Mitochondrial SLC25 Carriers: Novel Targets for Cancer Therapy
Abstract
1. Introduction
2. The SLC25 Mitochondrial Inner Membrane Family of Proteins
2.1. Characteristics of SLC25
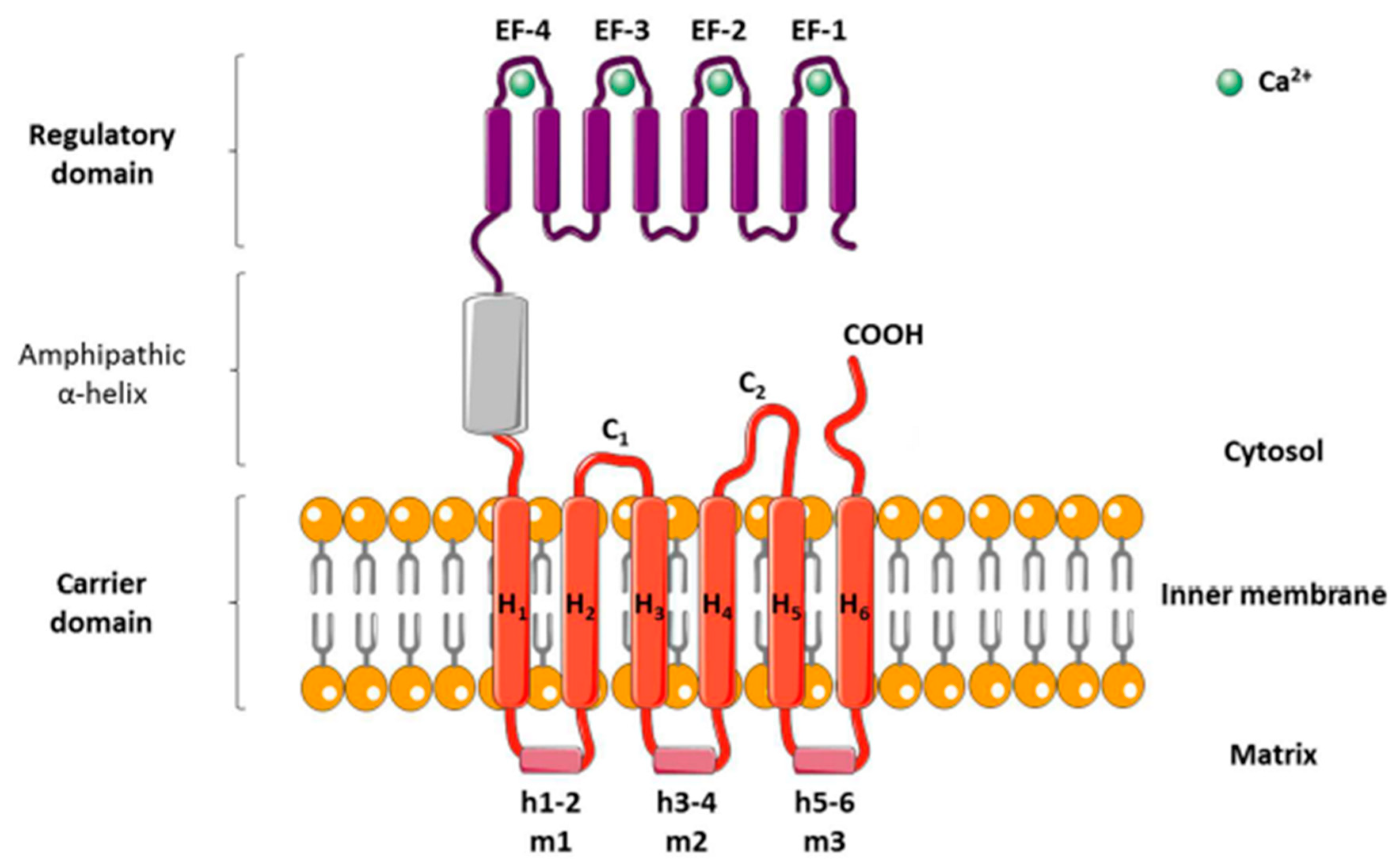
2.1.1. Role of Calcium and Magnesium in Relation to Mitochondrial SLC25 Proteins
2.1.2. Metabolic Role of Mitochondrial SLC25 Proteins
Mitochondrial Phosphate Carrier (PiC)
SLC25A23/ SLC25A24 /SLC25A25 and Homeostasis
2.2. Cellular Iron and Mitochondrial SLC25 Proteins
3. Mitochondria and the Transport of Substrates
3.1. Mitochondrial Bioenergetics and Metabolic Functions
3.2. Mitochondrial Oxidative Phosphorylation (OXPHOS) Process
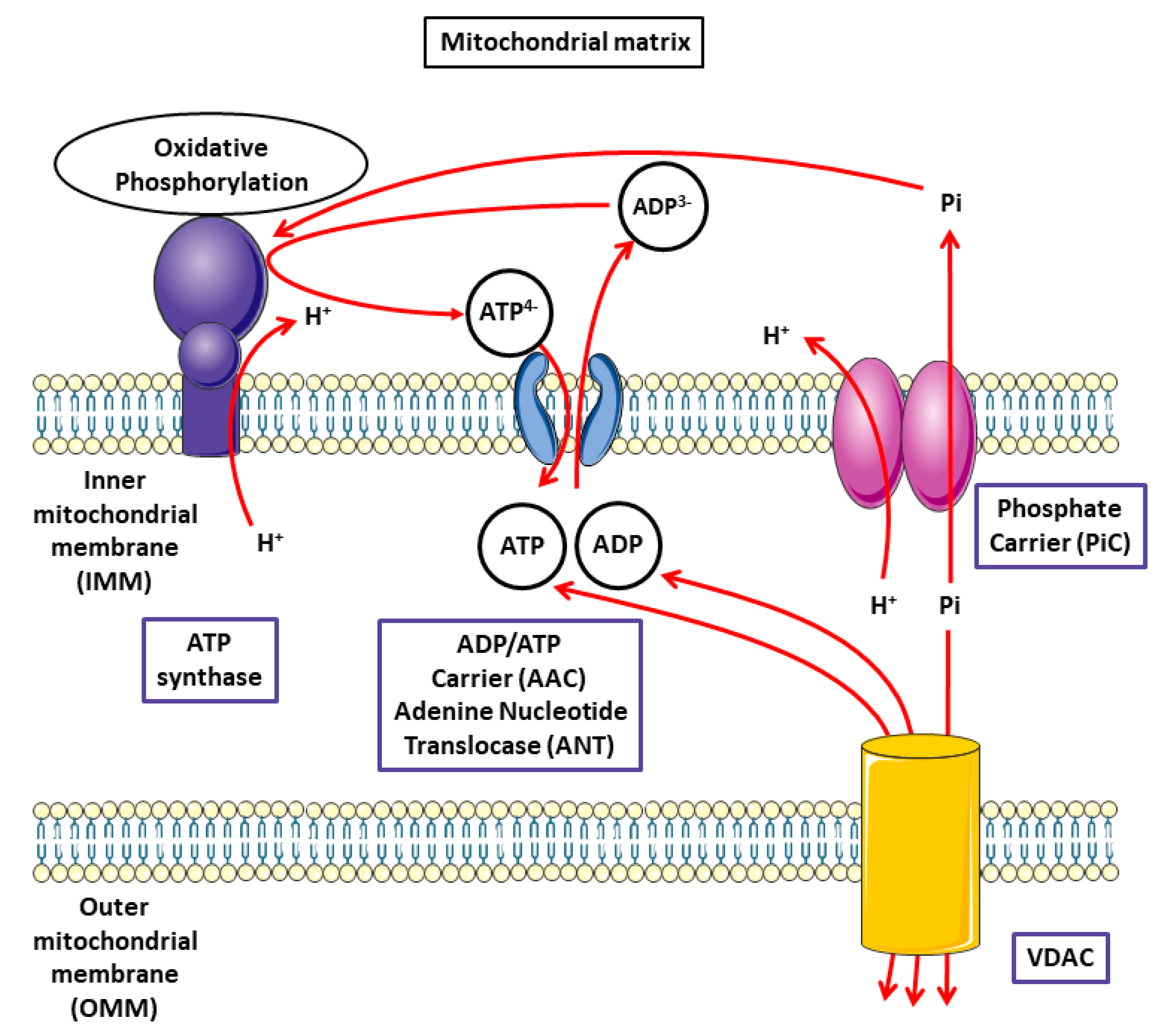
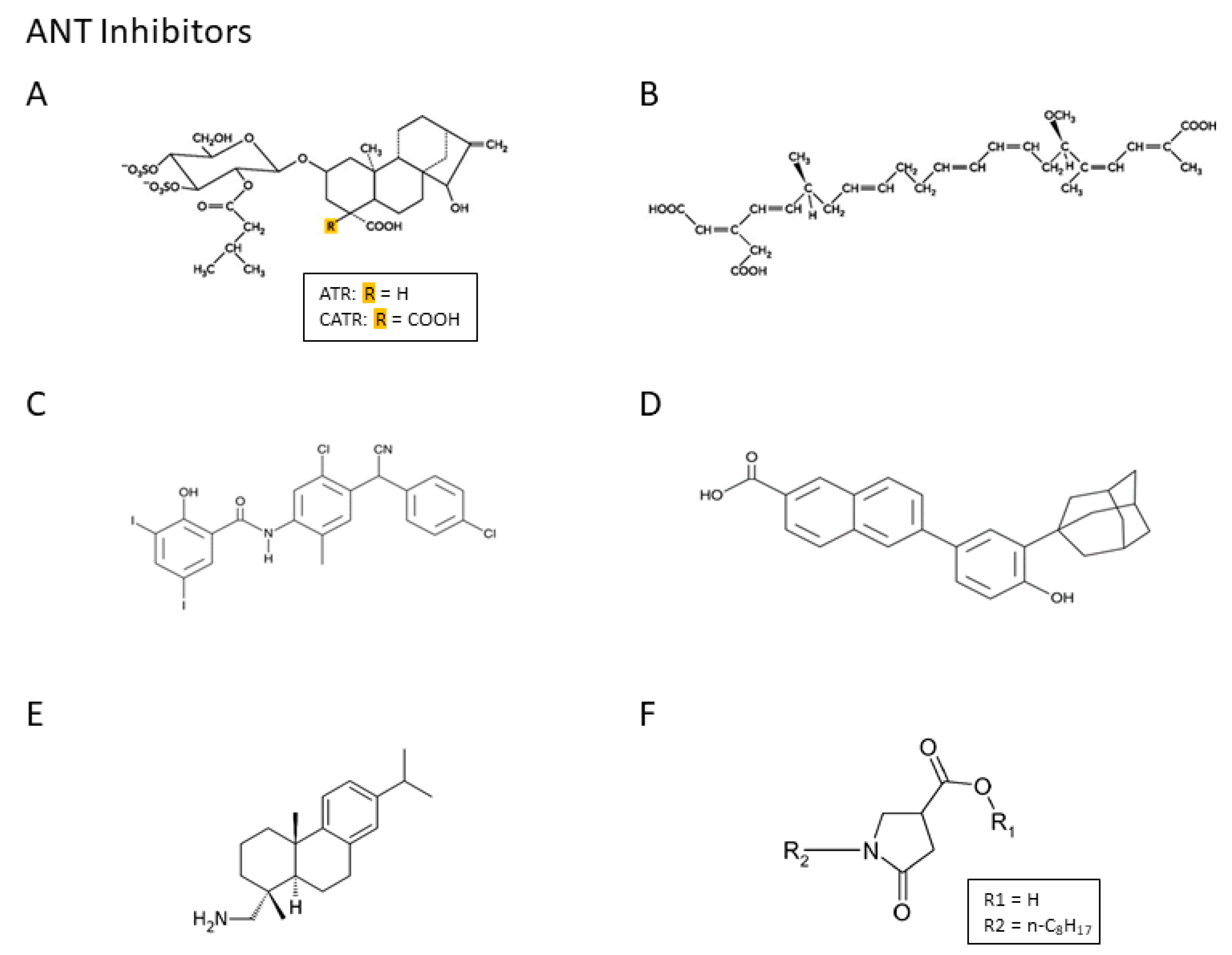
3.3. Characterization of Pi Transport in Mitochondria: Role of the PiC and Adenine Nucleotide Translocase (ANT) in Oxidative Phosphorylation
3.4. Role of Gasotransmitters on Mitochondrial Carriers
3.5. Role of ATP-Mg/Pi Carrier in Cellular Functions and Disease
3.5.1. Overview of the Regulation of ATP-Mg/Pi Carrier
3.5.2. SLC25 Family and Cancer
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Hahn, A.; Zuryn, S. The Cellular Mitochondrial Genome Landscape in Disease. Trends Cell Boil. 2019, 29, 227–240. [Google Scholar] [CrossRef]
- Perland, E.; Fredriksson, R. Classification Systems of Secondary Active Transporters. Trends Pharmacol. Sci. 2017, 38, 305–315. [Google Scholar] [CrossRef]
- Palmieri, F. The mitochondrial transporter family SLC25: Identification, properties and physiopathology. Mol. Asp. Med. 2013, 34, 465–484. [Google Scholar] [CrossRef]
- Ruprecht, J.J.; Kunji, E.R. The SLC25 Mitochondrial Carrier Family: Structure and Mechanism. Trends Biochem. Sci. 2020, 45, 244–258. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, F.; Monné, M. Discoveries, metabolic roles and diseases of mitochondrial carriers: A review. Biochim. et Biophys. Acta (BBA) Bioenerg. 2016, 1863, 2362–2378. [Google Scholar] [CrossRef] [PubMed]
- Nishi, Y.; Fujimoto, S.; Sasaki, M.; Mukai, E.; Sato, H.; Sato, Y.; Tahara, Y.; Nakamura, Y.; Inagaki, N. Role of mitochondrial phosphate carrier in metabolism–secretion coupling in rat insulinoma cell line INS-1. Biochem. J. 2011, 435, 421–430. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Satrustegui, J.; Pardo, B.; Del Arco, A. Mitochondrial Transporters as Novel Targets for Intracellular Calcium Signaling. Physiol. Rev. 2007, 87, 29–67. [Google Scholar] [CrossRef]
- Harborne, S.P.; Ruprecht, J.; Kunji, E.R. Calcium-induced conformational changes in the regulatory domain of the human mitochondrial ATP-Mg/Pi carrier. Biochim. Biophys. Acta (BBA) Bioenerg. 2015, 1847, 1245–1253. [Google Scholar] [CrossRef]
- Mayr, J.A.; A Zimmermann, F.; Horvath, R.; Schneider, H.-C.; Schoser, B.; Holinski-Feder, E.; Czermin, B.; Freisinger, P.; Sperl, W. Deficiency of the mitochondrial phosphate carrier presenting as myopathy and cardiomyopathy in a family with three affected children. Neuromuscul. Disord. 2011, 21, 803–808. [Google Scholar] [CrossRef]
- Sethi, I.; Romano, R.-A.; Glück, C.; Smalley, K.; Vojtesek, B.; Buck, M.J.; Sinha, S. A global analysis of the complex landscape of isoforms and regulatory networks of p63 in human cells and tissues. BMC Genom. 2015, 16, 584. [Google Scholar] [CrossRef]
- Calvello, R.; Cianciulli, A.; Panaro, M.A. Unusual structure and splicing pattern of the vertebrate mitochondrial solute carrier SLC25A3 gene. J. Genet. 2018, 97, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Boulet, A.; Vest, K.E.; Maynard, M.K.; Gammon, M.G.; Russell, A.C.; Mathews, A.T.; Cole, S.E.; Zhu, X.; Phillips, C.B.; Kwong, J.Q.; et al. The mammalian phosphate carrier SLC25A3 is a mitochondrial copper transporter required for cytochrome c oxidase biogenesis. J. Boil. Chem. 2017, 293, 1887–1896. [Google Scholar] [CrossRef] [PubMed]
- Baughman, J.M.; Perocchi, F.; Girgis, H.S.; Plovanich, M.; Belcher-Timme, C.A.; Sancak, Y.; Bao, X.R.; Strittmatter, L.; Goldberger, O.; Bogorad, R.L.; et al. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature 2011, 476, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Amigo, I.; Traba, J.; Gonzalez-Barroso, M.D.M.; Rueda, C.; Fernández, M.; Rial, E.; Sánchez, A.; Satrustegui, J.; Del Arco, A. Glucagon Regulation of Oxidative Phosphorylation Requires an Increase in Matrix Adenine Nucleotide Content through Ca2+ Activation of the Mitochondrial ATP-Mg/Pi Carrier SCaMC-3*. J. Boil. Chem. 2013, 288, 7791–7802. [Google Scholar] [CrossRef]
- Qiu, J.; Tan, Y.-W.; Hagenston, A.M.; Martel, M.-A.; Kneisel, N.; Skehel, P.A.; Wyllie, D.J.A.; Bading, H.; Hardingham, G.E. Mitochondrial calcium uniporter Mcu controls excitotoxicity and is transcriptionally repressed by neuroprotective nuclear calcium signals. Nat. Commun. 2013, 4, 4. [Google Scholar] [CrossRef]
- Anunciado-Koza, R.P.; Jingying, Z.; Jozef, U.; Sudip, B.; Koza, R.A.; Rogers, R.C.; Cefalu, W.T.; Mynatt, R.L.; Kozak, L.P. Inactivation of the mitochondrial carrier SLC25A25 (ATP-Mg2+/Pi transporter) reduces physical endurance and metabolic efficiency in mice. J. Biol. Chem. 2011, 286, 11659–11671. [Google Scholar] [CrossRef]
- Rueda, C.; Traba, J.; Amigo, I.; Llorente-Folch, I.; González-Sánchez, P.; Pardo, B.; Esteban, J.A.; Del Arco, A.; Satrustegui, J. Mitochondrial ATP-Mg/Pi Carrier SCaMC-3/Slc25a23 Counteracts PARP-1-Dependent Fall in Mitochondrial ATP Caused by Excitotoxic Insults in Neurons. J. Neurosci. 2015, 35, 3566–3581. [Google Scholar] [CrossRef]
- Writzl, K.; Maver, A.; Kovačič, L.; Martinez-Valero, P.; Contreras, L.; Satrustegui, J.; Castori, M.; Faivre, L.; Lapunzina, P.; van Kuilenburg, A.B.P.; et al. De Novo Mutations in SLC25A24 Cause a Disorder Characterized by Early Aging, Bone Dysplasia, Characteristic Face, and Early Demise. Am. J. Hum. Genet. 2017, 101, 844–855. [Google Scholar] [CrossRef]
- Hirota, K. An intimate crosstalk between iron homeostasis and oxygen metabolism regulated by the hypoxia-inducible factors (HIFs). Free. Radic. Boil. Med. 2019, 133, 118–129. [Google Scholar] [CrossRef]
- Gutierrez-Aguilar, M.; Baines, C.P. Physiological and pathological roles of mitochondrial SLC25 carriers. Biochem. J. 2013, 454, 371–386. [Google Scholar] [CrossRef]
- Philpott, C.C.; Ryu, M.-S. Special delivery: Distributing iron in the cytosol of mammalian cells. Front. Pharmacol. 2014, 5, 173. [Google Scholar] [CrossRef]
- Rochette, L.; Gudjoncik, A.; Guenancia, C.; Zeller, M.; Cottin, Y.; Vergely, C. The iron-regulatory hormone hepcidin: A possible therapeutic target? Pharmacol. Ther. 2015, 146, 35–52. [Google Scholar] [CrossRef]
- Colombini, M. The VDAC channel: Molecular basis for selectivity. Biochim. Biophys. Acta (BBA) Bioenerg. 2016, 1863, 2498–2502. [Google Scholar] [CrossRef] [PubMed]
- Rochette, L.; Lorin, J.; Zeller, M.; Guilland, J.-C.; Lorgis, L.; Cottin, Y.; Vergely, C. Nitric oxide synthase inhibition and oxidative stress in cardiovascular diseases: Possible therapeutic targets? Pharmacol. Ther. 2013, 140, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Pfanner, N.; Warscheid, B.; Wiedemann, N. Mitochondrial proteins: From biogenesis to functional networks. Nat. Rev. Mol. Cell Boil. 2019, 20, 267–284. [Google Scholar] [CrossRef] [PubMed]
- Quiros, P.M.; Mottis, A.; Auwerx, J. Mitonuclear communication in homeostasis and stress. Nat. Rev. Mol. Cell Boil. 2016, 17, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Rochette, L.; Zeller, M.; Cottin, Y.; Vergely, C. Diabetes, oxidative stress and therapeutic strategies. Biochim. et Biophys. Acta (BBA) Gen. Subj. 2014, 1840, 2709–2729. [Google Scholar] [CrossRef] [PubMed]
- E Formosa, L.; Ryan, M.T. Mitochondrial OXPHOS complex assembly lines. Nature 2018, 20, 511–513. [Google Scholar] [CrossRef]
- Zhao, R.; Jiang, S.; Zhang, L.; Yu, Z. Mitochondrial electron transport chain, ROS generation and uncoupling (Review). Int. J. Mol. Med. 2019, 44, 3–15. [Google Scholar] [CrossRef]
- Iacobazzi, V.; Infantino, V.; Costanzo, P.; Izzo, P.; Palmieri, F. Functional analysis of the promoter of the mitochondrial phosphate carrier human gene: Identification of activator and repressor elements and their transcription factors. Biochem. J. 2005, 391, 613–621. [Google Scholar] [CrossRef]
- Kwong, J.Q.; Davis, J.; Baines, C.P.; A Sargent, M.; Karch, J.; Wang, X.; Huang, T.; Molkentin, J.D. Genetic deletion of the mitochondrial phosphate carrier desensitizes the mitochondrial permeability transition pore and causes cardiomyopathy. Cell Death Differ. 2014, 21, 1209–1217. [Google Scholar] [CrossRef] [PubMed]
- Mayr, J.A.; Merkel, O.; Kohlwein, S.D.; Gebhardt, B.R.; Böhles, H.; Fötschl, U.; Koch, J.; Jaksch, M.; Lochmüller, H.; Horvath, R.; et al. Mitochondrial Phosphate–Carrier Deficiency: A Novel Disorder of Oxidative Phosphorylation. Am. J. Hum. Genet. 2007, 80, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Clémençon, B.; Babot, M.; Trézéguet, V. The mitochondrial ADP/ATP carrier (SLC25 family): Pathological implications of its dysfunction. Mol. Asp. Med. 2013, 34, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Willis, W.; Miranda-Grandjean, D.; Hudgens, J.; Willis, E.; Finlayson, J.; De Filippis, E.; Bustos, R.Z.; Langlais, P.; Mielke, C.; Mandarino, L.J. Dominant and sensitive control of oxidative flux by the ATP-ADP carrier in human skeletal muscle mitochondria: Effect of lysine acetylation. Arch. Biochem. Biophys. 2018, 647, 93–103. [Google Scholar] [CrossRef]
- Zhang, Y.; Tian, D.; Matsuyama, H.; Hamazaki, T.; Shiratsuchi, T.; Terada, N.; Hook, D.J.; A Walters, M.; Georg, G.I.; Hawkinson, J.E. Human Adenine Nucleotide Translocase (ANT) Modulators Identified by High-Throughput Screening of Transgenic Yeast. J. Biomol. Screen. 2016, 21, 381–390. [Google Scholar] [CrossRef][Green Version]
- Allouche, M.; Pertuiset, C.; Robert, J.-L.; Martel, C.; Veneziano, R.; Henry, C.; El Dein, O.S.; Saint, N.; Brenner, C.; Chopineau, J.C. ANT-VDAC1 interaction is direct and depends on ANT isoform conformation in vitro. Biochem. Biophys. Res. Commun. 2012, 429, 12–17. [Google Scholar] [CrossRef]
- Yang, M.; Xu, Y.; Heisner, J.S.; Sun, J.; Stowe, D.; Kwok, W.-M.; Camara, A.K. Peroxynitrite nitrates adenine nucleotide translocase and voltage-dependent anion channel 1 and alters their interactions and association with hexokinase II in mitochondria. Mitochondrion 2019, 46, 380–392. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.J. Adenine Nucleotide Translocase, Mitochondrial Stress, and Degenerative Cell Death. Oxidative Med. Cell. Longev. 2013, 2013, 1–10. [Google Scholar] [CrossRef]
- Leung, A.W.C.; Varanyuwatana, P.; Halestrap, A.P. The Mitochondrial Phosphate Carrier Interacts with Cyclophilin D and May Play a Key Role in the Permeability Transition. J. Boil. Chem. 2008, 283, 26312–26323. [Google Scholar] [CrossRef]
- Varanyuwatana, P.; Halestrap, A.P. The roles of phosphate and the phosphate carrier in the mitochondrial permeability transition pore. Mitochondrion 2011, 12, 120–125. [Google Scholar] [CrossRef]
- Burke, M.A.; Cook, S.A.; Seidman, J.G.; Seidman, C.E. Clinical and Mechanistic Insights into the Genetics of Cardiomyopathy. J. Am. Coll. Cardiol. 2016, 68, 2871–2886. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, C.; Nussbaum, B.; Calzia, E.; Radermacher, P.; Wepler, M. Gaseous Mediators and Mitochondrial Function: The Future of Pharmacologically Induced Suspended Animation? Front. Physiol. 2017, 8, 691. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Shen, Z.; Luo, S.; Guo, W.; Zhu, Y.Z. The Cardioprotective Effects of Hydrogen Sulfide in Heart Diseases: From Molecular Mechanisms to Therapeutic Potential. Oxidative Med. Cell. Longev. 2015, 2015, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Gorgoglione, R.; Porcelli, V.; Santoro, A.; Daddabbo, L.; Vozza, A.; Monné, M.; Di Noia, M.A.; Palmieri, L.; Fiermonte, G.; Palmieri, F. The human uncoupling proteins 5 and 6 (UCP5/SLC25A14 and UCP6/SLC25A30) transport sulfur oxyanions, phosphate and dicarboxylates. Biochim. Biophys. Acta (BBA) Bioenerg. 2019, 1860, 724–733. [Google Scholar] [CrossRef] [PubMed]
- Rochette, L.; Zeller, M.; Cottin, Y.; Vergely, C. Redox Functions of Heme Oxygenase-1 and Biliverdin Reductase in Diabetes. Trends Endocrinol. Metab. 2018, 29, 74–85. [Google Scholar] [CrossRef]
- Long, R.; Salouage, I.; Berdeaux, A.; Motterlini, R.; Morin, D. CORM-3, a water soluble CO-releasing molecule, uncouples mitochondrial respiration via interaction with the phosphate carrier. Biochim. et Biophys. Acta (BBA) Gen. Subj. 2014, 1837, 201–209. [Google Scholar] [CrossRef]
- Fiermonte, G.; Dolce, V.; Palmieri, F. Expression inEscherichia coli, Functional Characterization, and Tissue Distribution of Isoforms A and B of the Phosphate Carrier from Bovine Mitochondria. J. Boil. Chem. 1998, 273, 22782–22787. [Google Scholar] [CrossRef]
- Fiermonte, G.; De Leonardis, F.; Todisco, S.; Palmieri, F.; Lasorsa, F.; Palmieri, F. Identification of the Mitochondrial ATP-Mg/PiTransporter. J. Boil. Chem. 2004, 279, 30722–30730. [Google Scholar] [CrossRef]
- Ruprecht, J.; Kunji, E.R. Structural changes in the transport cycle of the mitochondrial ADP/ATP carrier. Curr. Opin. Struct. Boil. 2019, 57, 135–144. [Google Scholar] [CrossRef]
- Dörner, A.; Giessen, S.; Gaub, R.; Siestrup, H.G.; Schwimmbeck, P.L.; Hetzer, R.; Poller, W.; Schultheiss, H.-P. An isoform shift in the cardiac adenine nucleotide translocase expression alters the kinetic properties of the carrier in dilated cardiomyopathy. Eur. J. Hear. Fail. 2005, 8, 81–89. [Google Scholar] [CrossRef]
- Fernandez, H.R.; Gadre, S.M.; Tan, M.; Graham, G.; Mosaoa, R.; Ongkeko, M.S.; Kim, K.A.; Riggins, R.B.; Parasido, E.; Petrini, I.; et al. The mitochondrial citrate carrier, SLC25A1, drives stemness and therapy resistance in non-small cell lung cancer. Cell Death Differ. 2018, 25, 1239–1258. [Google Scholar] [CrossRef] [PubMed]
- Catalina-Rodriguez, O.; Kolukula, V.K.; Tomita, Y.; Preet, A.; Palmieri, F.; Wellstein, A.; Byers, S.; Giaccia, A.J.; Glasgow, E.; Albanese, C.; et al. The mitochondrial citrate transporter, CIC, is essential for mitochondrial homeostasis. Oncotarget 2012, 3, 1220–1235. [Google Scholar] [CrossRef] [PubMed]
- Kolukula, V.K.; Sahu, G.; Wellstein, A.; Rodriguez, O.C.; Preet, A.; Iacobazzi, V.; D’Orazi, G.; Albanese, C.; Palmieri, F.; Avantaggiati, M.L. SLC25A1, or CIC, is a novel transcriptional target of mutant p53 and a negative tumor prognostic marker. Oncotarget 2014, 5, 1212–1225. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Paredes, J.; Krishnan, S.; Curbo, S.; Karlsson, A. The mitochondrial carrier SLC25A10 regulates cancer cell growth. Oncotarget 2015, 6, 9271–9283. [Google Scholar] [CrossRef] [PubMed]
- Jia, D.; Park, J.H.; Jung, K.H.; Levine, H.; Kaipparettu, B. Elucidating the Metabolic Plasticity of Cancer: Mitochondrial Reprogramming and Hybrid Metabolic States. Cells 2018, 7, 21. [Google Scholar] [CrossRef]
- Ivanova, D.; Bakalova, R.; Lazarova, D.; Gadjeva, V.; Zhelev, Z. The impact of reactive oxygen species on anticancer therapeutic strategies. Adv. Clin. Exp. Med. 2014, 22, 899–908. [Google Scholar]
- Fonken, L.; Nelson, R.J. The Effects of Light at Night on Circadian Clocks and Metabolism. Endocr. Rev. 2014, 35, 648–670. [Google Scholar] [CrossRef]
- Takahashi, J.S. Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet. 2016, 18, 164–179. [Google Scholar] [CrossRef]
- Cai, T.; Hua, B.; Luo, D.; Xu, L.; Cheng, Q.; Yuan, G.; Yan, Z.; Sun, N.; Hua, L.; Lu, C. The circadian protein CLOCK regulates cell metabolism via the mitochondrial carrier SLC25A10. Biochim. Biophys. Acta (BBA) Bioenerg. 2019, 1866, 1310–1321. [Google Scholar] [CrossRef]
- Cho, H.; Zhao, X.; Hatori, M.; Yu, R.T.; Barish, G.D.; Lam, M.T.; Chong, L.-W.; DiTacchio, L.; Atkins, A.R.; Glass, C.K.; et al. Regulation of circadian behaviour and metabolism by REV-ERB-α and REV-ERB-β. Nature 2012, 485, 123–127. [Google Scholar] [CrossRef]
- Kojetin, U.J.; Burris, T.P. REV-ERB and ROR nuclear receptors as drug targets. Nat. Rev. Drug Discov. 2014, 13, 197–216. [Google Scholar] [CrossRef] [PubMed]
- Puig, L.S.; Valera-Alberni, M.; Cantó, C.; Pillon, N. Circadian Rhythms and Mitochondria: Connecting the Dots. Front. Genet. 2018, 9, 452. [Google Scholar] [CrossRef] [PubMed]
- Monné, M.; Vozza, A.; Lasorsa, F.; Porcelli, V.; Palmieri, F. Mitochondrial Carriers for Aspartate, Glutamate and Other Amino Acids: A Review. Int. J. Mol. Sci. 2019, 20, 4456. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, L.B.; Luengo, A.; Danai, L.V.; Bush, L.N.; Diehl, F.F.; Hosios, A.M.; Lau, A.N.; Elmiligy, S.; Malstrom, S.; Lewis, C.A.; et al. Aspartate is an endogenous metabolic limitation for tumour growth. Nature 2018, 20, 782–788. [Google Scholar] [CrossRef] [PubMed]
- Infantino, V.; Dituri, F.; Convertini, P.; Santarsiero, A.; Palmieri, F.; Todisco, S.; Mancarella, S.; Giannelli, G.; Iacobazzi, V. Epigenetic upregulation and functional role of the mitochondrial aspartate/glutamate carrier isoform 1 in hepatocellular carcinoma. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2019, 1865, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Kwok, K.H.-H.; Ho, P.W.-L.; Chu, A.C.-Y.; Ho, J.W.-M.; Liu, H.-F.; Yiu, D.C.-W.; Chan, K.H.; Kung, M.H.-W.; Ramsden, D.B.; Ho, S.-L. Mitochondrial UCP5 is neuroprotective by preserving mitochondrial membrane potential, ATP levels, and reducing oxidative stress in MPP+ and dopamine toxicity. Free. Radic. Boil. Med. 2010, 49, 1023–1035. [Google Scholar] [CrossRef]
- Tina, E.; Lindqvist, B.M.; Gabrielson, M.; Lubovac-Pilav, Z.; Wegman, P.; Wingren, S. The mitochondrial transporter SLC25A43 is frequently deleted and may influence cell proliferation in HER2-positive breast tumors. BMC Cancer 2012, 12, 350. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Zhang, P.-Y. Mitochondria targeting nano agents in cancer therapeutics. Oncol. Lett. 2016, 12, 4887–4890. [Google Scholar] [CrossRef]
- Rochette, L.; Guenancia, C.; Gudjoncik, A.; Hachet, O.; Zeller, M.; Cottin, Y.; Vergely, C. Anthracyclines/trastuzumab: New aspects of cardiotoxicity and molecular mechanisms. Trends Pharmacol. Sci. 2015, 36, 326–348. [Google Scholar] [CrossRef]
- Hlouschek, J.; Ritter, V.; Wirsdörfer, F.; Klein, D.; Jendrossek, V.; Matschke, J.; Julian, H.; Violetta, R.; Florian, W.; Diana, K.; et al. Targeting SLC25A10 alleviates improved antioxidant capacity and associated radioresistance of cancer cells induced by chronic-cycling hypoxia. Cancer Lett. 2018, 439, 24–38. [Google Scholar] [CrossRef]
- Poncet, D.; Pauleau, A.-L.; Szabadkai, G.; Vozza, A.; Scholz, S.R.; Le Bras, M.; Brière, J.-J.; Jalil, A.; Le Moigne, R.; Brenner, C.; et al. Cytopathic effects of the cytomegalovirus-encoded apoptosis inhibitory protein vMIA. J. Cell Boil. 2006, 174, 985–996. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zhou, X.; Curbo, S.; Karlsson, A. Metformin downregulates the mitochondrial carrier SLC25A10 in a glucose dependent manner. Biochem. Pharmacol. 2018, 156, 444–450. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rochette, L.; Meloux, A.; Zeller, M.; Malka, G.; Cottin, Y.; Vergely, C. Mitochondrial SLC25 Carriers: Novel Targets for Cancer Therapy. Molecules 2020, 25, 2417. https://doi.org/10.3390/molecules25102417
Rochette L, Meloux A, Zeller M, Malka G, Cottin Y, Vergely C. Mitochondrial SLC25 Carriers: Novel Targets for Cancer Therapy. Molecules. 2020; 25(10):2417. https://doi.org/10.3390/molecules25102417
Chicago/Turabian StyleRochette, Luc, Alexandre Meloux, Marianne Zeller, Gabriel Malka, Yves Cottin, and Catherine Vergely. 2020. "Mitochondrial SLC25 Carriers: Novel Targets for Cancer Therapy" Molecules 25, no. 10: 2417. https://doi.org/10.3390/molecules25102417
APA StyleRochette, L., Meloux, A., Zeller, M., Malka, G., Cottin, Y., & Vergely, C. (2020). Mitochondrial SLC25 Carriers: Novel Targets for Cancer Therapy. Molecules, 25(10), 2417. https://doi.org/10.3390/molecules25102417




