Antifungal Activity of Gentamicin B1 against Systemic Plant Mycoses
Abstract
1. Introduction
2. Results
2.1. Antifusarial Effect of Gentamicins and Aminoglycoside Derivatives
2.2. Antifungal Agents against Plant Pathogenic Fungi
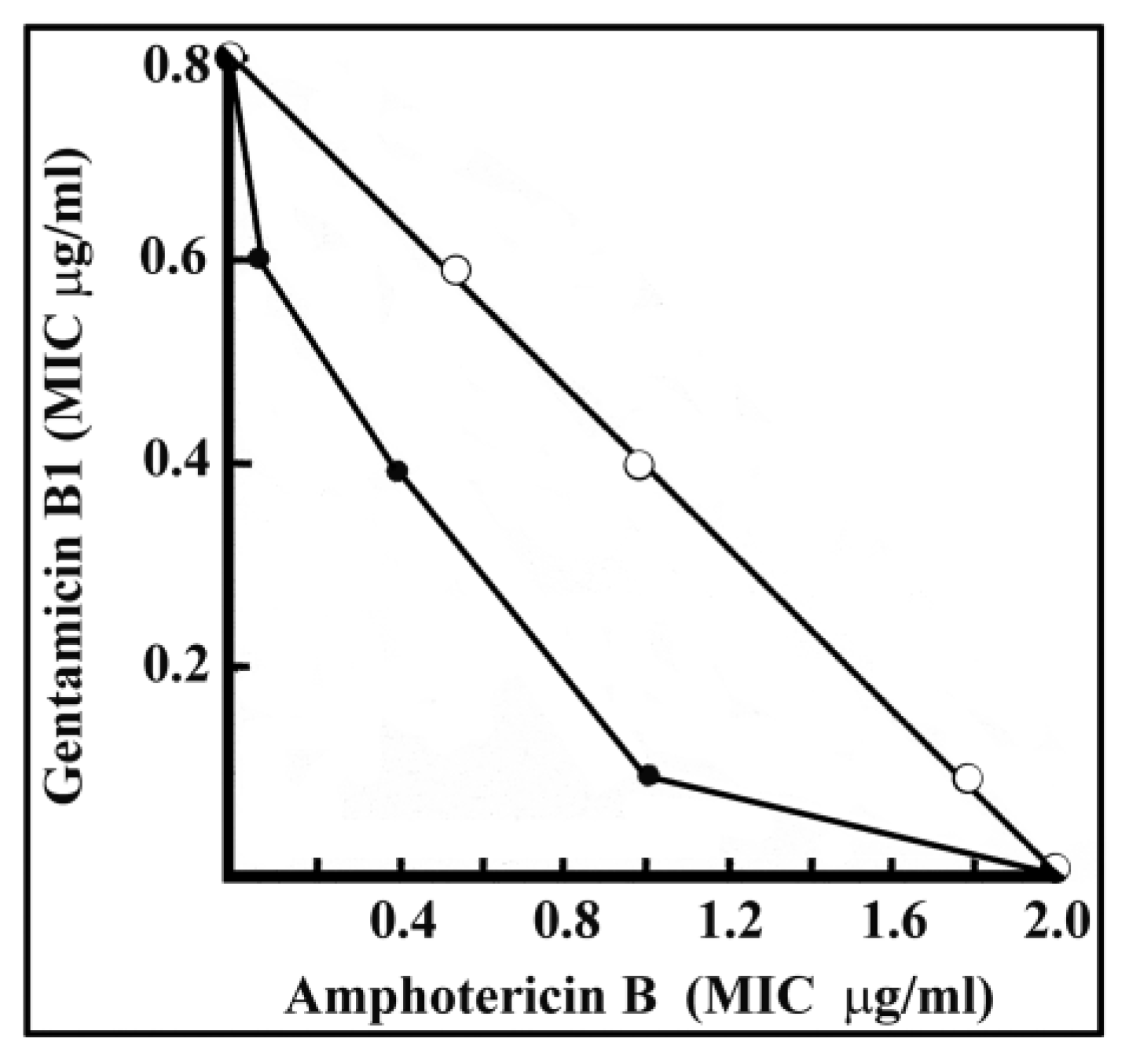
2.3. Synergy between Amphotericin B and Gentamicin B1 against C. Neoformans
- (a)
- 1 µg/mL amphotericin B and 0.1 µg/mL gentamicin B1,
- (b)
- 0.4 µg/mL amphotericin B and 0.4 µg/mL gentamicin B1, or
- (c)
- 0.1 µg/mL amphotericin B + 0.6 µg/mL gentamicin B1,
2.4. Suppression of Antifungal Activity
2.5. In Vivo Antifungal Effect
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Sabouraud Broth
4.3. Inocula
4.4. In Vitro Tests
4.5. Animal Experiments
Funding
Acknowledgments
Conflicts of Interest
References
- Nucci, F.; Nouér, S.A.; Capone, D.; Anaissie, E.; Nucci, M. Fusariosis. Semin. Respir. Crit. Care Med. 2015, 36, 706–714. [Google Scholar] [CrossRef]
- Cramer, R.A.; Rivera, A.; Hohl, T.M. Immune responses against Aspergillusfumigatus: What have we learned? Curr. Opin. Infect. Dis. 2011, 24, 315–322. [Google Scholar] [CrossRef]
- Rafferty, P.; Biggs, B.–A.; Crompton, G.K.; Grant, I.W. What happens to patients with pulmonary aspergilloma? Analysis of 23 cases. Thorax 1983, 38, 579–583. [Google Scholar] [CrossRef]
- Morgan, J.; Wannemuehler, K.; Marr, K.A.; Hadley, S.; Kontoyiannis, D.P.; Walsh, T.J.; Fridkin, S.K.; Pappas, P.G.; Warnock, D.W. Incidence of invasive aspergillosis following hematopoietic stem cell and solid organ transplantation: Interim results of a prospective multicenter surveillance program. Med. Mycol. 2005, 43, 49–58. [Google Scholar] [CrossRef]
- Banfalvi, G. Retrospective evaluation of in vitro effect of gentamicin B1 against Fusarium species. Appl. Microbiol. Biotechnol. 2018, 102, 10353–10359. [Google Scholar] [CrossRef]
- Kaufmann, C.A. Pulmonary and invasive fungal infections. Semin. Respir. Crit. Care Med. 2015, 36, 639–640. [Google Scholar] [CrossRef]
- Pham, C.D.; Ahn, S.; Turner, L.A.; Wohrle, R.; Lockhart, S.R. Development and validation of benomyl birdseed agar for the isolation of Cryptococcus neoformans and Cryptococcus gattii from environmental samples. Med. Mycol. 2014, 52, 417–421. [Google Scholar] [CrossRef]
- Chang, C.-W.T.; Takemoto, J.Y. Antifungal amphiphilic aminoglycosides. MedChemComm 2014, 5, 1048–1057. [Google Scholar] [CrossRef]
- Fosso, M.Y.; Shrestha, S.K.; Green, K.D.; Garneau-Tsodikova, S. Synthesis and Bioactivities of Kanamycin B-Derived Cationic Amphiphiles. J. Med. Chem. 2015, 58, 9124–9132. [Google Scholar] [CrossRef]
- Kawakami, H.; Inuzuka, H.; Hori, N.; Takahashi, N.; Ishida, K.; Mochizuki, K.; Ohkusu, K.; Muraosa, Y.; Watanabe, A.; Kamei, K. Inhibitory effects of antimicrobial agents againstFusariumspecies. Med. Mycol. 2015, 53, 603–611. [Google Scholar] [CrossRef]
- Zhang, W.; Xue, B.; Li, M.; Mu, Y.; Chen, Z.; Li, J.; Shan, A. Screening a strain of Aspergillusniger and optimization of fermentation conditions for degradation of aflatoxin B1. Toxins 2014, 6, 3157–3172. [Google Scholar] [CrossRef]
- Almeida, E.J.; Corso, C.R. Comparative study of toxicity of azo dye Procion Red MX-5B following biosorption and biodegradation treatments with the fungi Aspergillusniger and Aspergillusterreus. Chemosphere 2014, 112, 317–322. [Google Scholar] [CrossRef]
- Liu, Y.; Chang, H.; Li, Z.; Zhang, C.; Feng, Y.; Cheng, D. Gentamicin removal in submerged fermentation using the novel fungal strain Aspergillusterreus FZC3. Sci. Rep. 2016, 6, 35856. [Google Scholar] [CrossRef]
- Bidou, L.; Bugaud, O.; Belakhov, V.; Baasov, T.; Namy, O. Characterization of new-generation aminoglycoside promoting premature termination codon readthrough in cancer cells. RNA Boil. 2017, 14, 378–388. [Google Scholar] [CrossRef]
- Baradaran-Heravi, A.; Balgi, A.D.; Zimmerman, C.; Choi, K.; Shidmoossavee, F.S.; Tan, J.S.; Bergeaud, C.; Krause, A.; Flibotte, S.; Shimizu, Y.; et al. Novel small molecules potentiate premature termination codon readthrough by aminoglycosides. Nucleic Acids Res. 2016, 44, 6583–6598. [Google Scholar] [CrossRef]
- Baradaran-Heravi, A.; Niesser, J.; Balgi, A.D.; Choi, K.; Zimmerman, C.; South, A.P.; Anderson, H.J.; Strynadka, N.C.; Bally, M.B.; Roberge, M. Gentamicin B1 is a minor gentamicin component with major nonsense mutation suppression activity. Proc. Natl. Acad. Sci. USA 2017, 114, 3479–3484. [Google Scholar] [CrossRef]
- Summerbell, R.C. Aspergillus, Fusarium, Sporothrix, Piedraia, and their relatives. In Pathogenic Fungi in Humans and Animals; Howard, D.H., Ed.; Marcel Dekker, Inc.: New York, NY, USA, 2003; pp. 237–498. [Google Scholar]
- Buchanan, K.L.; Murphy, J.W. What makes Cryptococcus neoformans a pathogen? Emerg. Infect. Dis. 1998, 4, 71–83. [Google Scholar] [CrossRef]
- Berdicevsky, I.; Grossowicz, N. Reversal by calcium ion of the growth inhibition of Debaromycesnicotianae caused by antifungal polyene antibiotics. Antimicrobial. Agents Chemother. 1972, 2, 1–7. [Google Scholar] [CrossRef][Green Version]
- Davis, S.D.; Ianetta, A. Antagonistic effect of calcium in serum on the activ.ity of tobramycin against Pseudomonas. Antimicrob. Agents Chemother. 1972, 1, 466–469. [Google Scholar] [CrossRef]
- Raab, W.P.; Windisch, J. Antagonism of neomycin by heparin. Further observations on the anaphylactoid activity of neomycin. Arzneimittelforschung 1973, 23, 1326–1328. [Google Scholar]
- Subedi, Y.P.; AlFindee, M.N.; Takemoto, J.Y.; Chang, C.W. Antifungal amphiphilickanamycins: New life for an old drug. Med. Chem. Comm. 2018, 9, 909–919. [Google Scholar] [CrossRef] [PubMed]
- Subedi, Y.P.; Pandey, U.; Alfindee, M.N.; Montgomery, H.; Roberts, P.; Wight, J.; Nichols, G.; Grilley, M.; Takemoto, J.Y.; Chang, C.–W.T. Scalable and cost-effective tosylation-mediated synthesis of antifungal and fungal diagnostic 6″-modified amphiphilickanamycins. Eur. J. Med. Chem. 2019, 182. [Google Scholar] [CrossRef] [PubMed]
- Alfindee, M.N.; Subedi, Y.P.; Grilley, M.M.; Takemoto, J.Y.; Chang, C.-W.T. Antifungal activities of 4′’, 6′’-disubstituted amphiphilic kanamycins. Molecules 2019, 24, 1882. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-W.T.; Fosso, M.; Kawasaki, Y.; Shrestha, S.; Bensaci, M.F.; Wang, J.; Evans, C.K.; Takemoto, J.Y. Antibacterial to antifungal conversion of neamine aminoglycosides through alkyl modification. Strategy for reviving old drugs into agrofungicides. J. Antibiot. 2010, 63, 667–672. [Google Scholar] [CrossRef][Green Version]
- Mingeot-Leclercq, M.-P.; Glupczynski, Y.; Tulkens, P.M. Aminoglycosides: Activity and Resistance. Antimicrob. Agents Chemother. 1999, 43, 727–737. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, M.J.; Luedemann, G.M.; Oden, E.M.; Wagman, G.H.; Rosselet, J.P.; Marquez, J.A.; Coniglio, C.T.; Charney, W.; Herzog, H.L.; Black, J. Gentamicin,1a New Antibiotic Complex from Micromonospora. J. Med. Chem. 1963, 6, 463–464. [Google Scholar] [CrossRef] [PubMed]
- Gadó, I.; Bérdy, J.; Koczka, I.; Horváth, I.; Járay, M.; Zlatos, G. Gentamicin Antibiotics. Hungarian Patent1975, 30 December 1973. [Google Scholar]
- Bérdy, J.; Pauncz, J.K.; Vajna, Z.M.; Horváth, G.; Gyimesi, J.; Koczka, I. Metabolites of gentamicin-producing Micromonospora species I. Isolation and identification of metabolites. J. Antibiot. 1977, 30, 945–954. [Google Scholar] [CrossRef]
Data and Materials Availability: All data needed to evaluate the conclusions are present in the paper. Additional data related to this paper may be requested from the author. |
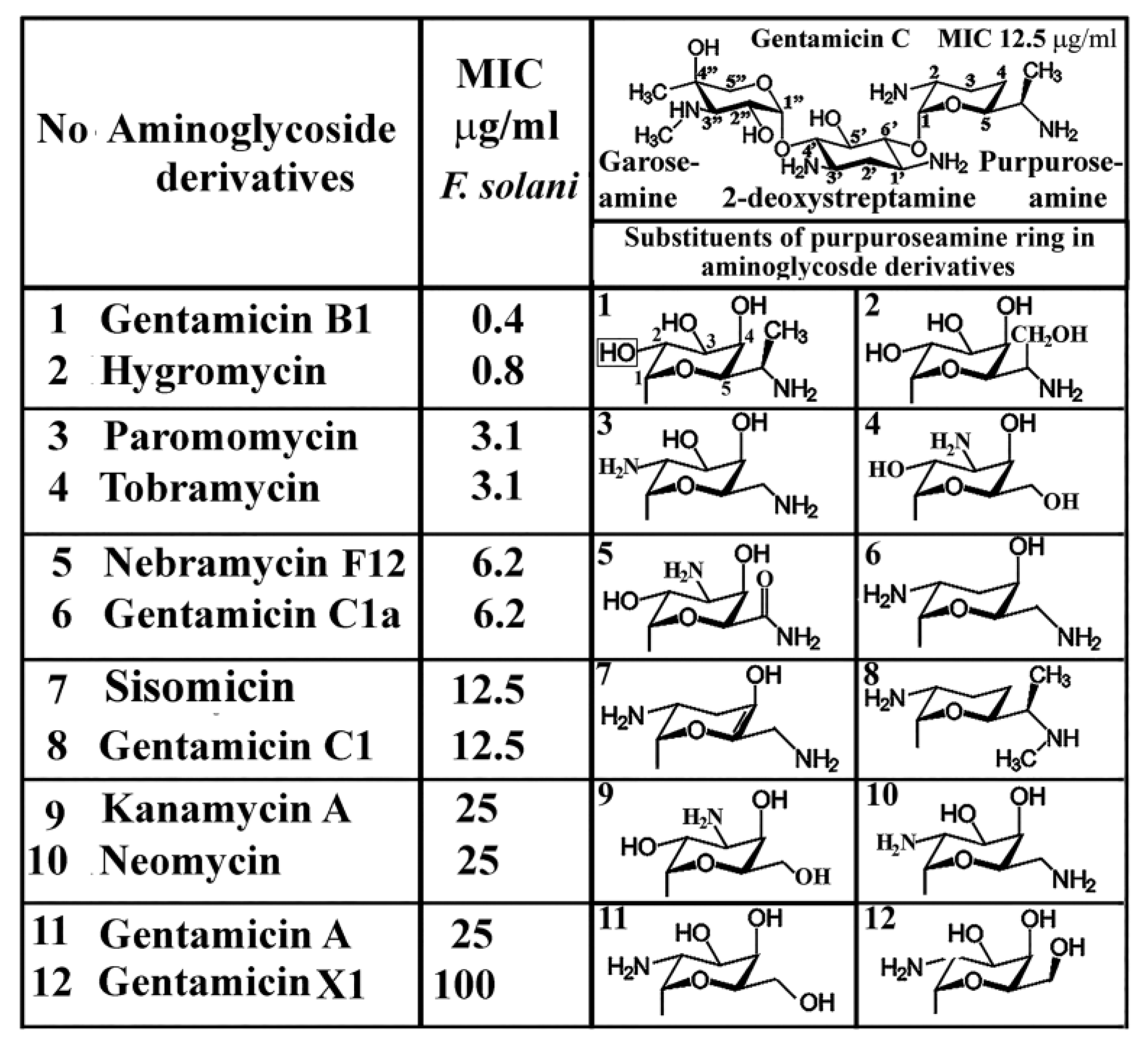
| Compound | Fungal Strain | MIC (µg/mL) |
|---|---|---|
| Gentamicin B1 | Fusarium solani | 0.4 |
| Gentamicin B1 | Aspergillus flavus | 0.4 |
| Gentamicin B1 | Aspergillus niger | 3.1 |
| Clotrimazol | Fusarium solani | 12.5 |
| Amphotericin B | Fusarium solani | 12.5 |
| Nystatin | Fusarium solani | 50 |
| Griseofulvin | Fusarium solani | 100 |
| Gentamicin B1 | Microsporon gypseum | 3.1 |
| Clotrimazol | Microsporon gypseum | 6.2 |
| Amphotericin B | Microsporon gypseum | 50 |
| Nystatin | Microsporon gypseum | 50 |
| Griseofulvin | Microsporon gypseum | 100 |
| Gentamicin B1 | C. neoformans A-15053 | 0.4 |
| Clotrimazol | C. neoformans A-15053 | 0.4 |
| Amphotericin B | C. neoformans A-15053 | 1.6 |
| Nystatin | C. neoformans A-15053 | 12.5 |
| Griseofulvin | C. neoformans A-15053 | 50 |
| Clotrimazol | Trychophyton gypseum | 0.4 |
| Amphotericin B | Trychophyton gypseum | 6.2 |
| Nystatin | Trychophyton gypseum | 12.5 |
| Gentamicin B1 | Trychophyton gypseum | 25 |
| Griseofulvin | Trychophyton gypseum | 50 |
| Amphotericin B | Candida albicans IAM 4888 | 1.6 |
| Clotrimazol | Candida albicans IAM 4888 | 3.1 |
| Nystatin | Candida albicans IAM 4888 | 12.5 |
| Gentamicin B1 | Candida albicans IAM 4888 | 50 |
| Griseofulvin | Candida albicans IAM 4888 | 100 |
| Antifungal Agent | Suppression of Antifungal Activity (µg/mL) | |||||||
|---|---|---|---|---|---|---|---|---|
| Fusarium solani | Cryptococcus neoformans | |||||||
| Control | CaCl2 | Heparin | Human Serum | Control | CaCl2 | Heparin | Human Serum | |
| 1 mg/mL | 1 mg/mL | 20% v/v | 1 mg/mL | 1 mg/mL | 20% v/v | |||
| Gentamicin B1 | 0.4 | 25 | 50 | 3.1 | 0.4 | 25 | 50 | 0.8 |
| Hygromycin B | 0.8 | 25 | 12.5 | 1.6 | 6.2 | 50 | 6.2 | 6.2 |
| Polymixin B | 1.6 | 50 | 50 | 3.1 | 1.6 | 25 | >50 | 6.2 |
| Clotrimazol | 12.5 | 25 | 50 | 25 | 0.4 | 0.4 | 0.4 | 0.8 |
| Amphotericin B | 12.5 | >50 | 12.5 | 50 | 0.8 | 0.8 | 1.6 | 0.8 |
| No. | Antifungal Agent | Type of Treatment | Dose mg/kg | No. of Mice | No. of Animals Survived (Days) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 6 | 8 | 10 | 14 | |||||
| 1 | Control | None | 0 | 10 | 10 | 6 | 2 | 0 | |||
| 2 | Amphotericin B | i.v. | 1 | 10 | 10 | 10 | 7 | 3 | 0 | ||
| 3 | i.v. | 5 | 10 | 10 | 10 | 10 | 7 | 4 | 3 | 0 | |
| 4 | Amphotericin B | s.c. | 2 | 10 | 10 | 9 | 6 | 0 | |||
| 5 | s.c. | 10 | 10 | 10 | 10 | 10 | 5 | 5 | 4 | 2 | |
| 6 | Gentamicin B1 | i.v. | 5 | 10 | 10 | 6 | 5 | 1 | 0 | ||
| 7 | i.v. | 25 | 10 | 10 | 2 | 1 | 0 | ||||
| 8 | Gentamicin B1 | s.c. | 10 | 10 | 10 | 6 | 4 | 0 | |||
| 9 | s.c. | 50 | 10 | 10 | 6 | 6 | 3 | 2 | 1 | 1 | |
| 10 | Gentamicin B1 + Amphotericin B | i.v. | 5 | 10 | 10 | 10 | 10 | 10 | 10 | 5 | 4 |
| i.v. | 5 | ||||||||||
| 11 | Gentamicin B1 + Amphotericin B | s.c. | 10 | 10 | 10 | 10 | 4 | 3 | 0 | ||
| i.v. | 1 | ||||||||||
| 12 | Gentamicin B1 + Amphotericin B | s.c. | 50 | 10 | 10 | 10 | 5 | 3 | 2 | 0 | |
| i.v. | 1 | ||||||||||
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banfalvi, G. Antifungal Activity of Gentamicin B1 against Systemic Plant Mycoses. Molecules 2020, 25, 2401. https://doi.org/10.3390/molecules25102401
Banfalvi G. Antifungal Activity of Gentamicin B1 against Systemic Plant Mycoses. Molecules. 2020; 25(10):2401. https://doi.org/10.3390/molecules25102401
Chicago/Turabian StyleBanfalvi, Gaspar. 2020. "Antifungal Activity of Gentamicin B1 against Systemic Plant Mycoses" Molecules 25, no. 10: 2401. https://doi.org/10.3390/molecules25102401
APA StyleBanfalvi, G. (2020). Antifungal Activity of Gentamicin B1 against Systemic Plant Mycoses. Molecules, 25(10), 2401. https://doi.org/10.3390/molecules25102401






