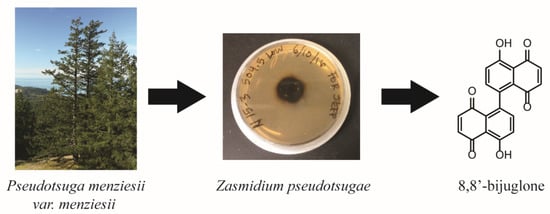
Chemical, Bioactivity, and Biosynthetic Screening of Epiphytic Fungus Zasmidium pseudotsugae
Abstract
1. Introduction
2. Results and Discussion
2.1. Isolation and Characterization of 8,8′-Bijuglone (1)
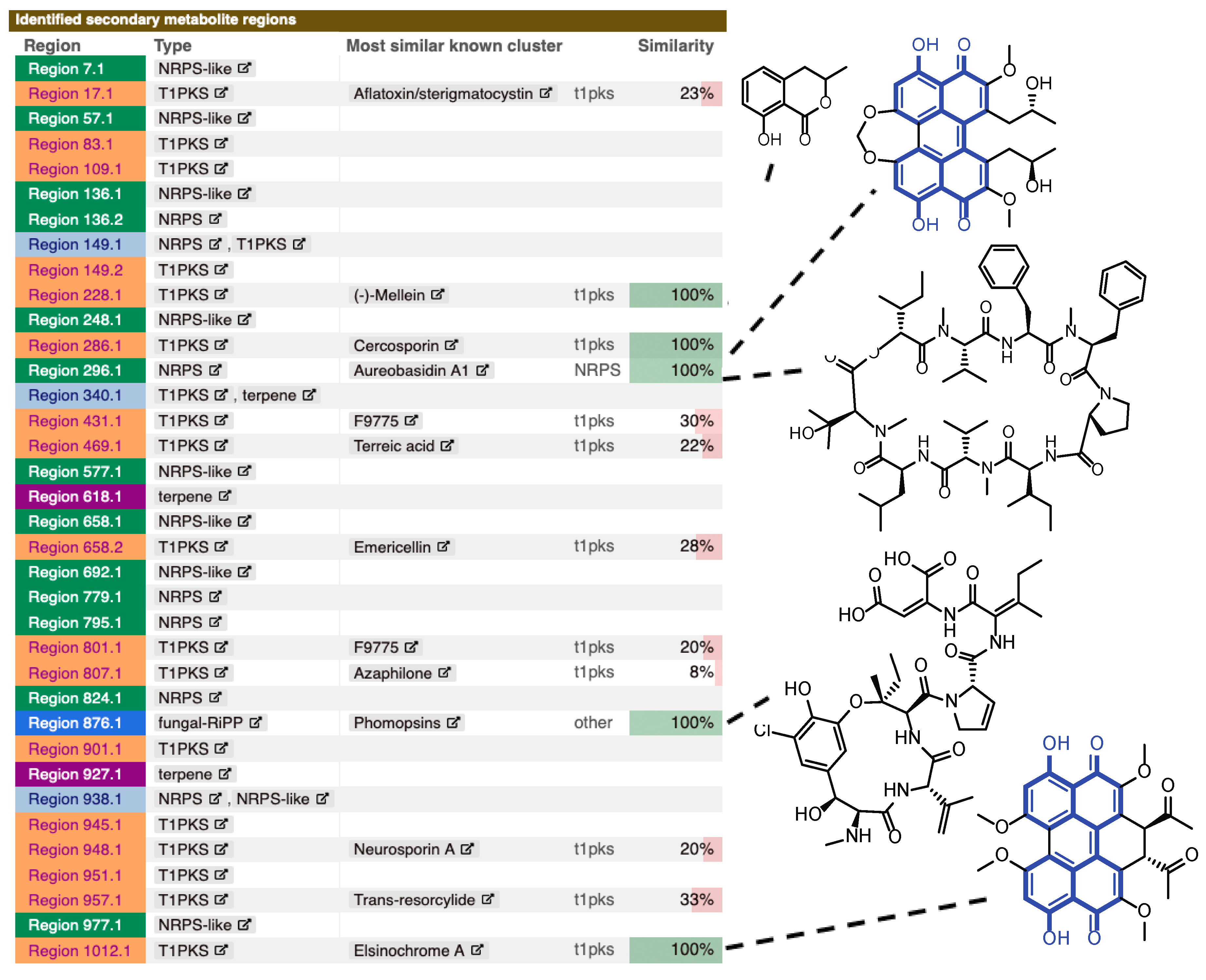
2.2. Genome Analysis of Z. pseudotsugae
2.3. Antimicrobial Activity
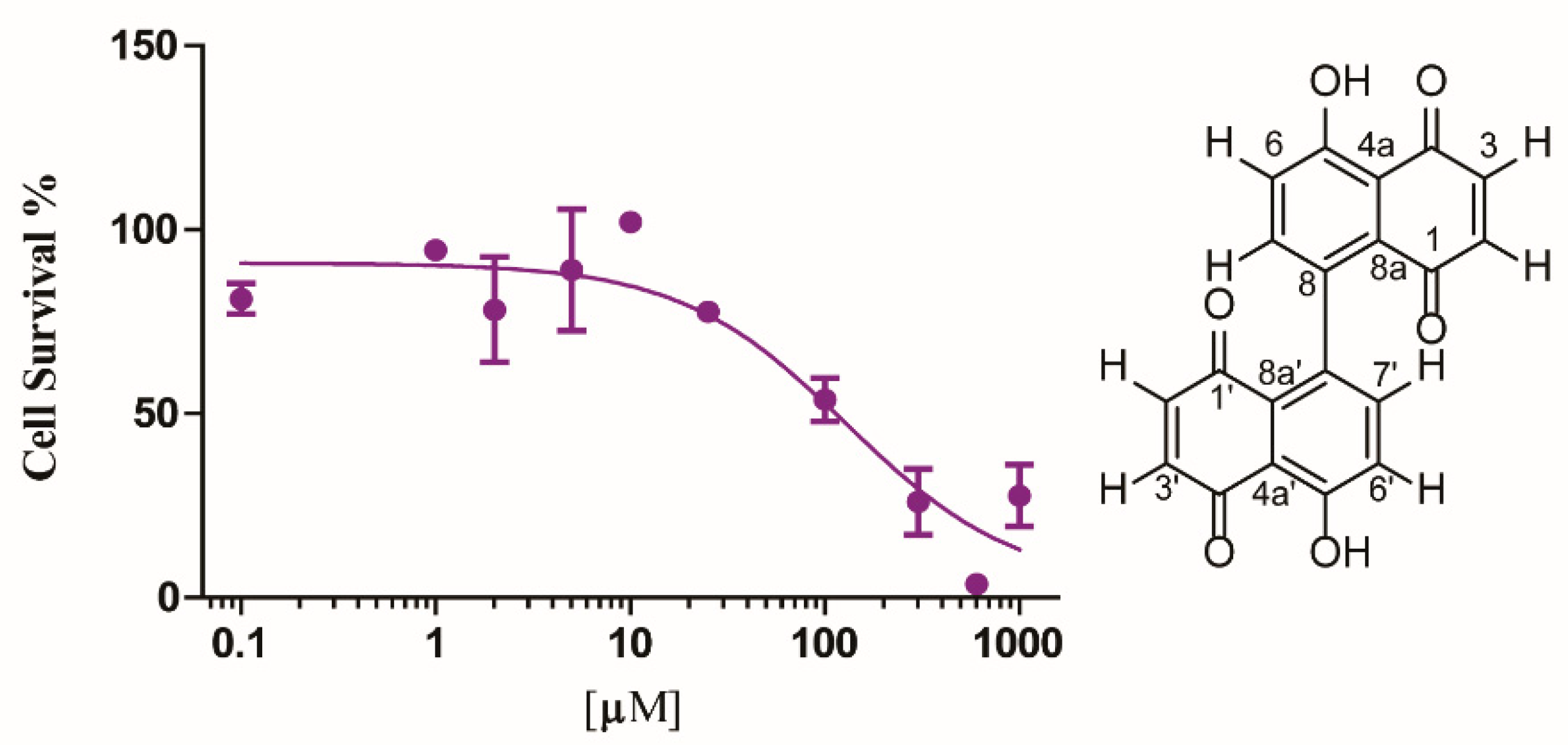
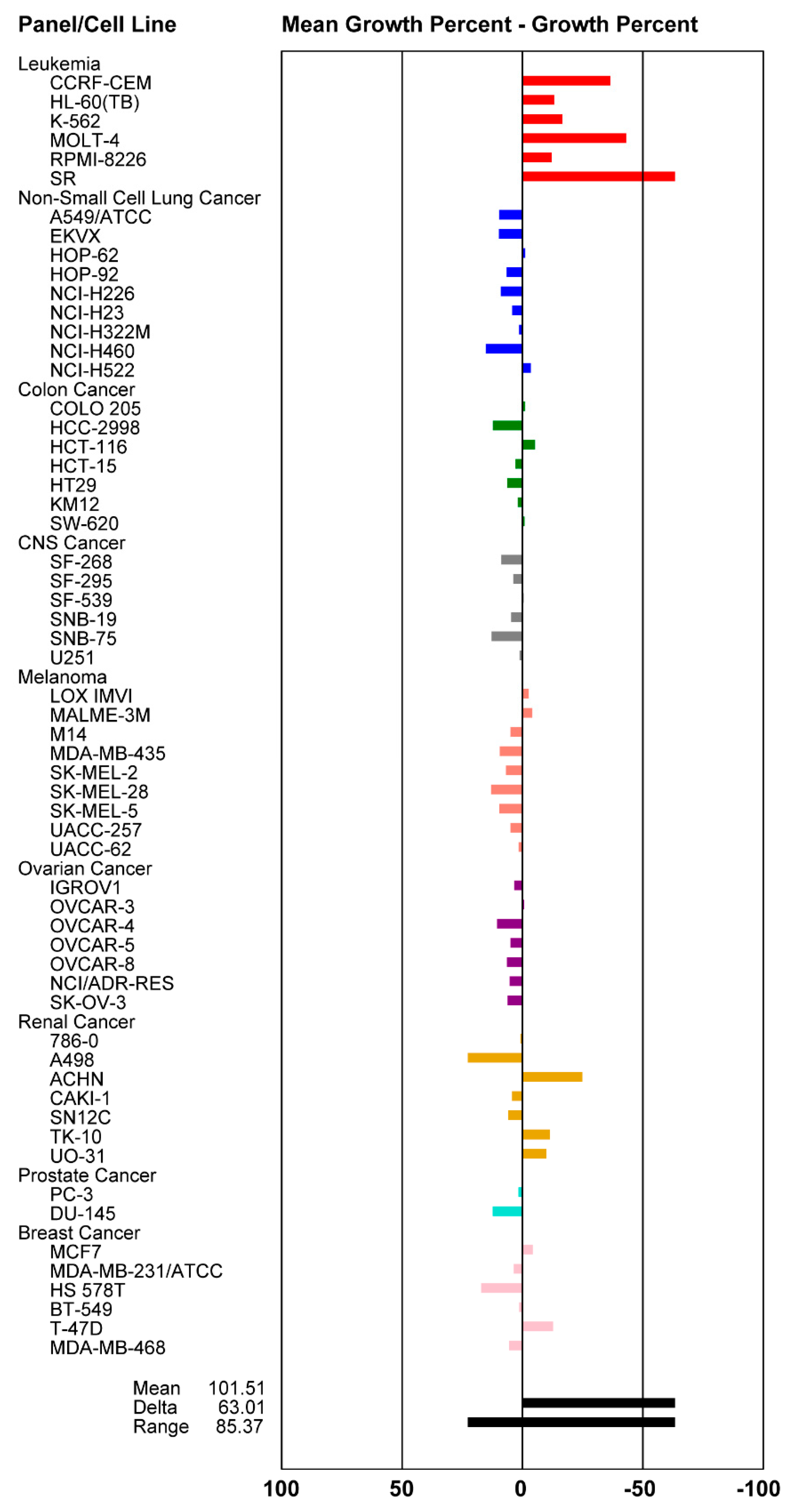
2.4. Cytotoxicity Activity
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Foliage Sampling
3.3. Culture Media
3.4. Preparation of Organic Extracts and Vacuum Liquid Chromatography
3.5. Isolation and Physiochemical Properties of 8,8′-Bijuglone (1)
3.6. Genome Assembly and Bio-Informatics Analysis
3.7. Antimicrobial Assays
3.8. Cytotoxicity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Carroll, G. Fungal endophytes in stems and leaves: From latent pathogen to mutualistic symbiont. Ecology 1988, 69, 2–9. [Google Scholar] [CrossRef]
- Stone, J.K.; Capitano, B.R.; Kerrigan, J.L. The histopathology of Phaeocryptopus gaeumannii on Douglas-fir needles. Mycologia 2008, 100, 431–444. [Google Scholar] [CrossRef] [PubMed]
- Hansen, E.; Stone, J.; Capitano, B.; Rosso, P.; Sutton, W.; Winton, L.; Kanaskie, A.; McWilliams, M. Incidence and impact of Swiss needle cast in forest plantations of Douglas-fir in coastal Oregon. Plant Dis. 2000, 84, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Kimberley, M.O.; Hood, I.A.; Knowles, R.L. Impact of Swiss needle-cast on growth of Douglas-fir. Phytopathology 2011, 101, 583–593. [Google Scholar] [CrossRef]
- Winton, L.; Manter, D.; Stone, J.; Hansen, E. Comparison of biochemical, molecular, and visual methods to quantify Phaeocryptopus gaeumannii in Douglas-Fir foliage. Phytopathology 2003, 93, 121–126. [Google Scholar] [CrossRef]
- Winton, L.M.; Stone, J.K.; Hansen, E.M.; Shoemaker, R. The systematic position of Phaeocryptopus gaeumannii. Mycologia 2007, 99, 240–252. [Google Scholar] [CrossRef]
- Bennett, P.; Stone, J. Assessments of population structure, diversity, and phylogeography of the Swiss needle cast fungus (Phaeocryptopus gaeumannii) in the US Pacific Northwest. Forests 2016, 7, 14. [Google Scholar] [CrossRef]
- Ritóková, G.; Shaw, D.; Filip, G.; Kanaskie, A.; Browning, J.; Norlander, D. Swiss needle cast in western Oregon Douglas-fir plantations: 20-year monitoring results. Forests 2016, 7, 155. [Google Scholar] [CrossRef]
- Bradshaw, R. Dothistroma (red-band) needle blight of pines and the dothistromin toxin: A review. For. Pathol. 2004, 34, 163–185. [Google Scholar] [CrossRef]
- Ma, L.-J.; Geiser, D.M.; Proctor, R.H.; Rooney, A.P.; O’Donnell, K.; Trail, F.; Gardiner, D.M.; Manners, J.M.; Kazan, K. Fusarium pathogenomics. Annu. Rev. Microbiol. 2013, 67, 399–416. [Google Scholar] [CrossRef]
- de Wit, P.J. Cladosporium fulvum effectors: Weapons in the arms race with tomato. Annu. Rev. Phytopathol. 2016, 54, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Videira, S.; Groenewald, J.; Nakashima, C.; Braun, U.; Barreto, R.W.; de Wit, P.J.; Crous, P. Mycosphaerellaceae–chaos or clarity? Stud. Mycol. 2017, 87, 257–421. [Google Scholar] [CrossRef] [PubMed]
- Noar, R.D.; Daub, M.E. Bioinformatics prediction of polyketide synthase gene clusters from Mycosphaerella fijiensis. PLoS ONE 2016, 11, e0158471. [Google Scholar] [CrossRef]
- Medina, R.; Lucentini, C.G.; Franco, M.E.; Petroselli, G.; Rosso, J.A.; Erra-Balsells, R.; Balatti, P.A.; Saparrat, M.C. Identification of an intermediate for 1, 8-dihydroxynaphthalene-melanin synthesis in a race-2 isolate of Fulvia fulva (syn. Cladosporium fulvum). Heliyon 2018, 4, e01036. [Google Scholar] [CrossRef] [PubMed]
- Noar, R.D.; Daub, M.E. Transcriptome sequencing of Mycosphaerella fijiensis during association with Musa acuminata reveals candidate pathogenicity genes. BMC Genom. 2016, 17, 690. [Google Scholar] [CrossRef] [PubMed]
- Muria-Gonzalez, M.J.; Chooi, Y.H.; Breen, S.; Solomon, P.S. The past, present and future of secondary metabolite research in the D othideomycetes. Mol. Plant Pathol. 2015, 16, 92–107. [Google Scholar] [CrossRef]
- Adpressa, D.A.; Loesgen, S. Bioprospecting chemical diversity and bioactivity in a marine derived Aspergillus terreus. Chem. Biodivers. 2016, 13, 253–259. [Google Scholar] [CrossRef]
- Mandelare, P.E.; Adpressa, D.A.; Kaweesa, E.N.; Zakharov, L.N.; Loesgen, S. Coculture of Two Developmental Stages of a Marine-Derived Aspergillus alliaceus Results in the Production of the Cytotoxic Bianthrone Allianthrone A. J. Nat. Prod. 2018, 81, 1014–1022. [Google Scholar] [CrossRef]
- Gunatilaka, A.L. Natural products from plant-associated microorganisms: Distribution, structural diversity, bioactivity, and implications of their occurrence. J. Nat. Prod. 2006, 69, 509–526. [Google Scholar] [CrossRef]
- Arnold, A.E. Understanding the diversity of foliar endophytic fungi: Progress, challenges, and frontiers. Fungal Biol. Rev. 2007, 21, 51–66. [Google Scholar] [CrossRef]
- Kusari, S.; Hertweck, C.; Spiteller, M. Chemical ecology of endophytic fungi: Origins of secondary metabolites. Chem. Biol. 2012, 19, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Gouda, S.; Das, G.; Sen, S.K.; Shin, H.-S.; Patra, J.K. Endophytes: A Treasure House of Bioactive Compounds of Medicinal Importance. Front. Microbiol. 2016, 7, 1538. [Google Scholar] [CrossRef] [PubMed]
- Sieber, C.M.; Lee, W.; Wong, P.; Münsterkötter, M.; Mewes, H.-W.; Schmeitzl, C.; Varga, E.; Berthiller, F.; Adam, G.; Güldener, U. The Fusarium graminearum genome reveals more secondary metabolite gene clusters and hints of horizontal gene transfer. PLoS ONE 2014, 9, e110311. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.C.; Grijseels, S.; Prigent, S.; Ji, B.; Dainat, J.; Nielsen, K.F.; Frisvad, J.C.; Workman, M.; Nielsen, J. Global analysis of biosynthetic gene clusters reveals vast potential of secondary metabolite production in Penicillium species. Nat. Microbiol. 2017, 2, 17044. [Google Scholar] [CrossRef] [PubMed]
- Lyu, H.-N.; Liu, H.-W.; Keller, N.P.; Yin, W.-B. Harnessing diverse transcriptional regulators for natural product discovery in fungi. Nat. Prod. Rep. 2019. [Google Scholar] [CrossRef] [PubMed]
- Laatsch, H. Dimere Naphthochinone, XVI. Synthese von Maritinon und anderen 8, 8′-Bijuglonen. Liebigs Annalen der Chemie 1985, 1985, 2420–2442. [Google Scholar] [CrossRef]
- Bao, N.; Ou, J.; Shi, W.; Li, N.; Chen, L.; Sun, J. Highly Efficient Synthesis and Structure–Activity Relationships of a Small Library of Substituted 1, 4-Naphthoquinones. Eur. J. Org. Chem. 2018, 2018, 2254–2258. [Google Scholar] [CrossRef]
- Tezuka, M.; Takahashi, C.; Kuroyanagi, M.; Satake, M.; Yoshihira, K.; Natori, S. New naphthoquinones from Diospyros. Phytochemistry 1973, 12, 175–183. [Google Scholar] [CrossRef]
- Aldemir, H.; Richarz, R.; Gulder, T.A. The biocatalytic repertoire of natural biaryl formation. Angew. Chem. Int. Ed. 2014, 53, 8286–8293. [Google Scholar] [CrossRef]
- Gu, J.-Q.; Graf, T.N.; Lee, D.; Chai, H.-B.; Mi, Q.; Kardono, L.B.S.; Setyowati, F.M.; Ismail, R.; Riswan, S.; Farnsworth, N.R.; et al. Cytotoxic and Antimicrobial Constituents of the Bark of Diospyros maritima Collected in Two Geographical Locations in Indonesia. J. Nat. Prod. 2004, 67, 1156–1161. [Google Scholar] [CrossRef]
- Higa, M.; Takashima, Y.; Yokaryo, H.; Harie, Y.; Suzuka, T.; Ogihara, K. Naphthoquinone Derivatives from Diospyros maritima. Chem. Pharm. Bull. 2017, 65, 739–745. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lunardelli Negreiros de Carvalho, P.; de Oliveira Silva, E.; Aparecida Chagas-Paula, D.; Honorata Hortolan Luiz, J.; Ikegaki, M. Importance and implications of the production of phenolic secondary metabolites by endophytic fungi: A mini-review. Mini Rev. Med. Chem. 2016, 16, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Blin, K.; Shaw, S.; Steinke, K.; Villebro, R.; Ziemert, N.; Lee, S.Y.; Medema, M.H.; Weber, T. antiSMASH 5.0: Updates to the secondary metabolite genome mining pipeline. Nucleic Acids Res. 2019, 47, W81–W87. [Google Scholar] [CrossRef] [PubMed]
- Medema, M.H.; Kottmann, R.; Yilmaz, P.; Cummings, M.; Biggins, J.B.; Blin, K.; De Bruijn, I.; Chooi, Y.H.; Claesen, J.; Coates, R.C. Minimum information about a biosynthetic gene cluster. Nat. Chem. Biol. 2015, 11, 625. [Google Scholar] [CrossRef] [PubMed]
- Jiao, W.; Liu, L.; Zhou, R.; Xu, M.; Xiao, D.; Xue, C. Elsinochrome phytotoxin production and pathogenicity of Elsinoë arachidis isolates in China. PLoS ONE 2019, 14, e0218391. [Google Scholar] [CrossRef] [PubMed]
- Chooi, Y.H.; Zhang, G.; Hu, J.; Muria-Gonzalez, M.J.; Tran, P.N.; Pettitt, A.; Maier, A.G.; Barrow, R.A.; Solomon, P.S. Functional genomics-guided discovery of a light-activated phytotoxin in the wheat pathogen Parastagonospora nodorum via pathway activation. Environ. Microbiol. 2017, 19, 1975–1986. [Google Scholar] [CrossRef]
- Hu, J.; Sarrami, F.; Li, H.; Zhang, G.; Stubbs, K.A.; Lacey, E.; Stewart, S.G.; Karton, A.; Piggott, A.M.; Chooi, Y.-H. Heterologous biosynthesis of elsinochrome A sheds light on the formation of the photosensitive perylenequinone system. Chem. Sci. 2019, 10, 1457–1465. [Google Scholar] [CrossRef]
- Görner, H. Photoreactions of p-Quinones with Dimethyl Sulfide and Dimethyl Sulfoxide in Aqueous Acetonitrile. Photochem. Photobiol. 2006, 82, 71–77. [Google Scholar] [CrossRef]
- Zhu, X.-Q.; Wang, C.-H. Accurate estimation of the one-electron reduction potentials of various substituted quinones in DMSO and CH3CN. J. Org. Chem. 2010, 75, 5037–5047. [Google Scholar] [CrossRef]
- Widhalm, J.R.; Rhodes, D. Biosynthesis and molecular actions of specialized 1,4-naphthoquinone natural products produced by horticultural plants. Hortic. Res. 2016, 3, 16046. [Google Scholar] [CrossRef]
- Elhabiri, M.; Sidorov, P.; Cesar-Rodo, E.; Marcou, G.; Lanfranchi, D.A.; Davioud-Charvet, E.; Horvath, D.; Varnek, A. Electrochemical properties of substituted 2-methyl-1,4-naphthoquinones: Redox behavior predictions. Chem.-A Eur. J. 2015, 21, 3415–3424. [Google Scholar] [CrossRef] [PubMed]
- Salae, A.-W.; Karalai, C.; Ponglimanont, C.; Kanjana-Opas, A.; Yuenyongsawad, S. Naphthalene derivatives from Diospyros wallichii. Can. J. Chem. 2010, 88, 922–927. [Google Scholar] [CrossRef]
- McGaw, L.J.; Lall, N.; Hlokwe, T.M.; Michel, A.L.; Meyer, J.J.M.; Eloff, J.N. Purified compounds and extracts from Euclea species with antimycobacterial activity against Mycobacterium bovis and fast-growing mycobacteria. Biol. Pharm. Bull. 2008, 31, 1429–1433. [Google Scholar] [CrossRef] [PubMed]
- Shoemaker, R.H. The NCI60 human tumour cell line anticancer drug screen. Nat. Rev. Cancer 2006, 6, 813. [Google Scholar] [CrossRef] [PubMed]
- Babij, N.R.; McCusker, E.O.; Whiteker, G.T.; Canturk, B.; Choy, N.; Creemer, L.C.; Amicis, C.V.D.; Hewlett, N.M.; Johnson, P.L.; Knobelsdorf, J.A. NMR chemical shifts of trace impurities: Industrially preferred solvents used in process and green chemistry. Org. Process Res. Dev. 2016, 20, 661–667. [Google Scholar] [CrossRef]
- Simpson, J.T.; Wong, K.; Jackman, S.D.; Schein, J.E.; Jones, S.J.; Birol, I. ABySS: A parallel assembler for short read sequence data. Genome Res. 2009, 19, 1117–1123. [Google Scholar] [CrossRef]
- Plitzko, B.; Kaweesa, E.N.; Loesgen, S. The natural product mensacarcin induces mitochondrial toxicity and apoptosis in melanoma cells. J. Biol. Chem. 2017, 292, 21102–21116. [Google Scholar] [CrossRef]
- Eide, C.A.; O’Hare, T. Chronic myeloid leukemia: Advances in understanding disease biology and mechanisms of resistance to tyrosine kinase inhibitors. Curr. Hematol. Malig. Rep. 2015, 10, 158–166. [Google Scholar] [CrossRef]
Sample Availability: Enriched extract is available on request. |
| Compound | 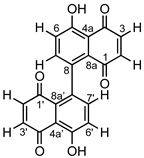 | ||
|---|---|---|---|
| Position | δC | Type | δH, mult (J in Hz) |
| 1, 1′ | 184.9 | C | - |
| 2, 2′ | 140.6 | CH | 6.71, d (10.2 Hz) |
| 3, 3′ | 138.0 | CH | 6.92, d (10.2 Hz) |
| 4, 4′ | 190.8 | C | - |
| 4a, 4a′ | 115.5 | C | - |
| 5, 5′ | 161.9 | C | - |
| 6, 6′ | 124.8 | CH | 7.31, d (8.7 Hz) |
| 7, 7′ | 138.7 | CH | 7.24, d (8.7 Hz) |
| 8, 8′ | 135.2 | C | - |
| 8a, 8a′ | 128.3 | C | - |
| 5-OH, 5′-OH | - | - | 12.49, s |
| Sample 1 | Antibacterial 2 | Antifungal 2 | ||||
|---|---|---|---|---|---|---|
| MRSA | Bacillus subtilis | Mycobacterium smegmatis | Escherichia coli | Pseudomonas aeruginosa | Candida albicans | |
| 8,8′-bijuglone | 29.3% | 32.6% | 91.5% | 97.1% | 86.0% | 99.5% |
| positive control | 0.0% vancomycin | 15.1% chloramphenicol | 1.5% rifampicin | 11.6% ampicillin | 0.2% kanamycin | 23.6% amphotericin B |
| negative control | > 100% ethanol | 68% ethanol | 100% DMSO | 89.0% ethanol | 92.4% ethanol | 100% ethanol |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Montiel, G.A.; Kaweesa, E.N.; Feau, N.; Hamelin, R.C.; Stone, J.K.; Loesgen, S. Chemical, Bioactivity, and Biosynthetic Screening of Epiphytic Fungus Zasmidium pseudotsugae. Molecules 2020, 25, 2358. https://doi.org/10.3390/molecules25102358
González-Montiel GA, Kaweesa EN, Feau N, Hamelin RC, Stone JK, Loesgen S. Chemical, Bioactivity, and Biosynthetic Screening of Epiphytic Fungus Zasmidium pseudotsugae. Molecules. 2020; 25(10):2358. https://doi.org/10.3390/molecules25102358
Chicago/Turabian StyleGonzález-Montiel, Gisela A., Elizabeth N. Kaweesa, Nicolas Feau, Richard C. Hamelin, Jeffrey K. Stone, and Sandra Loesgen. 2020. "Chemical, Bioactivity, and Biosynthetic Screening of Epiphytic Fungus Zasmidium pseudotsugae" Molecules 25, no. 10: 2358. https://doi.org/10.3390/molecules25102358
APA StyleGonzález-Montiel, G. A., Kaweesa, E. N., Feau, N., Hamelin, R. C., Stone, J. K., & Loesgen, S. (2020). Chemical, Bioactivity, and Biosynthetic Screening of Epiphytic Fungus Zasmidium pseudotsugae. Molecules, 25(10), 2358. https://doi.org/10.3390/molecules25102358