Sulfur Compounds Inhibit High Glucose-Induced Inflammation by Regulating NF-κB Signaling in Human Monocytes
Abstract
1. Introduction
2. Results
2.1. Sulfur Compounds Inhibit the High Glucose-Induced Expression of TLRs
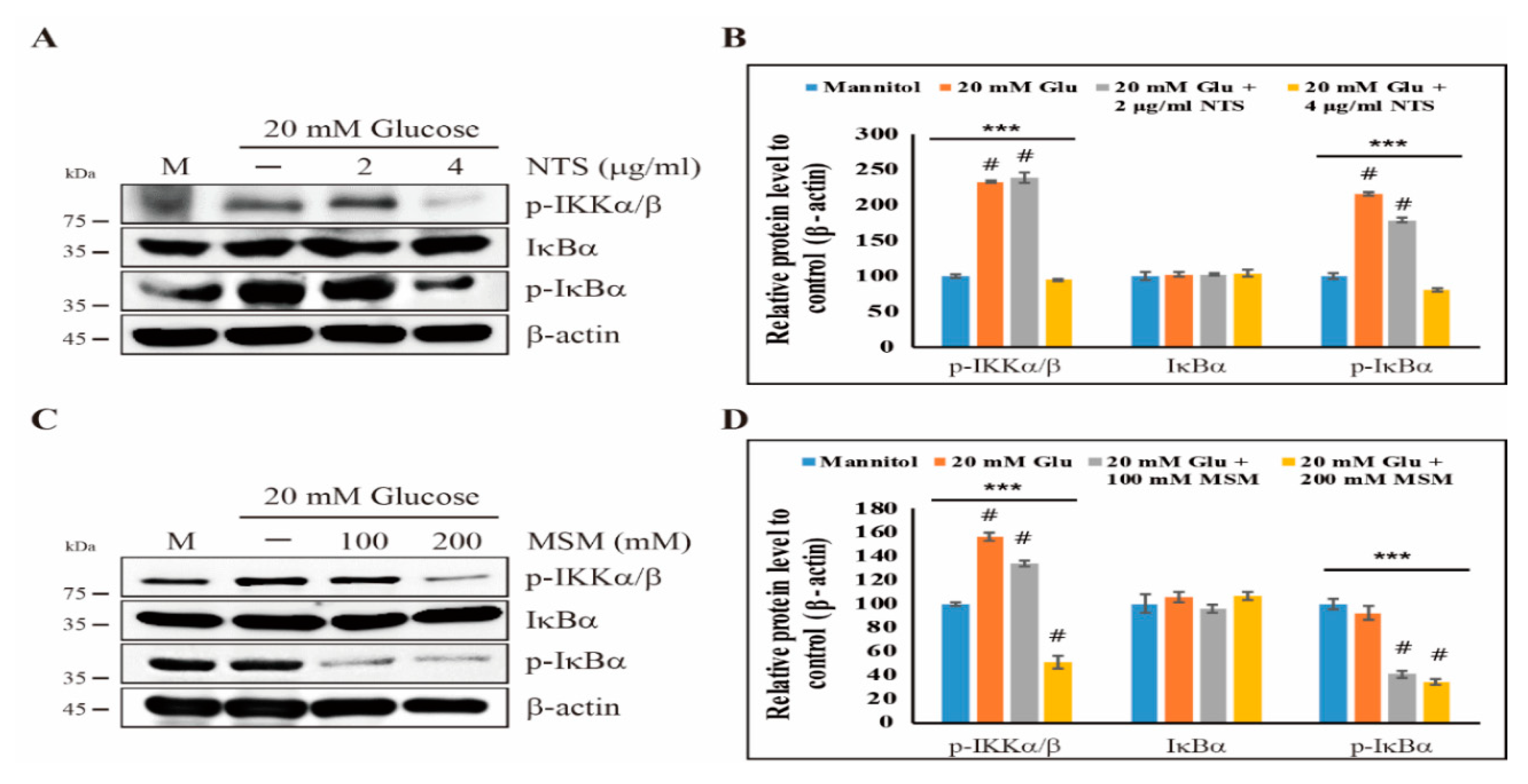
2.2. Downregulation of the Canonical NF-κB Pathway by Sulfur Compounds
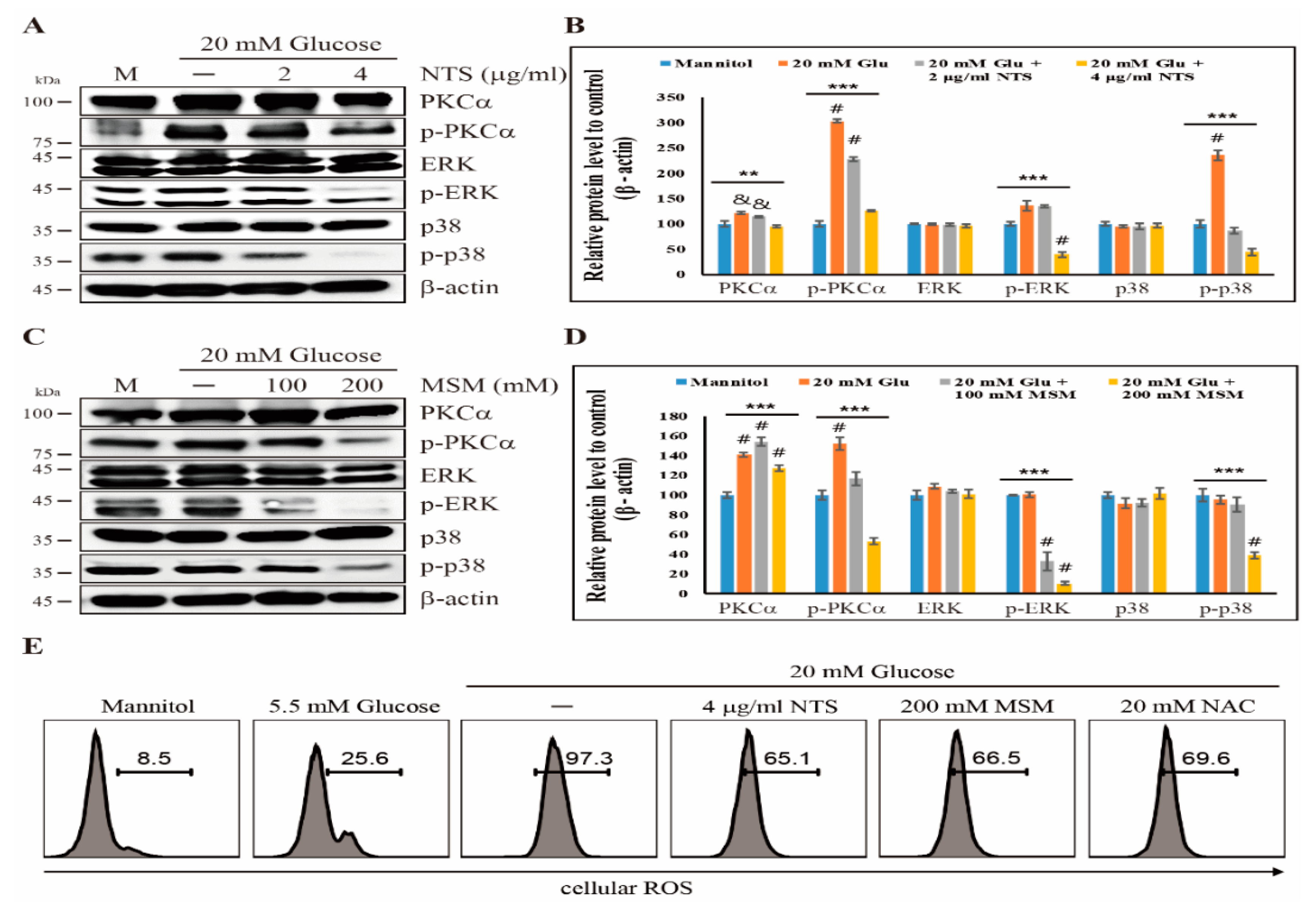
2.3. Sulfur Compounds Downregulates ROS-Dependent PKC-Mediated Upstream Targets of NF-κB
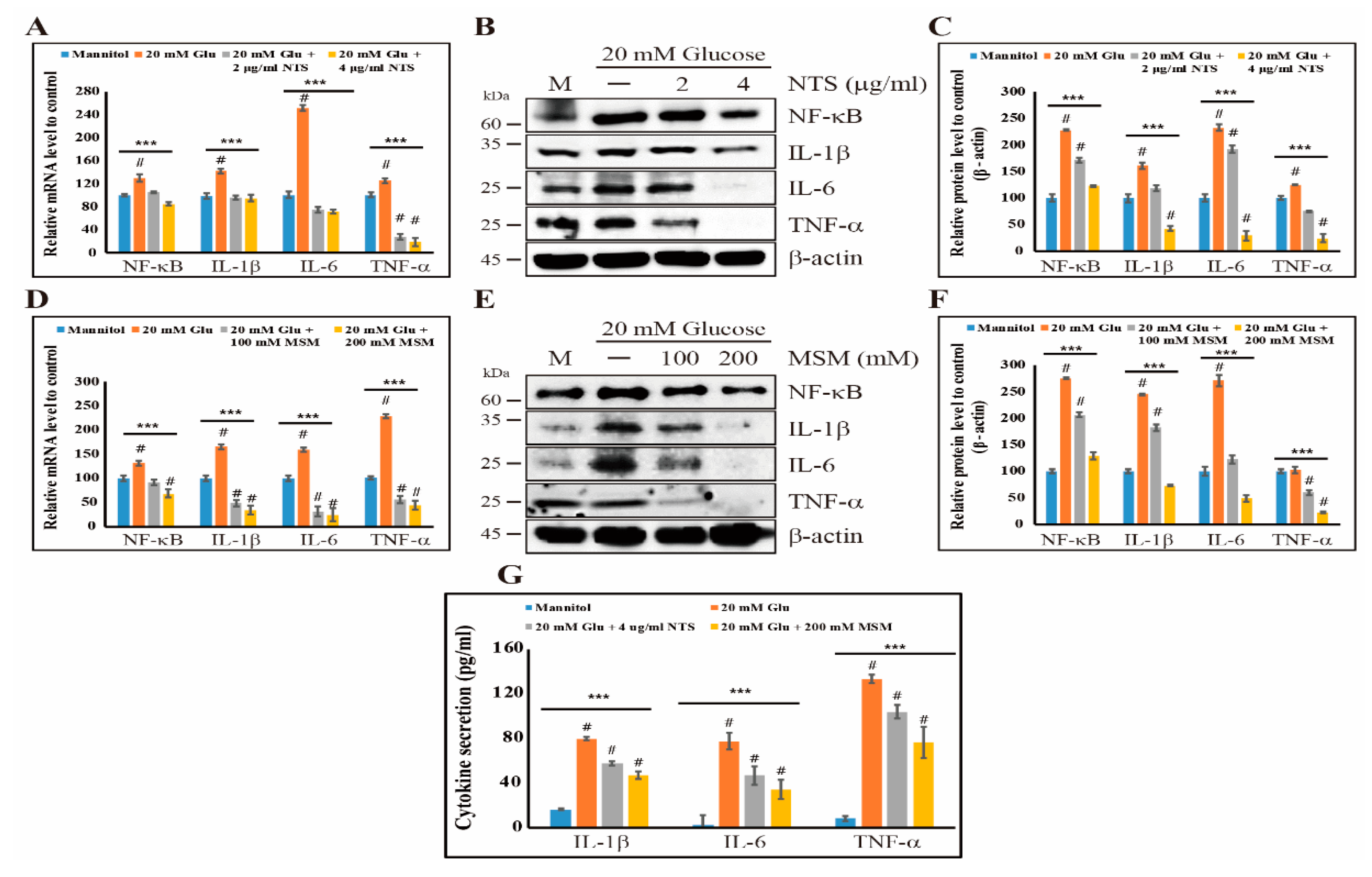
2.4. Sulfur Compounds Induce Anti-Inflammation Effects by Inhibiting NF-κB and its Proinflammatory Cytokines
2.5. Inhibition of NF-κB and Its Binding to Proinflammatory Cytokines by Sulfur Compounds
3. Discussion
4. Materials and Methods
4.1. Antibodies and Reagents
4.2. Cell Culture and Treatment
4.3. Cell Vaibility Assay
4.4. Real-Time qPCR
4.5. Western Blotting
4.6. Flow Cytometry Analysis
4.7. ELISA
4.8. Nuclear Extraction Assay and NF-kB Detection
4.9. ChIP Assay
4.10. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Nathan, D.M.; Cleary, P.A.; Backlund, J.-Y.C.; Genuth, S.M.; Lachin, J.M.; Orchard, T.J.; Raskin, P.; Zinman, B. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group Intensive Diabetes Treatment and Cardiovascular Disease in Patients with Type 1 Diabetes. N. Engl. J. Med. 2005, 353, 2643–2653. [Google Scholar] [CrossRef] [PubMed]
- Ruderman, N.B.; Haudenschild, C. Diabetes as an atherogenic factor. Prog. Cardiovasc. Dis. 1984, 26, 373–412. [Google Scholar] [CrossRef]
- Chait, A.; Bornfeldt, K.E. Diabetes and atherosclerosis: Is there a role for hyperglycemia? J. Lipid Res. 2008, 50, S335–S339. [Google Scholar] [CrossRef] [PubMed]
- Mazzone, T.; Chait, A.; Plutzky, J. Cardiovascular disease risk in type 2 diabetes mellitus: Insights from mechanistic studies. Lancet 2008, 371, 1800–1809. [Google Scholar] [CrossRef]
- Brownlee, M. The Pathobiology of Diabetic Complications: A Unifying Mechanism. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Baker, R.G.; Hayden, M.S.; Ghosh, S. NF-kappaB, inflammation, and metabolic disease. Cell Metab. 2011, 13, 11–22. [Google Scholar] [CrossRef]
- Uematsu, S.; Akira, S. The role of Toll-like receptors in immune disorders. Expert Opin. Boil. Ther. 2006, 6, 203–214. [Google Scholar] [CrossRef]
- Kumar, H.; Kawai, T.; Akira, S. Toll-like receptors and innate immunity. Biochem. Biophys. Res. Commun. 2009, 388, 621–625. [Google Scholar] [CrossRef]
- Beutler, B. Inferences, questions and possibilities in Toll-like receptor signalling. Nature 2004, 430, 257–263. [Google Scholar] [CrossRef]
- Devaraj, S.; Dasu, M.R.; Rockwood, J.; Winter, W.; Griffen, S.C.; Jialal, I. Increased toll-like receptor (TLR) 2 and TLR4 expression in monocytes from patients with type 1 diabetes: Further evidence of a proinflammatory state. J. Clin. Endocrinol. Metab. 2007, 93, 578–583. [Google Scholar] [CrossRef] [PubMed]
- Tobias, P.; Curtiss, L.K. Toll-like receptors in atherosclerosis. Biochem. Soc. Trans. 2007, 35, 1453–1455. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Sun, B. Toll-like receptor 4 in atherosclerosis. J. Cell. Mol. Med. 2007, 11, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Mullick, A.; Tobias, P.S.; Curtiss, L.K. Modulation of atherosclerosis in mice by Toll-like receptor 2. J. Clin. Investig. 2005, 115, 3149–3156. [Google Scholar] [CrossRef]
- Björkbacka, H.; Kunjathoor, V.V.; Moore, K.J.; Koehn, S.; Ordija, C.M.; Lee, M.A.; Means, T.; Halmen, K.; Luster, A.D.; Golenbock, U.T.; et al. Reduced atherosclerosis in MyD88-null mice links elevated serum cholesterol levels to activation of innate immunity signaling pathways. Nat. Med. 2004, 10, 416–421. [Google Scholar] [CrossRef]
- Mohammad, M.K.; Morran, M.; Slotterbeck, B.; Leaman, D.W.; Sun, Y.; Von Grafenstein, H.; Hong, S.-C.; McInerney, M.F. Dysregulated Toll-like receptor expression and signaling in bone marrow-derived macrophages at the onset of diabetes in the non-obese diabetic mouse. Int. Immunol. 2006, 18, 1101–1113. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, S. NF-κB in inflammation and renal diseases. Cell Biosci. 2015, 5, 63. [Google Scholar] [CrossRef]
- Sun, S.C. The noncanonical NF-kappaB pathway. Immunol. Rev. 2012, 246, 125–140. [Google Scholar] [CrossRef]
- Sun, S.C.; Liu, Z.G. A special issue on NF-kappaB signaling and function. Cell Res. 2011, 21, 1–2. [Google Scholar] [CrossRef]
- Karin, M.; Delhase, M. The IκB kinase (IKK) and NF-κB: Key elements of proinflammatory signalling. Semin. Immunol. 2000, 12, 85–98. [Google Scholar] [CrossRef]
- Israel, A. The IKK complex, a central regulator of NF-kappaB activation. Cold Spring Harb. Perspect. Biol. 2010, 2, a000158. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Ley, S.C. New insights into NF-κB regulation and function. Trends Immunol. 2008, 29, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-W.; Chu, W.; Hu, Y.; Delhase, M.; Deerinck, T.; Ellisman, M.; Johnson, R.S.; Karin, M. The IKKβ Subunit of IκB Kinase (IKK) is Essential for Nuclear Factor κB Activation and Prevention of Apoptosis. J. Exp. Med. 1999, 189, 1839–1845. [Google Scholar] [CrossRef] [PubMed]
- Beg, A.A.; Sha, W.C.; Bronson, R.T.; Baltimore, D. Constitutive NF-kappa B activation, enhanced granulopoiesis, and neonatal lethality in I kappa B alpha-deficient mice. Genome Res. 1995, 9, 2736–2746. [Google Scholar] [CrossRef]
- Dasu, M.R.; Devaraj, S.; Jialal, I. High glucose induces IL-1beta expression in human monocytes: Mechanistic insights. Am. J. Physiol. Metab. 2007, 293, E337–E346. [Google Scholar] [CrossRef]
- Volpe, C.M.O.; Villar-Delfino, P.H.; Dos Anjos, P.M.F.; Nogueira-Machado, J.A. Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell Death Dis. 2018, 9, 119. [Google Scholar] [CrossRef]
- Igarashi, M.; Wakasaki, H.; Takahara, N.; Ishii, H.; Jiang, Z.-Y.; Yamauchi, T.; Kuboki, K.; Meier, M.; Rhodes, C.J.; King, G.L. Glucose or diabetes activates p38 mitogen-activated protein kinase via different pathways. J. Clin. Investig. 1999, 103, 185–195. [Google Scholar] [CrossRef]
- Yamakawa, T.; Eguchi, S.; Matsumoto, T.; Yamakawa, Y.; Numaguchi, K.; Miyata, I.; Reynolds, C.M.; Motley, E.D.; Inagami, T. Intracellular Signaling in Rat Cultured Vascular Smooth Muscle Cells: Roles of Nuclear Factor-κB and p38 Mitogen-Activated Protein Kinase on Tumor Necrosis Factor-α Production*. Endocrinology 1999, 140, 3562–3572. [Google Scholar] [CrossRef]
- Olson, C.M.; Hedrick, M.N.; Izadi, H.; Bates, T.C.; Olivera, E.R.; Anguita, J. p38 Mitogen-Activated Protein Kinase Controls NF-κB Transcriptional Activation and Tumor Necrosis Factor Alpha Production through RelA Phosphorylation Mediated by Mitogen- and Stress-Activated Protein Kinase 1 in Response to Borrelia burgdorferi Antigens. Infect. Immun. 2006, 75, 270–277. [Google Scholar] [CrossRef]
- Koh, E.; Surh, J. Influence of Sulfur Fertilization on the Antioxidant Activities of Onion Juices Prepared by Thermal Treatment. Prev. Nutr. Food Sci. 2016, 21, 160–164. [Google Scholar] [CrossRef]
- Caron, J.; Bannon, M.; Rosshirt, L.; Luis, J.; Monteagudo, L.; Caron, J.M.; Sternstein, G.M. Methyl Sulfone Induces Loss of Metastatic Properties and Reemergence of Normal Phenotypes in a Metastatic Cloudman S-91 (M3) Murine Melanoma Cell Line. PLoS ONE 2010, 5, e11788. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.Y.; Sp, N.; Jo, E.S.; Kim, H.D.; Kim, I.H.; Bae, S.W.; Jang, K.; Yang, Y.M. Non-toxic sulfur enhances growth hormone signaling through the JAK2/STAT5b/IGF-1 pathway in C2C12 cells. Int. J. Mol. Med. 2019, 45, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Sp, N.; Darvin, P.; Yoo, Y.; Joung, Y.H.; Kang, D.Y.; Kim, D.N.; Hwang, T.S.; Kim, S.Y.; Kim, W.S.; Lee, H.K.; et al. The combination of methylsulfonylmethane and tamoxifen inhibits the Jak2/STAT5b pathway and synergistically inhibits tumor growth and metastasis in ER-positive breast cancer xenografts. BMC Cancer 2015, 15, 474. [Google Scholar] [CrossRef]
- Lim, E.J.; Hong, D.Y.; Park, J.H.; Joung, Y.H.; Darvin, P.; Kim, S.Y.; Na, Y.M.; Hwang, T.S.; Ye, S.-K.; Moon, E.-S.; et al. Methylsulfonylmethane Suppresses Breast Cancer Growth by Down-Regulating STAT3 and STAT5b Pathways. PLoS ONE 2012, 7, e33361. [Google Scholar] [CrossRef] [PubMed]
- Joung, Y.H.; Darvin, P.; Kang, D.Y.; Sp, N.; Byun, H.J.; Lee, C.-H.; Lee, H.K.; Yang, Y.M. Methylsulfonylmethane Inhibits RANKL-Induced Osteoclastogenesis in BMMs by Suppressing NF-κB and STAT3 Activities. PLoS ONE 2016, 11, e0159891. [Google Scholar] [CrossRef]
- Preetha, N.S.; Kang, D.Y.; Darvin, P.; Kim, D.N.; Joung, Y.H.; Kim, S.Y.; Cho, K.H.; Do, C.H.; Park, K.D.; Lee, J.; et al. Induction ofin vitroketosis condition and suppression using methylsulfonylmethane by altering ANGPTL3 expression through STAT5b signaling mechanism. Anim. Cells Syst. 2015, 19, 30–38. [Google Scholar] [CrossRef]
- Miller, L.E. Methylsulfonylmethane decreases inflammatory response to tumor necrosis factor-α in cardiac cells. Am. J. Cardiovasc. Dis. 2018, 8, 31–38. [Google Scholar]
- Kang, D.Y.; Darvin, P.; Yoo, Y.B.; Joung, Y.H.; Sp, N.; Byun, H.J.; Yang, Y.M. Methylsulfonylmethane inhibits HER2 expression through STAT5b in breast cancer cells. Int. J. Oncol. 2015, 48, 836–842. [Google Scholar] [CrossRef]
- Tsilidis, K.K.; Kasimis, J.C.; Lopez, D.S.; Ntzani, E.E.; Ioannidis, J.P.A. Type 2 diabetes and cancer: Umbrella review of meta-analyses of observational studies. BMJ 2015, 350, g7607. [Google Scholar] [CrossRef]
- Tsilidis, K.K.; Allen, N.E.; Appleby, P.N.; Rohrmann, S.; Nöthlings, U.; Arriola, L.; Gunter, M.J.; Chajès, V.; Rinaldi, S.; Romieu, I.; et al. Diabetes mellitus and risk of prostate cancer in the EuropeanProspectiveInvestigation into Cancer and Nutrition. Int. J. Cancer 2014, 136, 372–381. [Google Scholar] [CrossRef]
- Mudaliar, H.; Pollock, C.; Ma, J.; Wu, H.; Chadban, S.; Panchapakesan, U. The Role of TLR2 and 4-Mediated Inflammatory Pathways in Endothelial Cells Exposed to High Glucose. PLoS ONE 2014, 9, e108844. [Google Scholar] [CrossRef] [PubMed]
- Moscat, J.; Diaz-Meco, M.T.; Rennert, P. NF-κB activation by protein kinase C isoforms and B-cell function. EMBO Rep. 2003, 4, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Dasu, M.R.; Devaraj, S.; Zhao, L.; Hwang, D.H.; Jialal, I. High Glucose Induces Toll-Like Receptor Expression in Human Monocytes: Mechanism of Activation. Diabetes 2008, 57, 3090–3098. [Google Scholar] [CrossRef] [PubMed]
- Ramana, K.V.; Friedrich, B.; Bhatnagar, A.; Srivastava, S.K.; Srivastava, S.K. Activation of Nulcear Factor- B by Hyperglycemia in Vascular Smooth Muscle Cells Is Regulated by Aldose Reductase. Diabetes 2004, 53, 2910–2920. [Google Scholar] [CrossRef]
- Chen, L.; Chen, R.; Wang, H.; Liang, F.-X. Mechanisms Linking Inflammation to Insulin Resistance. Int. J. Endocrinol. 2015, 2015, 1–9. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds, NTS and MSM are available or not from the authors. |



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, E.S.; Sp, N.; Kang, D.Y.; Rugamba, A.; Kim, I.H.; Bae, S.W.; Liu, Q.; Jang, K.-J.; Yang, Y.M. Sulfur Compounds Inhibit High Glucose-Induced Inflammation by Regulating NF-κB Signaling in Human Monocytes. Molecules 2020, 25, 2342. https://doi.org/10.3390/molecules25102342
Jo ES, Sp N, Kang DY, Rugamba A, Kim IH, Bae SW, Liu Q, Jang K-J, Yang YM. Sulfur Compounds Inhibit High Glucose-Induced Inflammation by Regulating NF-κB Signaling in Human Monocytes. Molecules. 2020; 25(10):2342. https://doi.org/10.3390/molecules25102342
Chicago/Turabian StyleJo, Eun Seong, Nipin Sp, Dong Young Kang, Alexis Rugamba, Il Ho Kim, Se Won Bae, Qing Liu, Kyoung-Jin Jang, and Young Mok Yang. 2020. "Sulfur Compounds Inhibit High Glucose-Induced Inflammation by Regulating NF-κB Signaling in Human Monocytes" Molecules 25, no. 10: 2342. https://doi.org/10.3390/molecules25102342
APA StyleJo, E. S., Sp, N., Kang, D. Y., Rugamba, A., Kim, I. H., Bae, S. W., Liu, Q., Jang, K.-J., & Yang, Y. M. (2020). Sulfur Compounds Inhibit High Glucose-Induced Inflammation by Regulating NF-κB Signaling in Human Monocytes. Molecules, 25(10), 2342. https://doi.org/10.3390/molecules25102342






