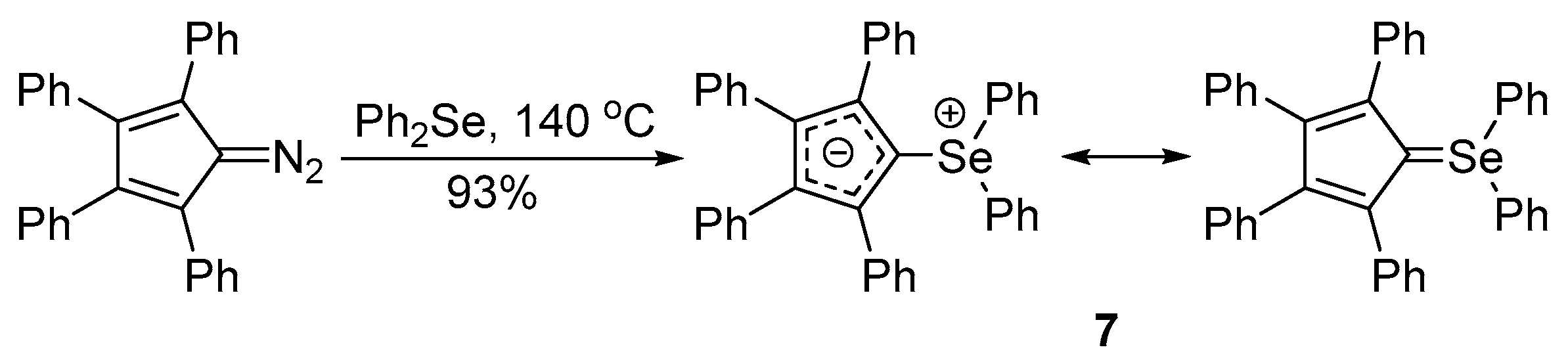
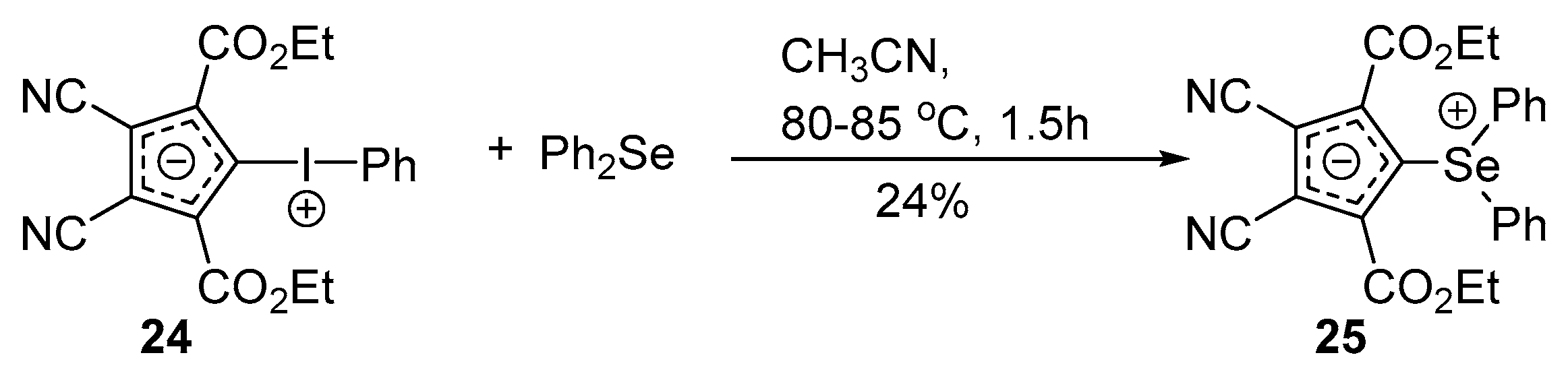
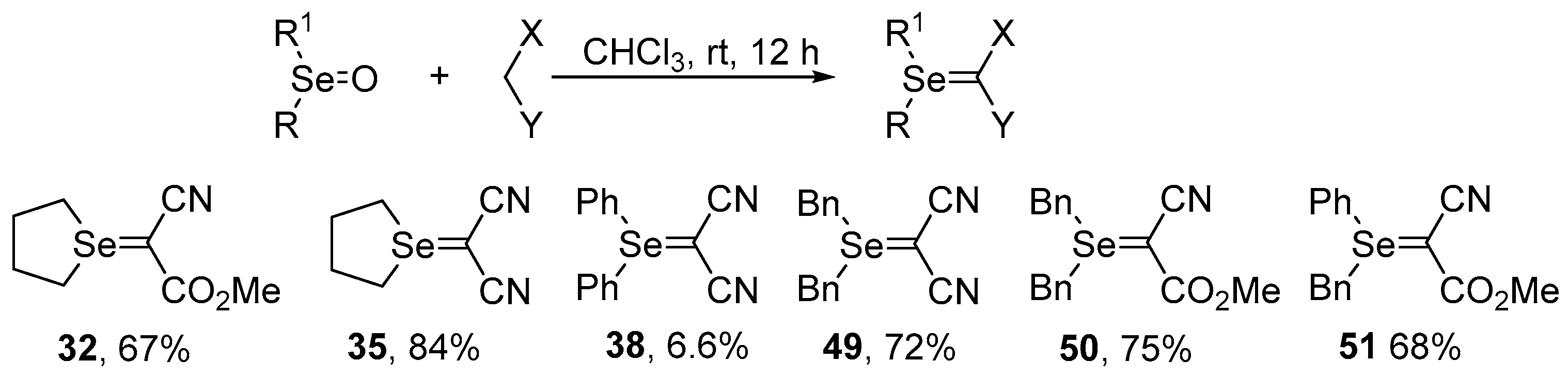
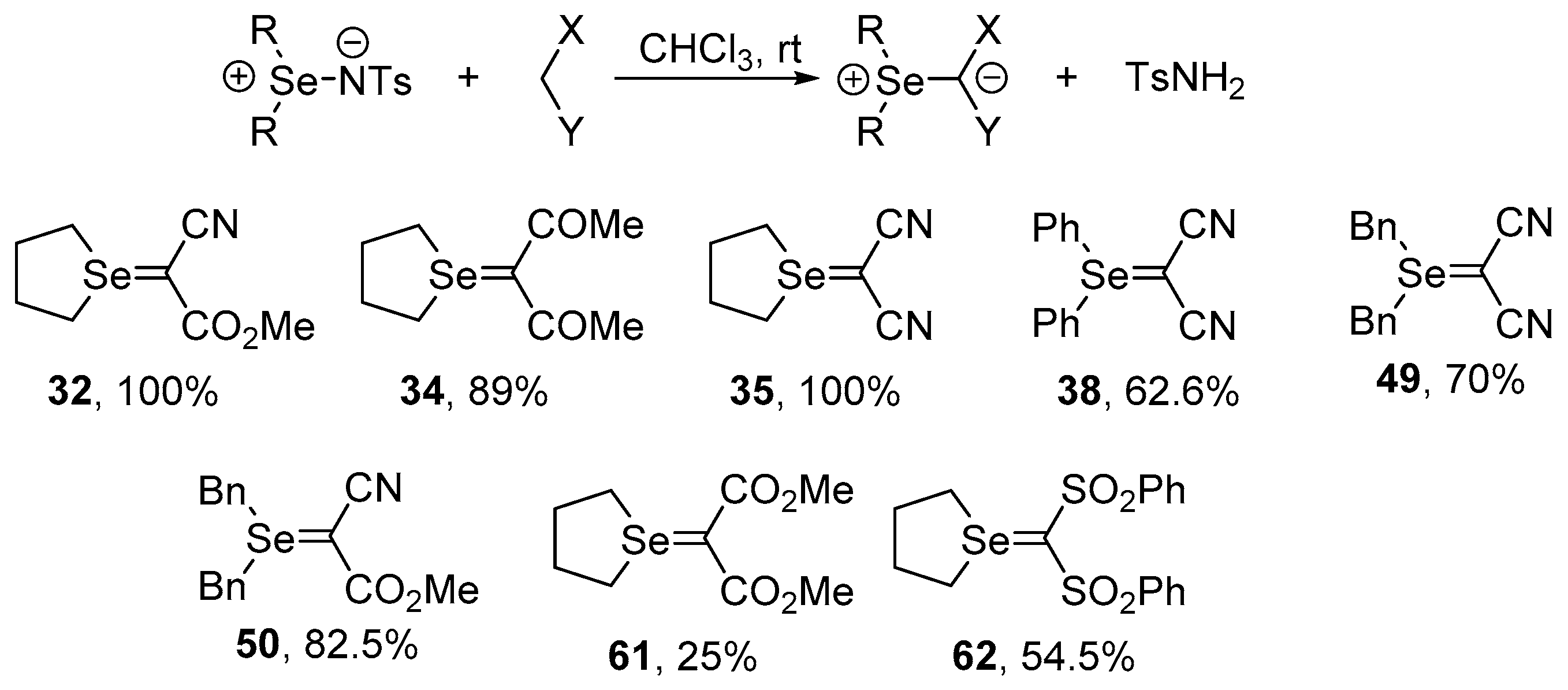
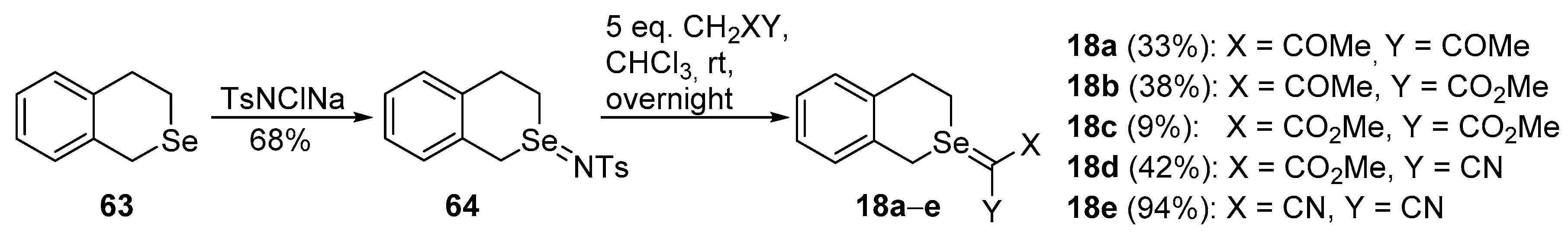
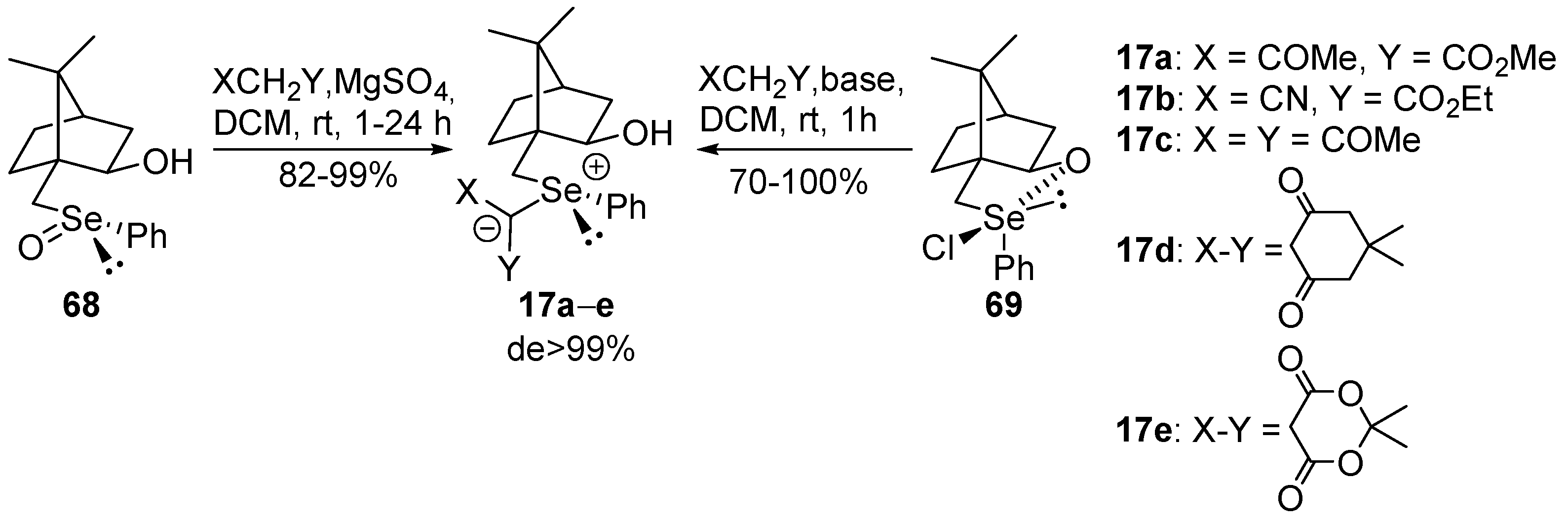
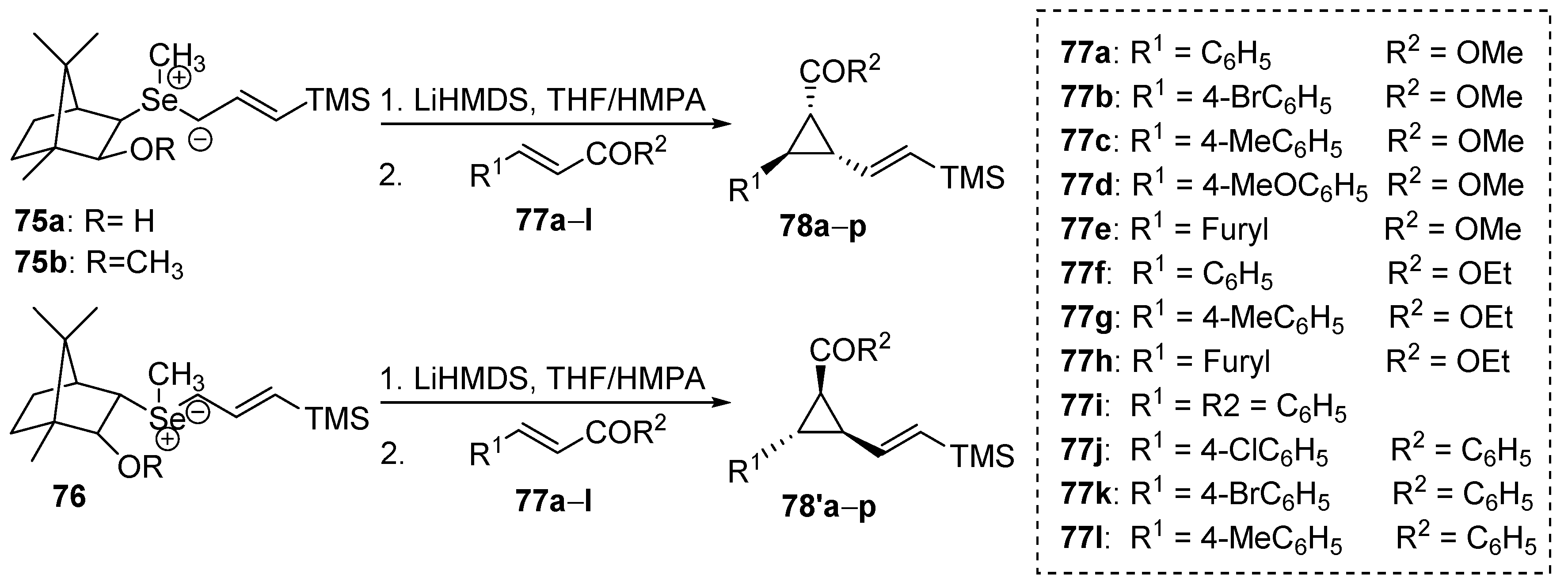
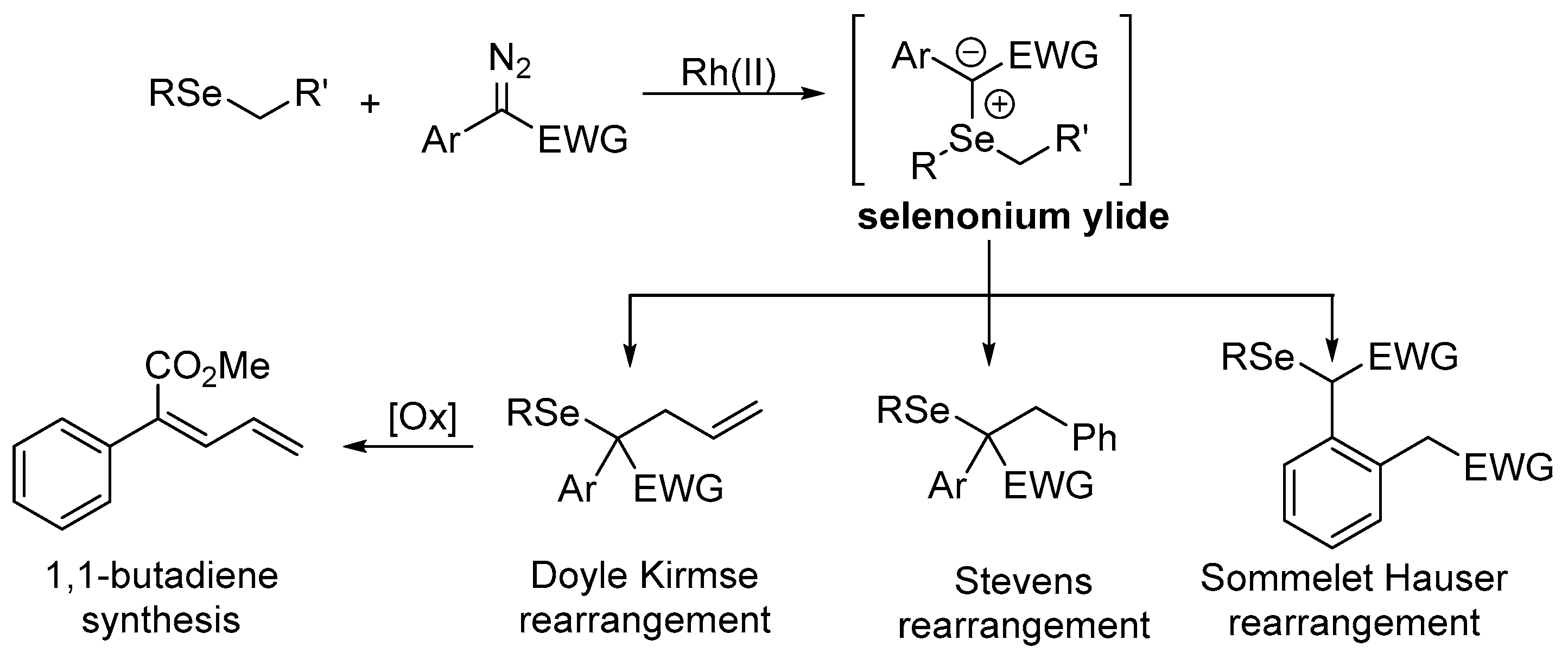
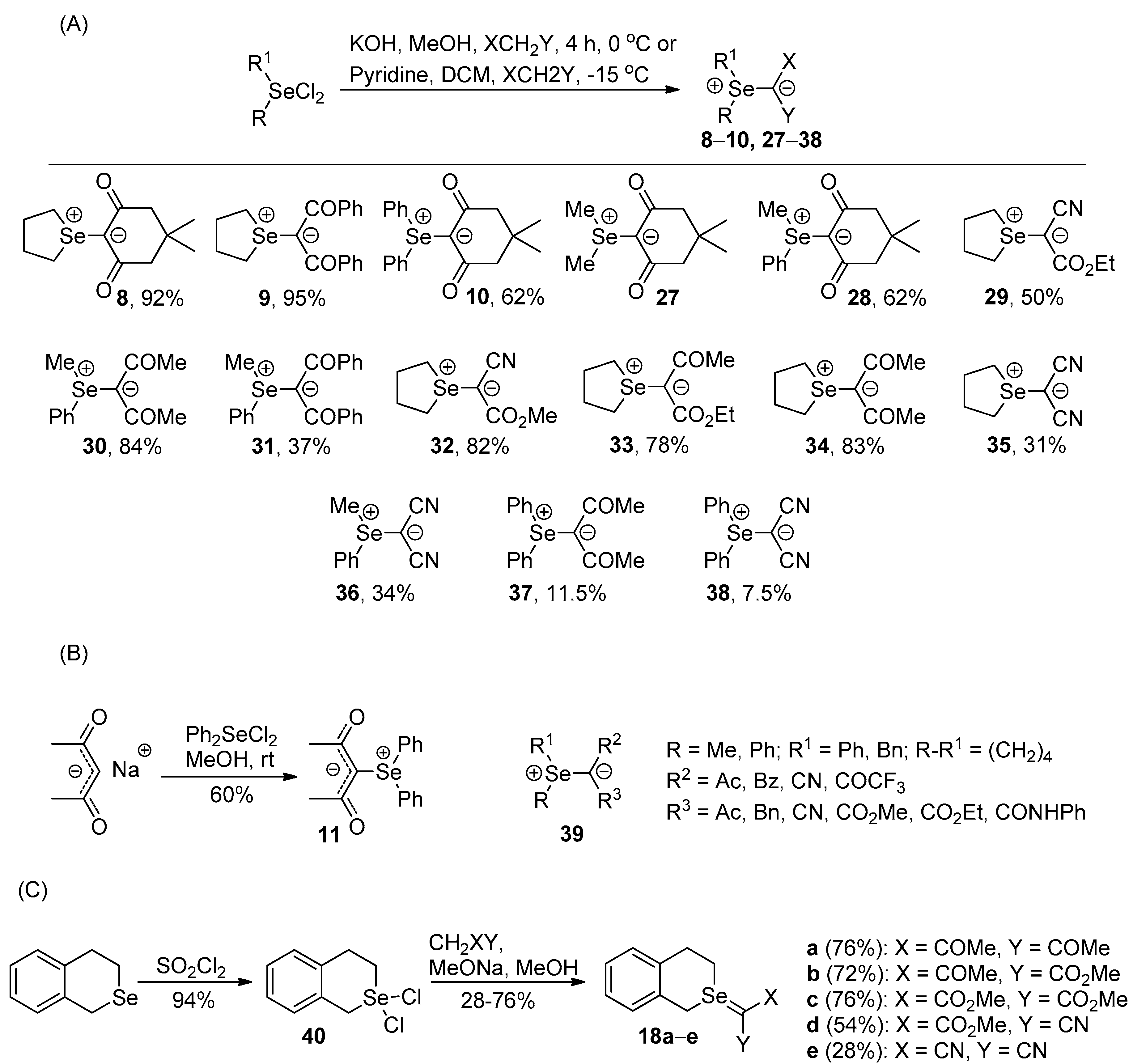Selenium-containing compounds are highly valuable reagents and catalysts with widespread application in the organic chemistry (i.e., synthesis of complex natural products, pharmaceutical ingredients, new materials, and as a tool for the introduction of new functional groups). Due to the specific chemical reactivity, it could be expected that selenonium ylides should serve as useful synthetic reagents. Until now, their synthetic applicability, mainly to induce several types of C–C bond formation reactions, is still limited. In this part of the review, we comprehensively discuss such applications to the cyclopropanation, epoxidation reactions, synthesis of α,β-unsaturated ketones, and sigmatropic rearrangements.
4.2. Epoxidation Reactions
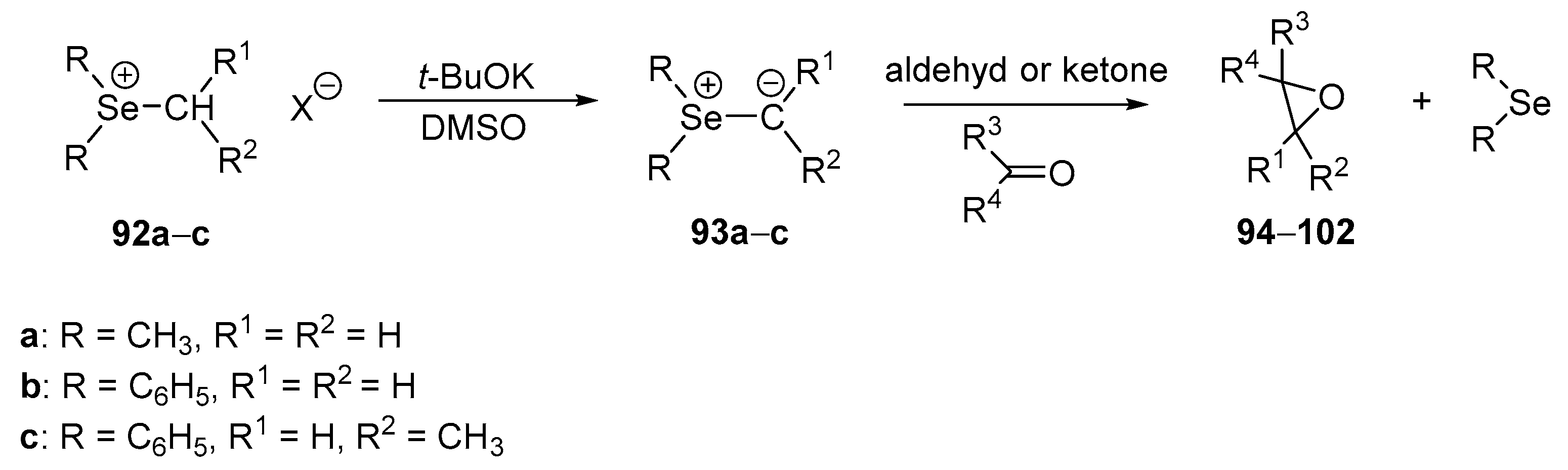
In 1974, Krief and his group reported the first example of the reaction of selenium ylides with carbonyl compounds, which provides epoxides [
63]. Selenonium ylides
93a–
c generated in situ from selenium salts
92a–
c using potassium
tert-butoxide as a base were found to react with aldehydes or ketones to form the corresponding epoxides
94–
102 in good to high yields (50–90%) (
Scheme 25,
Table 6).
It is worth noting that in the reaction of selenonium salts with enolizable carbonyl compounds (i.e., heptanal, cyclohexanone, methyl ethyl ketone, and acetophenone), the corresponding epoxides were not formed. Such a result is caused by the formation of an acetophenone anion, which is subsequently methylated by the selenonium salt. The generation of the selenonium ylides prior to the addition of acetophenone allowed the avoidance of the methylation reaction.
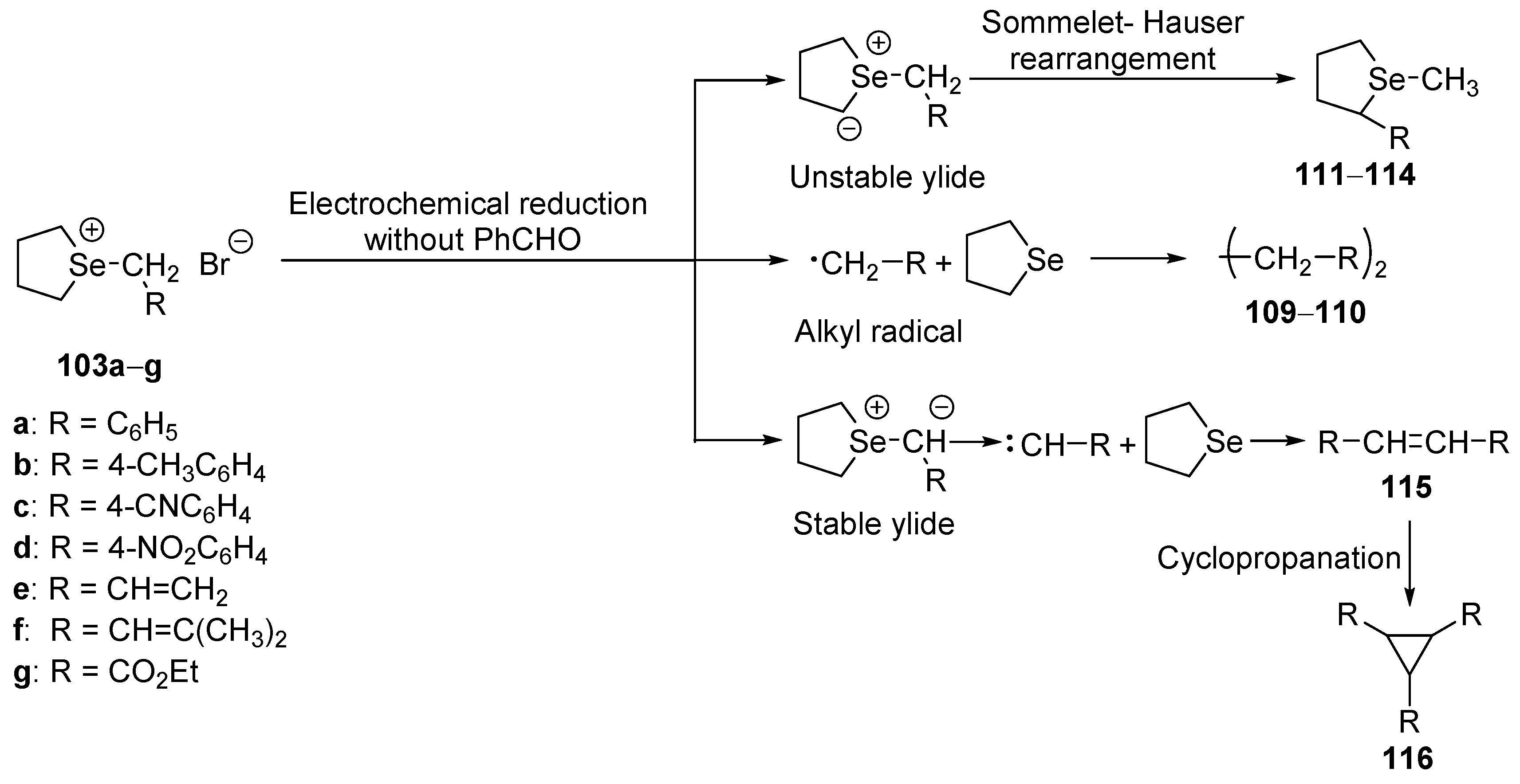
Morihara’s group reported the generation of the selenonium ylides by electrochemical reduction of the cyclic five-membered selenonium salts
103a–
g carried out in the presence or without benzaldehyde (
Scheme 26 and
Scheme 27) [
64].
When electrochemical reduction of selenonium salts
103a–
g was carried out in the presence of acetophenone, the main reaction was epoxidation (products
104–
108); only in two cases, a product of a radical coupling (
109–
110) was formed. In most cases, moderate to good yields were observed (
Table 7).
For electrochemical reduction carried out in the absence of benzaldehyde, the reaction course depends on the
R substituent in the selenonium salt (
Scheme 27). When the reactions were conducted on selenonium salts bearing a benzyl or allyl substituent, the products of the [2,3]-sigmatropic rearrangement (
111–
114) or radical coupling (
109–
110) were formed. In the case of a selenonium salt bearing a carbonyl group, formation of carbene led to olefins
115 or cyclopropanes
116 as products (
Table 8).
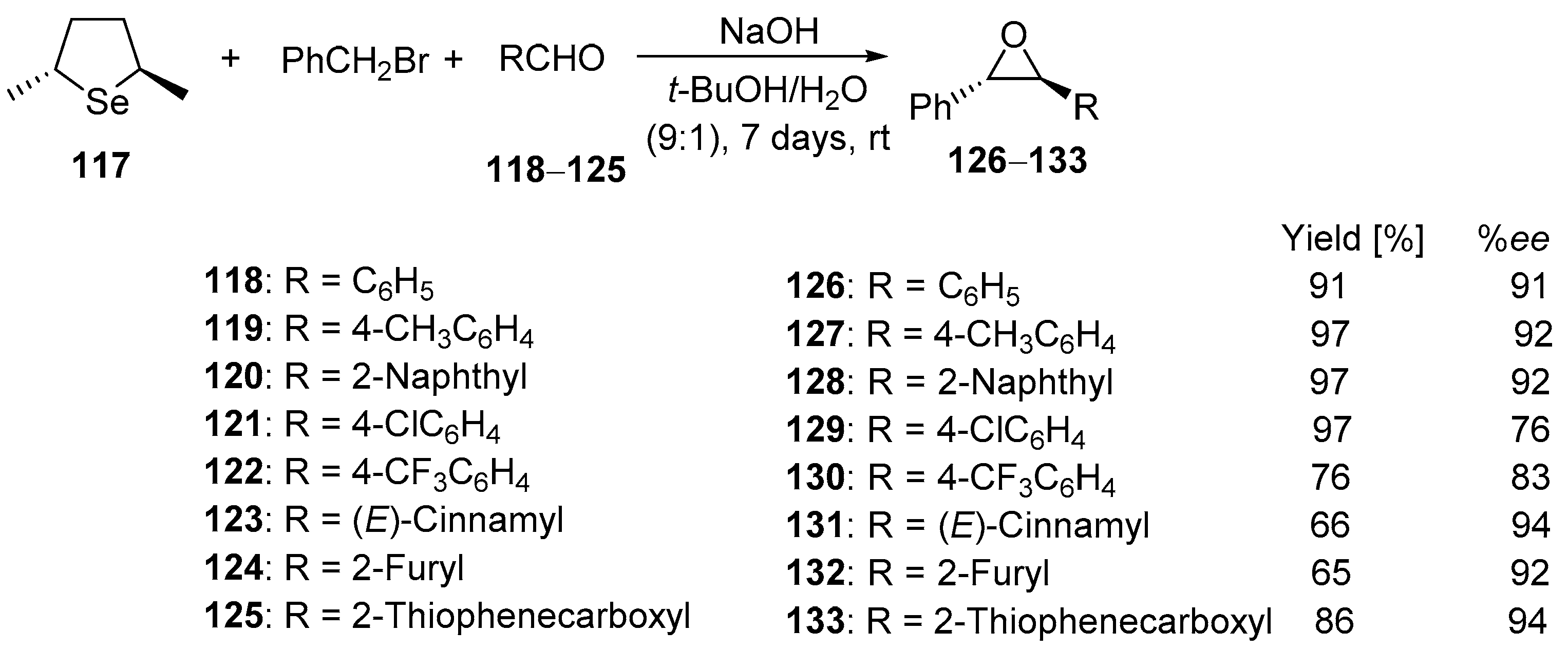
In 2001, Metzner and coworkers reported the first application of an optically active selenonium ylide in the asymmetric epoxidation reaction [
65]. The appropriate enantioenriched selenonium ylide was generated by the addition of benzyl bromide to C
2-symmetric (2
R,5
R)-2,5-dimethylselenolane
117 in the presence of NaOH. Its reaction with a variety of aldehydes
118–
125 led to the corresponding epoxides. Thus, the reaction of aldehydes
118–
120 with the stoichiometric amount of selenide
117 afforded the corresponding epoxides
126–
128 in the yield from 71% to 97% and with excellent enantiomeric excesses (92–93%) (
Scheme 28).
In turn, the use of a catalytic amount of selenolane
117 (20% mol) in reaction with aldehydes
118–
125 at ambient temperature led to the corresponding epoxides in good to excellent yields (65–97%). For more reactive heteroaromatic aldehydes (
124 and
125), the reaction time was optimized to 4 h. In most cases, enantiomeric excesses were in the range 91–94%, except for aldehydes
121 and
122, which bear electron-withdrawing groups (
Scheme 29).
Four years later, Metzner’s group also proved that (2
R,5
R)-2,5-dimethylselenolane (
117) is an efficient catalyst for the benzylidenation of aromatic aldehydes [
66]. The authors noted that as compared to the sulfur analogues, the selennium-based system leads to enhanced reactivity and higher asymmetric induction, with the same absolute configuration. The reaction of selenolane
117 with benzaldehyde
118 led to the epoxide
126, which was formed, surprisingly, with a lack of diastereoselectivity and with an enantiomeric excess higher than 90%. The authors explained [
67,
68] that the formation of equal amounts of
trans and
cis diastereomers is caused by a reversible betaine formation, leading to the
cis epoxide, which is formed from the betaine
syn conformer (
Scheme 30).
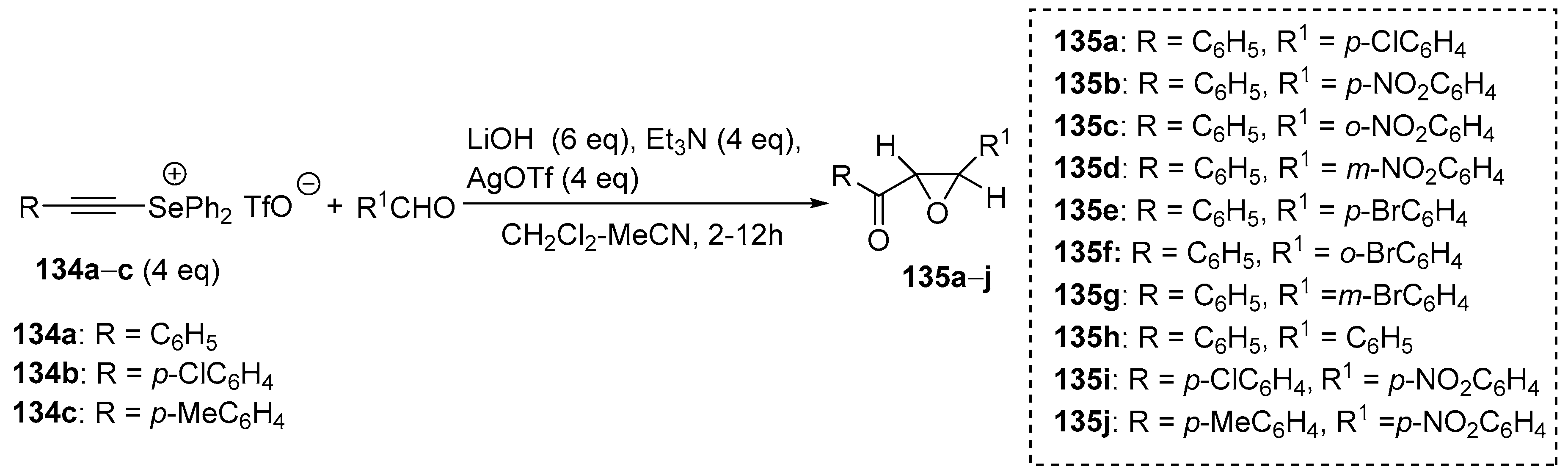
Watanabe and Kataoka described the use of ketodiphenylselenonium ylides generated from the corresponding alkynylselenonium salt for the synthesis of oxiranylketones [
69]. The reaction of alkynylselenonium salt
134a–
c with various aromatic aldehydes in the presence of LiOH, silver triflate, and triethylamine gave oxiranylketones
135a–
j just as a
trans-isomer in moderate to good yields (40–92%) (
Scheme 31,
Table 9).
In the case of aliphatic aldehydes, the desired oxiranylketones
136a–
e were formed in moderate yields (18–54%) under the same reaction conditions (
Scheme 32,
Table 10).
In turn, the reaction of alkynylselenonium salt
134a with aromatic aldehydes in the presence of sodium
p-toluenesulfonamide instead of LiOH led to the corresponding benzoyl aziridine derivatives
137a–
d in moderate yields (
Scheme 33,
Table 11). The comparison of the coupling constants values with literature data indicates that these isomers have the
cis geometry (
JHH the methine protons on the aziridine ring is 7–8 Hz) [
70].
Scheme 34 shows the plausible mechanism for the reactions of alkynylselenonium salt
134a with aldehydes and the hydroxyl ion in the presence of a silver salt and triethylamine (
Scheme 34). In the first step, the triple bond of the selenonium salt is activated by silver cation, and the hydroxyl ion attacks the β-carbon atom to form the vinyl ylide
138. In the presence of Et
3N, the ylide
138 is transformed into the ketodiphenylselenonium ylide
139, which reacts with an aldehyde to form appropriate oxiranylketones (route A). In turn, diphenyl selenoxide is formed by the attack of hydroxyl ion on a selenonium cation without activation of a triple bond by a silver ion (route B) [
71,
72].
4.4. Sigmatropic Rearrangements
The reactions of dibenzylselenonium dicyanomethylide
145 and dibenzylselenonium cyanomethoxycarbonylmethylide
146 with triphenylphosphine were examined by Tamagaka and coworkers [
74]. They found that the reaction of dibenzylselenonium dicyanomethylide
145 carried out at room temperature led to the mixture of products: Triphenylphosphine selenide
147 (87%), 2-benzyl-2-cyano-3-phenylpropanenitrile
148 (53%) and a small amount of dibenzyl diselenide (11%). The treatment of dibenzylselenonium cyanomethoxycarbonylmethylide
146 with triphenylphosphine afforded methyl 2-benzyl-2-cyano-3-phenylpropanoate
149, triphenylphosphine selenide
147, and dibenzyl diselenide in 29%, 49%, and 28% yields, respectively. In both cases, products obtained in the reactions were formed via the deselenization reaction and no dibenzyl selenide was observed (
Scheme 38).
On the contrary, the reaction of dibenzylselenonium
N-tosylimide
150 with triphenylphosphine at room temperature led to the formation of dibenzyl selenide (81%) as the main product, triphenyl
N-tosylphosphimine
152 (54%), and only a trace of triphenylphosphine selenide (6%) (
Scheme 39). The isolation of the phosphine selenide indicates that the selenonium imide
150 also undergoes deselenization but as a result of a minor process. In turn, the reaction of dibenzyl selenoxide with triphenylphosphine was found to be much more facile and led quantitatively to dibenzyl selenide and triphenylphosphine oxide
153. No deselenization products were observed. Therefore, the reactivity order for the reduction into dibenzyl selenide is selenoxide > selenonium imide > selenonium ylide.
It was found that the addition of acetic acid or water to the reaction system changes the product distribution and reaction modes. The reaction of compounds 145 and 146 under these conditions gave dibenzyl diselenides as the main product. Meanwhile, the corresponding selenonium imide 150 and selenoxide 151 afforded dibenzyl selenide.
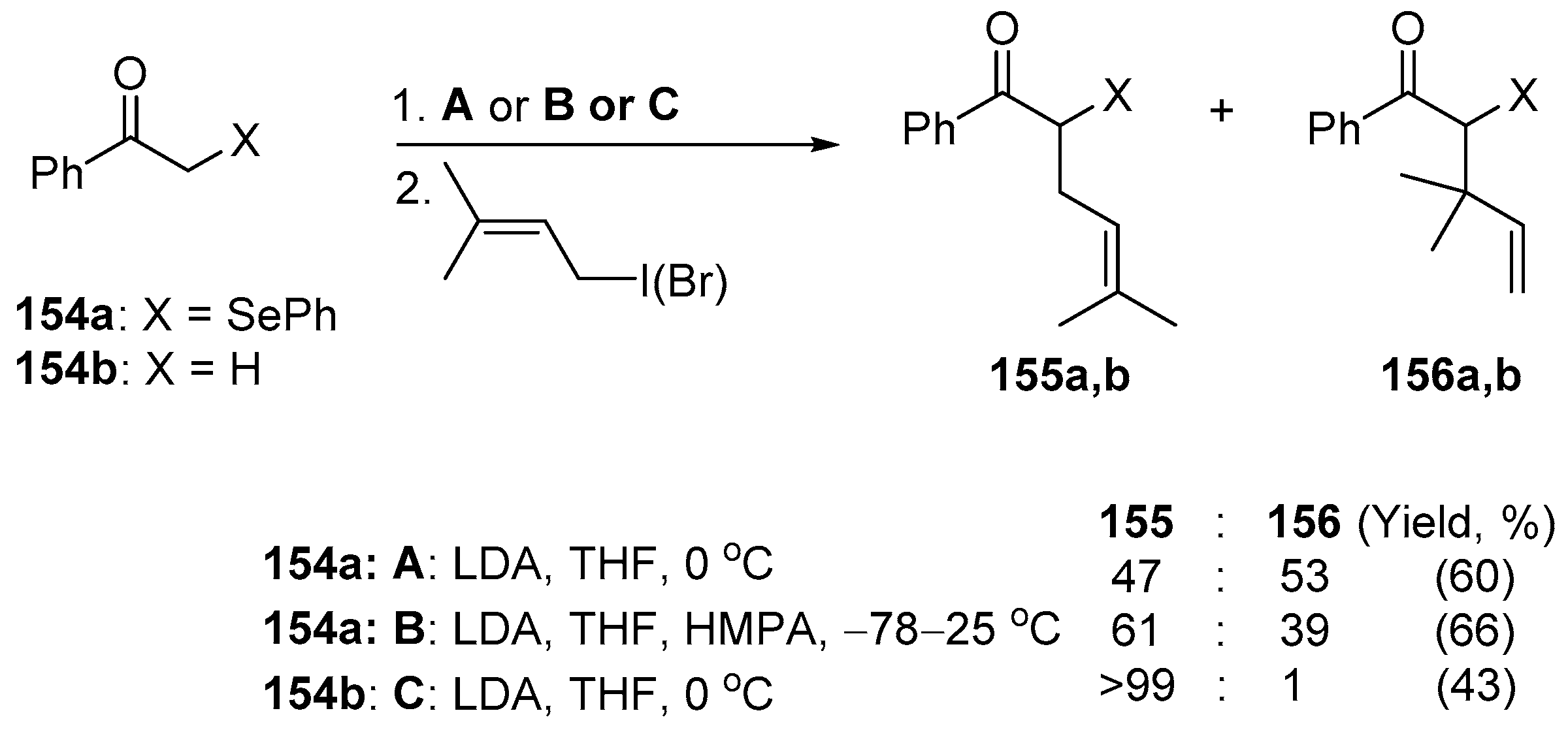
Reich and Cohen reported that the seleno-substituted ketone enolates can undergo an alkylation at the heteroatom [
75]. Treatment of the appropriate enolate of α-(phenylseleno)acetophenone
154a with prenyl iodide or bromide led to the alkylation product
155a and unexpected product
156a (
Scheme 40).
The formation of compound
156a may result from two reaction pathways. The first one is the
O-alkylation of enolate to give
157, followed by the [
3,
3]-sigmatropic rearrangement, while the second one assumes alkylation at the selenium atom to form the ylide
158 and the subsequent [2,3]-sigmatropic rearrangement (
Figure 16).
To confirm the ylide mechanism, the alkylation of α-allylselenoacetophenone
159 was performed. One of the reaction products was
C-allyl product
160, which must be formed by the [2,3]-sigmatropic rearrangement of the intermediate
161 capable of allyl migration [
76] (
Scheme 41).
Krief’s group reported the [2,3]-sigmatropic rearrangement of allylic selenonium ylides and the application of the rearrangement to the synthesis of functionalized alkylidene cyclopropanes [
77]. The alkylation reaction (method A, B, or C) of the corresponding 1-seleno-l-vinyl cyclopropanes
162a–
c led to the selenonium salts, which in the reaction with
t-BuOK afforded appropriate selenonium ylide as an intermediate, which subsequently rearranged to alkylidenecyclopropanes
164a–
c (
Scheme 42,
Table 13).
It was found that the homoallyl selenides
164a and
165 are good precursors of dienes. The alkylation reaction of the selenium atom (method A) followed by treatment with
t-BuOK/DMSO (method D) led to the dienes
167 and
169 by an elimination reaction in the regioselective way (
Scheme 43).
Braverman and coworkers reported a base-catalyzed [2,3]-sigmatropic rearrangement of bis-γ-substituted propargylic selenonium salts [
78]. The reaction of ethyl bis-γ-cyclohexenylpropargyl selenium tetrafluoroborate
170a,b with 1,8-diazabicyklo(5.4.0)undek-7-en (DBU) led to the appropriate selenonium ylide, which underwent a spontaneous [2,3]-sigmatropic rearrangement and gave the selenonium derivatives
171a,b. The obtained compounds underwent a subsequent [
1,
3] shift to the appropriate dienynes
172a,b (
Scheme 44).
Similarly, the selenonium salt
173 in the presence of CH
3ONa undergoes [2,3]-sigmatropic rearrangements leading to the corresponding selenides
175 and
176. Using weaker bases like 1,4-diazabicyclo[2.2.2]octane (DABCO) leads to mixtures of S
N2 products (
Scheme 45). This result indicates the relatively high sensitivity of selenonium salts to nucleophilic displacement with respect to the corresponding sulfonium salts, which may be explained by the relatively higher polarizability of the selenium atom as well as the weaker Se-C bond [
37].
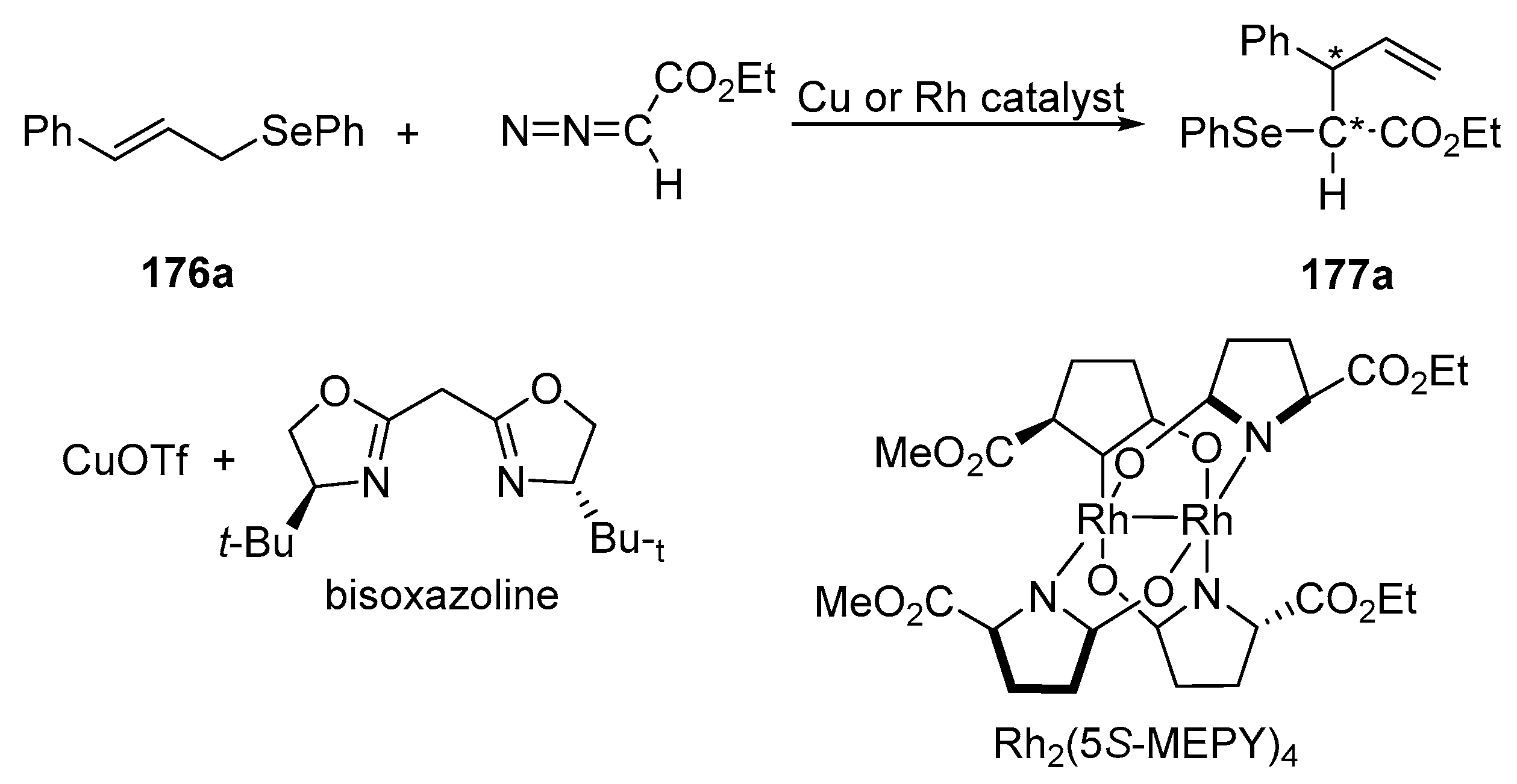
Uemura and coworkers described the first example of enantioselective addition of the carbenoid derived from ethyl diazoacetate to chalcogen atoms of aryl cinnamyl chalcogenides, which proceed via a [2,3]-sigmatropic rearrangement of the chalcogen ylide [
79]. The reaction of (
E)-cinnamyl aryl selenides
176a–
c with ethyl diazoacetate in the presence of a catalytic amount of copper or rhodium catalyst led to the mixture of diastereomeric ethyl 2-arylchalcogeno-3-phenylpent-4-enoates
177a–
c. When the reaction was carried out on (
E)-cinnamyl phenyl selenide
176a with a copper (I) salt and bisoxazoline, the desired products were obtained with an enantioselectivity up to 34%. It was found that the introduction of an electron-withdrawing group (
o-nitro or
o-trifluoromethyl) into the phenylselenium moiety improved the enantioselectivity of the corresponding products, while the introduction of an electron-donating group (i.e.,
o-methoxy, ferrocenylselenium) inhibited the reaction completely. Analogously, the reactions of (
E)-cinnamyl phenyl selenide
176a with the Doyle catalyst [Rh
2(5S-MEPY)
4 (1 mol%)] were carried out. At 40 °C, the reaction proceeded smoothly with slightly better but still low enantioselectivity (up to 41%
ee) in comparison to that obtained in the reaction with a copper catalyst (
Scheme 46,
Table 14).
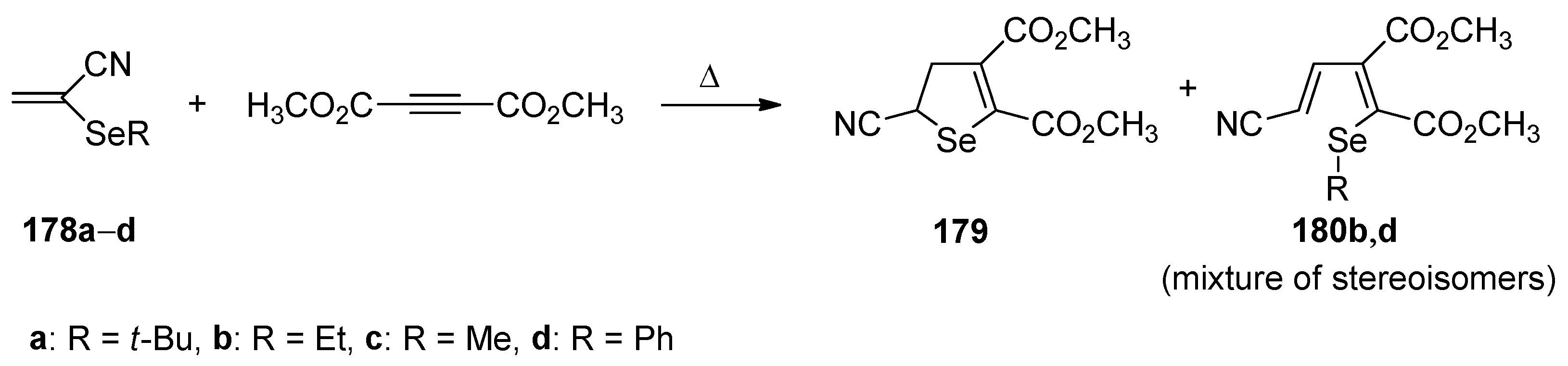
The reaction of (2-
tert-butylseleno)propenenitrile
178a with an excess of dimethylethynedicarboxylate provided dimethyl 5-cyano-4,5-dihydroselenophene-2,3-dicarboxylate (
179) in a 78% yield [
80]. In turn, the reaction carried out with 2-(ethylseleno)-, 2-(methylseleno)- and 2-(phenylseleno)propenenitrile
178b–
d and dimethylethynedicarboxylate led to polysubstituted butadienes
180b–
d in 63%, 34%, and 46% yields, respectively (
Scheme 47).
Two different reaction pathways leading to compound
179 and/or
180b–
d were postulated. In both cases, the intermediate selenonium ylides
181a–
d formed in the reaction of
178a–
d and dimethylethynedicarboxylate are involved. When the R group on Se in substrate
178a–
d is an alkyl unit, alkene can be eliminated (isobutene from
178a, ethene from
178b). In the case of
178a, elimination of isobutene appears very facile and compound
179 is formed as the only product. On the other hand, elimination of ethene from
178b is retarded by ring opening to
180b, via intermediate
182b, which results in the formation of a mixture of products. In turn, for derivatives
178c,
d, no olefin can be eliminated and thus the ring opening occurred and led to
180c,
d (
Scheme 48).
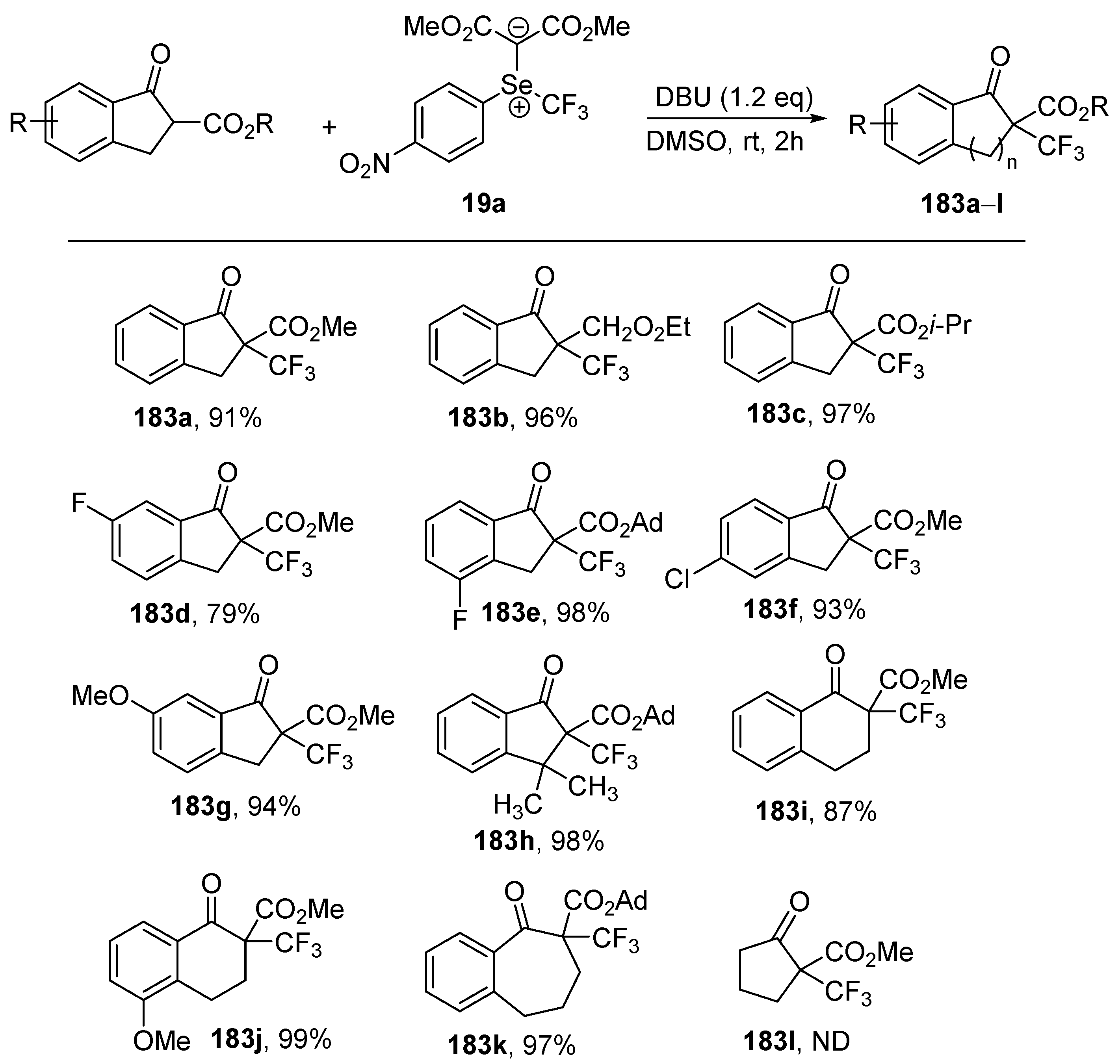
Shen and coworkers described the application of trifluoromethyl-substituted selenonium ylide
19a as an electrophilic reagent in the reactions with various nucleophiles, including β-ketoesters and silyl enol ethers, aryl/heteroaryl boronic acids, electron-rich heteroarenes, and sulfonates [
38]. The reactions of various β-ketoesters derived from indanone, tetralone, or 1-benzosuberone with
19a in the presence of DBU led to the corresponding trifluoromethylated compound
183a–
k in high yields (79–99%). The reactions of non-phenyl fused or open-chain β-ketoesters were much slower and the formation of the desired products were not observed (
Scheme 49).
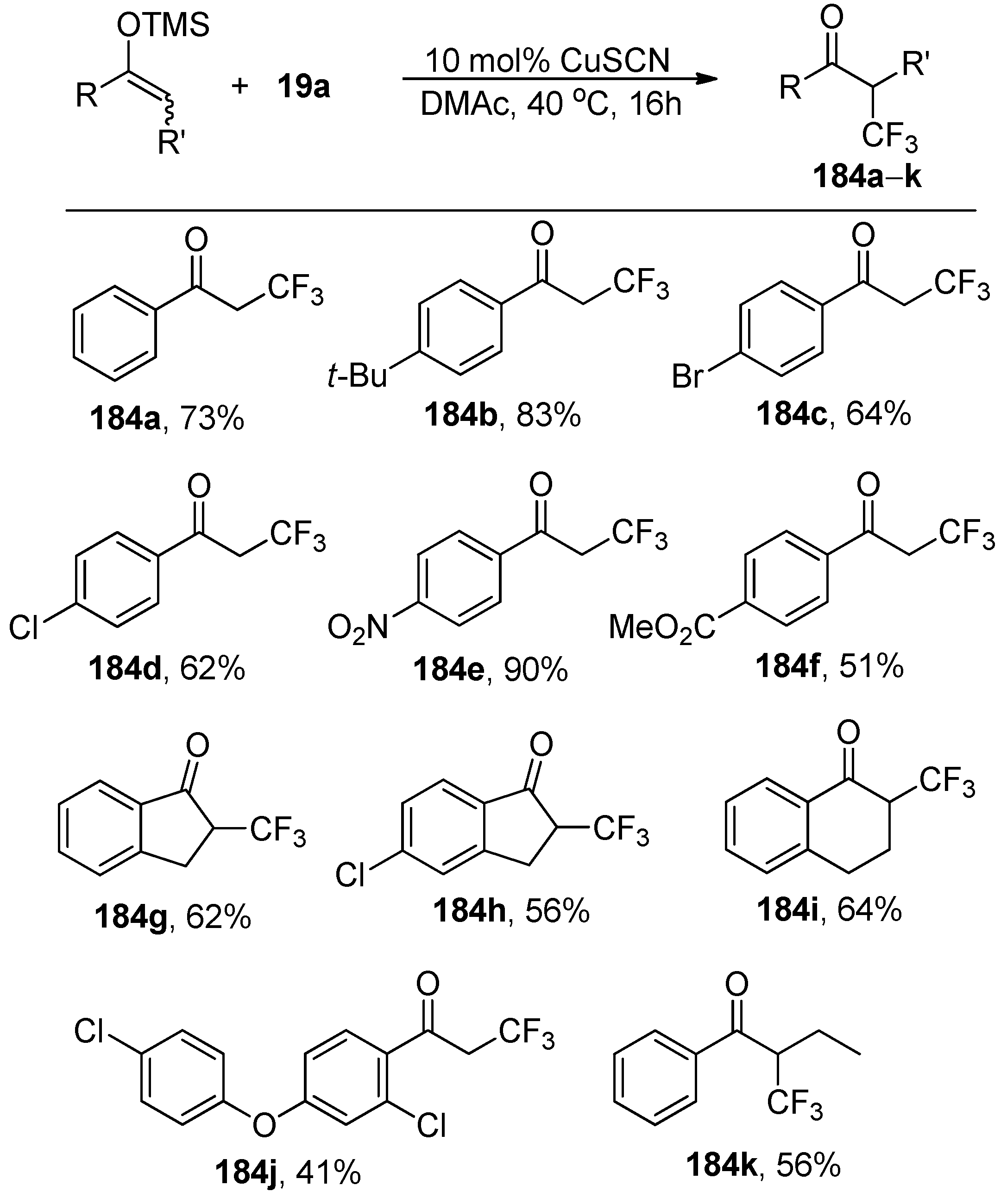
The reaction of trifluoromethyl-substituted selenium ylide
19a with various silyl enolethers in the presence of 10 mol% CuSCN afforded the α-trifluoromethyl ketones
184a–
k in high yields (
Scheme 50). The obtained results showed that
19a is a good electrophilic trifluoromethylating reagent not only for β-ketoesters but also for silyl enol ethers.
Compound
19a was also tested in the reaction with various arylboronic acids in the presence of a copper catalyst. The highest yields of
185a–
r were obtained when the reactions were carried out with the use of 1.2 equiv. of CuCl and 0.8 equiv. of Cs
2CO
3 in DMF at room temperature (
Scheme 51). It is worth noting that trifluoromethylated heteroarenes are important structural units in many agrochemicals and they can be used in the preparation of drugs.
The application of reagent
19a as a precursor of the trifluoromethyl radical was also studied. The obtained results showed that under the irradiation of blue LED light, reagent
19a reacted with electron-rich indole or pyrrole derivatives in the presence of 1.5 equiv. of DABCO to give the
m-trifluoromethylated indoles or
o-trifluoromethylated pyrroles
186a–
q in high yields (
Scheme 52). Similar reactions with the corresponding electron-rich arenes were less successful. It was found that the addition of DABCO played a key role in promoting the reaction since the absence of DABCO significantly decreased the yield to 40%.
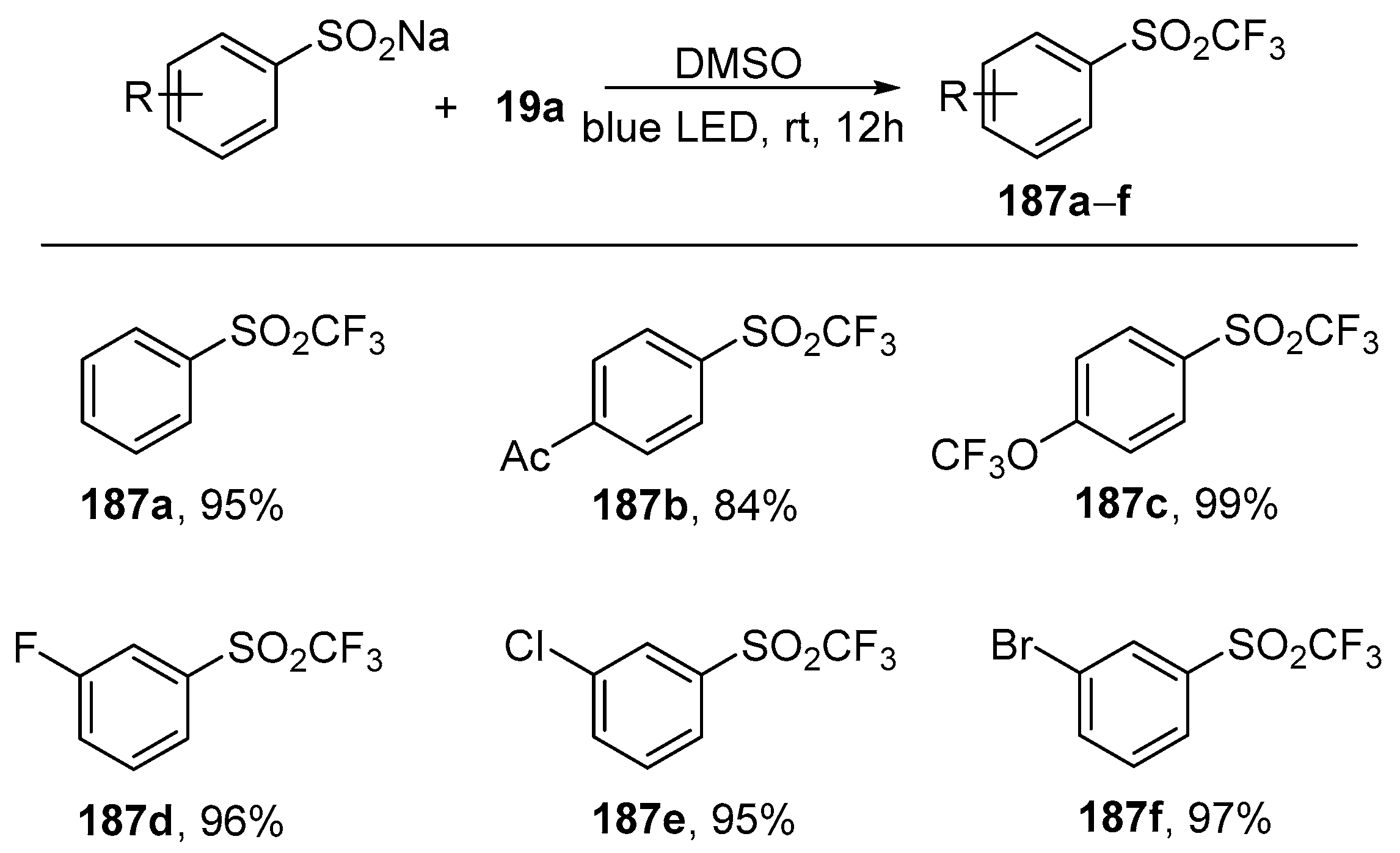
Additionally, it was found that the trifluoromethyl radical could be generated in the presence of sodium benzenesulfinate instead of an amine. Under the irradiation with visible light of
19a, the complex collapsed to generate the trifluoromethyl radical, which reacts with the sulfinate radical cation to form the trifluoromethylated sulfone derivatives. Under these conditions, various benzenesulfinate derivatives were trifluoromethylated to give trifluoromethylated sulfones
187a–
f in excellent yields (
Scheme 53).
Another group discovered that the reaction of trifluoromethyl aryl selenonium ylides with aryl halides in the presence of copper salts provided diaryl selenides in good yields rather than the expected trifluoromethylated arenes [
38].
Trifluoromethyl aryl selenonium ylides
19a–
f were suitable for the Cu-mediated arylselenylation of 4-iodo-1,1′-biphenyl (
Scheme 54). A number of selenium ylides containing different substituents on the aromatic ring were tested. In most cases, the desired products were obtained in good to high yields (54–95%).
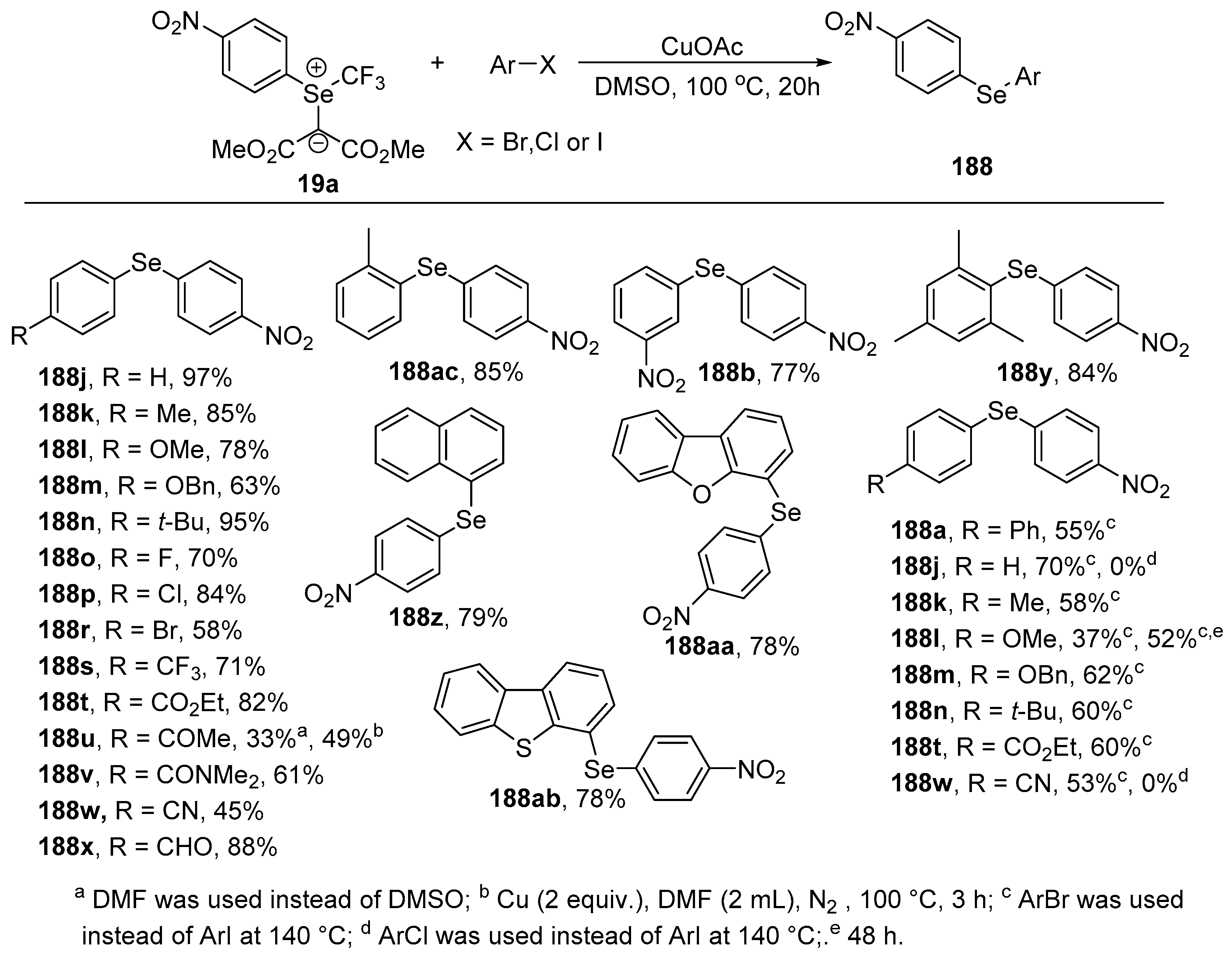
The reaction of dimethyl 2-((
p-nitrophenyl)(trifluoromethyl)-λ
4-selanylidene)malonate
19a with various aryl iodides or bromides bearing electron-donating groups, electron-withdrawing groups on the aryl rings led to the corresponding arylselenylated products
188a,
b and
j-ac in good to high yields (
Scheme 55). When the reaction was carried out with aryl chlorides, such as chlorobenzene and
p-chlorobenzonitrile, no desired products were obtained.
On the basis of the experiments and previous reports [
81,
82], a plausible reaction mechanism for the copper-mediated arylselenylation of aryl halides with trifluoromethyl aryl selenonium ylides was proposed (
Scheme 56). In the first step, the reduction of trifluoromethyl aryl selenonium ylide by copper via single electron transfer takes place and a radical intermediate (
I) is formed. Next, the intermediate (
I) rapidly decomposes to release a
•CF
3 radical and form an anion intermediate (
II). Then, α-elimination of an intermediate (
II) leads to an Ar
1Se
− anion, which combines with Cu(I) salt to generate the Ar
1SeCu complex (
III) and release tetramethyl ethene-1,1,2,2-tetracarboxylate (path a). It is also possible that intermediate (
II) undergoes protonation by traces of moisture in the solvent, which leads to dimethyl 2-(arylselanyl)malonate (
IV) (path b). Reduction of compound
IV by Cu afforded (phenylselanyl)copper (
III) and/or diaryl diselenide (
V). In the last step, Ar
1SeCu complex (
III) reacts with aryl halides via oxidative addition to give a Cu(III) complex (
VI), which undergoes reductive elimination to form diaryl selenide and regenerate Cu(I) species. It is worth noting that the
•CF
3 radical can be reduced via single electron transfer to the
−CF
3 anion, which bonds to Cu(I) and forms CuCF
3. Coupling of CuCF
3 with aryl halides via oxidative addition and reductive elimination led to the trifluoromethylated product. Under standard reaction conditions, this pathway might be very slow and may not be the main process because only trace amounts of ArCF
3 were detected.
Koengis and Jana recently reported the first study on rhodium-catalyzed generation and sigmatropic rearrangement of selenonium ylides and their synthetic applications. One of these applications allows access to important 1,1-disubstituted butadienes (
Scheme 57) [
83].
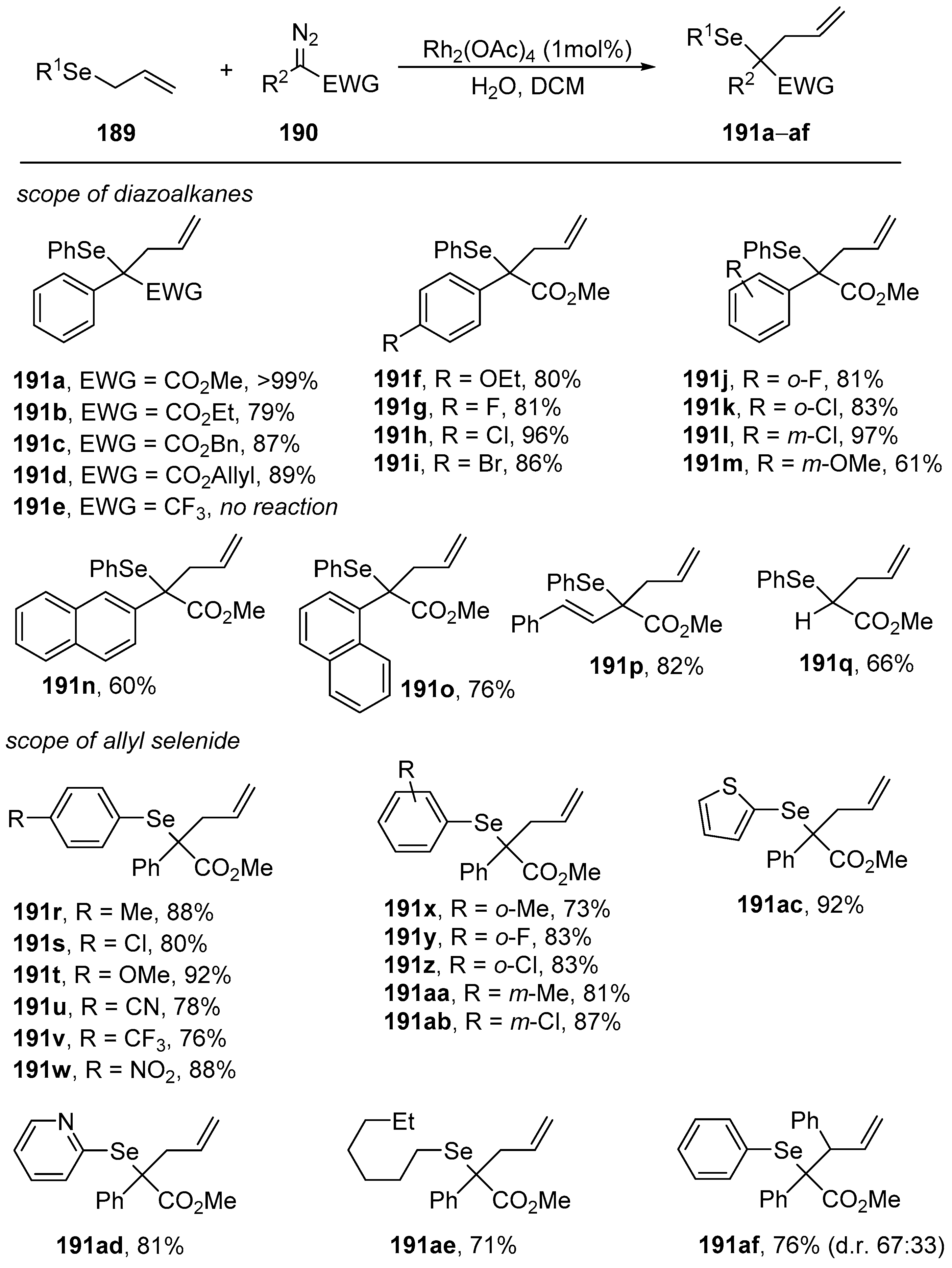
The most general application based on the generation of ylides in the reactions of allyl selenide
189 with different diazoesters
190 led to the desired homoallylic selenides
191 in excellent yields (
Scheme 58). It was found that the type of substituent in the aromatic ring had little influence on the reaction yield, as various electron-donating groups as well as halides were compatible with the reaction. Additionally, other allylic selenides with a broad range of functional groups, such as nitro, cyano, and trifluoromethyl substituents, were investigated (
Scheme 58). In this case, the seleno-Doyle–Kirmse reaction led to homoallylic selenides in good to high yields [
83]. Thiophene and pyridine heterocycles were also compatible under these reaction conditions, and no poisoning of the rhodium catalyst occurred. The corresponding heterocycle-functionalized homoallylic selenides the rearrangement product in good yield but with only low diastereoselectivity, which is comparable to the diastereoselectivity range for the classic Doyle–Kirmse reactions [
84,
85,
86].
Under optimized conditions, the reaction of propargyl selenides
192 with α-aryldiazoacetates
193 afforded the desired allenyl selenides
194a–
l in excellent yields (
Scheme 59).
The use of ethyl 2-(phenylselanyl)acetate
195a led to the α-seleno-substituted esters
196a, as products of Sommelet−Hauser or [2,3]-sigmatropic rearrangement. The reaction product corresponds to a formal
o-C-H functionalization of the aromatic substituent of
190. This transformation also took place for the cyano-substituted selenide
195b and afforded the products
o-C-H functionalization in good to high yields. In turn,
meta-substituted diazoalkanes reacted in a highly regioselective manner to form the corresponding trisubstituted phenyl acetic acid ester
196i in high yield (
Scheme 60).
In the case of benzylic selenide
197, the desired product of [
1,
2]-sigmatropic or Stevens rearrangement was obtained as a complex mixture. A change of the solvent used in the reaction to only water resulted in the rearrangement product
198a–
f in a good isolated yield. Similarly, the use of different donor−acceptor diazoesters smoothly led to the Stevens rearrangement product in a selective way (
Scheme 61).
A one-pot protocol consisting of rhodium-catalyzed generation and rearrangement of selenonium ylides and a subsequent oxidation of the formed selenides in the synthesis of olefins is shown in
Scheme 62. Thus, a two-step reaction of allyl selenide
189 with α-aryldiazoacetates
193 afforded
Z-configured 1,1-disubstituted butadiene
199 in a 56% yield. A consecutive reaction protocol led to
199 in a similar yield (60%). The stereochemical outcome of this
syn elimination was rationalized by the transition state in which the vinyl group and the phenyl ring have a
trans conformation that results in the
Z-configuration of the butadiene product (
Scheme 62c). The stereochemistry of this reaction is complementary to that of the reaction of diazoalkanes with electrophilic palladium-allyl complexes [
87]. A similar protocol was applied to the synthesis of trisubstituted olefin
200 as the product of the Stevens rearrangement of the generated ylide. The reaction led to the mixture of diastereoisomers in a 1:1 ratio (
Scheme 62b), which is a result of the similar steric hindrance in both transition states (
Scheme 62c).
In 2019, Anbarasan and coworkers described the rhodium-catalyzed rearrangement of selenonium ylides and their use in the synthesis of substituted vinylogous carbonates [
88]. The reaction of various α-selenoesters
201 with diazocompound
202 in the presence of 2 %mol of Rh
2(OAc)
4 led to the desired products
203a–
e in a good yield (62–72%) (
Scheme 63). Only in the case of ethyl α-phenylselenopropionate, the desired product
203f was not observed, most probably due to the steric effect.
Hori and coworkers reported the reactions of α-selenanaphthalene stabilized by the cyano group, i.e., l-cyano-2-methyl-2-selenanaphthalene (
20a) [
43]. Its reaction with dimethyl acetylenedicarboxylate (DMAD) in benzene at room temperature gave benzocycloheptene derivative
204 in a 61% yield, whereas the same reaction in sulfolane led to the mixture of naphthalene derivatives
205 (37%) and
204. Furthermore, the reaction was carried out in acetonitrile produced
204 (17%) and bisbenzocycloheptenyl derivative
206 (56%).
Scheme 64 shows a plausible mechanism for the formation of the benzocycloheptene
204 and the naphthalene
205.
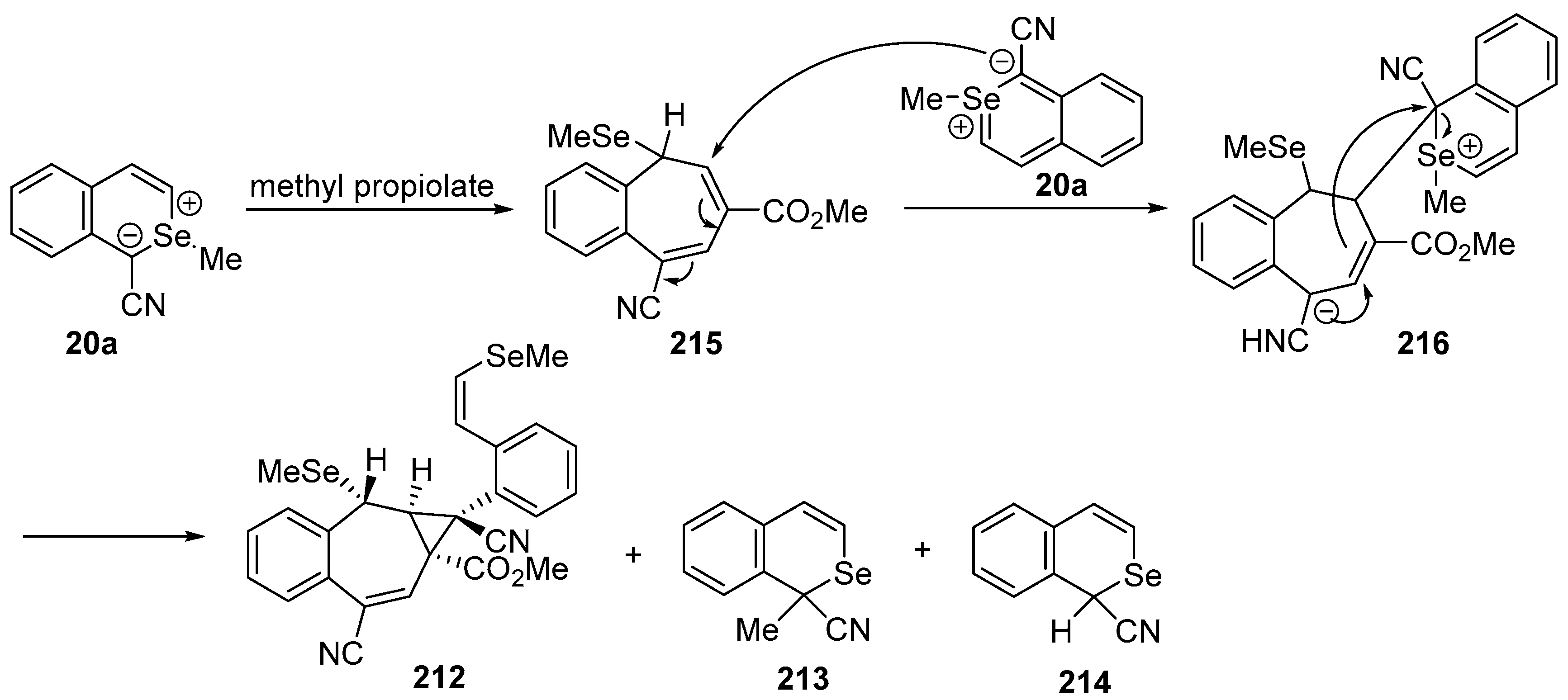
In contrast to the reaction with DMAD, reaction of
20a with methyl propiolate afforded a mixture of adduct
212, rearranged product
213, and demethylated product
214 (
Scheme 65).
Moreover, the reactions of selenanaphthalene
20a with olefins were examined (
Table 15). The obtained results showed that the reaction of
20a did not occur with styrene, dimethyl fumarate, and vinyl sulfones. However, when the reaction was conducted with vinyl sulfones, such as
trans-styryl tolyl sulfone and 3-(
p-tosyl)sulfolene, the yield of the 1,2-rearranged product
213 was reasonable.
Scheme 66 shows the reaction of selenanaphthalene
20a with monosubstituted olefins. The reaction with acrylonitrile afforded
r-l,
t-2-dicyano-l-[2-(
cis-2-methylselenovinyl)-phenyl] cyclopropane (
217) and the
r-l,
c-2-dicyano isomer (
218) in 29% and 53% yields, respectively. Methyl acrylate and methyl vinyl ketone reacted similarly with
7 to afford
cis-
trans mixtures of the cyclopropane derivatives
219,
220,
221, and
222, respectively.