Comparative Antiviral Activity of Remdesivir and Anti-HIV Nucleoside Analogs against Human Coronavirus 229E (HCoV-229E)
Abstract
1. Introduction
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. General Reagents
4.2. Anti-Coronavirus Cytoprotection Assay
4.2.1. Cell Preparation
4.2.2. Virus Preparation
4.2.3. Efficacy and Toxicity
4.2.4. Data Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lim, Y.X.; Ng, Y.L.; Tam, J.P.; Liu, D.X. Human Coronaviruses: A Review of Virus-Host Interactions. Diseases 2016, 4, 26. [Google Scholar] [CrossRef] [PubMed]
- Pene, F.; Merlat, A.; Vabret, A.; Rozenberg, F.; Buzyn, A.; Dreyfus, F.; Cariou, A.; Freymuth, F.; Lebon, P. Coronavirus 229E-related pneumonia in immunocompromised patients. Clin. Infect. Dis. 2003, 37, 929–932. [Google Scholar] [CrossRef] [PubMed]
- Woo, P.C.; Lau, S.K.; Tsoi, H.W.; Huang, Y.; Poon, R.W.; Chu, C.M.; Lee, R.A.; Luk, W.K.; Wong, G.K.; Wong, B.H.; et al. Clinical and molecular epidemiological features of coronavirus HKU1-associated community-acquired pneumonia. J. Infect. Dis. 2005, 192, 1898–1907. [Google Scholar] [CrossRef] [PubMed]
- Poutanen, S.M. Human coronaviruses. Princ. Pract. Pediatric Infect. Dis. 2012, 4, 1117–1120. [Google Scholar]
- Vassilara, F.; Spyridaki, A.; Pothitos, G.; Deliveliotou, A.; Papadopoulos, A. A Rare Case of Human Coronavirus 229E Associated with Acute Respiratory Distress Syndrome in a Healthy Adult. Case. Rep. Infect. Dis. 2018, 2018, 6796839. [Google Scholar] [CrossRef] [PubMed]
- de Wit, E.; Feldmann, F.; Cronin, J.; Jordan, R.; Okumura, A.; Thomas, T.; Scott, D.; Cihlar, T.; Feldmann, H. Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proc. Natl. Acad. Sci. USA 2020, 117, 6771–6776. [Google Scholar] [CrossRef] [PubMed]
- Sheahan, T.P.; Sims, A.C.; Graham, R.L.; Menachery, V.D.; Gralinski, L.E.; Case, J.B.; Leist, S.R.; Pyrc, K.; Feng, J.Y.; Trantcheva, I.; et al. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci. Transl. Med. 2017, 9, eaal3653. [Google Scholar] [CrossRef]
- Grein, J.; Ohmagari, N.; Shin, D.; Diaz, G.; Asperges, E.; Castagna, A.; Feldt, T.; Green, G.; Green, M.L.; Lescure, F.X.; et al. Compassionate Use of Remdesivir for Patients with Severe Covid-19. N Engl. J. Med. 2020. [Google Scholar] [CrossRef]
- Agostini, M.L.; Andres, E.L.; Sims, A.C.; Graham, R.L.; Sheahan, T.P.; Lu, X.; Smith, E.C.; Case, J.B.; Feng, J.Y.; Jordan, R.; et al. Coronavirus Susceptibility to the Antiviral Remdesivir (GS-5734) Is Mediated by the Viral Polymerase and the Proofreading Exoribonuclease. mBio 2018, 9, e00221-18. [Google Scholar] [CrossRef]
- Morse, J.S.; Lalonde, T.; Xu, S.; Liu, W.R. Learning from the Past: Possible Urgent Prevention and Treatment Options for Severe Acute Respiratory Infections Caused by 2019-nCoV. Chembiochem 2020, 21, 730–738. [Google Scholar] [CrossRef]
- Ko, W.C.; Rolain, J.M.; Lee, N.Y.; Chen, P.L.; Huang, C.T.; Lee, P.I.; Hsueh, P.R. Arguments in favour of remdesivir for treating SARS-CoV-2 infections. Int. J. Antimicrob. Agents. 2020, 55, 105933. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.J.; Won, J.J.; Graham, R.L.; Dinnon, K.H., 3rd; Sims, A.C.; Feng, J.Y.; Cihlar, T.; Denison, M.R.; Baric, R.S.; Sheahan, T.P. Broad spectrum antiviral remdesivir inhibits human endemic and zoonotic deltacoronaviruses with a highly divergent RNA dependent RNA polymerase. Antiviral. Res. 2019, 169, 104541. [Google Scholar] [CrossRef] [PubMed]
- Tzertzinis, G.; Tabor, S.; Nichols, N.M. RNA-dependent DNA polymerases. Curr. Protoc. Mol. Biol. 2008, 3, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Mulato, A.S.; Cherrington, J.M. Anti-HIV activity of adefovir (PMEA) and PMPA in combination with antiretroviral compounds: In vitro analyses. Antiviral. Res. 1997, 36, 91–97. [Google Scholar] [CrossRef]
- Robbins, B.L.; Srinivas, R.V.; Kim, C.; Bischofberger, N.; Fridland, A. Anti-human immunodeficiency virus activity and cellular metabolism of a potential prodrug of the acyclic nucleoside phosphonate 9-R-(2-phosphonomethoxypropyl)adenine (PMPA), Bis(isopropyloxymethylcarbonyl)PMPA. Antimicrob. Agents. Chemother. 1998, 42, 612–617. [Google Scholar] [CrossRef]
- Ohrui, H.; Kohgo, S.; Hayakawa, H.; Kodama, E.; Matsuoka, M.; Nakata, T.; Mitsuya, H. 2′-deoxy-4′-C-ethynyl-2-fluoroadenosine: A nucleoside reverse transcriptase inhibitor with highly potent activity against wide spectrum of HIV-1 strains, favorable toxic profiles, and stability in plasma. Nucleosides Nucleotides Nucleic Acids 2007, 26, 1543–1546. [Google Scholar] [CrossRef]
- Kageyama, M.; Miyagi, T.; Yoshida, M.; Nagasawa, T.; Ohrui, H.; Kuwahara, S. Concise synthesis of the anti-HIV nucleoside EFdA. Biosci. Biotechnol. Biochem. 2012, 76, 1219–1225. [Google Scholar] [CrossRef]
- Herdewijn, P.; Balzarini, J.; De Clercq, E.; Pauwels, R.; Baba, M.; Broder, S.; Vanderhaeghe, H. 3′-substituted 2′,3′-dideoxynucleoside analogues as potential anti-HIV (HTLV-III/LAV) agents. J. Med. Chem. 1987, 30, 1270–1278. [Google Scholar] [CrossRef]
- Parang, K.; Knaus, E.E.; Wiebe, L.I. Synthesis, in vitro anti-HIV activity, and biological stability of 5′-O-myristoyl analogue derivatives of 3′-fluoro-2′,3′-dideoxythymidine (FLT) as potential bifunctional prodrugs of FLT. Nucleosides Nucleotides 1998, 17, 987–1008. [Google Scholar] [CrossRef]
- Parang, K.; Wiebe, L.I.; Knaus, E.E.; Huang, J.S.; Tyrrell, D.L. In vitro anti-hepatitis B virus activities of 5”-O-myristoyl analogue derivatives of 3”-fluoro-2”,3”-dideoxythymidine (FLT) and 3”-azido-2”,3”-dideoxythymidine (AZT). J Pharm Pharm Sci 1998, 1, 108–114. [Google Scholar]
- Massard, J.; Benhamou, Y. Treatment of chronic hepatitis B in HIV co-infected patients. Gastroenterol. Clin. Biol. 2008, 32, S20–S24. [Google Scholar] [CrossRef]
- Saag, M.S. Emtricitabine, a new antiretroviral agent with activity against HIV and hepatitis B virus. Clin. Infect. Dis. 2006, 42, 126131. [Google Scholar]
- Nelson, M.; Schiavone, M. Emtricitabine (FTC) for the treatment of HIV infection. Int. J. Clin. Pract. 2004, 58, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, H.K.D.G.; Parang, K. Synthesis and anti-HIV activities of phosphate triester derivatives of 3′-fluoro-2′,3′-dideoxythymidine and 3′-azido-2′,3′-dideoxythymidine. Tetrahedron Lett. 2008, 49, 4905–4907. [Google Scholar] [CrossRef]
- Agarwal, H.K.; Loethan, K.; Mandal, D.; Doncel, G.F.; Parang, K. Synthesis and biological evaluation of fatty acyl ester derivatives of 2′,3′-didehydro-2′,3′-dideoxythymidine. Bioorg. Med. Chem. Lett. 2011, 21, 19171721. [Google Scholar] [CrossRef]
- Agarwal, H.K.; Chhikara, B.S.; Hanley, M.J.; Ye, G.; Doncel, G.F.; Parang, K. Synthesis and biological evaluation of fatty acyl ester derivatives of (-)-2′,3′-dideoxy-3′-thiacytidine. J. Med. Chem. 2012, 55, 4861–4871. [Google Scholar] [CrossRef]
- Agarwal, H.K.; Chhikara, B.S.; Bhavaraju, S.; Mandal, D.; Doncel, G.F.; Parang, K. Emtricitabine prodrugs with improved anti-HIV activity and cellular uptake. Mol. Pharm. 2013, 10, 467–476. [Google Scholar] [CrossRef]
- Gao, Y.; Yan, L.; Huang, Y.; Liu, F.; Zhao, Y.; Cao, L.; Wang, T.; Sun, Q.; Ming, Z.; Zhang, L.; et al. Structure of the RNA-dependent RNA polymerase from COVID-19 virus. Science 2020, 368, 779–782. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
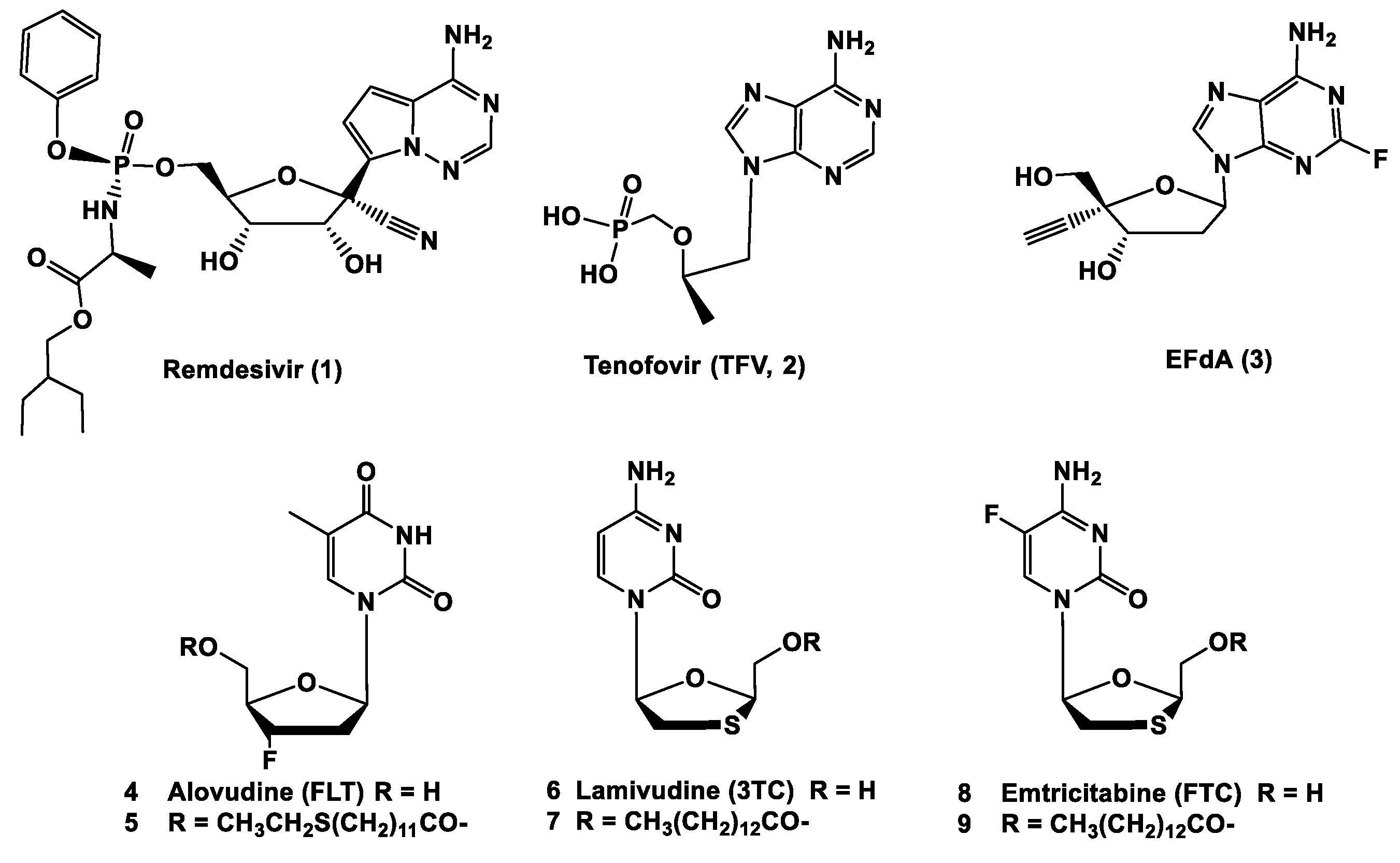

| MRC-5/HCoV-229E | |||
|---|---|---|---|
| Compound | EC50 a (µM) | TC50 b (µM) | Therapeutic Index c |
| Remdesivir (1) | 0.07 | > 2.0 | > 28.6 |
| TFV (2) | > 100 | > 100 | ----- |
| EFdA (3) | > 55.3 | 55.3 | ----- |
| FLT (4) | > 100 | > 100 | ----- |
| 5′-O-(12-thioethydodecanoyl)FLT (5) | > 45.4 | 45.4 | ----- |
| 3TC (6) | > 100 | > 100 | ----- |
| 5′-O-(tetradecanoyl)3TC (7) | > 47.5 | 47.5 | ----- |
| FTC (8) | > 100 | > 100 | ----- |
| 5′-O-(tetradecanoyl)FTC (9) | 72.8 | 87.5 | 1.20 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parang, K.; El-Sayed, N.S.; Kazeminy, A.J.; Tiwari, R.K. Comparative Antiviral Activity of Remdesivir and Anti-HIV Nucleoside Analogs against Human Coronavirus 229E (HCoV-229E). Molecules 2020, 25, 2343. https://doi.org/10.3390/molecules25102343
Parang K, El-Sayed NS, Kazeminy AJ, Tiwari RK. Comparative Antiviral Activity of Remdesivir and Anti-HIV Nucleoside Analogs against Human Coronavirus 229E (HCoV-229E). Molecules. 2020; 25(10):2343. https://doi.org/10.3390/molecules25102343
Chicago/Turabian StyleParang, Keykavous, Naglaa Salem El-Sayed, Assad J. Kazeminy, and Rakesh K. Tiwari. 2020. "Comparative Antiviral Activity of Remdesivir and Anti-HIV Nucleoside Analogs against Human Coronavirus 229E (HCoV-229E)" Molecules 25, no. 10: 2343. https://doi.org/10.3390/molecules25102343
APA StyleParang, K., El-Sayed, N. S., Kazeminy, A. J., & Tiwari, R. K. (2020). Comparative Antiviral Activity of Remdesivir and Anti-HIV Nucleoside Analogs against Human Coronavirus 229E (HCoV-229E). Molecules, 25(10), 2343. https://doi.org/10.3390/molecules25102343








