1. Introduction
The mono- and bis-pyridinium oximes, as quaternized derivatives of pyridyl oximes, are known as pharmacologically important agents [
1]. Explicitly, the deprotonation of the oxime group produces the oximate form, >C=N-O
−, a powerful nucleophile used in the cleavage of either phosphoric and carboxylic acid esters or amides. A nitrogen atom with an unshared electron pair adjacent to the oxy-anionic nucleophilic center makes pyridinium-4-oximes α-nucleophiles that exhibit a much higher reactivity than common oxygen nucleophiles of similar basicity. The ability of oximes to act as esterolytic agents had already been recognized in the late 1950s and various terms like oximolysis, esterolysis, thiocholine ester hydrolysis and cholinesterase pseudo-activity have been adopted to describe the phenomenon [
2,
3,
4,
5,
6,
7,
8,
9,
10]. This reflects their biological role, making them especially effective reagents involved in the reactivation of acetylcholinesterase (AcChE) initially inhibited by organophosphorus poisons (pesticides, nerve gases) [
1] or catalysts, which mimic the catalytic mode of hydrolases, as well as excellent Michael donors in nucleophilic additions [
11]. The biological importance and pharmacological application of pyridinuim oximes arises from their abilities to reactivate AcChE, the essential enzyme involved in neurotransmission, inhibited by organophosphorus poisons. The phosphylation of serine hydroxyl group in the active site of AcChE results in the accumulation of acetylcholine (AcCh
+) in the synaptic clefts, leading to overstimulation of cholinergic receptor sites. Even though the antidotal potency of pyridinium oximes is primarily attributed to their ability to displace the phosphoryl moiety from the enzyme active site by virtue of their powerful nucleophilicity, they can also bind to AcChE either at the active site or at the allosteric site acting as reversible inhibitors, thus explaining their mechanism of AcChE protection. In addition, so-called direct pharmacological effects such as direct reaction with organophosphorus nerve poisons, anticholinergic effect and properties that decrease the amount of liberated AcCh
+ into synaptic cleft are also known [
1]. The in vitro AcChE binding and reactivation capability of novel lipophilic pyridinium oxime derivatives and their structural analogues are still extensively studied in a search for universal antidote capable to cross the blood-brain barrier [
12,
13]. However, these pharmacological compounds possess multiple modes of action and alternative bioactivities are an active research topic. A particular focus is placed on electron and charge transfer processes where pyridinium-4-oxime cations are recognized as new electron acceptors for the formation of colored, supramolecular, inter-ionic charge-transfer complexes with hexacyanoferrate(II) anion [
14].
Beside the ubiquity of thioesters in living systems playing central roles in metabolism, the use of acetylthiocholine (AcSCh
+), the O→S substituted analogue of the AcChE natural substrate, AcCh
+, dates from the mid-twentieth century. A standard spectrophotometric protocol utilizing AcSCh
+, developed by Ellman, is used in various assays for probing the presence and activity of AcChE in biological fluids, cell suspensions, tissues and so forth [
15]. An alternative to AcChE use in biosensing approaches comprises the application of bioinspired AcChE-based models in which pseudo AcChE-molecules promote hydrolysis of AcCh
+ or AcSCh
+ and organophosphorus esters or carbamates [
16]. The carboxylic acid thio- and oxy-esters are rapidly cleaved during metabolism by hydrolytic enzymes that catalyze either direct water attack on the ester linkage or a double-displacement reaction in which the enzyme and the substrate form an acyl-enzyme intermediate that subsequently undergoes hydrolysis (such as AcChE) [
17]. Non-enzymatic hydrolysis of esters containing the acetyl group, beside the nucleophilic attack by H
2O, termed neutral or “water” (or pH-independent or uncatalyzed) hydrolysis comprises acid and base catalyzed hydrolysis. In the acid catalyzed mechanism, that is, catalysis by H
+, if the proton source is hydronium ion (H
3O
+), (and the ester is already protonated in the rate-limiting step of the reaction) the catalysis is termed specific acid catalysis. Specific base catalysis by HO
− ions is similar in that the base is hydroxide and the ester is attacked by hydroxide ion in the rate-limiting step of the reaction (there are no other bases, for example, such as the conjugate base of an acid, in the rate-limiting step). Each of these processes occurs independently of the others. Catalysis also occurs when an undissociated acid is present in the rate-limiting step of the reaction. Such a catalysis is termed general acid catalysis and the transfer of the proton to the substrate occurs in the rate-limiting step of the mechanism. Catalysis that involves the conjugate base of an acid in the slow step of the reaction is termed general base catalysis. In this mechanism the conjugate base of the acid deprotonates water, which simultaneously attacks the substrate. The process is often called general base assisted nucleophilic attack. Kinetics and mechanism of carboxylic acid esters hydrolysis are generally well understood including the influence of both, electronic and steric effects on their reactivity [
18,
19,
20,
21]. AcCh
+ is more susceptible to neutral and base-catalyzed hydrolysis than ethyl acetate but less susceptible to acid-catalyzed hydrolysis [
8], while majority of thioesters undergoes both acid and base-catalyzed hydrolysis [
10]. Kinetic studies indicated that there are two distinct types of thioesters—one which possesses an activated carbonyl group due to the presence of electron-withdrawing substituents on the α-carbon and second type which lacks an activated carbonyl—unactivated thioesters such as AcSCh
+ [
11]. Unactivated thioesters display three distinct regions in the pH-rate profile—HO
−-ion catalysis above pH 7, neutral (uncatalyzed) hydrolysis between pH 2–7 and H
+-ion catalysis below pH 2 [
11].
The esterolytic potencies of pyridinium oximes in the inhibited, usually phosphylated, AcChE reactivation kinetics or in the non-enzymatic AcSCh
+ hydrolysis reaction were widely studied [
8,
9,
22,
23]. In these pyridinium oxime-(thio/phospho)ester reactions, an oximate anion, as a nucleophile, attacks the carbonyl carbon or phosphorus atom of the (thio/phospho)ester, respectively, promoting the acylation/phosphylation of the pyridinium oxime. Certainly, in a competing process thioester slowly spontaneously hydrolyzes in water (
t1/2 ≈ 210 min at 24 °C,
kw = 3.3 × 10
−3 min
−1 for unactivated thioesters [
19]), while phosphylation of the active site serine in the inhibited AcChE results in the uncharged adduct which is unreactive toward spontaneous hydrolysis [
23]. More importantly, the further fate, structural and energetic features of the produced pyridinium oxime-ester (specifically acetylated pyridinium oximes formed in the reaction with AcSCh
+) is crucial for understanding the whole thermodynamic and catalytic cycle but usually undeveloped in the available literature.
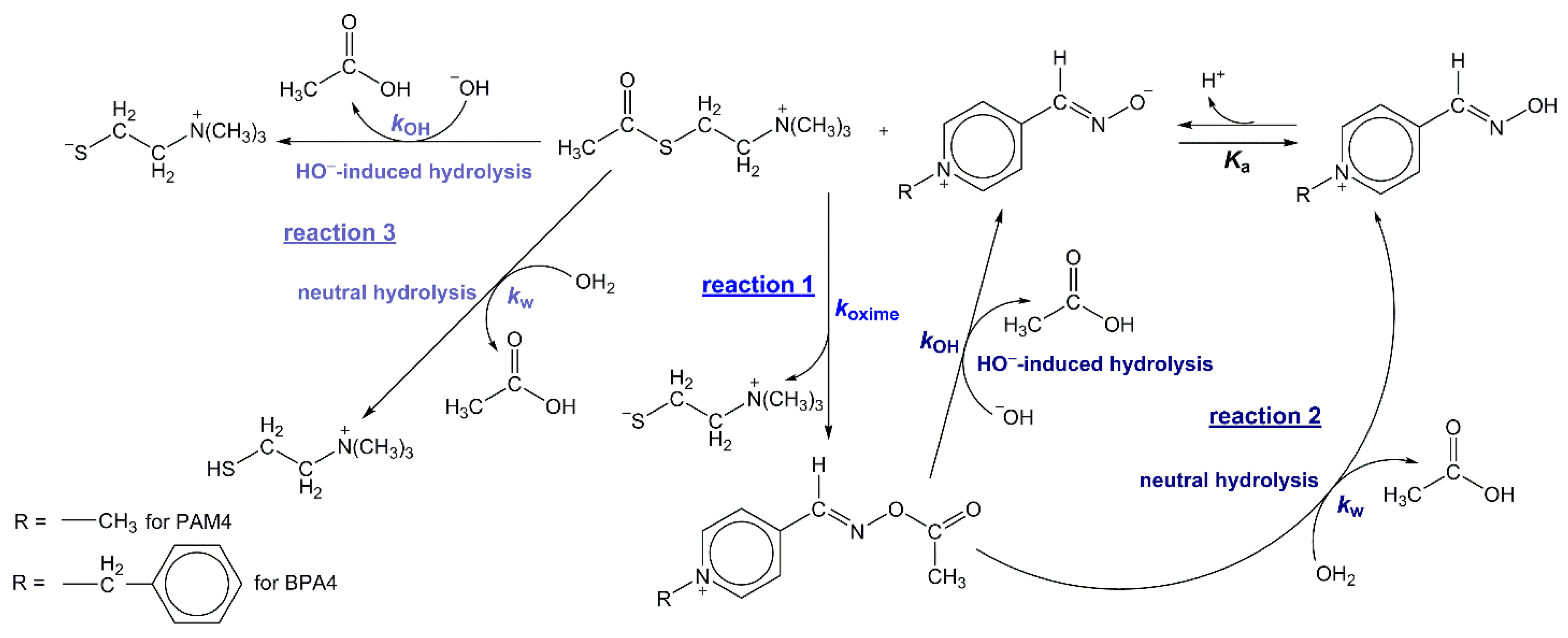
To provide insights into the role of pyridinium oximes such as pseudo-hydrolases (thioesterases), two well-defined pyridinium-4-oximes,
N-methylpyridinium-4-oxime iodide (PAM4
+I
−) and
N-benzylpyridinium-4-oxime chloride (BPA4
+Cl
−) [
14,
24], have been tested as oximolytic agents toward the AcSCh
+. To complete the cycle of the pyridinium-4-oximate promoted reactions, the hydrolytic susceptibility of the newly synthetized pyridinium-4-oxime-ester,
O-acetyl-
N-methylpyridinium-4-oxime iodide (AcPAM4
+I
−), has been separately investigated. Herein, we report for the first time the synthetic pathway and isolation of this novel oxime-ester, along with its physico-chemical characterization. The combined kinetic and computational mechanistic studies were undertaken and relevant kinetic and thermodynamic parameters revealed. The investigated reaction system (
Scheme 1) included three pH-dependent reactions—(1) the oxime-thioester reaction between oximate ion and AcSCh
+, that is, oxime-assisted thioesterolysis of AcSCh
+—termed oximolysis; (2) the consecutive neutral or HO
−-catalyzed hydrolysis of pyridinium-4-oxime ester (AcOxime
+) formed by oximolysis and (3) the competitive neutral or HO
−-catalyzed AcSCh
+ hydrolysis.
2. Results and Discussion
The investigated reaction system is presented on
Scheme 1. The reactions 1, 2 and 3 were independently studied spectrophotometrically and rate constants,
koxime,
kw and
kOH were evaluated over a convenient range of pH at
I = 0.1 M and 25 °C. In addition, the density functional theory (DFT) computational analysis provided a mechanistic insight into these reactions along with kinetic
and thermodynamic (Δ
GR COMP) parameters.
The nucleophilic ability of the examined pyridinium-4-oximes to promote AcSCh
+ esterolysis was investigated by measuring the rates of reactions between individual pyridinium-4-oxime and AcSCh
+ at different pH. In each set of experiments, the pH was kept constant by means of an external buffer (
I = 0.1 M, 25 °C) and the concentration of the reactive deprotonated pyridinium-4-oxime (oximate) was defined according to the ionization equilibrium (
Scheme 1) as:
To determine second-order rate constant
koxime for oximolysis over a convenient range of pH, the pseudo-first-order rate constants,
kobs, were evaluated. In a series of experiments, conducted with a large molar excess of AcSCh
+ over the concentration of the oximate, the rates of disappearance of oximate ions were followed at the wavelength of the absorption maximum of the reactive oximate (see experimental for details). The application of the derivated rate law to reaction 1 gives the Equation (2):
Accordingly, the combination of Equations (1) and (2) results in the following expression:
where
k0 represents possible competing pathway leading to oximate formation at the specified pH. The rates of oximolysis exceeded hydrolysis of AcSCh
+ present in high molar excess such that the reaction 3 on
Scheme 1 had a negligible contribution to the consumption of thioester in our experiments (see text below). The rates of consumption of the oximate correspond to the difference of the rates of oximolysis (reaction 1) and the neutral (H
2O) and HO
−-induced hydrolysis of the produced pyridinium-4-oxime-ester, AcOxime
+, (reaction 2) which can be expressed as:
The second-order rate constant,
koxime, was determined from the slopes of the linear plots in
Figure 1 and
Figure S1 at 25 °C and
I = 0.1 M and was found to be pH-independent in accordance with Equation (3).
The matching values of
koxime for both pyridinium-4-oximes, 3.81 M
−1∙s
−1 for PAM4 and 3.97 M
−1∙s
−1 for BPA4 (
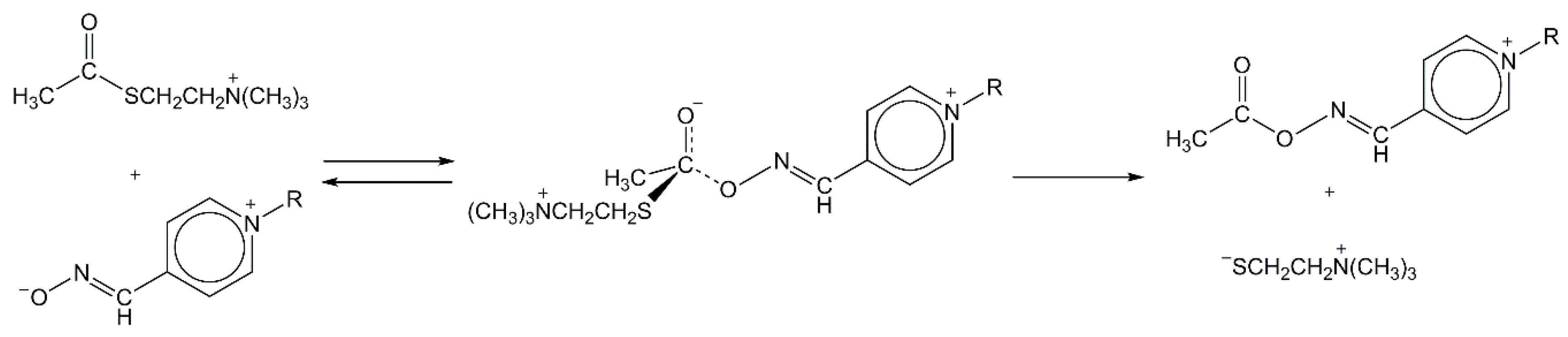
I = 0.1 M, 25 °C), are found to be in accordance with the mechanistic insight provided by the DFT computational studies. The computational analysis of reaction 1 points to the most common stepwise mechanism (
Scheme 2) [
21] and the formation of tetrahedral intermediate as the rate-limiting step with the
of 68.6 kJ∙mol
−1 for PAM4 and 69.0 kJ∙mol
−1 for BPA4.
Thermodynamic activation parameters
(kJ∙mol
−1),
(J∙K
−1·mol
−1) and
(kJ∙mol
−1) for the AcSCh
+ oximolysis by PAM4 were determined over the temperature range 25–37 °C in BR buffer at pH = 8.3 and
I = 0.1 M (
Figure S1). The evaluated value of 69.45 kJ∙mol
−1 for
, along with negative
supports the theoretical mechanistic study.
The above described acetylation reaction stage (oximolysis) indeed mimics only the first stage of the catalytic process of the serine hydrolases, such as AcChE, but the complete enzyme catalyzed process includes two successive stages, acetylation and deacetylation, that is, hydrolysis of seryl-acetyl-ester formed in active site of the AcChE [
25]. Assuming that oximate promotes the hydrolysis of AcSCh
+ acting as pseudo-hydrolase/thioesterase, further investigation of the fate of pyridinium-4-oxime-ester (acetylated oxime) was necessary to provide mechanistic features of the second, deacetylation, stage of the total catalytic process. For that purpose,
O-acetyl-
N-methylpyridinium-4-oxime iodide (AcPAM4
+I
−) was synthesized and the independent kinetic study of the AcPAM4
+ hydrolysis was performed (reaction 2 on
Scheme 1). The progress of AcPAM4
+ overall hydrolysis was followed spectrophotometrically at 25 °C and
I = 0.1 M over the pH range from 6 to 12 where the rate of hydrolysis is dominated by neutral (H
2O) and HO
− specific components, explicitly:
The lowest value of the
kobs obtained in pure water (
kobs,hydrolysis = 1.5 × 10
−5 s
−1) was taken as the value for
kw (
kw >
kOH·[HO
−] and hence
kobs,hydrolysis kw), see
Figure S2. The kinetic barrier, that is, the free energy of activation
(kJ∙mol
−1) for the neutral hydrolysis was calculated according to transition state theory [
26] as follows:
where
kB and
h are Boltzmann’s and Planck’s constants, respectively,
R is the gas constant.
Equation (6) gives
= 100.5 kJ∙mol
−1 at 25 °C and along with the value of
kw indicates insignificant contribution of the neutral AcPAM4
+ hydrolysis to the observed oximolysis rate constant (see Equations (3) and (4)). Profile of the observed rates of AcPAM4
+ hydrolysis vs. pH is presented in
Figure S3a. The HO
−-catalyzed hydrolysis appears to be the major hydrolytic mode above the pH 8.5 (since,
kOH·[HO
−] >>
kw, the
kobs,hydrolysis kOH·[HO
−]). Therefore, the second-order rate constant,
kOH, value of 30.3 M
−1∙s
−1 was evaluated, in the pH range from 9 to 11, as the slope of the straight line resulting from plot of the observed rate of hydrolysis (
kobs,hydrolysis/s
−1) against the concentration of HO
− (
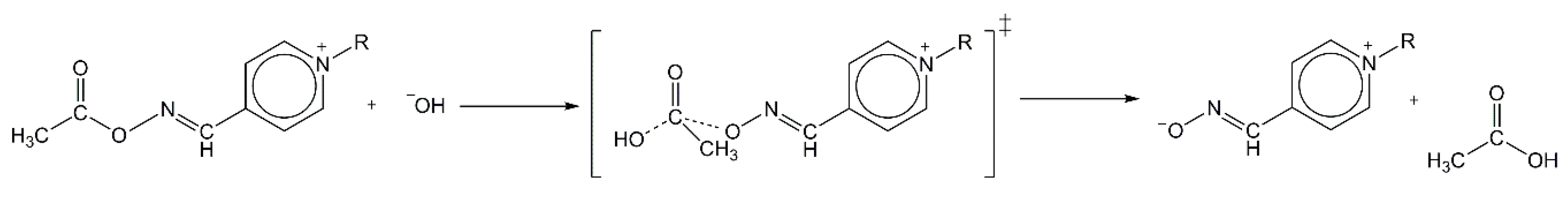
Figure S3b). The computational analysis of HO
−-catalyzed AcPAM4
+ hydrolysis points to the concerted mechanism and the formation of tetrahedral transition state with the kinetic barrier (
) of 35.1 kJ∙mol
−1. It is reasonable to assume that the concerted mechanism is valid for hydrolysis of both pyridinium-4-oxime-esters and in the alkaline media can be presented as in
Scheme 3.
The competitive reactions to AcSCh
+ oximolysis by pyridinium-4-oximes are neutral and HO
−-induced hydrolysis of AcSCh
+ (overall hydrolysis, that is, reaction 3 on
Scheme 1). To determine the influence of hydrolysis as a deleterious side reaction, the separate kinetic experiments were performed. The reactions were followed spectrophotometrically using the Ellman’s reagent [
15] within the pH range from 7 to 9 at 25 °C and
I = 0.1 M. We were forced to use the mild alkaline conditions due to the instability of Ellman’s reagent above pH 9 (see experimental for details). The rate constants
kw = 2.5 × 10
−5 s
−1 and
kOH = 59.0 M
−1∙s
−1 for hydrolysis of AcSCh
+ were evaluated analogously as was described for hydrolysis of AcPAM4
+, by using Equation (5). Profile of the observed rates of hydrolysis vs. pH and the data for the HO
−-catalyzed hydrolysis are plotted in
Figure S4. Again, the HO
−-catalyzed hydrolysis appears to be the major hydrolytic mode above the pH 8.5 (
Figure S4b). The
kw value for the neutral AcSCh
+ hydrolysis agrees reasonable with the value reported for the neutral hydrolysis of formylthiocholine (
kw = 2.25 × 10
−5 s
−1 at 25 °C and
I = 0.2 M) [
20]. The mechanistic study also agrees with the stepwise reaction mechanism suggested for neutral formylthiocholine hydrolysis [
20,
21] and Equation (6) gives the kinetic barrier,
= 99.3 kJ∙mol
−1. The computational analysis of HO
–-assisted AcSCh
+ hydrolysis suggested the concerted mechanism with the kinetic barrier (
) of 26.8 kJ∙mol
−1.
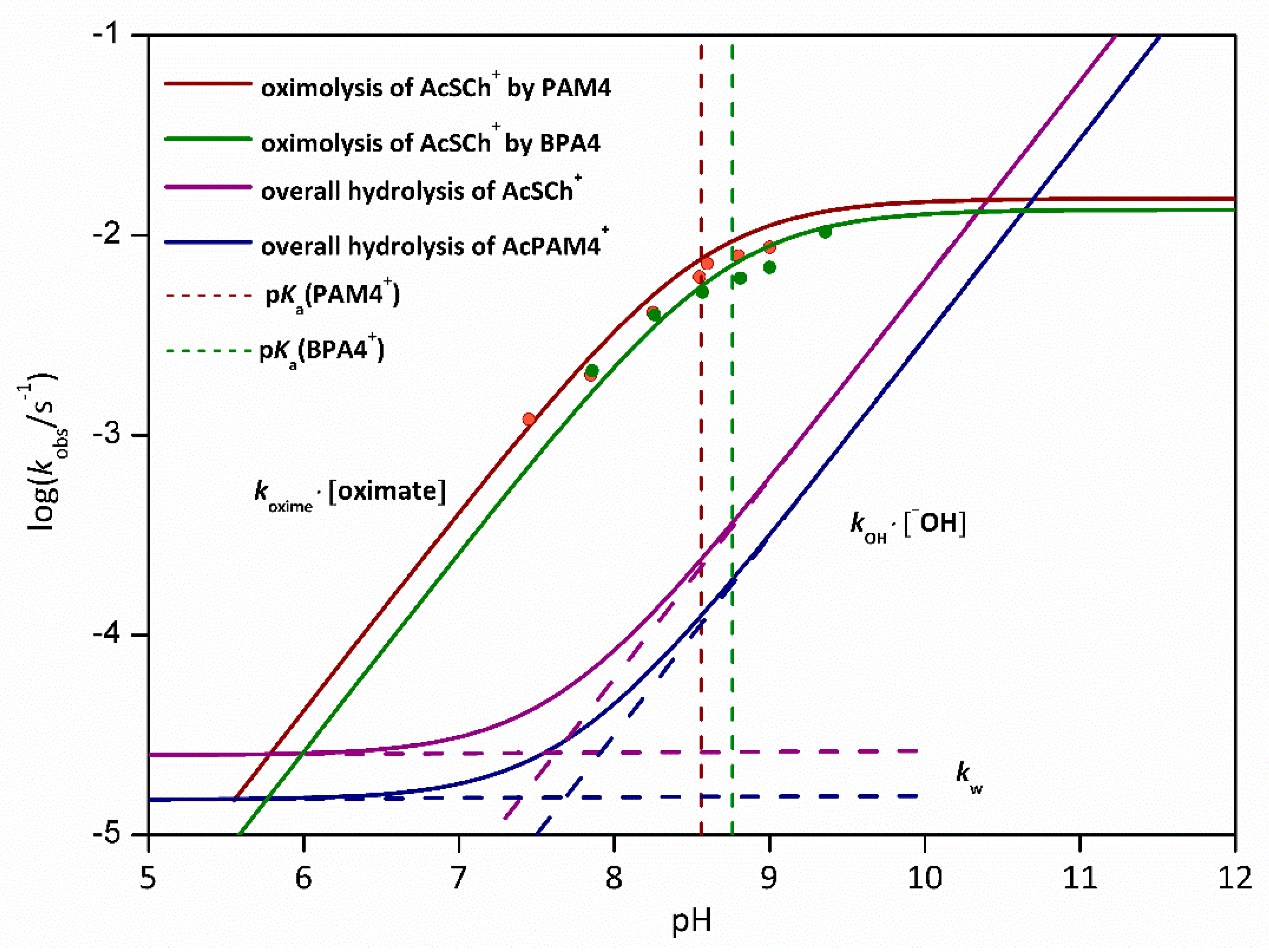
The experimentally determined kinetic parameters are summarized in
Table 1. Based on the values of kinetic constants, the pH-rate profiles are constructed and presented in
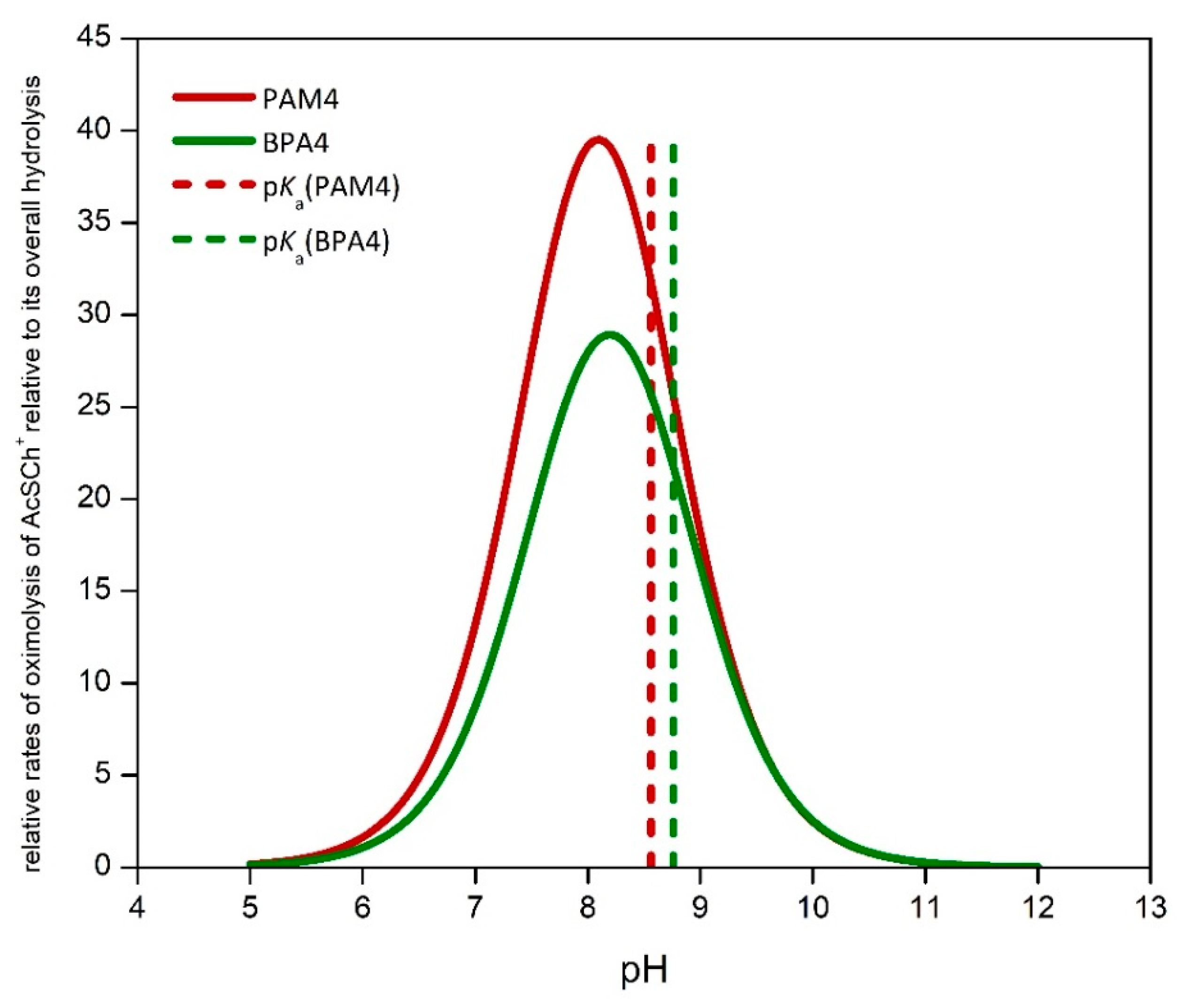
Figure 2, illustrating several key features of the reaction systems.
The shapes of the plots, that is, the rates of oximolysis are similar for both pyridinium-4-oximes suggesting that a phenyl substituent, distant from the reaction center, exerts practically no influence on the reaction outcomes. The rates of oximolysis scale with the concentrations of oximate and that is why rates plateau when pH reaches values of ~2 pH units higher than p
Ka(oxime). The rates of neutral hydrolysis of AcSCh
+ and AcPAM4
+ are constant, while the rates of HO
−-induced hydrolysis scale with the concentrations of HO
−. The HO
−-catalyzed hydrolysis appears to be a predominant hydrolytic mode for both, AcSCh
+ and AcPAM4
+, above the pH of 8.5 (
Figure 2,
Figures S3 and S4). The minimum observed rates are in the pH regions below 6.5 of the pH-rate profiles and account for neutral hydrolysis of AcSCh
+ and AcPAM4
+. The pH-profile of the observed pseudo-first-order oximolysis rate constants relative to the overall (neutral and HO
−-induced) observed rate of AcSCh
+ hydrolysis is presented in
Figure 3. The rates of AcSCh
+ oximolysis by PAM4 or BPA4 are maximized relative to AcSCh
+ overall hydrolysis at pH value of 8.1 and 8.2, respectively, where the oximolysis by PAM4 is ~40 times and by BPA4 ~ 30 times faster. A neutral and mildly basic aqueous media would have favored oximolysis with more acidic pyridinium-4-oximes providing the higher concentration of reactive nucleophile. Oximolysis by PAM4
+ with the p
Ka of 8.6, regardless the smaller value of
koxime (
koxime = 3.81 M
−1∙s
−1), is more favored than oximolysis by BPA4
+ with the p
Ka of 8.8 and
koxime = 3.97 M
−1∙s
−1.
When considering the potential pseudo-hydrolase action of explored pyridinium-4-oximes, the corresponding catalytic cycle should be composed of two stages where, in the first acetylation one, (herein reaction 1) the thioester bond of AcSCh
+ is oximolytically cleaved, while in the second deacetylation one (herein reaction 2) the ester bond of formed AcOxime
+ is cleaved hydrolytically. In addition, to ensure the free progress of the catalytic cycles and appropriate regeneration of the oximate, the rates of reaction 1 and 2 must be comparable. Since the neutral AcOxime
+ hydrolysis is very slow in comparison to its HO
−-catalyzed hydrolysis and in regard to reaction 1, it can be assumed that the oximolysis and the formation of AcOxime
+ is predominately followed by the HO
–-catalyzed hydrolysis of AcOxime
+ and restoration of the corresponding oximate, ending the catalytic cycle according to reactions 1 and 2 presented on
Scheme 1. Accordingly, at the optimal pH value of 8.1 for the oximolysis by the PAM4 over the AcSCh
+ overall hydrolysis (maximum of the curve in
Figure 3,
kobs,oximolysis = 3.9 × 10
−3 s
−1, Equation (3)) the consecutive observed hydrolysis of AcPAM4
+ proceeds almost 75-fold slower (
kobs,AcPAM4+ hydrolysis = 5.3 × 10
−5 s
−1), recovering 1.36% of the oximate
. This is further validated by the spectrophotometric study of the time-dependent electronic absorption spectral changes within reaction system containing PAM4 and 200-fold excess of AcSCh
+ at pH around 8,
I = 0.1 M and 25 °C (
Figure S5).
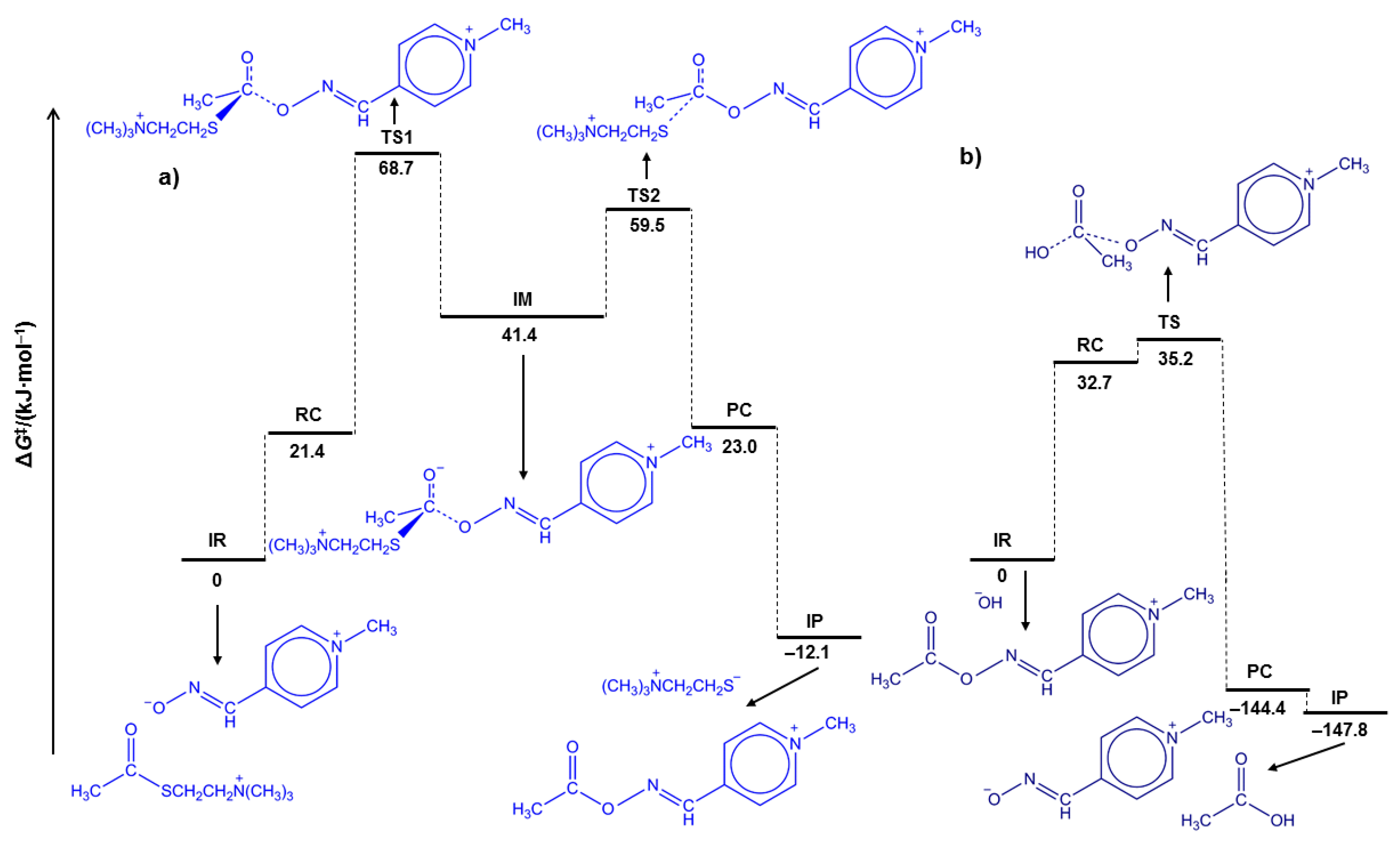
The computational mechanistic study was performed using the (CPCM)/M06–2X/6–311++G(2df,2pd)//(CPCM)/M06–2X/6–31+G(d) model and the mechanistic data are summarized in
Table 2 and
Figure 4. The key geometric parameters for the reactant complex (RC), transition state (TS), intermediate (IM) and product complex (PC) of the acetylation and deacetylation stages of AcSCh
+ hydrolysis are listed in
Table S1.
First, let us observe that computational results indicate that the total Gibbs free energy of the reactant complex in reactions 1 and 2 is higher than the sum of the isolated reactants (
Table 2). Nevertheless, these were found to be rather small, being in the range up to 33 kJ∙mol
−1 and contribute to the calculated activation free energies. Yet, even then, the calculated
values do not exceed 70 kJ∙mol
−1 allowing these reactions to proceed under normal conditions. The latter is also facilitated by the fact that all reactions reported in
Table 2 have negative reaction Gibbs energies and are favored thermodynamically, with the HO
−-mediated AcSCh
+ and AcPAM4
+ hydrolysis being highly exergonic. Analogous to the mechanism of the AChE-catalyzed process, the oxime-mediated AcSCh
+ hydrolysis consists of two successive stages—first, the oximolysis, that is, the acetylation stage characterized by the stepwise mechanism (
Figure 4a) and second, the deacetylation stage, characterized by the concerted HO
−-mediated AcOxime
+ hydrolysis (
Figure 4b). This is in accordance with the stepwise and concerted mechanisms found for acyl-transfer reactions of esters having weakly basic leaving groups [
27]. Based on the calculated activation free Gibbs energies for the acetylation stage, the formation of TS1 by nucleophilic attack of an oximate to the carbonyl carbon atom of AcSCh
+ represents the rate-determining step characterized with a kinetic parameter
koxime. The close resemblance between Δ
G‡ values, calculated (
= 68.7 kJ∙mol
−1) and experimentally determined (
= 69.5 kJ∙mol
−1) for the AcSCh
+ oximolysis by PAM4, strongly support these computations. The changes of bond lengths and bond angles during the oximolysis (
Figure 4a and
Table S1) point to the formation of quasi-tetrahedral transition states (TS1 and TS2) and the intermediate (IM) in which the partial double character of the carbonyl bond is continuously preserved. Interestingly, the structure of the IM might suggest an intramolecular charge-charge stabilization, which would allow to expect the energy of this stationary point to be significantly lower than 41.1 kJ∙mol
−1 above isolated reactants (IR) presented in
Figure 4. However, our calculations show that the charged cationic amino group and the anionic carboxylate are located distant from each other during the course of the reaction, likely because of both (i) the resonance stabilization within the carboxylate, which dislocates anionic charge over several atoms and (ii) steric crowding surrounding the quaternary cationic amine, which hinders the exposure of the positive charge. As a result, there is not intramolecular interaction between these two fragments, additionally disfavored by the presence of the highly dielectric aqueous environment, thus the high-energy lying structure of the IM. Moreover, the structures of the first transition state (TS1) and IM indicate that the formation of the C
carbonyl-O
oxime bond causes the substantial elongation of C-S rather than the carbonyl bond. Although the formation of the fully resolved tetrahedral intermediate within the stepwise mechanism should be expected and has been recognized for the methoxide- and AcChE-catalyzed AcSCh
+ hydrolysis [
25,
28], our result could be rationalized with the postulation that the course of oximolysis is predominantly controlled by the cation-cation repulsive forces between thiocholine leaving group and pyridinium part of zwitterionic pyridinium-4-oximate. In the case of the second—deacetylation—stage characterized by the concerted HO
−-mediated AcOxime
+ hydrolysis (
Figure 4b), the transition state (TS) structurally has a close resemblance to the initially formed reactant complex (RC) with a small degree of cleavage of the C
carbonyl-O
oxime bond caused by the formation of C
carbonyl--O
hydroxide close contact. Furthermore, the theoretical study points to the substantial mechanistic differences between the PAM4- and AcChE-mediated AcSCh
+ hydrolysis. In the AcChE-mediated AcSCh
+ hydrolysis both stages, acetylation and deacetylation, indeed occur through the stepwise mechanism, where within rate-limiting deacetylation stage the H
2O as a nucleophile attacks the carbonyl carbon of the acetylated seryl-residue within the enzyme active site [
25]. On the contrary, the stepwise acetylation stage of the PAM4-mediated AcSCh
+ hydrolysis is rate-limiting process followed by the concerted attack of HO
− to the carbonyl carbon of the AcPAM4
+ in the second, deacetylation stage. The involvement of H
2O as a nucleophile in the implied stepwise deacetylation stage is ruled out, due to the evaluated high energy barrier (
= 100.5 kJ∙mol
−1) for the neutral AcPAM4
+ hydrolysis when compared with HO
−-catalyzed reaction (
= 35.2 kJ∙mol
−1). Our results of kinetic and theoretical mechanistic studies clearly show that the acetylation stage of AcSCh
+ hydrolysis by PAM4 or BPA4 represents the rate-limiting stage of the investigated, overall, process.
The potential mimetic enzymatic performance of pyridinium-4-oximes toward AcSCh
+ hydrolysis was further validated by taking in account that the neutral and pyridinium-4-oxime-catalyzed hydrolysis of AcSCh
+ presumably occur via the equivalent stepwise mechanism. The experimentally determined kinetic parameters
koxime/M
−1∙s
−1 and
/s
−1 were used for the calculation of the rate enhancement expressed as
koxime/
, where
= 4.5 × 10
−7 M
−1·s
−1, accounts for the second-order rate constant of neutral AcSCh
+ hydrolysis (
=
/55.56 M). The 8.5 × 10
6- and 8.8 × 10
6-fold rate increase of the PAM4- and BPA4-catalyzed AcSCh
+ hydrolysis, respectively, puts the pyridinium-4-oximes into the category of very efficient and potent esterolytic agents. While important, this rate enhancement is modest in comparison to the 10
13-fold catalytic power manifested by cholinesterases [
16,
17].
The presented results of AcSCh
+ oximolysis by PAM4 and BPA4 reflect their equal nucleophilicity and insignificant effect of the benzene ring within BPA4. Moreover, the reaction with both oximes is exergonic (Δ
GR COMP = −12.1 kJ·mol
−1 for PAM4 and Δ
GR COMP = −10.0 kJ∙mol
−1 for BPA4), the difference being only 2.1 kJ∙mol
−1 in favor of PAM4. The similar reactivity, that is, electronic properties of these two pyridinium-4-oximes has been recently established by the analysis of the corresponding frontier orbitals where it becomes clear that no significant electron density is observed in the additional phenyl ring in the LUMO of BPA4
+, while the majority of the electron density is located in the pyridinium-4-oxime moiety, just as in PAM4
+ [
14]. As was previously recognized for the reactivity of pyridinium-oximes toward the AcSCh
+, the reactivity differences did not origin from different electron density on the oxygen of the oximate but was caused with the steric hindrance of the oxime group by the rest of the molecule, resulting in higher reactivity of the oxime group in para-position than those in ortho-position [
8,
9,
22]. It should be noted, that even though the greater acidity of oxime functional group ensures higher concentration of nucleophilic active oximate form in neutral solutions, that is, at physiological pH, the significant contribution on the nucleophilicity and consequent esterolytic activity has the steric effect, whether through structural characteristic of electrophilic center or nucleophile itself.