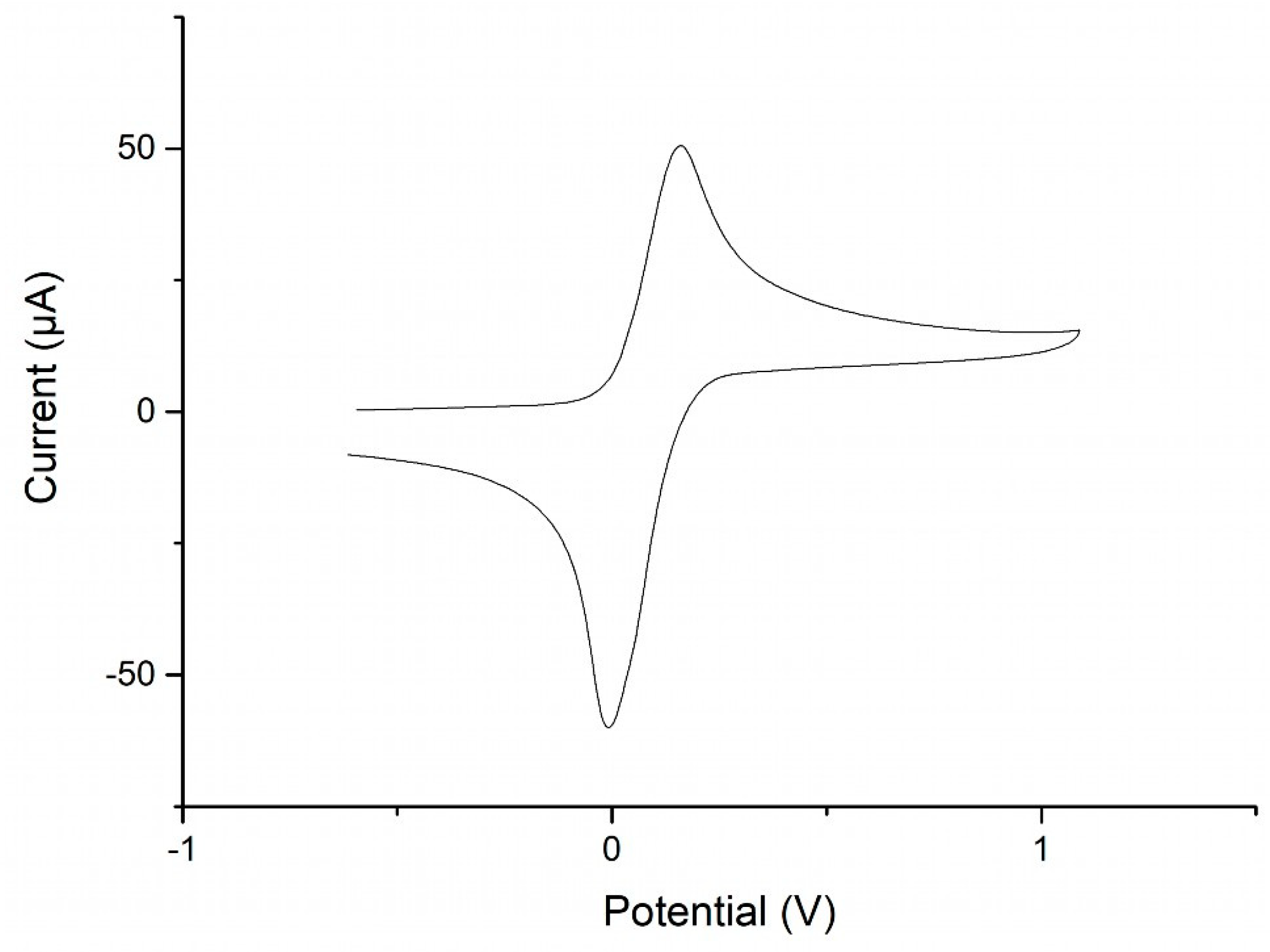
2.5. Redox Behavior of MVF
3. Materials and Methods
3.1. General Procedures
3.2. Synthesis of Poly[(Methyl Methacrylate)-co-(Vinyl Ferrocene)] (MVF)
3.3. Imprint Procedure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nguyen, P.; Gomez-Elipe, P.; Manners, I. Organometallic Polymers with Transition Metals in the Main Chain. Chem. Rev. 1999, 99, 1515–1548. [Google Scholar] [CrossRef] [PubMed]
- Manners, I. Putting metals into polymers. Science 2001, 294, 1664–1666. [Google Scholar] [CrossRef] [PubMed]
- Manners, I. Polymer science with main group elements and transition metals. Macromol. Symp. 2003, 196, 57–62. [Google Scholar] [CrossRef]
- Abd-El-Aziz, A.; Manners, I. Neutral and Cationic Macromolecules based on Iron Sandwich Complexes. J. Inorg. Organomet. Polym. Mater. 2005, 15, 157–195. [Google Scholar]
- Gao, Y.; Shreeve, J.M. Ferrocene-containing Liquid Crystalline Polymers. J. Inorg. Organomet. Polym. Mater. 2007, 17, 19–36. [Google Scholar] [CrossRef]
- Pietschnig, R. Polymers with pendant ferrocenes. Chem. Soc. Rev. 2016, 45, 5216–5231. [Google Scholar] [CrossRef]
- Herbert, D.E.; Mayer, U.F.; Manners, I. Strained metallocenophanes and related organometallic rings containing p-hydrocarbon ligands and transition-metal centers. Angew. Chem. (Int. Ed.) 2007, 46, 5060–5081. [Google Scholar] [CrossRef]
- Bellas, V.; Rehahn, M. Polyferrocenylsilane-based polymer systems. Angew. Chem. (Int. Ed.) 2007, 46, 5082–5104. [Google Scholar] [CrossRef]
- Elbert, J.; Gallei, M.; Ruettiger, C.; Brunsen, A.; Didzoleit, H.; Stuehn, B.; Rehahn, M. Ferrocene Polymers for Switchable Surface Wettability. Organometallics 2013, 32, 5873–5878. [Google Scholar] [CrossRef]
- Neumann, P.; Dib, H.; Caminade, A.-M.; Hey-Hawkins, E. Redox Control of a Dendritic Ferrocenyl-Based Homogeneous Catalyst. Angew. Chem. (Int. Ed.) 2015, 54, 311–314. [Google Scholar] [CrossRef]
- Puzzo, D.P.; Arsenault, A.C.; Manners, I.; Ozin, G.A. Electroactive inverse opal: A single material for all colors. Angew. Chem. (Int. Ed.) 2009, 48, 943–947. [Google Scholar] [CrossRef] [PubMed]
- McDowell, J.J.; Zacharia, N.S.; Puzzo, D.; Manners, I.; Ozin, G.A. Electroactuation of Alkoxysilane-Functionalized Polyferrocenylsilane Microfibers. J. Am. Chem. Soc. 2010, 132, 3236–3237. [Google Scholar] [CrossRef] [PubMed]
- Hudson, Z.M.; Lunn, D.J.; Winnik, M.A.; Manners, I. Colour-tunable fluorescent multiblock micelles. Nat. Commun. 2014, 5, 3372. [Google Scholar] [CrossRef]
- Chou, S.Y.; Krauss, P.R.; Renstrom, P.J. Imprint Lithography with 25-Nanometer Resolution. Science 1996, 272, 85–87. [Google Scholar] [CrossRef]
- Chou, S.Y. Nanoimprint Lithography. U.S. Patent 5,772,905, 30 June 1998. [Google Scholar]
- Gates, B.D.; Xu, Q.; Stewart, M.; Ryan, D.; Willson, C.G.; Whitesides, G.M. New Approaches to Nanofabrication: Molding, Printing, and Other Techniques. Chem. Rev. 2005, 105, 1171–1196. [Google Scholar] [CrossRef]
- Herlihy, K.P.; Nunes, J.; DeSimone, J.M. Electrically Driven Alignment and Crystallization of Unique Anisotropic Polymer Particles. Langmuir 2008, 24, 8421–8426. [Google Scholar] [CrossRef]
- Xu, Z.; Wu, H.-Y.; Ali, U.; Jiang, J.; Cunningham, B.; Liu, L. Nanoreplicated positive and inverted sub-micron polymer pyramids array for surface enhanced Raman spectroscopy (SERS). J. Nanophot. 2011, 5, 053526. [Google Scholar] [CrossRef]
- Hauser, H.; Tucher, N.; Tokai, K.; Schneider, P.; Wellens, C.; Volk, A.; Seitz, S.; Benick, J.; Barke, S. Development of nanoimprint processes for photovoltaic applications. J. Micro/Nanolith. Mems Moems 2005, 14, 031210. [Google Scholar] [CrossRef]
- Reuter, S.; Smolarczyk, M.A.; Istock, A.; Ha, U.-M.; Schneider, O.; Worapattrakul, N.; Nazemroaya, S.; Hoang, H.; Gomer, L.; Pilger, F.; et al. Bending properties of two- and three-dimensional-shaped nanoparticles fabricated via substrate conformal imprint lithography. J. Nanoparticle Res. 2017, 19, 184. [Google Scholar] [CrossRef]
- Ha, U.; Kaban, B.; Tomita, A.; Krekić, K.; Klintuch, D.; Pietschnig, R.; Ehresmann, A.; Holzinger, D.; Hillmer, H. Multifunctional guest-host particles engineered by reversal nanoimprint lithography. Appl. Nanosci. 2018, 8, 1161–1169. [Google Scholar] [CrossRef]
- Roesler, F.; Kaban, B.; Klintuch, D.; Ha, U.-M.; Hillmer, H.; Pietschnig, R. Tailoring Phospholes for Imprint of Fluorescent 3D Structures. Eur. J. Inorg. Chem. 2019, 4820–4825. [Google Scholar]
- Acikgoz, C.; Vratzov, B.; Hempenius, M.A.; Vancso, G.J.; Huskens, J. Nanoscale Patterning by UV Nanoimprint Lithography Using an Organometallic Resist. Acs Appl. Mater. Interfaces 2009, 1, 2645–2650. [Google Scholar] [CrossRef]
- Dong, Q.; Li, G.; Ho, C.-L.; Faisal, M.; Leung, C.-W.; Pong, P.W.-T.; Liu, K.; Tang, B.-Z.; Manners, I.; Wong, W.-Y. A Polyferroplatinyne Precursor for the Rapid Fabrication of L10-FePt-type Bit Patterned Media by Nanoimprint Lithography. Adv. Mat. 2012, 24, 1034–1040. [Google Scholar] [CrossRef]
- Nunns, A.; Gwyther, J.; Manners, I. Inorganic block copolymer lithography. Polymer 2013, 54, 1269–1284. [Google Scholar] [CrossRef]
- Dong, Q.; Meng, Z.; Ho, C.-L.; Guo, H.; Yang, W.; Manners, I.; Xu, L.; Wong, W.-Y. A molecular approach to magnetic metallic nanostructures from metallopolymer precursors. Chem. Soc. Rev. 2018, 47, 4934–4953. [Google Scholar] [CrossRef]
- Wong, W.Y.; Meng, Z.; Li, G.; Yiu, S.-C.; Zhu, N.; Yu, Z.-Q.; Leung, C.-W.; Manners, I. Nanoimprint Lithography-Directed Self-Assembly of Heterobimetallic FeM (M = Pd, Pt) Complexes for Magnetic Patterning. Angew. Chem. (Int. Ed.) 2020. in print. [Google Scholar] [CrossRef]
- Arimoto, F.S.; Haven, A.C. Derivatives of Dicyclopentadienyliron. J. Am. Chem. Soc. 1955, 77, 6295–6297. [Google Scholar] [CrossRef]
- Subramanian, K. Synthesis and characterization of poly(vinyl ferrocene) grafted hydroxyl-terminated poly(butadiene): A propellant binder with a built-in burn-rate catalyst. J. Polym. Sci. Part A: Polym. Chem. 1999, 37, 4090–4099. [Google Scholar] [CrossRef]
- Tang, J.; Liu, X.-F.; Zhang, L.-Y.; Xu, X.-L.; Zhan, P.-R. A New Convenient Method for the Synthesis of Formyl Ferrocene with Triethyl Orthoformate and AlCl3. Synth. Commun. 2000, 30, 1657–1660. [Google Scholar] [CrossRef]
- Barry, K.P.; Nataro, C. A new synthesis and electrochemistry of 1,1′-bis(β-hydroxyethyl)ferrocene. Inorg. Chim. Acta 2009, 362, 2068–2070. [Google Scholar] [CrossRef]
- Robinson, K.L.; Lawrence, N.S. Vinylferrocene Homopolymer and Copolymers: An Electrochemical Comparison. Anal. Sci. 2008, 24, 339–343. [Google Scholar] [CrossRef][Green Version]
- Staff, R.H.; Gallei, M.; Mazurowski, M.; Rehahn, M.; Berger, R.; Landfester, K.; Crespy, D. Patchy Nanocapsules of Poly(vinylferrocene)-Based Block Copolymers for Redox-Responsive Release. Acs Nano 2012, 6, 9042–9049. [Google Scholar] [CrossRef]
- CRC Handbook of Chemistry and Physics, 100th ed.; Rumble, J., Ed.; CRC Press: Taylor & Francis: Boca Raton, FL, USA, 2019. [Google Scholar]
- Fulmer, G.R.; Miller, A.J.M.; Sherden, N.H.; Gottlieb, H.E.; Nudelman, A.; Stoltz, B.M.; Bercaw, J.E.; Goldberg, K.I. NMR Chemical Shifts of Trace Impurities: Common Laboratory Solvents, Organics, and Gases in Deuterated Solvents Relevant to the Organometallic Chemist. Organometallics 2010, 29, 2176–2179. [Google Scholar] [CrossRef]
- Noviandri, I.; Brown, K.N.; Fleming, D.S.; Gulyas, P.T.; Lay, P.A.; Masters, A.F.; Phillips, L. The Decamethylferrocenium/Decamethylferrocene Redox Couple: A Superior Redox Standard to the Ferrocenium/Ferrocene Redox Couple for Studying Solvent Effects on the Thermodynamics of Electron Transfer. J. Phys. Chem. B 1999, 103, 6713–6722. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Löber, D.; Dey, S.; Kaban, B.; Roesler, F.; Maurer, M.; Hillmer, H.; Pietschnig, R. 3D Micro/Nanopatterning of a Vinylferrocene Copolymer. Molecules 2020, 25, 2438. https://doi.org/10.3390/molecules25102438
Löber D, Dey S, Kaban B, Roesler F, Maurer M, Hillmer H, Pietschnig R. 3D Micro/Nanopatterning of a Vinylferrocene Copolymer. Molecules. 2020; 25(10):2438. https://doi.org/10.3390/molecules25102438
Chicago/Turabian StyleLöber, Dennis, Subhayan Dey, Burhan Kaban, Fabian Roesler, Martin Maurer, Hartmut Hillmer, and Rudolf Pietschnig. 2020. "3D Micro/Nanopatterning of a Vinylferrocene Copolymer" Molecules 25, no. 10: 2438. https://doi.org/10.3390/molecules25102438
APA StyleLöber, D., Dey, S., Kaban, B., Roesler, F., Maurer, M., Hillmer, H., & Pietschnig, R. (2020). 3D Micro/Nanopatterning of a Vinylferrocene Copolymer. Molecules, 25(10), 2438. https://doi.org/10.3390/molecules25102438