Liquid Chromatographic Tandem Mass Spectrometric (LC-MS/MS) Determination of Perfluorooctane Sulfonate (PFOS) and Perfluorooctanoic Acid (PFOA) in the Yolk of Poultry Eggs in Malaysia
Abstract
1. Introduction
2. Results
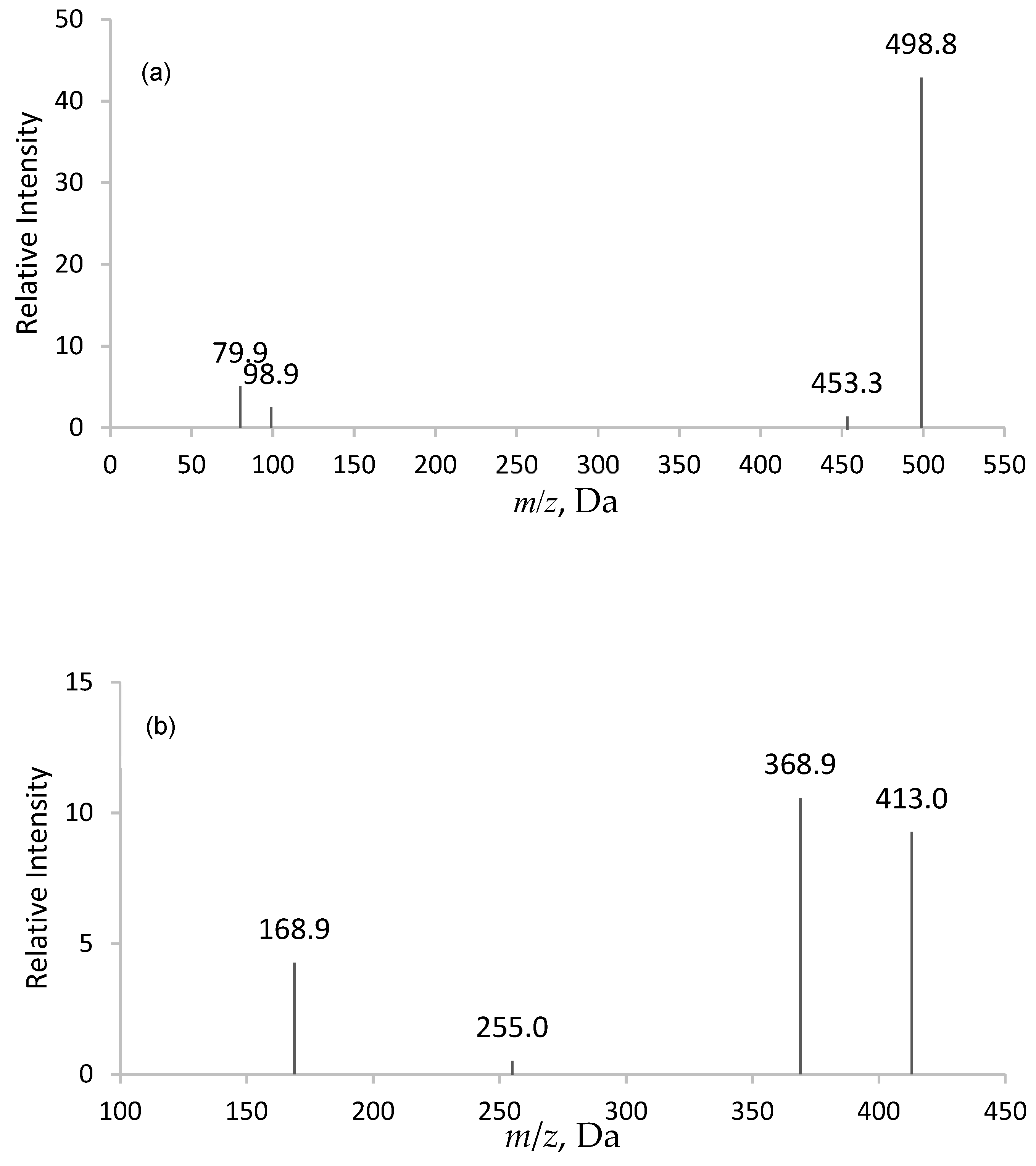
2.1. Selection of Deprotonated Ions
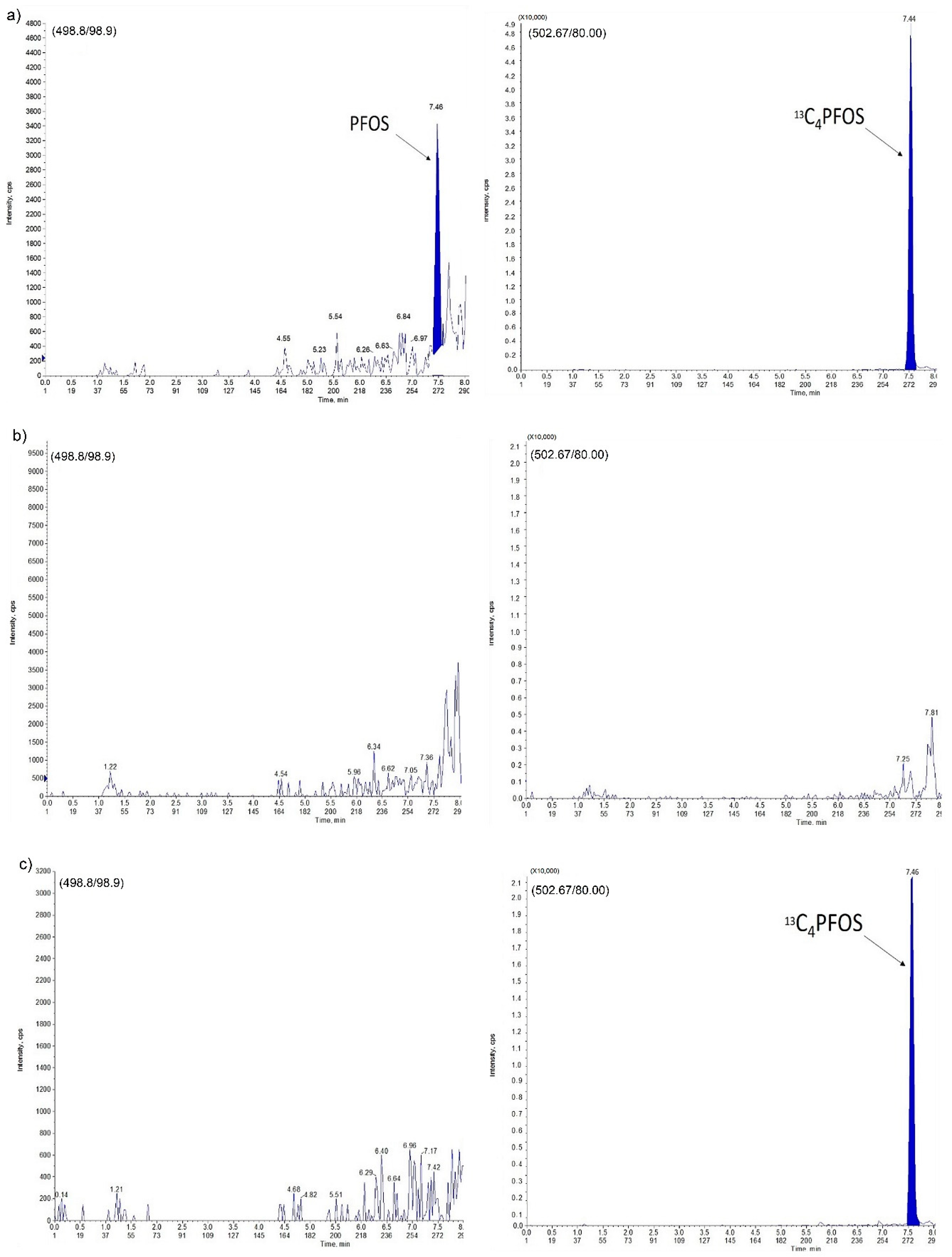
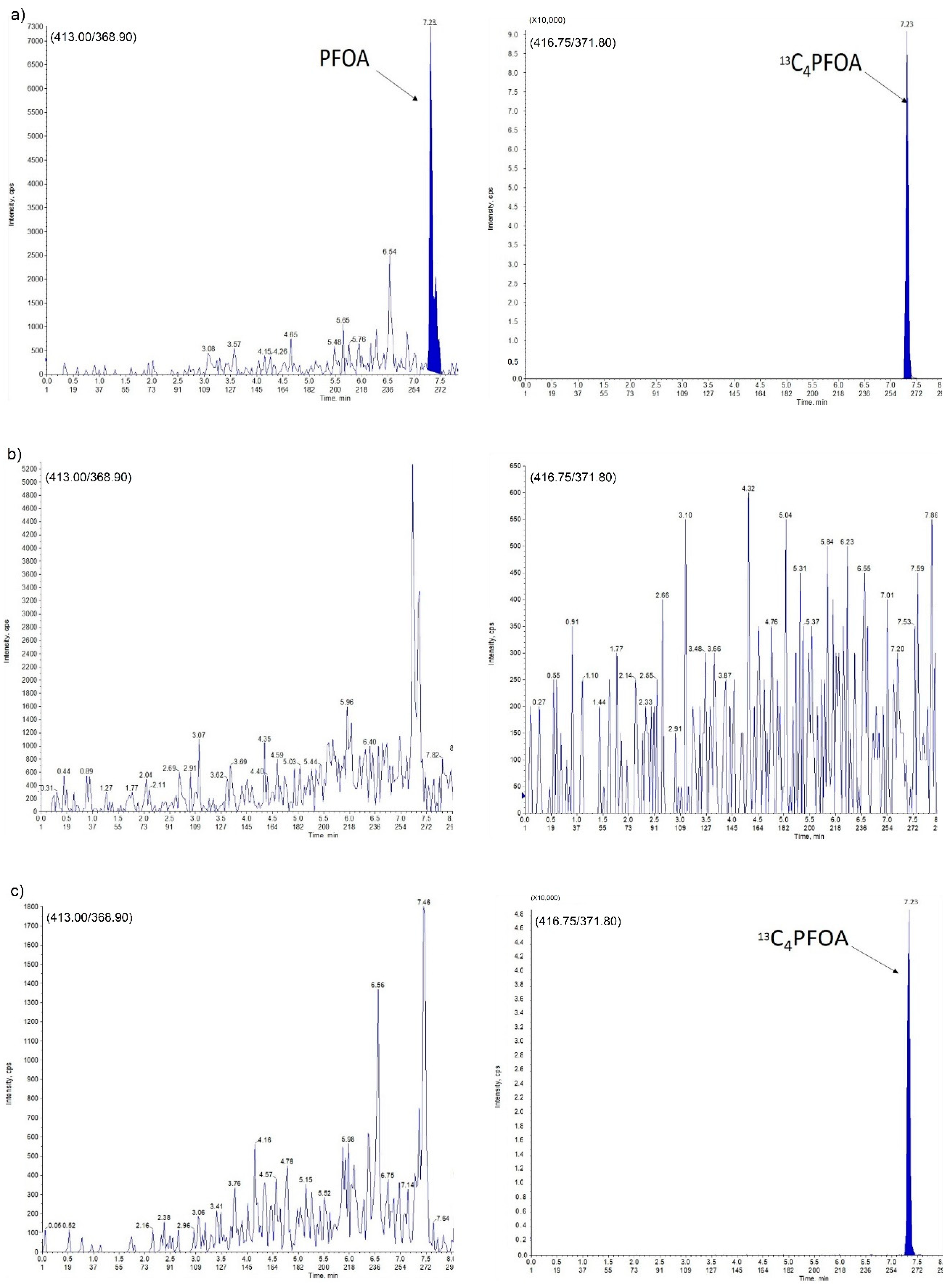
2.2. Assay Specificity and Selectivity
2.3. Calibration Curve and Sensitivity
2.4. Precision and Accuracy
2.5. Stability
2.6. Concentration of Perfluorinated Compounds in Yolk Samples
3. Discussion
3.1. Sample Preparation for LC-MS/MS Analysis
3.2. LC-MS/MS Method Validation
3.3. Concentration of Perfluorinated Compounds in Egg Yolk Samples
4. Materials and Methods
4.1. Chemicals
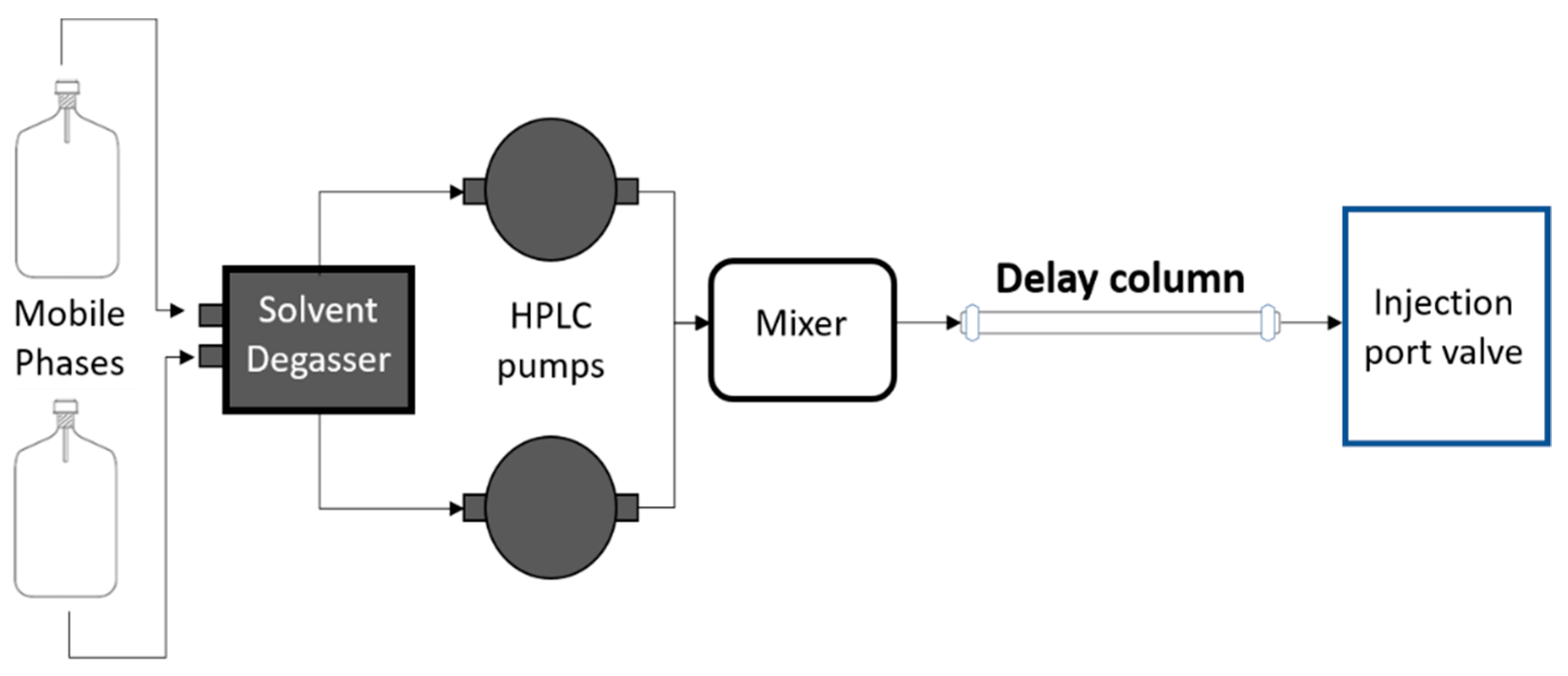
4.2. Instrumentation
4.3. Sample Collection and Preparation
4.4. Preparation of Standards and Quality Control Materials
4.5. Sample Extraction
4.6. Method Validation
4.6.1. Selectivity
4.6.2. Calibration Curve and Sensitivity
4.6.3. Accuracy and Precision
4.6.4. Recovery
4.6.5. Stability
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Xiao, F.; Simcik, M.F.; Halbach, T.R.; Gulliver, J.S. Perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in soils and groundwater of a US metropolitan area: Migration and implications for human exposure. Water Res. 2015, 72, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Zafeiraki, E.; Costopoulou, D.; Vassiliadou, I.; Leondiadis, L.; Dassenakis, E.; Hoogenboom, R.L.; van Leeuwen, S.P. Perfluoroalkylated substances (PFASs) in home and commercially produced chicken eggs from the Netherlands and Greece. Chemosphere 2016, 144, 2106–2112. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.-G.; Zhao, J.-L.; Liu, Y.-S.; Zhang, Q.-Q.; Chen, Z.-F.; Lai, H.-J.; Peng, F.-J.; Liu, S.-S.; Ying, G.-G. Bioaccumulation and risk assessment of per-and polyfluoroalkyl substances in wild freshwater fish from rivers in the Pearl River Delta region, South China. Ecotoxicol. Environ. Saf. 2014, 107, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Suja, F.; Pramanik, B.K.; Zain, S.M. Contamination, bioaccumulation and toxic effects of perfluorinated chemicals (PFCs) in the water environment: A review paper. Water Sci. Technol. 2009, 60, 1533–1544. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.D.; Hu, W.; De Coen, W.; Newsted, J.L.; Giesy, J.P. Binding of perfluorinated fatty acids to serum proteins. Environ. Toxicol. Chem. Int. J. 2003, 22, 2639–2649. [Google Scholar] [CrossRef] [PubMed]
- Kudo, N.; Kawashima, Y. Toxicity and toxicokinetics of perfluorooctanoic acid in humans and animals. J. Toxicol. Sci. 2003, 28, 49–57. [Google Scholar] [CrossRef]
- Sunderland, E.M.; Hu, X.C.; Dassuncao, C.; Tokranov, A.K.; Wagner, C.C.; Allen, J.G. A review of the pathways of human exposure to poly-and perfluoroalkyl substances (PFASs) and present understanding of health effects. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 131–147. [Google Scholar] [CrossRef]
- Christensen, K.Y.; Raymond, M.; Blackowicz, M.; Liu, Y.; Thompson, B.A.; Anderson, H.A.; Turyk, M. Perfluoroalkyl substances and fish consumption. Environ. Res. 2017, 154, 145–151. [Google Scholar] [CrossRef]
- Wang, Y.; Yeung, L.W.Y.; Yamashita, N.; Taniyasu, S.; So, M.K.; Murphy, M.B.; Lam, P.K.S. Perfluorooctane sulfonate (PFOS) and related fluorochemicals in chicken egg in China. Chin. Sci. Bull. 2008, 53, 501–507. [Google Scholar] [CrossRef]
- Lorenzo, M.; Campo, J.; Picó, Y. Analytical challenges to determine emerging persistent organic pollutants in aquatic ecosystems. TrAC Trends Anal. Chem. 2018, 103, 137–155. [Google Scholar] [CrossRef]
- Lindstrom, A.B.; Strynar, M.J.; Libelo, E.L. Polyfluorinated compounds: Past, present, and future. Environ. Sci. Technol. 2011, 45, 7954–7961. [Google Scholar] [CrossRef] [PubMed]
- Berger, U.; Langlois, I.; Oehme, M.; Kallenborn, R. Comparison of three types of mass spectrometer for high-performance liquid chromatography/mass spectrometry analysis of perfluoroalkylated substances and fluorotelomer alcohols. Eur. J. Mass Spectrom. 2004, 10, 579–588. [Google Scholar] [CrossRef]
- Nakayama, S.F.; Yoshikane, M.; Onoda, Y.; Nishihama, Y.; Iwai-Shimada, M.; Takagi, M.; Kobayashi, Y.; Isobe, T. Worldwide trends in tracing poly-and perfluoroalkyl substances (PFAS) in the environment. TrAC Trends Anal. Chem. 2019, 121, 1–20. [Google Scholar] [CrossRef]
- So, M.K.; Taniyasu, S.; Lam, P.K.; Zheng, G.J.; Giesy, J.P.; Yamashita, N. Alkaline digestion and solid phase extraction method for perfluorinated compounds in mussels and oysters from South China and Japan. Arch. Environ. Contam. Toxicol. 2006, 50, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Malinsky, M.D.; Jacoby, C.B.; Reagen, W.K. Determination of perfluorinated compounds in fish fillet homogenates: Method validation and application to fillet homogenates from the Mississippi River. Anal. Chim. Acta 2011, 683, 248–257. [Google Scholar] [CrossRef]
- Reagen, W.K.; Ellefson, M.E.; Kannan, K.; Giesy, J.P. Comparison of extraction and quantification methods of perfluorinated compounds in human plasma, serum, and whole blood. Anal. Chim. Acta 2008, 628, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Hemat, H.; Wilhelm, M.; Völkel, W.; Mosch, C.; Fromme, H.; Wittsiepe, J. Low serum levels of perfluorooctanoic acid (PFOA), perfluorooctane sulfonate (PFOS) and perfluorohexane sulfonate (PFHxS) in children and adults from Afghanistan. Sci. Total Environ. 2010, 408, 3493–3495. [Google Scholar] [CrossRef]
- Authority, E.F.S. Results of the monitoring of perfluoroalkylated substances in food in the period 2000–2009. EFSA J. 2011, 9, 2016. [Google Scholar]
- Shafique, U.; Dorn, V.; Paschke, A.; Schüürmann, G. Adsorption of perfluorocarboxylic acids at the silica surface. Chem. Commun. 2017, 53, 589–592. [Google Scholar] [CrossRef]
- Martin, J.; Gracia, A.R.; Asuero, A.G. Fitting Nonlinear Calibration Curves: No Models Perfect. J. Anal. Sci. Methods Instrum. 2017, 7, 1. [Google Scholar] [CrossRef]
- Rahman, M.F.; Peldszus, S.; Anderson, W.B. Behaviour and fate of perfluoroalkyl and polyfluoroalkyl substances (PFASs) in drinking water treatment: A review. Water Res. 2014, 50, 318–340. [Google Scholar] [CrossRef] [PubMed]
- Kärrman, A.; van Bavel, B.; Järnberg, U.; Hardell, L.; Lindström, G. Perfluorinated chemicals in relation to other persistent organic pollutants in human blood. Chemosphere 2006, 64, 1582–1591. [Google Scholar] [CrossRef] [PubMed]
- Hargitai, R.; Nagy, G.; Nyiri, Z.; Bervoets, L.; Eke, Z.; Eens, M.; Török, J. Effects of breeding habitat (woodland versus urban) and metal pollution on the egg characteristics of great tits (Parus major). Sci. Total Environ. 2016, 544, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhao, C.; Gao, Y.; Fu, J.; Gao, K.; Lv, K.; Wang, K.; Yue, H.; Lan, X.; Liang, Y. Protein-specific distribution patterns of perfluoroalkyl acids in egg yolk and albumen samples around a fluorochemical facility. Sci. Total Environ. 2019, 650, 2697–2704. [Google Scholar] [CrossRef] [PubMed]
- Ericson, I.; Martí-Cid, R.; Nadal, M.; Van Bavel, B.; Lindström, G.; Domingo, J.L. Human exposure to perfluorinated chemicals through the diet: Intake of perfluorinated compounds in foods from the Catalan (Spain) market. J. Agric. Food Chem. 2008, 56, 1787–1794. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shi, Y.; Pan, Y.; Cai, Y. Perfluorooctane sulfonate (PFOS) and other fluorochemicals in viscera and muscle of farmed pigs and chickens in Beijing, China. Chin. Sci. Bull. 2010, 55, 3550–3555. [Google Scholar] [CrossRef]
- Lim, W.Y.; Aris, A.Z.; Tengku Ismail, T.H. Spatial geochemical distribution and sources of heavy metals in the sediment of Langat River, Western Peninsular Malaysia. Environ. Forensics 2013, 14, 133–145. [Google Scholar] [CrossRef]
- Oliaei, F.; Kriens, D.; Weber, R.; Watson, A. PFOS and PFC releases and associated pollution from a PFC production plant in Minnesota (USA). Environ. Sci. Pollut. Res. 2013, 20, 1977–1992. [Google Scholar] [CrossRef]
- Tittlemier, S.A.; Pepper, K.; Seymour, C.; Moisey, J.; Bronson, R.; Cao, X.-L.; Dabeka, R.W. Dietary exposure of Canadians to perfluorinated carboxylates and perfluorooctane sulfonate via consumption of meat, fish, fast foods, and food items prepared in their packaging. J. Agric. Food Chem. 2007, 55, 3203–3210. [Google Scholar] [CrossRef]
- Jogsten, I.E.; Perelló, G.; Llebaria, X.; Bigas, E.; Martí-Cid, R.; Kärrman, A.; Domingo, J.L. Exposure to perfluorinated compounds in Catalonia, Spain, through consumption of various raw and cooked foodstuffs, including packaged food. Food Chem. Toxicol. 2009, 47, 1577–1583. [Google Scholar] [CrossRef]
- Clarke, D.B.; Bailey, V.A.; Routledge, A.; Lloyd, A.S.; Hird, S.; Mortimer, D.N.; Gem, M. Dietary intake estimate for perfluorooctanesulphonic acid (PFOS) and other perfluorocompounds (PFCs) in UK retail foods following determination using standard addition LC-MS/MS. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2010, 27, 530–545. [Google Scholar] [CrossRef] [PubMed]
- Zainuddin, K.; Zakaria, M.P.; Al-Odaini, N.A.; Bakhtiari, A.R.; Latif, P.A. Perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) in surface water from the Langat River, Peninsular Malaysia. Environ. Forensics 2012, 13, 82–92. [Google Scholar] [CrossRef]
- Food, U.; Administration, D. FDA Guidance for Industry: Bioanalytical Method Validation. In Rockv. MD; 2011. Available online: http://www.fda.gov/downloads/Drugs/Guidances/ucm070107.pdf (accessed on 15 May 2020).
Sample Availability: Samples of the compounds are not available from the authors. |
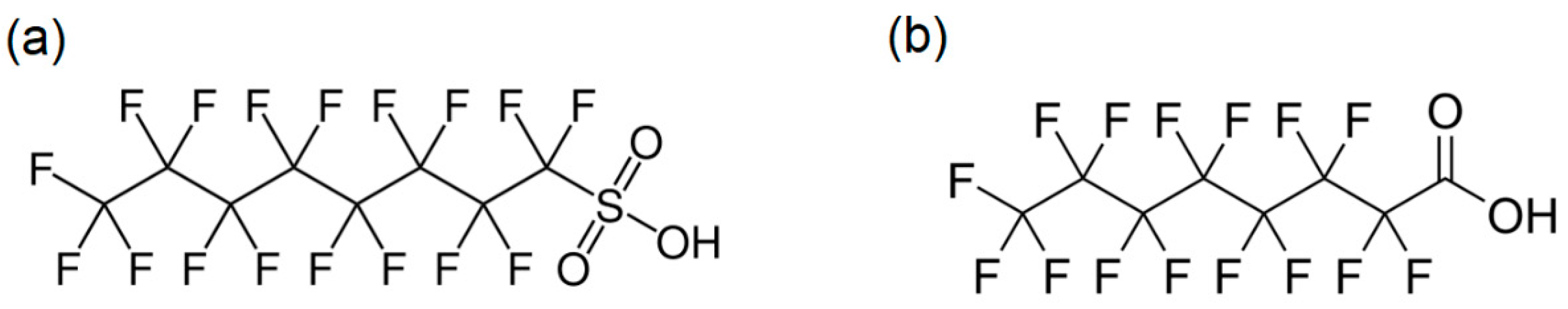
| Analyte | Concentration (ng g−1) | Intra-Assay (n = 5) | Inter-Assay (n = 15) | ||||
|---|---|---|---|---|---|---|---|
| Observed Concentration (ng g−1) | Accuracy | RSD (% CV) | Observed Concentration (ng g−1) | Accuracy | RSD (% CV) | ||
| PFOS | 0.50 | 0.54 ± 0.03 | 108 | 5.01 | 0.54 ± 0.03 | 108 | 6.35 |
| 0.75 | 0.81 ± 0.02 | 108 | 2.03 | 0.81 ± 0.05 | 108 | 6.05 | |
| 7.50 | 6.85 ± 0.12 | 91.3 | 1.77 | 7.28 ± 0.50 | 97.1 | 6.91 | |
| 12.5 | 11.5 ± 0.28 | 92.3 | 2.42 | 11.8 ± 0.40 | 94.3 | 3.38 | |
| PFOA | 0.10 | 0.11 ± 0.01 | 110 | 6.79 | 0.11 ± 0.01 | 110 | 6.25 |
| 0.75 | 0.67 ± 0.01 | 89.3 | 1.82 | 0.69 ± 0.03 | 92.0 | 5.06 | |
| 7.50 | 7.38 ± 0.82 | 98.4 | 11.1 | 8.93 ± 0.68 | 101 | 4.08 | |
| 12.5 | 12.3 ± 0.51 | 98.4 | 4.19 | 12.4 ± 0.51 | 99.1 | 4.08 | |
| Analyte | QC Concentration (ng g−1) | Time | Mean Measured Concentration, ng g−1 (n = 5) | Accuracy (%) | RSD (% CV) |
|---|---|---|---|---|---|
| PFOS | 0.75 | 0 hr | 0.81 ± 0.02 | 109 | 2.03 |
| 12 hr | 0.80 ± 0.07 | 106 | 9.29 | ||
| 24 hr | 0.83 ± 0.04 | 111 | 5.13 | ||
| 12.5 | 0 hr | 11.5 ± 0.28 | 92.3 | 2.42 | |
| 12 hr | 11.8 ± 0.42 | 94.2 | 3.57 | ||
| 24 hr | 12.1 ± 0.36 | 96.5 | 3.02 | ||
| PFOA | 0.75 | 0 hr | 0.67 ± 0.01 | 89.7 | 1.82 |
| 12 hr | 0.73 ± 0.06 | 97.8 | 8.13 | ||
| 24 hr | 0.70 ± 0.03 | 93.0 | 4.82 | ||
| 12.5 | 0 hr | 12.3 ± 0.51 | 98.4 | 4.19 | |
| 12 hr | 12.2 ± 0.57 | 97.6 | 4.67 | ||
| 24 hr | 12.7 ± 0.40 | 101 | 3.14 |
| Type | Source | Number of Yolk (n) | Concentration (ng g−1 ww) | |
|---|---|---|---|---|
| PFOS | PFOA | |||
| Chicken Eggs (Home Produced) | ||||
| 1 | 3 | 0.64 | <0.10 a | |
| 2 | 3 | <0.50 | <0.10 | |
| 3 | 3 | 0.50 | <0.10 a | |
| 4 | 3 | <0.50 a | <0.10 a | |
| 5 | 3 | 1.01 | <0.10 | |
| 6 | 3 | 0.53 | <0.10 a | |
| 7 | 3 | <0.50 a | <0.10 a | |
| 8 | 3 | <0.50 a | <0.10 | |
| 9 | 3 | 0.52 | <0.10 | |
| 10 | 3 | <0.50 | <0.10 | |
| Duck eggs | ||||
| 1 | 2 | <0.50 a | <0.10 | |
| 2 | 2 | <0.50 a | <0.10 | |
| 3 | 2 | <0.50 a | <0.10 a | |
| Quail eggs | ||||
| 1 | 5 | <0.50 a | <0.10 | |
| 2 | 5 | 0.69 | <0.10 | |
| 3 | 5 | <0.50 a | <0.10 a | |
| 4 | 5 | <0.50 a | <0.10 a | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tahziz, A.; Mohamad Haron, D.E.; Aziz, M.Y. Liquid Chromatographic Tandem Mass Spectrometric (LC-MS/MS) Determination of Perfluorooctane Sulfonate (PFOS) and Perfluorooctanoic Acid (PFOA) in the Yolk of Poultry Eggs in Malaysia. Molecules 2020, 25, 2335. https://doi.org/10.3390/molecules25102335
Tahziz A, Mohamad Haron DE, Aziz MY. Liquid Chromatographic Tandem Mass Spectrometric (LC-MS/MS) Determination of Perfluorooctane Sulfonate (PFOS) and Perfluorooctanoic Acid (PFOA) in the Yolk of Poultry Eggs in Malaysia. Molecules. 2020; 25(10):2335. https://doi.org/10.3390/molecules25102335
Chicago/Turabian StyleTahziz, Atiqah, Didi Erwandi Mohamad Haron, and Mohd Yusmaidie Aziz. 2020. "Liquid Chromatographic Tandem Mass Spectrometric (LC-MS/MS) Determination of Perfluorooctane Sulfonate (PFOS) and Perfluorooctanoic Acid (PFOA) in the Yolk of Poultry Eggs in Malaysia" Molecules 25, no. 10: 2335. https://doi.org/10.3390/molecules25102335
APA StyleTahziz, A., Mohamad Haron, D. E., & Aziz, M. Y. (2020). Liquid Chromatographic Tandem Mass Spectrometric (LC-MS/MS) Determination of Perfluorooctane Sulfonate (PFOS) and Perfluorooctanoic Acid (PFOA) in the Yolk of Poultry Eggs in Malaysia. Molecules, 25(10), 2335. https://doi.org/10.3390/molecules25102335






