Alkalinity and Its Consequences for the Performance of Steel-Reinforced Geopolymer Materials
Abstract
1. Introduction
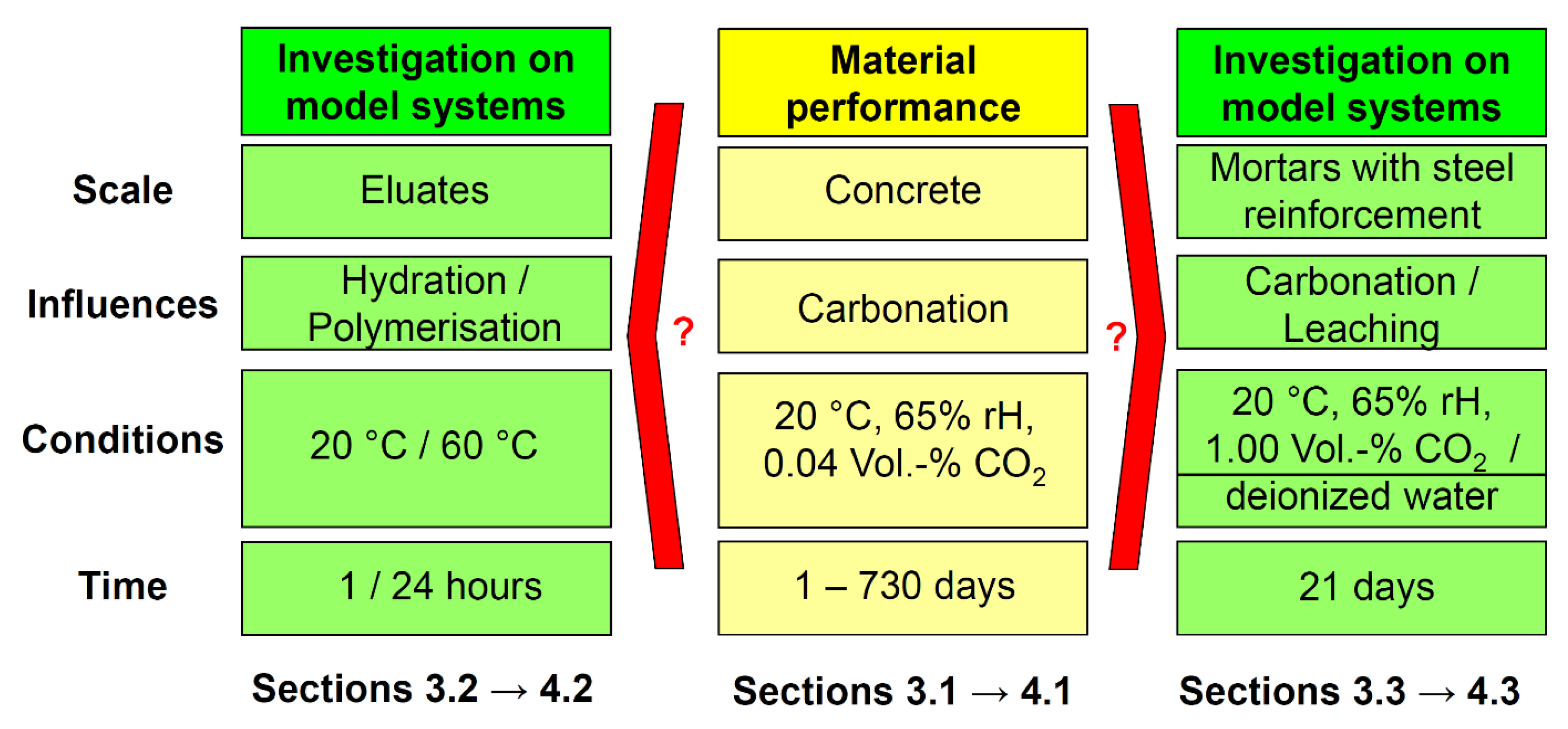
2. Results
2.1. Carbonation Resistance of Concretes under Normal Conditions
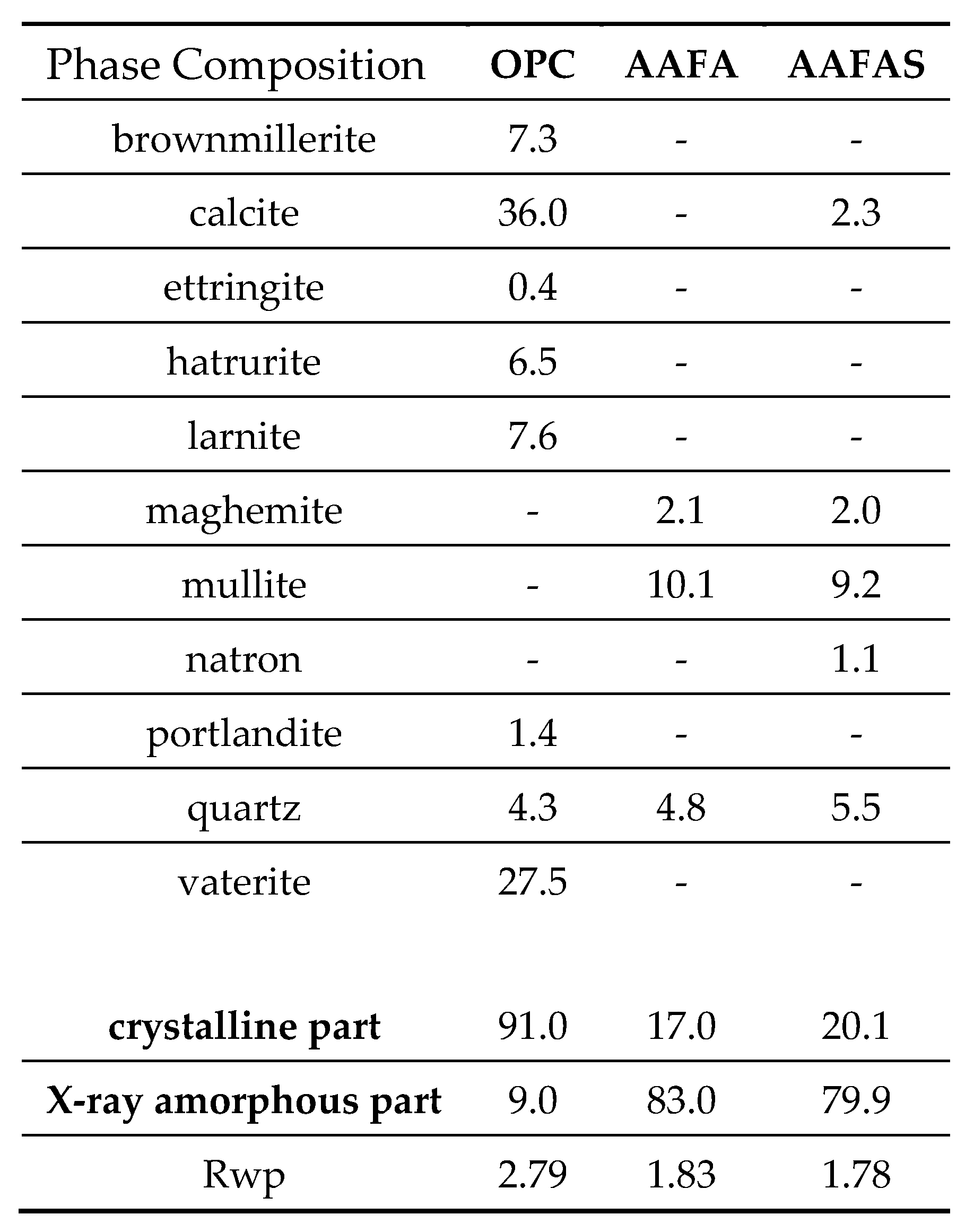
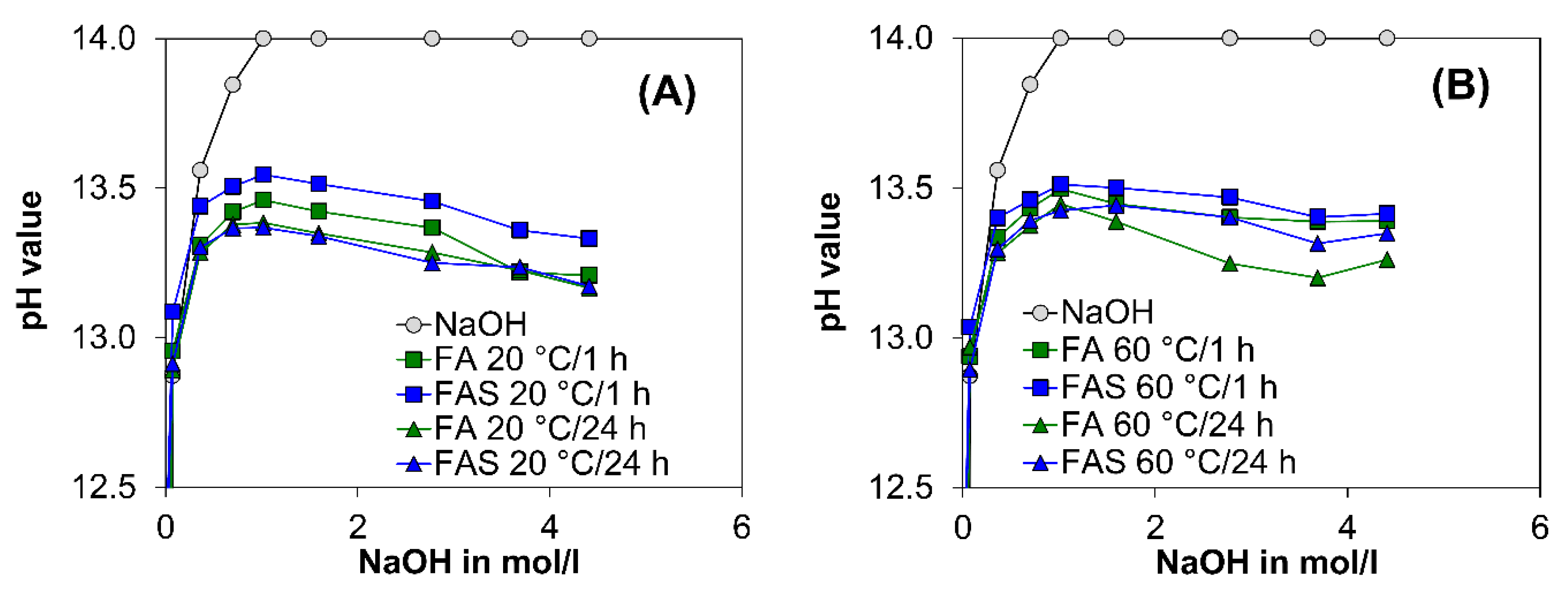
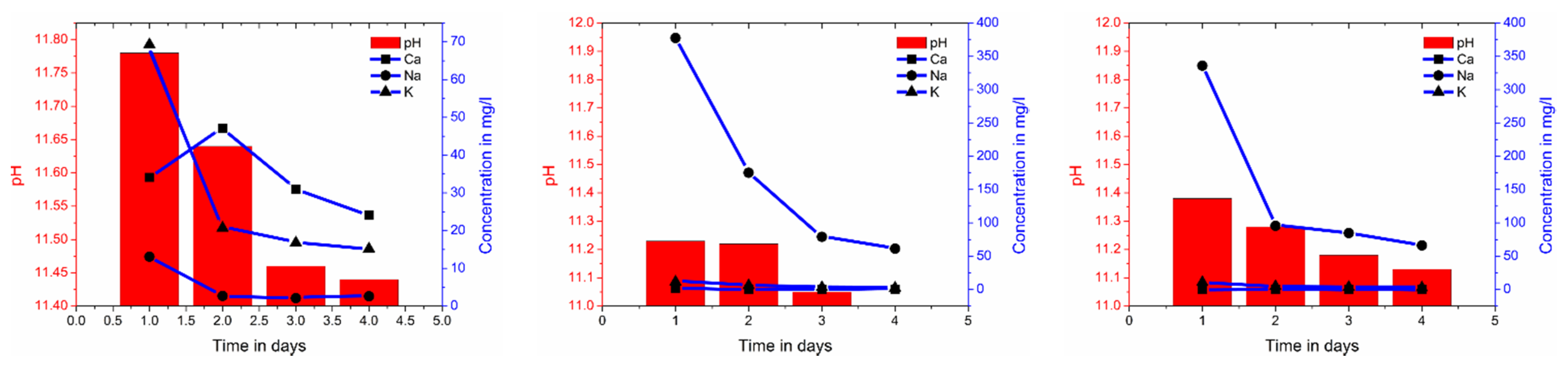
2.2. Eluate Monitoring Tests to Examine the Influence of the Hydration and Polymerisation Reaction on the Alkalinity
2.3. Accelerated Carbonation and Leaching Tests on Mortars with Steel Reinforcement to Examine Corrosion Behaviour
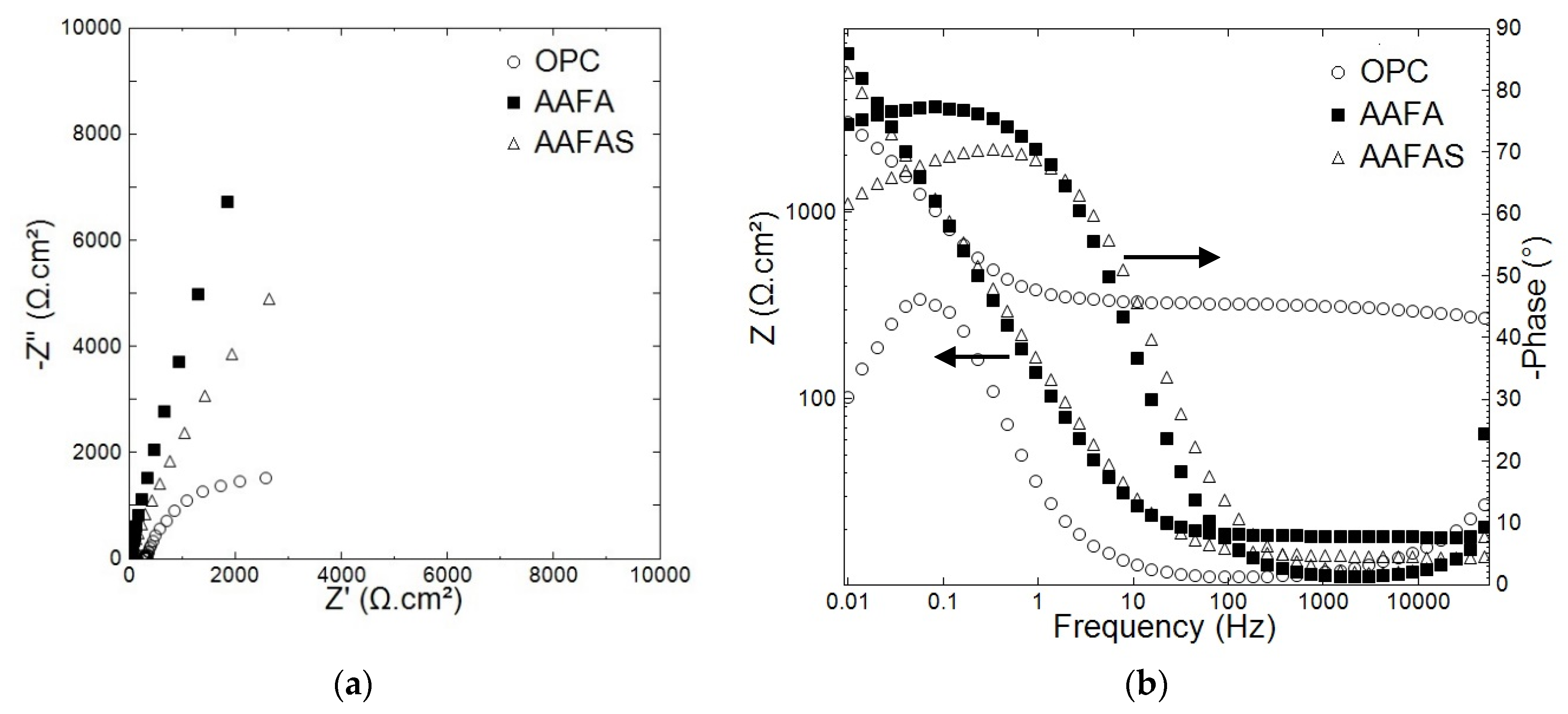
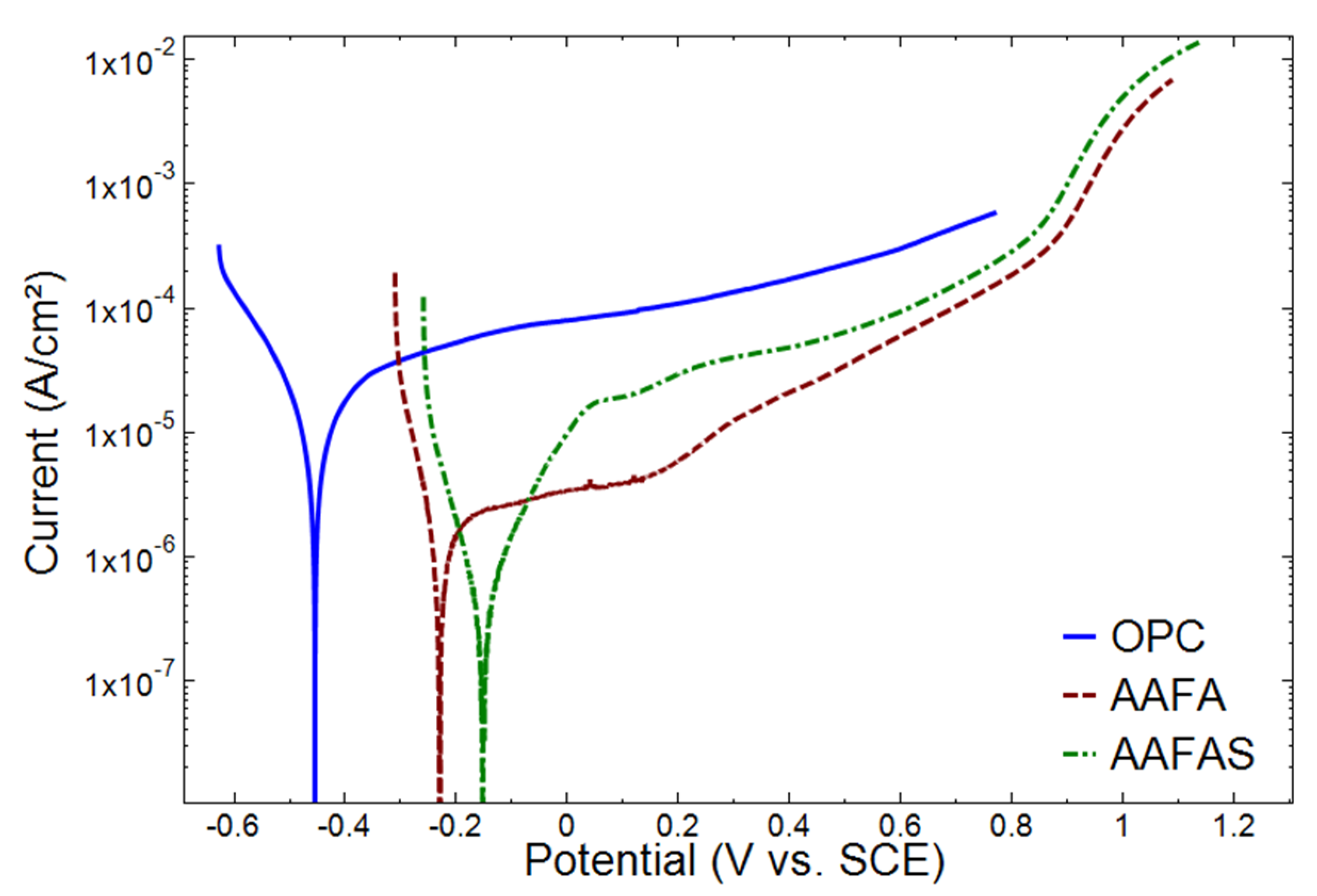
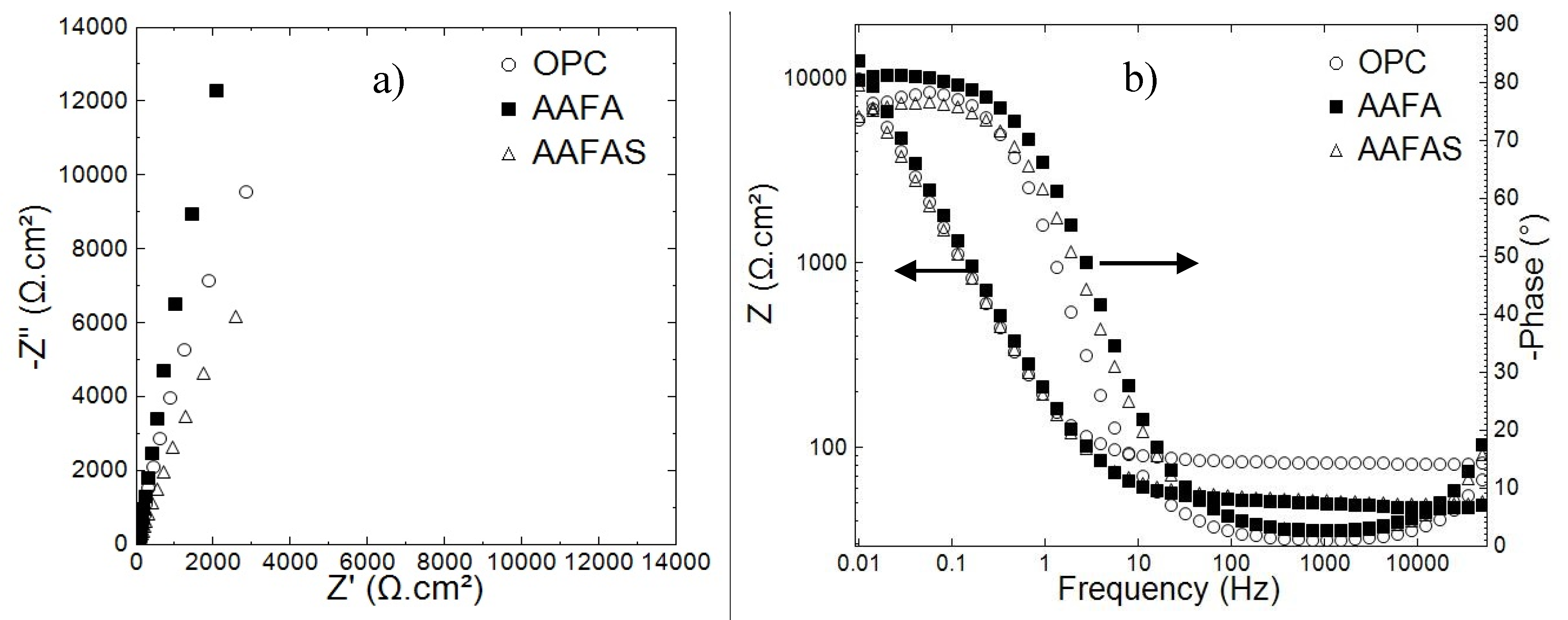
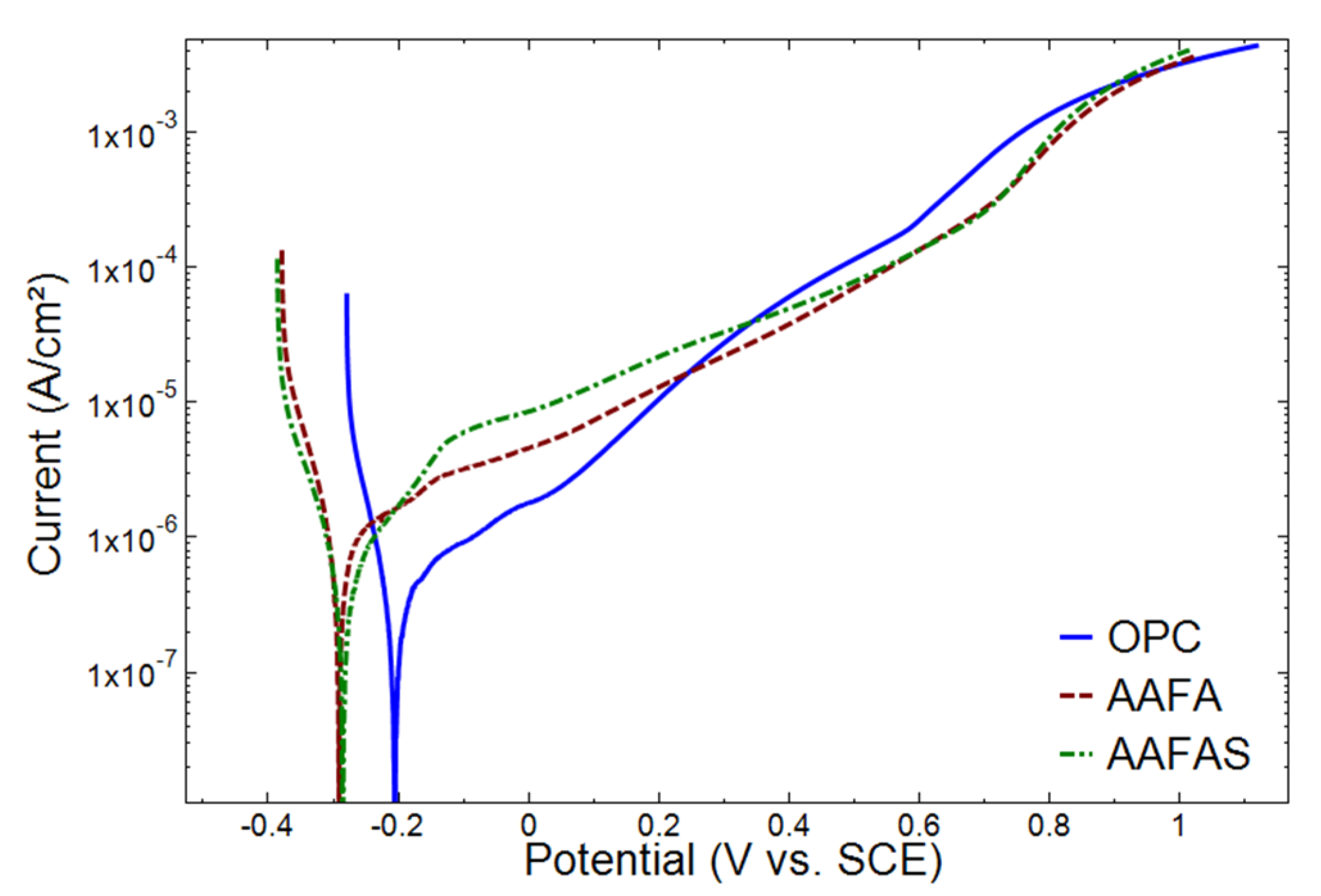
2.3.1. Electrochemical Investigations
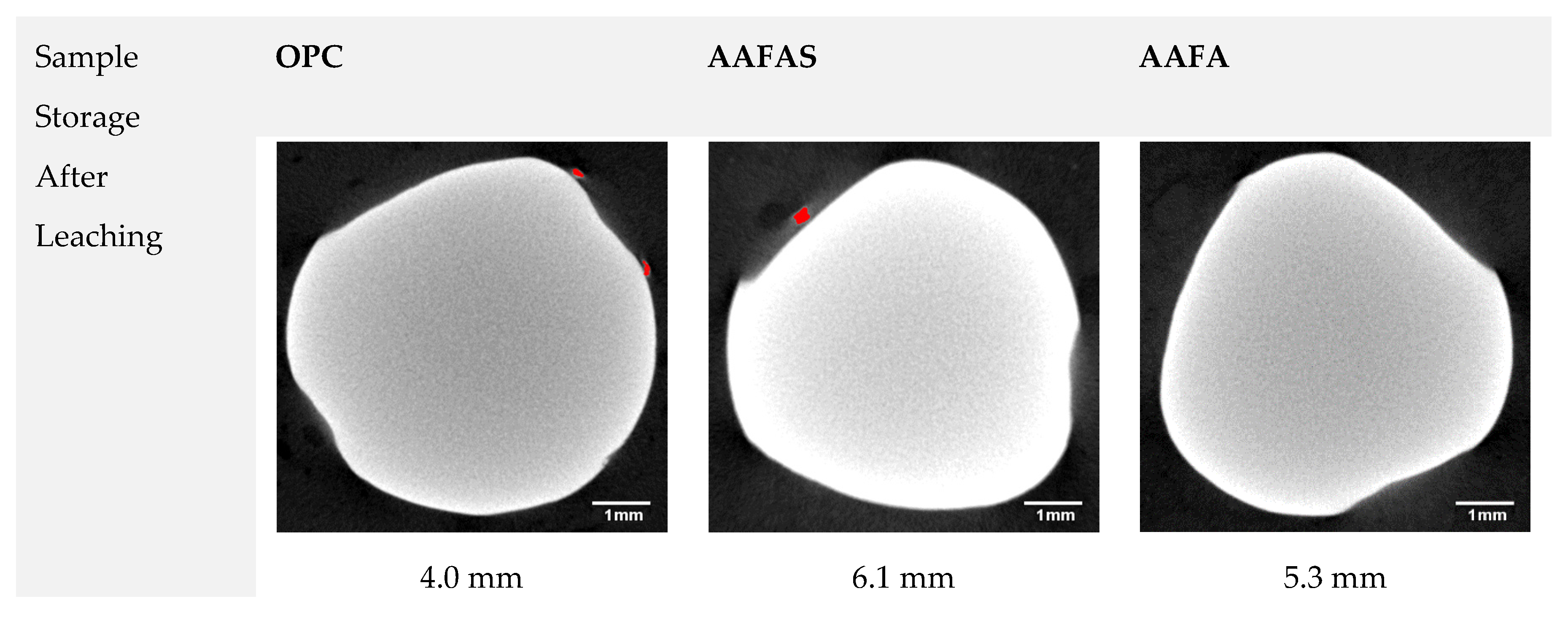
2.3.2. Comparative µXCT Investigations after Carbonation and Leaching Tests
3. Discussion
3.1. Eluate Tests
H2SiO42− + 2Na+ → Na2SiO3 + H2O
3.2. Concrete Carbonation Tests under Standard Climate Conditions
3.3. Accelerated Carbonation Tests on Mortars with Steel Reinforcement
4. Materials and Methods
4.1. Materials
4.2. Mixture Proportions
4.3. Test Procedure
4.3.1. Carbonation Resistance of Concretes under Normal Condition
4.3.2. Eluate Monitoring Tests
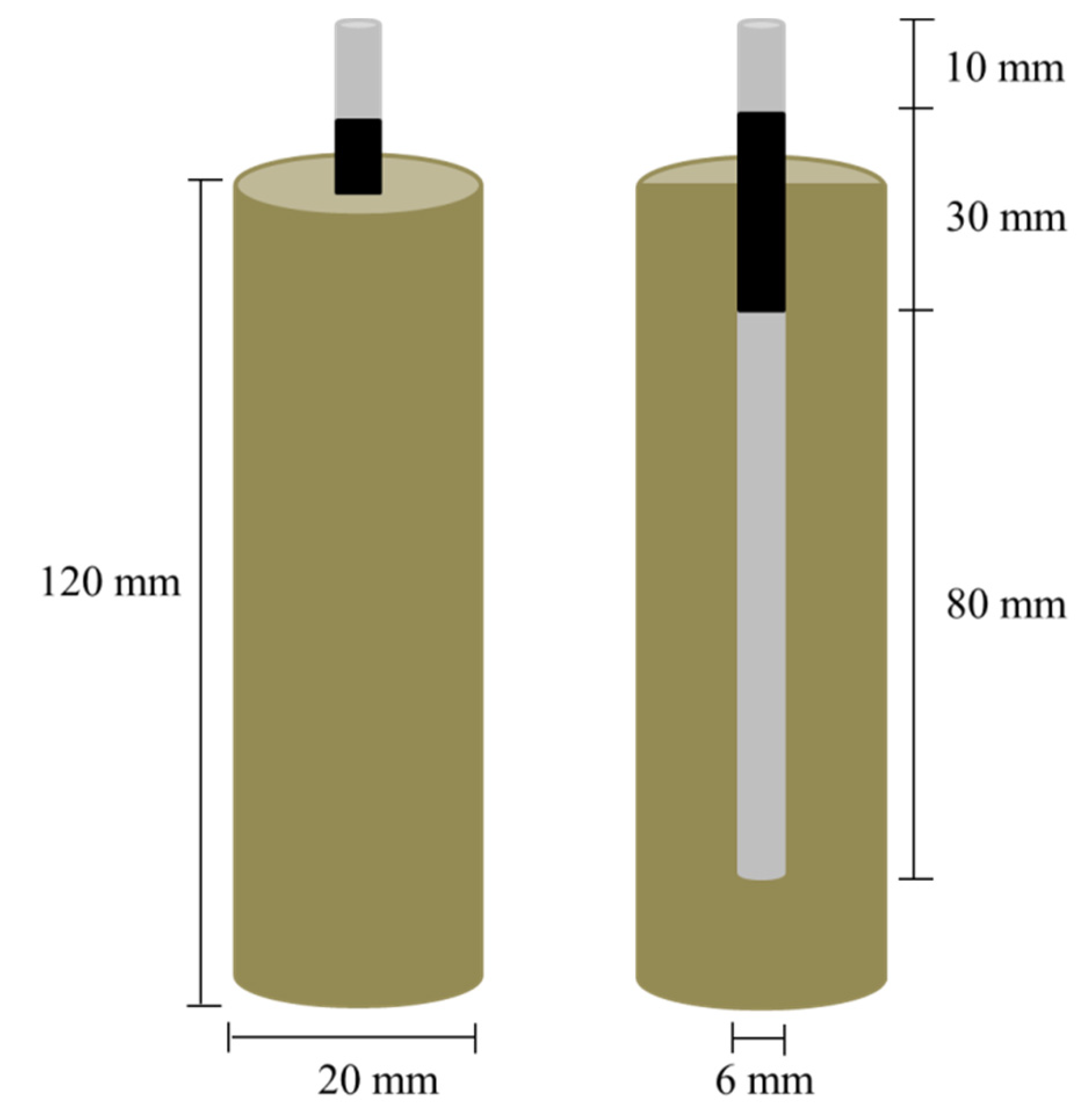
4.3.3. Accelerated Carbonation and Leaching Tests on Mortars with Steel Reinforcement
4.4. Methods
4.4.1. Micro-X-Ray Computer Tomography (µXCT)
4.4.2. X-Ray Diffraction (XRD)
4.4.3. Electrochemical Response of Reinforcement
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Herrmann, A.; Koenig, A.; Dehn, F. Structural concrete based on alkali-activated binders: Terminology, reaction mechanisms, mix designs and performance. Struct. Concr. 2018, 19, 918–929. [Google Scholar] [CrossRef]
- Provis, J.L.; van Deventer, J.S.J. Alkali Activated Materials: State-of-the-Art Report, RILEM TC 224-AAM; Springer: Dordrecht, The Netherlands, 2014. [Google Scholar]
- Turner, L.K.; Collins, G.C. Carbon dioxide equivalent (CO2-e) emissions: A comparison between geopolymer and OPC cement concrete. Constr. Build. Mater. 2013, 43, 125–130. [Google Scholar] [CrossRef]
- McLellan, B.C.; Williams, R.P.; Lay, J.; van Riessen, A.; Corder, G.D. Costs and carbon emissions for geopolymer pastes in comparison to ordinary portland cement. J. Clean. Prod. 2011, 19, 1080–1090. [Google Scholar] [CrossRef]
- Heath, A.; Paine, K.; McManus, M. Minimising the global warming potential of clay based geopolymers. J. Clean. Prod. 2014, 78, 75–83. [Google Scholar] [CrossRef]
- Mehta, A.; Siddique, R. Sulfuric acid resistance of fly ash based geopolymer concrete. Constr. Build. Mater. 2017, 146, 136–143. [Google Scholar] [CrossRef]
- Lee, N.K.; Lee, H.K. Influence of the slag content on the chloride and sulfuric acid resistances of alkali-activated fly ash/slag paste. Cem. Concr. Compos. 2016, 72, 168–179. [Google Scholar] [CrossRef]
- Koenig, A.; Herrmann, A.; Overmann, S.; Dehn, F. Resistance of alkali-activated binders to organic acid attack: Assessment of evaluation criteria and damage mechanisms. Constr. Build. Mater. 2017, 151, 405–413. [Google Scholar] [CrossRef]
- Bakhareva, T.; Sanjayana, J.G.; Cheng, Y.-B. Resistance of alkali-activated slag concrete to acid attack. Constr. Build. Mater. 2003, 33, 1607–1611. [Google Scholar] [CrossRef]
- Zhuang, H.J.; Zhang, H.Y.; Xu, H. Resistance of geopolymer mortar to acid and chloride attacks. Proc. Eng. 2017, 210, 126–131. [Google Scholar] [CrossRef]
- Ma, C.-K.; Awang, A.Z.; Omara, W. Structural and material performance of geopolymer concrete: A review. Constr. Build. Mater. 2018, 186, 90–102. [Google Scholar] [CrossRef]
- Koenig, A.; Herrmann, A.; Gattic, F.; Rossi, L.; Fuchs, F.; Fessel, D.; Dathe, F.; Dehn, F.; Minelli, F. Flexural behaviour of steel and macro-PP fibre reinforced concretes based on alkali-activated binders. Constr. Build. Mater. 2019, 211, 583–593. [Google Scholar] [CrossRef]
- Herrmann, A.; Koenig, A.; Dehn, F. Proposal for the classification of alkali-activated binders and Geopolymer binder. Cem. Int. 2015, 13, 63–69. [Google Scholar]
- Buchwald, A. The Influence of Calcium on the Condensation of (Alumino-) silicates in Alkali-Activated Binders; Habilitation: Weimar, Germany, 2012. [Google Scholar]
- Corrosion of Steel in Concrete: Prevention, Diagnosis, Repair, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2013.
- Committee 222. Corrosion of Metals in Concrete; Elsevier: Amsterdam, The Netherlands, 1985; pp. 3–32. [Google Scholar]
- Vollpracht, A.; Brameshuber, W. Binding and leaching of trace elements in Portland cement pastes. Cem. Concr. Res. 2016, 79, 76–92. [Google Scholar] [CrossRef]
- Engelsen, C.J.; van der Sloot, H.A.; Petkovic, G. Long-term leaching from recycled concrete aggregates applied as sub-base material in road construction. Sci. Total Environ. 2017, 587–588, 94–101. [Google Scholar] [CrossRef]
- Saha, A.K.; Sarker, P.K. Sustainable use of ferronickel slag fine aggregate and fly ash in structural concrete: Mechanical properties and leaching study. J. Clean. Prod. 2017, 162, 438–448. [Google Scholar] [CrossRef]
- Sorlini, S.; Collivignarelli, M.C.; Abbà, A. Leaching behaviour of municipal solid waste incineration bottom ash: From granular material to monolithic concrete. Waste Manag. Res. 2017, 35, 978–990. [Google Scholar] [CrossRef]
- Hewlett, P. Lea’s Chemistry of Cement and Concrete, 4th ed.; Elsevier Butterworth-Heinemann: Oxford, UK, 2008. [Google Scholar]
- Pouhet, R.; Cyr, M. Carbonation in the pore solution of metakaolin-based geopolymer. Cem. Concr. Res. 2016, 88, 227–235. [Google Scholar] [CrossRef]
- Revertegat, E.; Richet, C.; Gégout, P. Effect of pH on the durability of cement pastes. Cem. Concr. Res. 1992, 22, 259–272. [Google Scholar] [CrossRef]
- Jacques, D.; Wang, L.; Martens, E.; Mallants, D. Modelling chemical degradation of concrete during leaching with rain and soil water types. Cem. Concr. Res. 2010, 40, 1306–1313. [Google Scholar] [CrossRef]
- Rozière, E.; Loukili, A.; el Hachem, R.; Grondin, F. Durability of concrete exposed to leaching and external sulphate attacks. Cem. Concr. Res. 2009, 39, 1188–1198. [Google Scholar] [CrossRef]
- Koenig, A.; Dehn, F. Main considerations for the determination and evaluation of the acid resistance of cementitious materials. Mater. Struct. 2016, 49, 1693–1703. [Google Scholar] [CrossRef]
- Beddoe, R.E.; Dorner, H.W. Modelling acid attack on concrete: Part I. The essential mechanisms. Cem. Concr. Res. 2005, 35, 2333–2339. [Google Scholar] [CrossRef]
- Koenig, A.; Dehn, F. Acid Resistance of Ultra High-Performance Concrete (UHPC). In Nanotechnology in Construction (Nicom); Springer: Berlin, Germany, 2015; Volume 5, pp. 317–323. [Google Scholar]
- Mahmoud, H.; Sánchez, M.; Alonso, M.C. Ageing of the spontaneous passive state of 2304 duplex stainless steel in high-alkaline conditions with the presence of chloride. J. Solid State Electrochem. 2015, 19, 2961–2972. [Google Scholar] [CrossRef]
- Qiu, Q. A state-of-the-art review on the carbonation process in cementitious materials: Fundamentals and characterization techniques. Constr. Build. Mater. 2020, 247, 118503. [Google Scholar] [CrossRef]
- Ashraf, W. Carbonation of cement-based materials: Challenges and opportunities. Constr. Build. Mater. 2016, 120, 558–570. [Google Scholar] [CrossRef]
- Koenig, A.; Dehn, F. Biogenic acid attack on concretes in biogas plants. Biosyst. Eng. 2016, 147, 226–237. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Abdollahnejad, Z.; Camões, A.F.; Jamshidi, M.; Ding, Y. Durability of alkali-activated binders: A clear advantage over Portland cement or an unproven issue? Constr. Build. Mater. 2012, 30, 400–405. [Google Scholar] [CrossRef]
- Della Roy, M.; Jiang, W.; Silsbee, M.R. Chloride diffusion in ordinary, blended, and alkali-activated cement pastes and its relation to other properties. Cem. Concr. Res. 2000, 30, 1879–1884. [Google Scholar]
- Miranda, J.M.; Fernández-Jiménez, A.; González, J.A.; Palomo, A. Corrosion resistance in activated fly ash mortars. Cem. Concr. Res. 2005, 35, 1210–1217. [Google Scholar] [CrossRef]
- Saraswathy, V.; Muralidharan, S.; Thangavel, K.; Srinivasan, S. Influence of activated fly ash on corrosion-resistance and strength of concrete. Cem. Concr. Compos. 2003, 25, 673–680. [Google Scholar] [CrossRef]
- Aperador, W.; Mejia, R.; Bastidas, D.M. Steel corrosion behaviour in carbonated alkali-activated slag concrete. Corros. Sci. 2009, 51, 2027–2033. [Google Scholar] [CrossRef]
- Bernal, S.A.; de Gutiérrez, R.M.; Pedraza, A.L.; Provis, J.L.; Rodriguez, E.D.; Delvasto, S. Effect of binder content on the performance of alkali-activated slag concretes. Cem. Concr. Res. 2011, 41, 1–8. [Google Scholar] [CrossRef]
- Li, N.; Farzadnia, N.; Shi, C. Microstructural changes in alkali-activated slag mortars induced by accelerated carbonation. Cem. Concr. Res. 2017, 100, 214–226. [Google Scholar] [CrossRef]
- Palacios, M.; Puertas, F. Effect of Carbonation on Alkali-Activated Slag Paste. J Am. Ceram. Soc. 2006, 89, 3211–3221. [Google Scholar] [CrossRef]
- Shi, C.; Krivenko, P.V.; Roy, D. (Eds.) Alkali-Activated Cements and Concretes, 1st ed.; CRC Press: Taylor and Francis Group: Boca Raton, FL, USA, 2005. [Google Scholar]
- Pasupathy, K.; Berndt, M.; Castel, A.; Sanjayan, J.; Pathmanathan, R. Carbonation of a blended slag-fly ash geopolymer concrete in field conditions after 8years. Constr. Build. Mater. 2016, 125, 661–669. [Google Scholar] [CrossRef]
- Badar, M.S.; Kupwade-Patil, K.; Bernal, S.A.; Provis, J.L.; Allouche, E.N. Corrosion of steel bars induced by accelerated carbonation in low and high calcium fly ash geopolymer concretes. Constr. Build. Mater. 2014, 61, 79–89. [Google Scholar] [CrossRef]
- Bernal, S.A.; Provis, J.L.; Brice, D.G.; Kilcullen, A.; Duxson, P.; van Deventer, J.S.J. Accelerated carbonation testing of alkali-activated binders significantly underestimates service life: The role of pore solution chemistry. Cem. Concr. Res. 2012, 42, 1317–1326. [Google Scholar] [CrossRef]
- Nedeljković, M. Carbonation Mechanism of Alkali-Activated Fly Ash and Slag Materials: In View of Long-Term Performance Predictions. Ph.D. Thesis, TU Delft, Delft, The Netherlands, 2019. [Google Scholar]
- Neves, J.M. The Alkali-Silica Reaction in Alkali-Activated Fly Ash Concrete. Master’s Thesis, Pennsylvania State University, State College, PA, USA, 2016. [Google Scholar]
- Lloyd, R.R.; Provis, J.L.; van Deventer, J.S.J. Pore solution composition and alkali diffusion in inorganic polymer cement. Cem. Concr. Res. 2010, 40, 1386–1392. [Google Scholar] [CrossRef]
- Babaee, M.; Castel, A. Chloride-induced corrosion of reinforcement in low-calcium fly ash-based geopolymer concrete. Cem. Concr. Res. 2016, 88, 96–107. [Google Scholar] [CrossRef]
- Criado, M.; Sobrados, I.; Bastidas, J.M.; Sanz, J. Corrosion behaviour of coated steel rebars in carbonated and chloride-contaminated alkali-activated fly ash mortar. Prog. Org. Coat. 2016, 99, 11–22. [Google Scholar] [CrossRef]
- Tittarelli, F.; Mobili, A.; Giosuè, C.; Belli, A.; Bellezze, T. Corrosion behaviour of bare and galvanized steel in geopolymer and Ordinary Portland Cement based mortars with the same strength class exposed to chlorides. Corros. Sci. 2018, 134, 64–77. [Google Scholar] [CrossRef]
- Mundra, S.; Criado, M.; Bernal, S.A.; Provis, J.L. Chloride-induced corrosion of steel rebars in simulated pore solutions of alkali-activated concretes. Cem. Concr. Res. 2017, 100, 385–397. [Google Scholar] [CrossRef]
- Mundra, S.; Bernal, S.A.; Provis, J.L. Chloride-Induced Corrosion of Steel in Alkali Activated Cements: A Review. In RILEM Annual Week ICACMS in Chennai, India 3–8 September; 2017; pp. 147–155. Available online: https://www.diva-portal.org/smash/get/diva2:1142144/FULLTEXT01.pdf#page=172 (accessed on 18 May 2020).
- Chindaprasirt, P.; Chalee, W.; Chindaprasirt, P. Effect of sodium hydroxide concentration on chloride penetration and steel corrosion of fly ash-based geopolymer concrete under marine site. Constr. Build. Mater. 2014, 63, 303–310. [Google Scholar] [CrossRef]
- Monticelli, C.; Natali, M.E.; Balbo, A.; Chiavari, C.; Zanotto, F.; Manzi, S.; Bignozzi, M.C. Corrosion behavior of steel in alkali-activated fly ash mortars in the light of their microstructural, mechanical and chemical characterization. Cem. Concr. Res. 2016, 80, 60–68. [Google Scholar] [CrossRef]
- Gunasekara, C.; Bhuiyan, S.; Law, D.; Setunge, S.; Ward, L. Corrosion Resistance in Different Fly Ash Based Geopolymer Concretes. In HPC/CIC Tromsø; Norway, 2017; Available online: https://www.researchgate.net/profile/David_Law8/publication/314837264_Corrosion_resistance_in_different_fly_ash_based_geopolymer_concretes/links/58ca415792851c4b5e6e36ec/Corrosion-resistance-in-different-fly-ash-based-geopolymer-concretes.pdf (accessed on 18 May 2020).
- Farhana, Z.F.; Kamarudin, H.; Rahmat, A.; Abdullah, M.M.a.; Norainiza, S. Corrosion Performance of Reinforcement Bar in Geopolymer Concrete Compare with its Performance in Ordinary Portland Cement Concrete: A Short Review. Adv. Mater. Res. 2013, 795, 509–512. [Google Scholar] [CrossRef]
- Babaee, M.; Khan, M.S.H.; Castel, A. Passivity of embedded reinforcement in carbonated low-calcium fly ash-based geopolymer concrete. Cem. Concr. Compos. 2018, 85, 32–43. [Google Scholar] [CrossRef]
- Kamsuwan, T.; Srikhirin, T. Effect of Lignosulfonate on Mechanical and Setting Time Properties of Geopolymer Paste. 5th Conference in the Asian Region and Australasian Structural Engineering Conference. Sydney, Australia from 8–12 August 2010. Available online: https://www.researchgate.net/profile/Trithos_Kamsuwan/publication/228912027_EFFECT_OF_LIGNOSULFONATE_ON_MECHANICAL_AND_SETTING_TIME_PROPERTIES_OF_GEOPOLYMER_PASTE/links/0deec52d0355a0abc0000000/EFFECT-OF-LIGNOSULFONATE-ON-MECHANICAL-AND-SETTING-TIME-PROPERTIES-OF-GEOPOLYMER-PASTE.pdf (accessed on 18 May 2020).
- Andrade, C.; Alonso, C. Corrosion rate monitoring in the laboratory and on site. Constr. Build. Mater. 1996, 10, 315–328. [Google Scholar] [CrossRef]
- Li, D.; Chen, Y.; Shen, J.; Su, J.; Wu, X. The influence of alkalinity on activation and microstructure of fly ash. Cem. Concr. Res. 2000, 30, 881–886. [Google Scholar] [CrossRef]
- Tigges, V.E. Die Hydratation von Hüttensanden und Möglichkeiten ihrer Beeinflussung zur Optimierung von Hochofenzementeigenschafte. Ph.D. Thesis, TU Clausthal, Clausthal, Germany, 2009. [Google Scholar]
- Rodrigues, F.A.; Monteiro, P.J.M.; Sposito, G. The alkali-silica reaction: The effect of monovalent and bivalent cations on surface charge density of opal. Cem. Concr. Res. 2001, 31, 1549–1552. [Google Scholar] [CrossRef]
- Rivard, P.; Bérubé, M.A.; Ollivier, J.P.; Ballivy, G. Decrease of pore solution alkalinity in concrete tested for alkali-silica reaction. Mater. Struct. 2007, 40, 909–921. [Google Scholar] [CrossRef]
- Iler, R.K. The Chemistry of Silica—Solubility, Polymerization, Colloid and Surface Properties, and Biochemistry; Wiley & Sons Ltd.: Hoboken, NJ, USA, 2004. [Google Scholar]
- Song, S.; Jennings, H.M. Pore solution chemistry of alkali-activated ground granulated blast-furnace slag11This paper was originally submitted to Advanced Cement Based Materials. The paper was received at the Editorial Office of Cement and Concrete Research on 12 November 1998 and accepted in final form on 16 November 1998. Cem. Concr. Res. 1999, 29, 159–170. [Google Scholar]
- Puertas, F.; Fernández-Jiménez, A.; Blanco-Varela, M.T. Pore solution in alkali-activated slag cement pastes. Relation to the composition and structure of calcium silicate hydrate. Cem. Concr. Res. 2004, 34, 139–148. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, A.; Sobrados, I.; Sanz, J. The role played by the reactive alumina content in the alkaline activation of fly ashes. Microporous Mesoporous Mater. 2006, 91, 111–119. [Google Scholar] [CrossRef]
- Rahier, H.; van Mele, B.; Biesemans, M.; Wastiels, J.; Wu, X. Low-temperature synthesized aluminosilicate glasses. J. Mater. Sci. 1996, 31, 71–79. [Google Scholar] [CrossRef]
- Catalfamo, P.; di Pasquale, S.; Corigliano, F.; Mavilia, L. Influence of the calcium content on the coal fly ash features in some innovative applications, Resources. Conserv. Recycl. 1997, 20, 119–125. [Google Scholar] [CrossRef]
- Puligilla, S.; Mondal, P. Role of slag in microstructural development and hardening of fly ash-slag geopolymer. Cem. Concr. Res. 2013, 43, 70–80. [Google Scholar] [CrossRef]
- Garcia-Lodeiro, I.; Palomo, A.; Fernández-Jiménez, A.; Macphee, D.E. Compatibility studies between N-A-S-H and C-A-S-H gels. Study in the ternary diagram Na2O–CaO–Al2O3–SiO2–H2O. Cem. Concr. Res. 2011, 41, 923–931. [Google Scholar] [CrossRef]
- Meftah, M.; Oueslati, W.; Amara, A.B.H. Synthesis process of zeolite P using a poorly crystallized kaolinite. Phys. Procedia 2009, 2, 1081–1086. [Google Scholar] [CrossRef]
- Querol, X.; Moreno, N.; Umaña, J.C.; Alastuey, A.; Hernández, E.; López-Soler, A.; Plana, F. Synthesis of zeolites from coal fly ash: An overview. Int. J. Coal Geol. 2002, 50, 413–423. [Google Scholar] [CrossRef]
- Lin, C.-F.; Hsi, H.-C. Resource recovery of waste fly ash: Synthesis of zeolite-like materials. Environ. Sci. Technol. 1995, 29, 1109–1117. [Google Scholar] [CrossRef]
- Villain, G.; Thiery, M. Impact of carbonation on microstructure and transport properties of concrete. In Proceedings of the DBMC 10th International Conference on Durability, Lyon, France, 20 April 2005. [Google Scholar]
- Bente, K.; Berthold, C.; Keuper, M.; Gerdes, A.; Ansorge, J.; König, A. Die Korallenperlenkette aus Greifswald von um 1300-archäometrische Untersuchungen an Corallium rubrum aus einer mittelalterlichen Hansestadt: The coral pearl necklace from Greifswald from around 1300-archaeometric investigations on Corallium rubrum from a medieval Hanseatic town. In Archäologische Berichte aus Mecklenburg-Vorpommern; pp. 69–79. Available online: https://www.researchgate.net/profile/Klaus_Bente/publication/320211944_The_coral_pearl_necklace_from_Greifswald_from_around_1300_-_archaeometric_investigations_on_Corallium_rubrum_from_a_medieval_Hanseatic_town/links/59d4fb3caca2721f436ffbf1/The-coral-pearl-necklace-from-Greifswald-from-around-1300-archaeometric-investigations-on-Corallium-rubrum-from-a-medieval-Hanseatic-town.pdf (accessed on 18 May 2020).
- Ries, J.B. Review: Geological and experimental evidence for secular variation in seawater Mg/Ca (calcite-aragonite seas) and its effects on marine biological calcification. Biogeosciences 2010, 7, 2795–2849. [Google Scholar] [CrossRef]
- Railsback, L.B. Some Fundamentals of Mineralogy and Geochemistry, Internet-Book, University of Georgia; USA. 2006. Available online: http://railsback.org/FundamentalsIndex.html (accessed on 18 May 2020).
- Davidovits, J. Geopolymer Chemistry and Applications, 4th ed.; Geopolymer Institute: Saint-Quentin, France, 2015. [Google Scholar]
- Law, D.W.; Adam, A.A.; Molyneaux, T.K.; Patnaikuni, I.; Wardhono, A. Long term durability properties of class F fly ash geopolymer concrete. Mater. Struct. 2015, 48, 721–731. [Google Scholar] [CrossRef]
- Temuujin, J.; van Riessen, A.; Williams, R. Influence of calcium compounds on the mechanical properties of fly ash geopolymer pastes. J. Hazard. Mater. 2009, 167, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Castellote, M.; Fernandez, L.; Andrade, C.; Alonso, C. Chemical changes and phase analysis of OPC pastes carbonated at different CO2 concentrations. Mater. Struct. 2009, 42, 515–525. [Google Scholar] [CrossRef]
- Wang, W.; Chen, H.; Li, X.; Zhu, Z. Corrosion behavior of steel bars immersed in simulated pore solutions of alkali-activated slag mortar. Constr. Build. Mater. 2017, 143, 289–297. [Google Scholar] [CrossRef]
- Holleman, A.F.; Wiberg, E.; Wiberg, N. Lehrbuch der Anorganischen Chemie: [mit 188 Tabellen], 102nd ed.; de Gruyter: Berlin, Germany, 2007. [Google Scholar]
- Nguyen, A.D.; Škvára, F. The influence of ambient pH on fly ash-based geopolymer. Cem. Concr. Compos. 2016, 72, 275–283. [Google Scholar] [CrossRef]
- Koenig, A. Analysis of air voids in cementitious materials using micro X-ray computed tomography (µXCT). Constr. Build. Mater. 2020, 244, 118313. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |










| Raw Material | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | K2O | Na2O | LOI | SSA | X-Ray Amorphous |
|---|---|---|---|---|---|---|---|---|---|---|---|
| FA | 52.85 | 25.57 | 9.41 | 3.26 | 1.74 | 0.48 | 2.42 | 1.44 | 1.55 | 1.06 | 63.2 |
| S | 35.35 | 11.18 | 0.57 | 40.33 | 6.83 | 2.69 | 0.68 | 0.18 | - | 3.00 | 98.8 |
| OPC | 20.10 | 4.82 | 3.20 | 63.5 | 3.46 | 1.27 | 0.99 | 0.21 | 2.22 | 3.11 | 4.0 |
| Scale | Components | Concrete in kg/m3 | Mortar in g | ||||
|---|---|---|---|---|---|---|---|
| Components | OPC | AAFA | AAFAS | OPC | AAFA | AAFAS | |
| Binder | CEM I 42.5 R | 360 | - | - | 569 | - | - |
| Fly Ash | - | 488 | 443 | - | 724 | 654 | |
| Blast furnace slag | - | - | 45 | - | - | 73 | |
| Activator | Sodium silicate (Na2O/SiO2 = 0.9) | - | 43 | 43 | - | 182 | 182 |
| NaOH (30 %) | - | 20 | 20 | - | 99 | 99 | |
| Water | Total water | 162 | 142 | 142 | 256 | 210 | 210 |
| Additional water | 162 | 15 | 15 | 256 | 23 | 23 | |
| w/b-value | 0.45 | 0.27 | 0.35 | 0.45 | 0.27 | 0.35 | |
| SP | LSN | 0.7 | 2.5 | 12.2 | - | 1.5 | 5.0 |
| Aggregates | Sand 0–2 mm | 735 | 689 | 691 | 1203 | 1032 | 1040 |
| Gravel 2–8 mm | 551 | 517 | 518 | - | - | - | |
| Gravel 8–16 mm | 551 | 517 | 518 | - | - | - | |
| Properties | Units | OPC | AAFA | AAFAS |
|---|---|---|---|---|
| Fresh concretes | ||||
| Spread flow (EN 12350-5) | mm | 460 | 580 | 480 |
| Air content (EN 12350-7) | vol.-% | 2.6 | 2.2 | 2.6 |
| Bulk density (EN 12350-6) | kg/m3 | 2.358 | 2.325 | 2.341 |
| Hardened concretes (cubes 100 × 100 × 100 mm3) | ||||
| Compressive strength 28d | N/mm2 | 49.4 | 19.4 | 30.5 |
| Compressive strength 56d | N/mm2 | 56.5 | 27.0 | 34.7 |
| Compressive strength 112d | N/mm2 | 61.9 | 30.4 | 36.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koenig, A.; Mahmoud, H.; Baehre, O.; Dehn, F. Alkalinity and Its Consequences for the Performance of Steel-Reinforced Geopolymer Materials. Molecules 2020, 25, 2359. https://doi.org/10.3390/molecules25102359
Koenig A, Mahmoud H, Baehre O, Dehn F. Alkalinity and Its Consequences for the Performance of Steel-Reinforced Geopolymer Materials. Molecules. 2020; 25(10):2359. https://doi.org/10.3390/molecules25102359
Chicago/Turabian StyleKoenig, Andreas, Hitham Mahmoud, Oliver Baehre, and Frank Dehn. 2020. "Alkalinity and Its Consequences for the Performance of Steel-Reinforced Geopolymer Materials" Molecules 25, no. 10: 2359. https://doi.org/10.3390/molecules25102359
APA StyleKoenig, A., Mahmoud, H., Baehre, O., & Dehn, F. (2020). Alkalinity and Its Consequences for the Performance of Steel-Reinforced Geopolymer Materials. Molecules, 25(10), 2359. https://doi.org/10.3390/molecules25102359








