Chiral Phase Transfer Catalysis in the Asymmetric Synthesis of a 3,3-Disubstituted Isoindolinone and Determination of Its Absolute Configuration by VCD Spectroscopy
Abstract
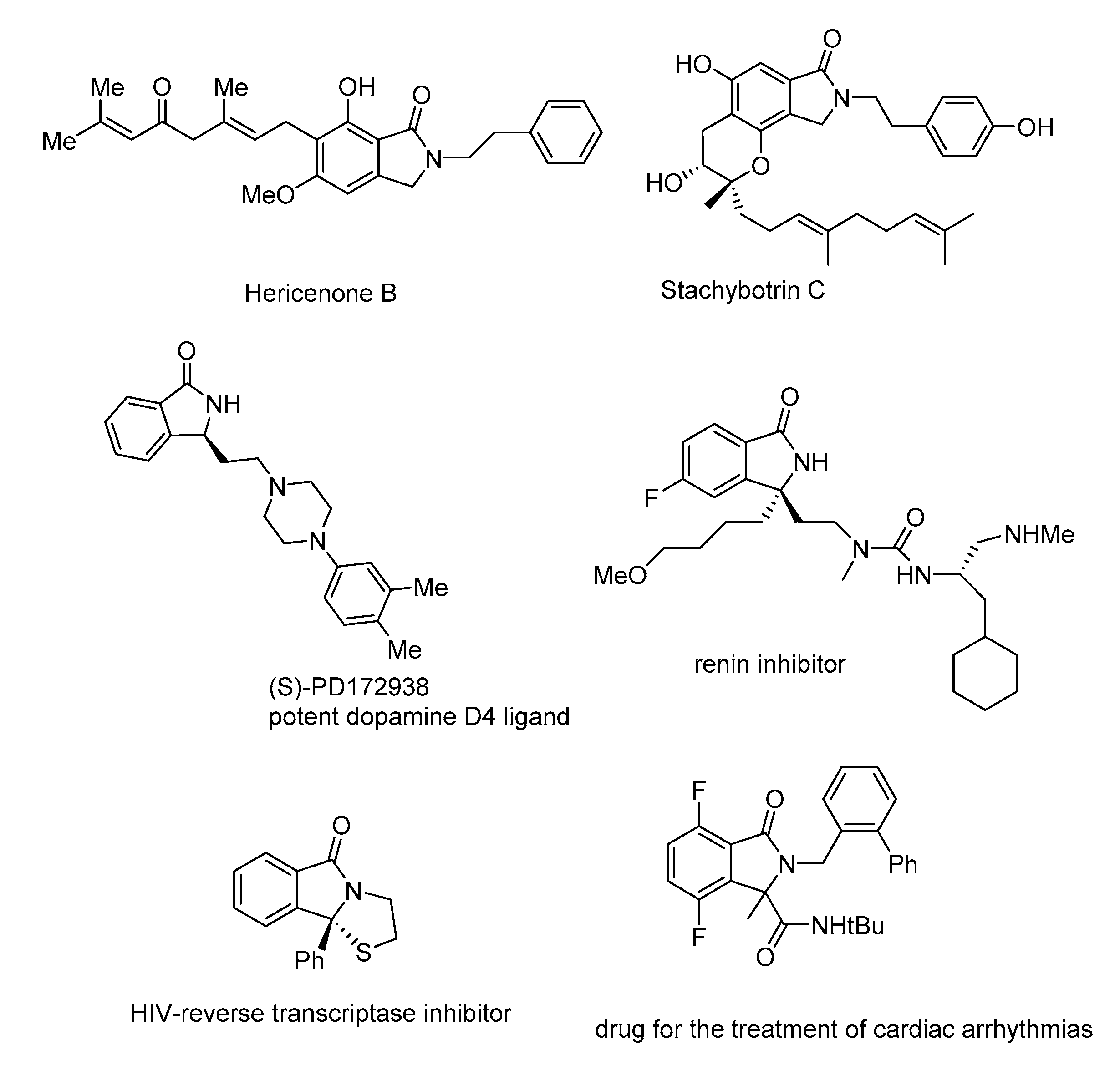
1. Introduction
2. Results and Discussion
2.1. Screening of Different Organocatalytic Systems
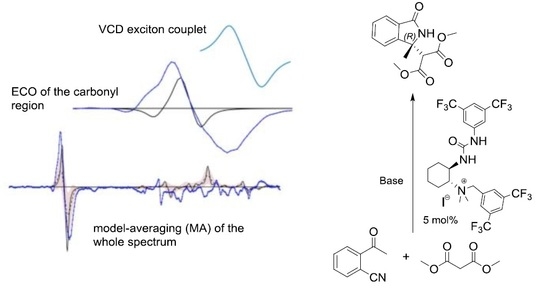
2.2. Determination of the Absolute Configuration by VCD
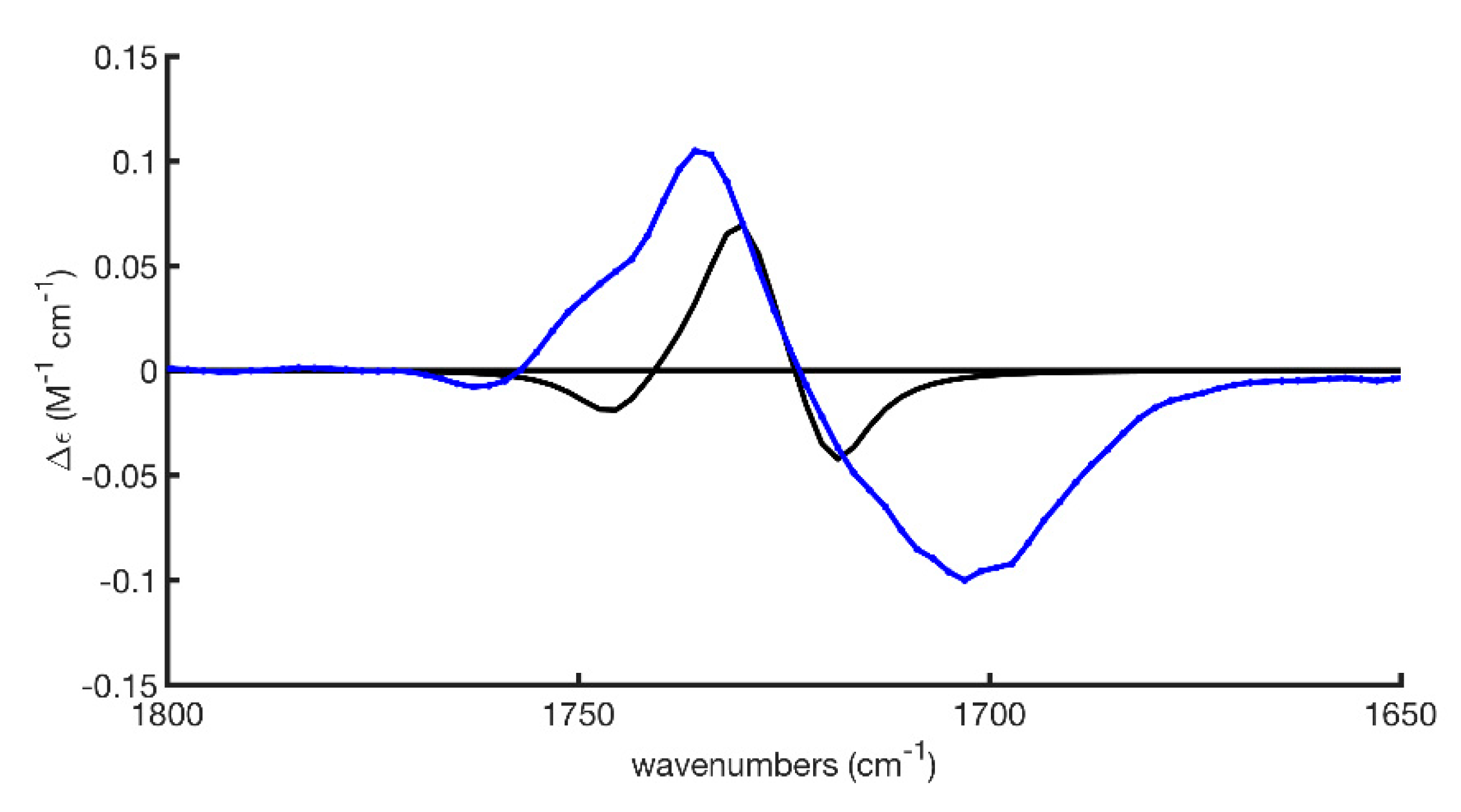
2.2.1. The Experimental Spectra
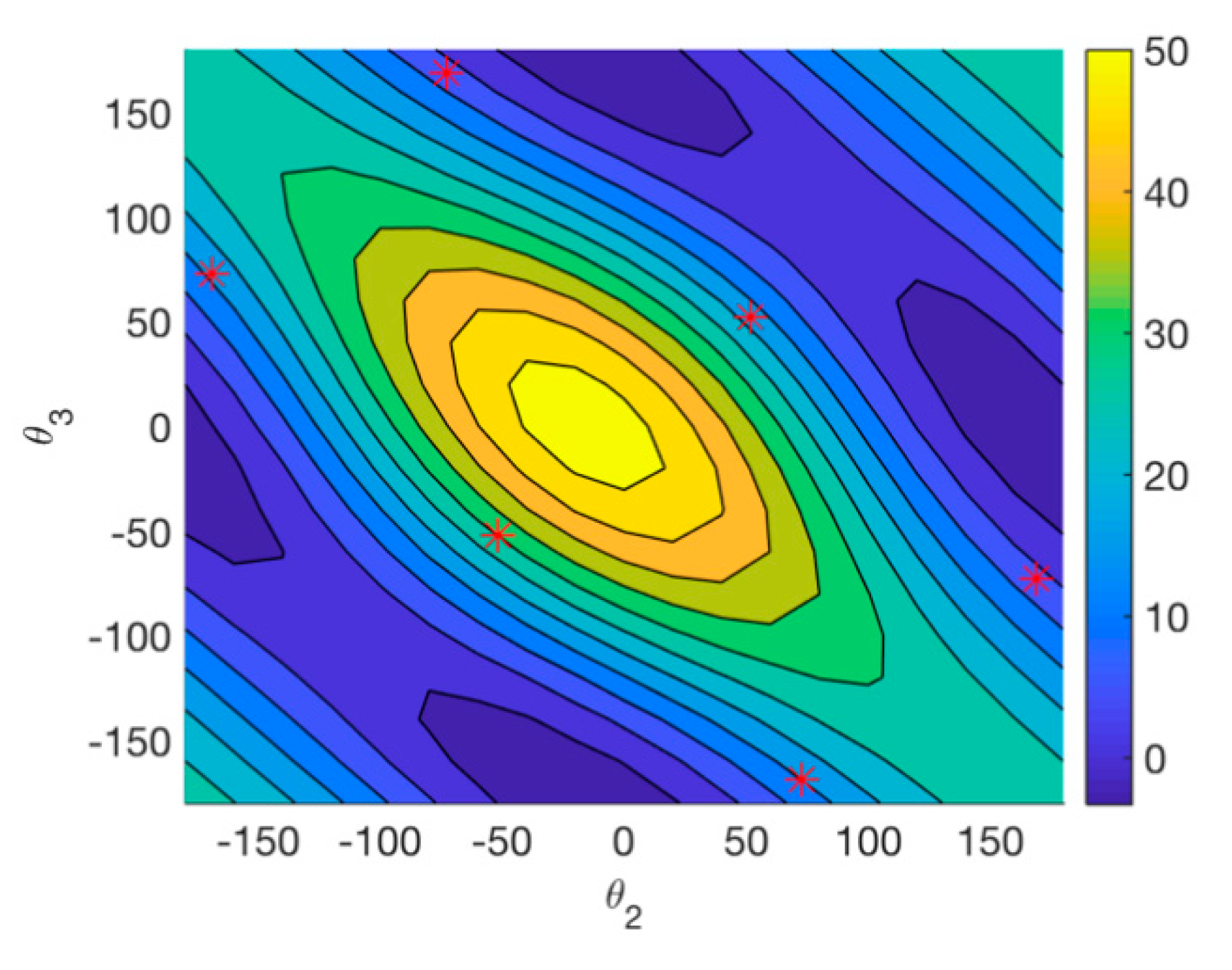
2.2.2. Conformational Analysis
2.2.3. AC Assignment through Model-Averaged DFT Calculations
2.2.4. AC Assignment through the ECO Model
2.2.5. Applicability of Taniguchi and Monde Approach
3. Materials and Methods
3.1. General Remarks
3.2. Procedure of Asymmetric Synthesis and Crystallization of 1
3.3. Determination of the Absolute Configuration by VCD
3.4. Computations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Speck, K.; Magauer, T. The Chemistry of Isoindole Natural Products. Beilstein J. Org. Chem. 2013, 9, 2048–2078. [Google Scholar] [CrossRef] [PubMed]
- Di Mola, A.; Palombi, L.; Massa, A. An Overview on Asymmetric Synthesis of 3-Substituted Isoindolinones. In Targets in Heterocyclic Systems: Chemistry and Properties; Attanasi, O.A., Ed.; Società Chimica Italiana: Rome, Italy, 2014; Volume 18, pp. 113–140. [Google Scholar]
- Gao, W.; Chen, M.; Ding, Q.; Peng, Y. Catalytic Asymmetric Synthesis of Isoindolinones. Chem. Asian J. 2019, 14, 1306–1322. [Google Scholar] [CrossRef] [PubMed]
- Mertens, A.; Zilch, J.H.; Koining, B.; Schafer, W.; Poll, T.; Kampe, W.; Seidel, H.; Leser, U.; Leinert, H. Selective non-nucleoside HIV-1 reverse transcriptase inhibitors. New 2,3-dihydrothiazolo[2,3-a]isoindol-5 (9bH)-ones and related compounds with anti-HIV-1 activity. J. Med. Chem. 1993, 36, 2526. [Google Scholar] [CrossRef] [PubMed]
- Bjoere, A.; Bostroem, J.; Davidsson, O.; Emtenaes, H.; Gran, H.; Iliefski, T.; Kajanus, J.; Olsson, R.; Sandberg, L.; Strandlund, G.; et al. Isoindoline Derivatives for the Treatment of Arrhythmias. WO/2008/008022, 17 January 2008. [Google Scholar]
- Baldwin, J.J.; Claremon, D.A.; Tice, C.M.; Cacatian, S.; Dillard, L.H.; Ishchenko, A.V.; Yuan, J.; Xu, Z.; Mcgeehan, G.; Zhao, W.; et al. Renin Inhibitors. WO/2008/156816, 24 December 2008. [Google Scholar]
- Yang, G.; Shen, C.; Zhang, W. An asymmetric aerobic aza-Wacker-type cyclization: Synthesis of isoindolinones bearing tetrasubstituted carbon stereocenters. Angew. Chem. Int. Ed. 2012, 51, 9141–9145. [Google Scholar] [CrossRef]
- Nishimura, T.; Noishiki, A.; Ebe, Y.; Hayashi, T. Hydroxorhodium/Chiral Diene Complexes as Effective Catalysts for the Asymmetric Arylation of 3-Aryl-3-hydroxyisoindolin-1-ones. Angew. Chem. Int. Ed. 2013, 52, 1777–1780. [Google Scholar] [CrossRef]
- Yu, X.; Wang, Y.; Wu, G.; Song, H.; Zhou, Z.; Tang, C. Organocatalyzed Enantioselective Synthesis of Quaternary Carbon-Containing Isoindolinon1-ones. Eur. J. Org. Chem. 2011, 2011, 3060–3066. [Google Scholar] [CrossRef]
- Scorzelli, F.; Di Mola, A.; De Piano, F.; Tedesco, C.; Palombi, L.; Filosa, R.; Waser, M.; Massa, A. A Systematic Study on the Use of Different Organocatalytic Activation Modes for Asymmetric Conjugated Addition Reactions of Isoindolinones. Tetrahedron 2017, 73, 819–828. [Google Scholar] [CrossRef]
- Bella, M.; Gasperi, T. Organocatalytic Formation of Quaternary Stereocenters. Synthesis 2009, 10, 1583–1614. [Google Scholar] [CrossRef]
- Di Mola, A.; Di Martino, M.; Capaccio, V.; Pierri, G.; Palombi, L.; Tedesco, C.; Massa, A. Synthesis of 2-Acetylbenzonitriles and Their Reactivity in Tandem Reactions with Carbon and Hetero Nucleophiles: Easy Access to 3,3-Disubstituted Isoindolinones. Eur. J. Org. Chem. 2018, 1699–1708. [Google Scholar] [CrossRef]
- Romano, F.; Di Mola, A.; Tiffner, M.; Waser, M.; Massa, A. Synthesis and Organocatalytic Asymmetric Nitro-Aldol Initiated Cascade Reactions of 2-Acylbenzonitriles Leading to 3,3-Disubstituted Isoindolinones. Catalysts 2019, 9, 327. [Google Scholar] [CrossRef]
- Hayashi, Y. Pot economy and one-pot synthesis. Chem. Sci. 2016, 7, 866–880. [Google Scholar] [CrossRef] [PubMed]
- Di Mola, A.; Tiffner, M.; Scorzelli, F.; Palombi, L.; Filosa, R.; De Caprariis, P.; Waser, M.; Massa, A. Bifunctional Phase Transfer Catalysis in the Asymmetric Synthesis of Biologically Active Isoindolinones. Beilstein J. Org. Chem. 2015, 11, 2591–2599. [Google Scholar] [CrossRef] [PubMed]
- Stephens, P.J.; Devlin, F.J.; Cheeseman, J.R. VCD Spectroscopy for Organic Chemists; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Polavarapu, P.L. Chiroptical Spectroscopy: Fundamentals and Applications; Taylor & Francis: Boca Raton, FL, USA, 2017. [Google Scholar]
- Monaco, G.; Aquino, F.; Zanasi, R.; Herrebout, W.; Bultinck, P.; Massa, A. Model-Averaging of Ab Initio Spectra for the Absolute Configuration Assignment via Vibrational Circular Dichroism. Phys. Chem. Chem. Phys. 2017, 19, 28028–28036. [Google Scholar] [CrossRef] [PubMed]
- Monaco, G.; Procida, G.; Di Mola, A.; Herrebout, W.; Massa, A. Error Bounds on Goodness of Fit Indicators in Vibrational Circular Dichroism Spectroscopy. Chem. Phys. Lett. 2020, 739, 137000. [Google Scholar] [CrossRef]
- Kuppens, T.; Vandyck, K.; Van der Eycken, J.; Herrebout, W.; van der Veken, B.J.; Bultinck, P. Determination of the Absolute Configuration of Three As-Hydrindacene Compounds by Vibrational Circular Dichroism. J. Org. Chem. 2005, 70, 9103–9114. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; De Gussem, E.; Abbaspour Tehrani, K.; Sergeyev, S.; Bultinck, P.; Herrebout, W. Stereochemistry of the Tadalafil Diastereoisomers: A Critical Assessment of Vibrational Circular Dichroism, Electronic Circular Dichroism, and Optical Rotatory Dispersion. J. Med. Chem. 2013, 56, 8903–8914. [Google Scholar] [CrossRef]
- Birke, S.S.; Agbaje, I.; Diem, M. Experimental and Computational Infrared CD Studies of Prototypical Peptide Conformations. Biochemistry 1992, 31, 450–455. [Google Scholar] [CrossRef]
- Massa, A.; Rizzo, P.; Monaco, G.; Zanasi, R. Absolute Configuration Assignment Made Easier by the VCD of Coupled Oscillating Carbonyls: The Case of (−)-Propanedioic Acids, 2-(2,3)-Dihydro-3-Oxo-1H-Isoindol-1-Yl)-1,3-Dimethyl Ester. Tetrahedron Lett. 2013, 54, 6242–6246. [Google Scholar] [CrossRef]
- Taniguchi, T.; Monde, K. Exciton Chirality Method in Vibrational Circular Dichroism. J. Am. Chem. Soc. 2012, 134, 3695–3698. [Google Scholar] [CrossRef]
- Covington, C.L.; Nicu, V.P.; Polavarapu, P.L. Determination of the Absolute Configurations Using Exciton Chirality Method for Vibrational Circular Dichroism: Right Answers for the Wrong Reasons? J. Phys. Chem. A 2015, 119, 10589–10601. [Google Scholar] [CrossRef]
- Abbate, S.; Mazzeo, G.; Meneghini, S.; Longhi, G.; Boiadjiev, S.E.; Lightner, D.A. Bicamphor: A Prototypic Molecular System to Investigate Vibrational Excitons. J. Phys. Chem. A. 2015, 119, 4261–4267. [Google Scholar] [CrossRef] [PubMed]
- Schörgenhumer, J.; Otte, S.; Haider, V.; Novacek, J.; Waser, M. A Flexible Strategy for the Synthesis of Bifunctional 6′-(Thio)-Urea Containing Cinchona Alkaloid Ammonium Salts. Tetrahedron 2019, 130816. [Google Scholar] [CrossRef]
- Novacek, J.; Izzo, J.A.; Vetticatt, M.J.; Waser, M. Bifunctional Ammonium Salt Catalyzed Asymmetric α-Hydroxylation of β-Ketoesters by Simultaneous Resolution of Oxaziridines. Chem. Eur. J. 2016, 22, 17339–17344. [Google Scholar] [CrossRef] [PubMed]
- Armarego, W.L.F.; Perrin, D.D. Purification of Laboratory Chemicals, 4th ed.; Butterworth Heinemann: Oxford, UK, 1996. [Google Scholar]
- Stephens, P.J.; Devlin, F.J.; Pan, J.-J. The Determination of the Absolute Configurations of Chiral Molecules Using Vibrational Circular Dichroism (VCD) Spectroscopy. Chirality 2008, 20, 643–663. [Google Scholar] [CrossRef] [PubMed]
- Krief, A.; Dunkle, M.; Bahar, M.; Bultinck, P.; Herrebout, W.; Sandra, P. Elucidation of the Absolute Configuration of Rhizopine by Chiral Supercritical Fluid Chromatography and Vibrational Circular Dichroism: Other Techniques. J. Sep. Sci. 2015, 38, 2545–2550. [Google Scholar] [CrossRef] [PubMed]
- Debie, E.; De Gussem, E.; Dukor, R.K.; Herrebout, W.; Nafie, L.A.; Bultinck, P. A Confidence Level Algorithm for the Determination of Absolute Configuration Using Vibrational Circular Dichroism or Raman Optical Activity. ChemPhysChem 2011, 12, 1542–1549. [Google Scholar] [CrossRef]
- Cherblanc, F.L.; Lo, Y.-P.; Herrebout, W.; Bultinck, P.; Rzepa, H.S.; Fuchter, M.J. Mechanistic and Chiroptical Studies on the Desulfurization of Epidithiodioxopiperazines Reveal Universal Retention of Configuration at the Bridgehead Carbon Atoms. J. Org. Chem. 2013, 78, 11646–11655. [Google Scholar] [CrossRef] [PubMed]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar] [CrossRef]
- Singhal, A. Modern Information Retrieval: A Brief Overview. IEEE Data Eng. Bull. 2001, 24, 35–43. [Google Scholar]
- Kolassa, S.; Schütz, W. Advantages of the MAD/Mean Ratio over the MAPE. Foresight Int. J. Appl. Forecast. 2007, 6, 40–43. [Google Scholar]
- Efron, B. Bootstrap Methods: Another Look at the Jackknife. Ann. Stat. 1979, 7, 1–26. [Google Scholar] [CrossRef]
- Dekking, F.M.; Kraaikamp, C.; Lopuhaä, H.P.; Meester, L.E. A Modern Introduction to Probability and Statistics: Understanding Why and How; Springer: London, UK, 2005. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- O’Boyle, N.M.; Vandermeersch, T.; Flynn, C.J.; Maguire, A.R.; Hutchison, G.R. Confab-Systematic Generation of Diverse Low-Energy Conformers. J. Cheminform. 2011, 3, 8. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples are not available. |
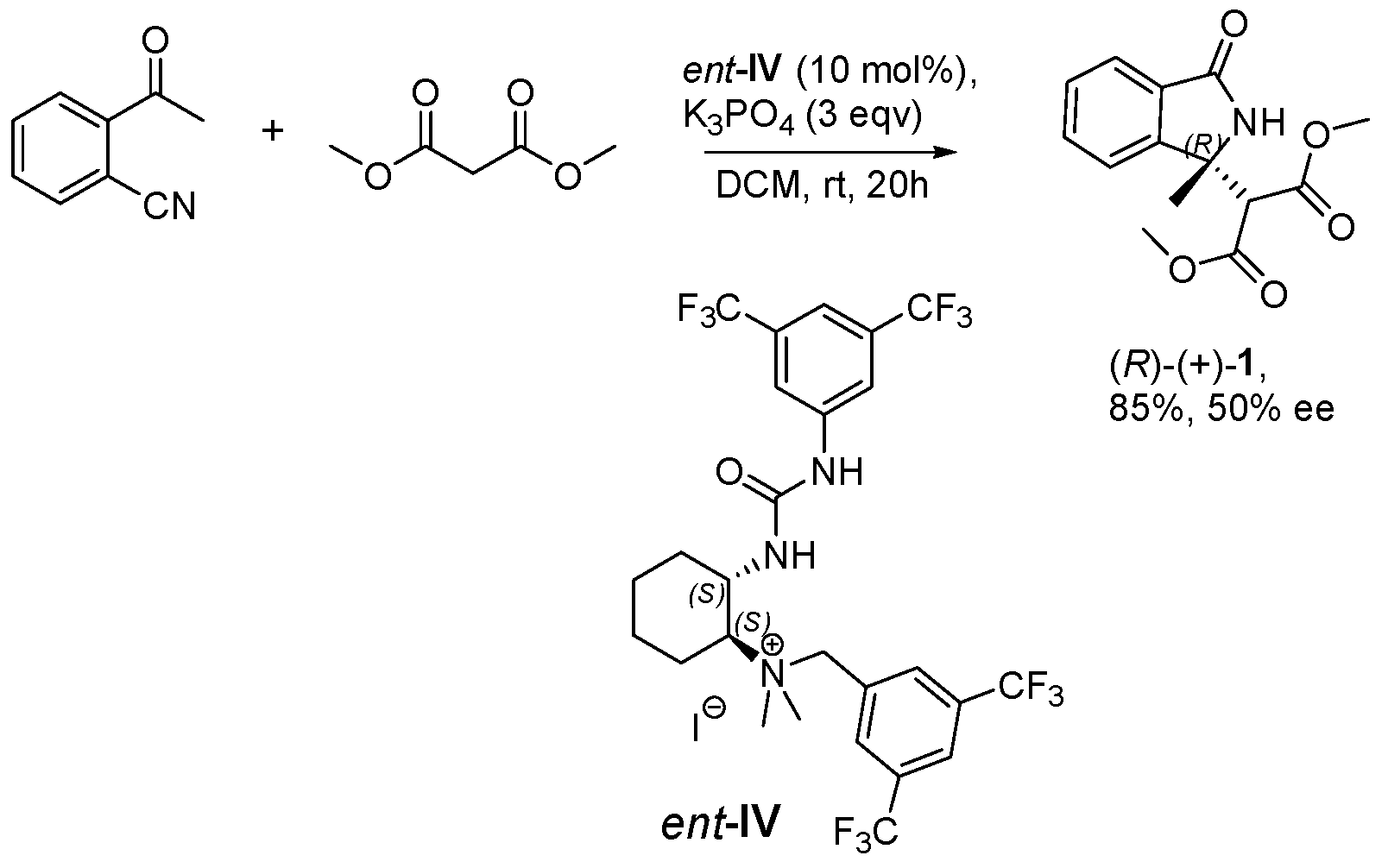
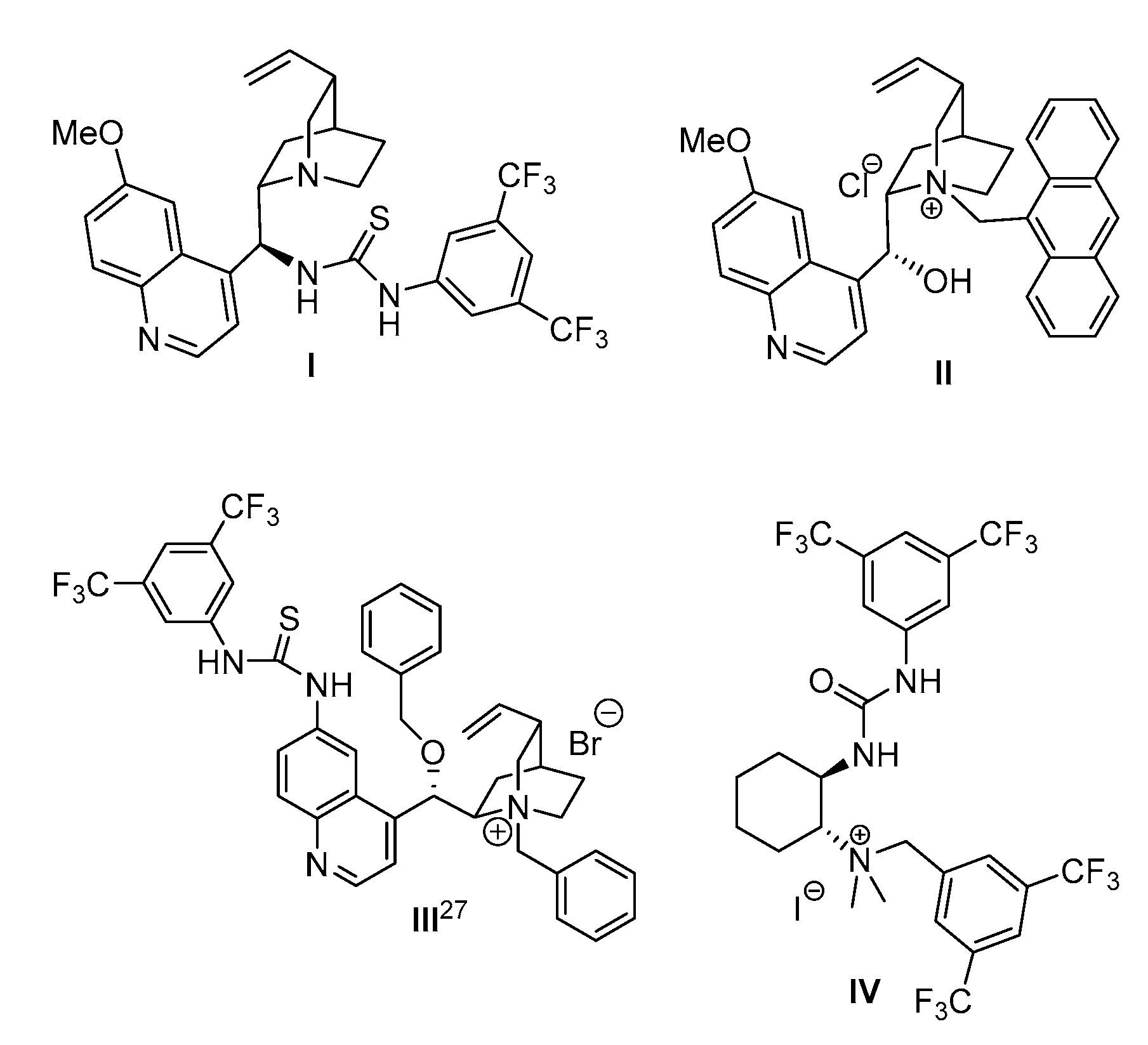
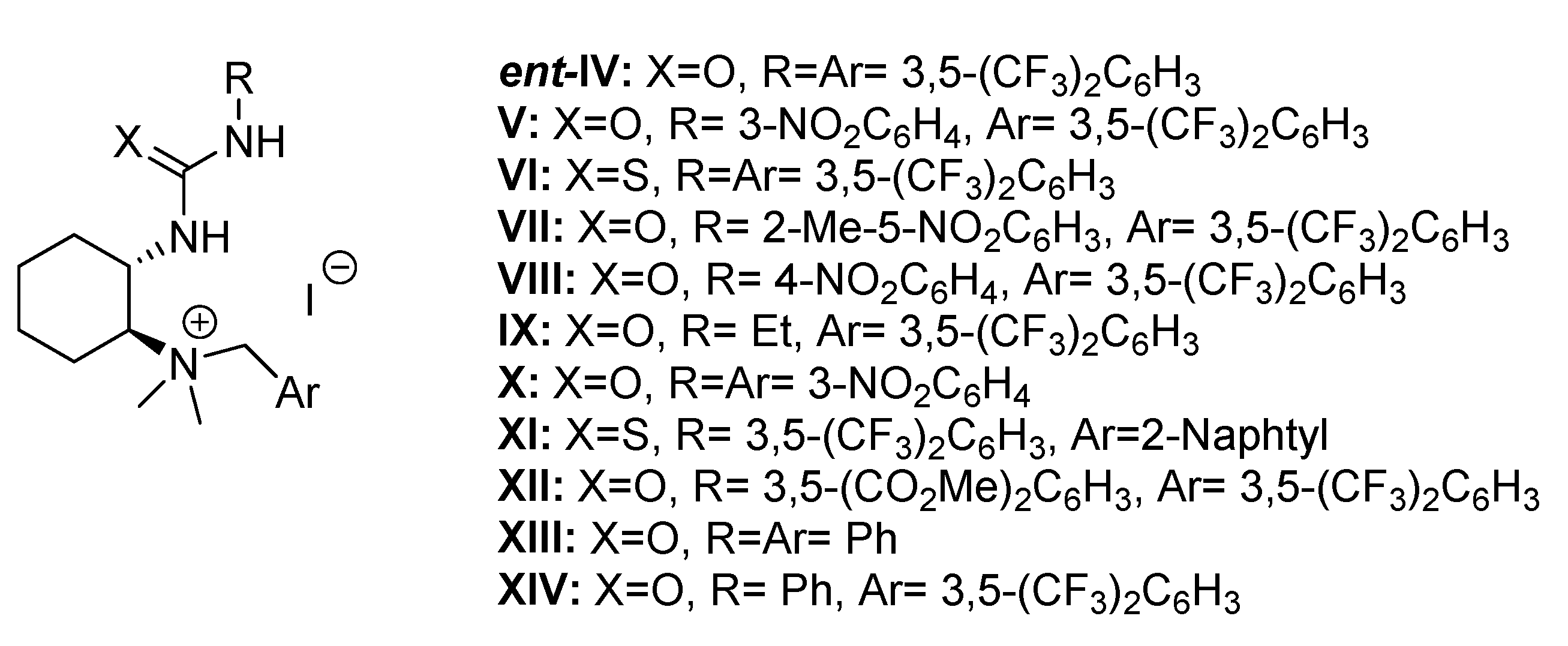
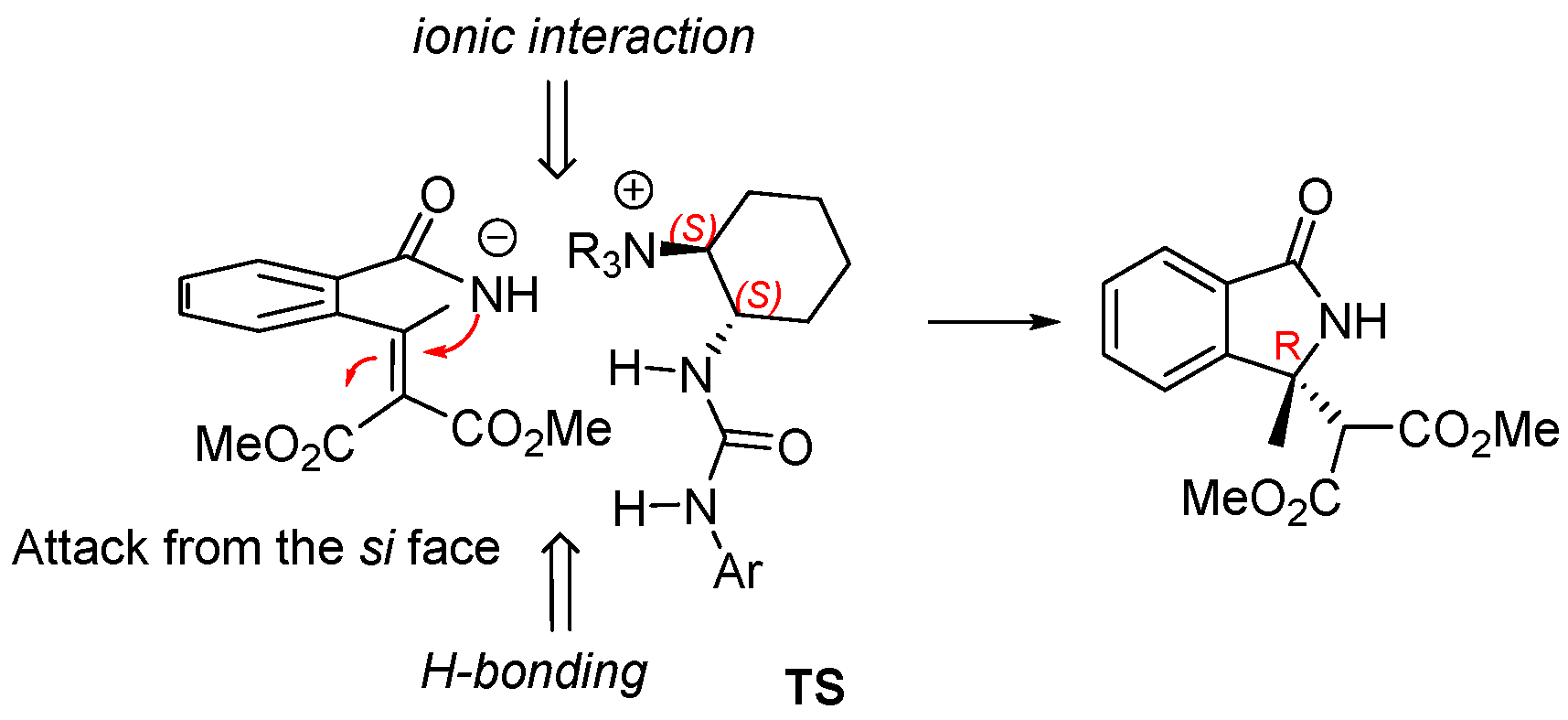

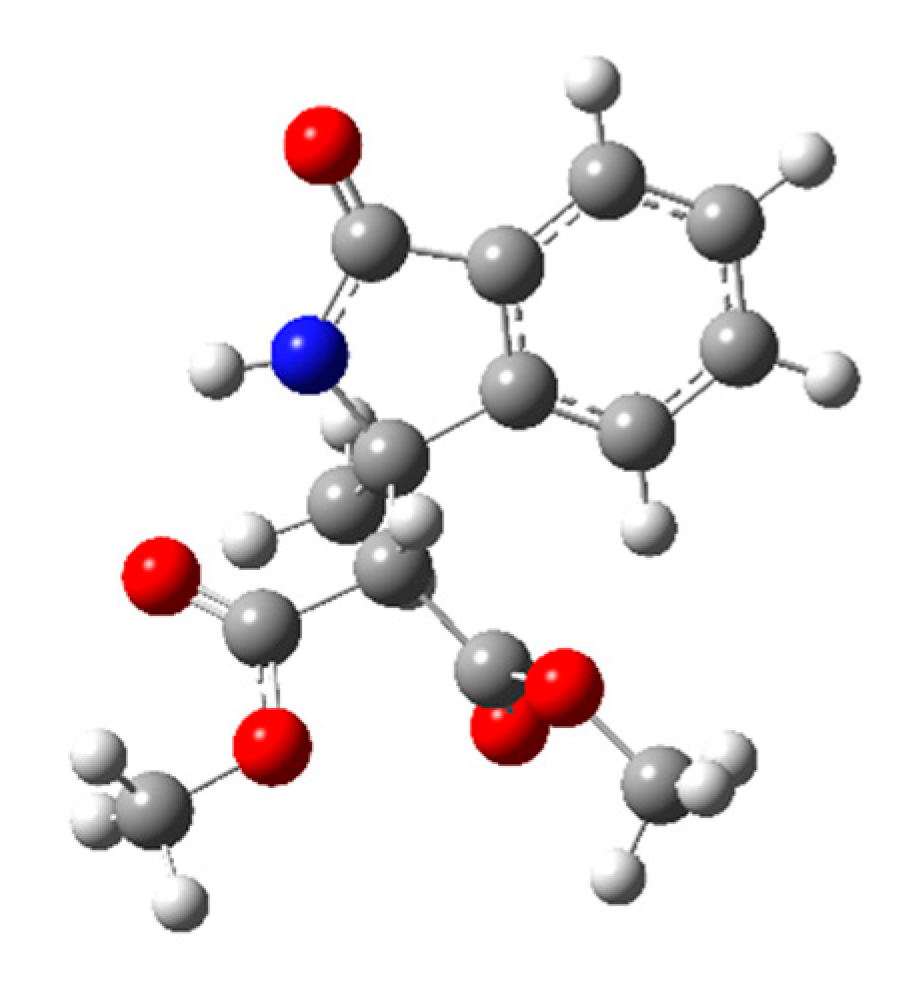
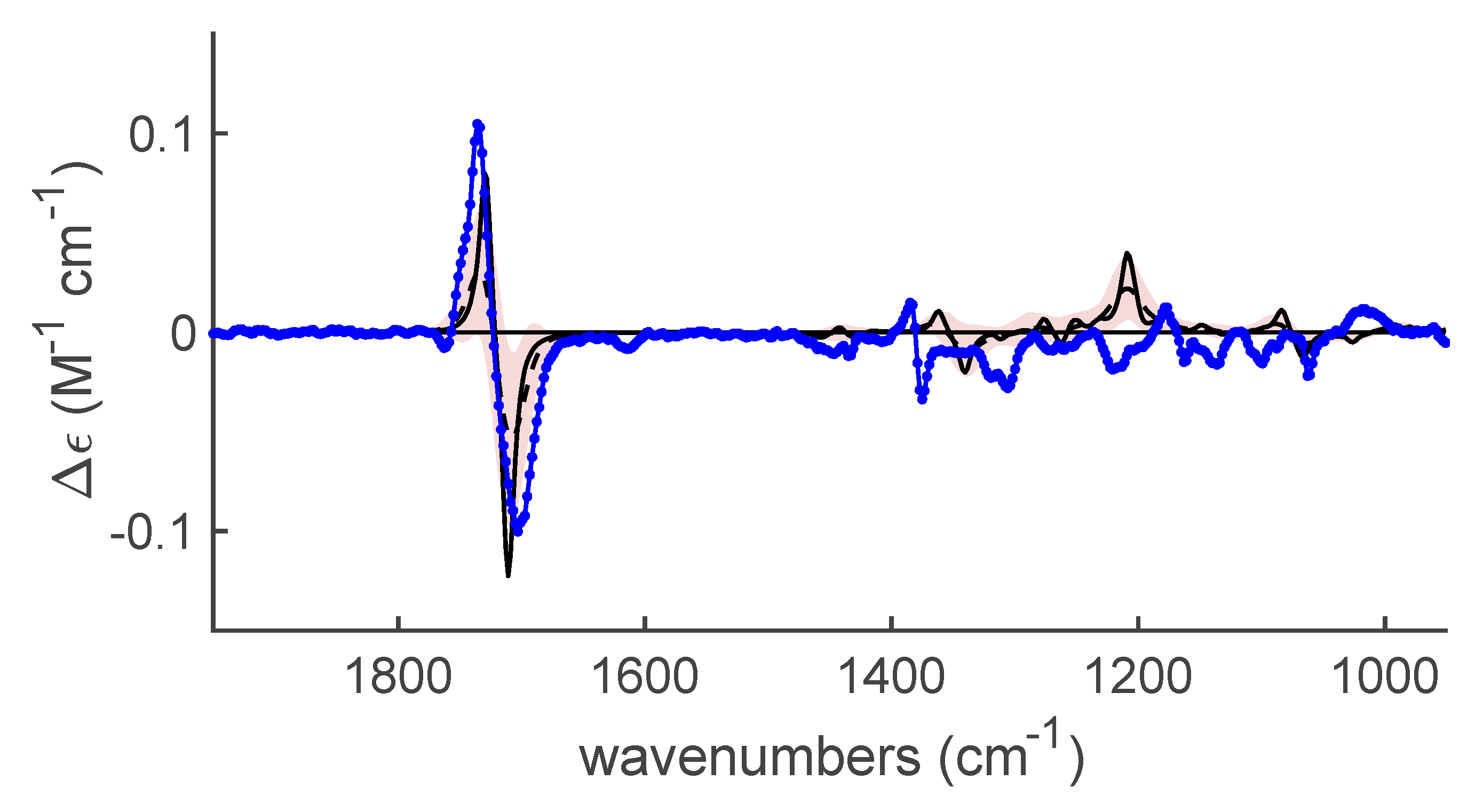
| Entry | Base (1 eq.) | Catalyst | Time (d) | Yield (%) a | ee (%) b |
|---|---|---|---|---|---|
| 1 | None | I | 7 d | No react. | -- |
| 2 | K2CO3 | II | 7 d | 78 | 10 |
| 3 | KOH | II | 7 d | 41 | Rac |
| 4 | K2CO3 | III | 3 d | 53 | Rac |
| 5 | K2CO3 | IV | 7 d | 90 | 48 |
| Entry | IV (mol%) | Base (eq) | Solvent | Time (h) | Yield (%) a | ee (%) b |
|---|---|---|---|---|---|---|
| 1 | 10 | K2CO3 (1) | DCM | 60h | 11 | 50 |
| 2 | 10 | K2CO3 (3) | DCM | 60h | 83 | 50 |
| 3 | 10 | K2CO3 (aq.50%) | DCM | 60h | 9 | 50 |
| 4 | 10 | Cs2CO3 (1) | DCM | 20 | 95 | 28 |
| 5 | 10 | K3PO4 (1) | DCM | 44 | 80 | 50 |
| 6 | 5 | K3PO4 (3) | DCM | 20 | 85 | 50 |
| 7 | 5 | K2HPO4 (3) | DCM | 20 | No react | -- |
| 8 | 5 | KH2PO4 (3) | DCM | 20 | No react | -- |
| 9 | 5 | K3PO4 (3) | toluene | 20 | 66 | 22 |
| 10 | 5 | K3PO4 (3) | MtBE | 20 | 78 | 34 |
| 11 | 5 | K3PO4 (3) | DCE | 20 | 75 | 50 |
| 12 c | 5 | K3PO4 (5) | DCM | 48 | 75 | 30 |
| Entry | Catalyst | Time (h) | Yield (%) b | ee (%) c |
|---|---|---|---|---|
| 1 | ent-IV | 20 | 85 | 50 |
| 2 | V | 72 | 83 | 48 |
| 3 | VI | 72 | 20 | 50 |
| 4 | VII | 20 | 85 | 46 |
| 5 | VIII | 72 | 82 | 52 |
| 6 | IX | 72 | 18 | 22 |
| 7 | X | 72 | 18 | 44 |
| 8 | XI | 72 | 19 | 26 |
| 9 | XII | 20 | 89 | 26 |
| 10 | XIII | 72 | 42 | 7 |
| 11 | XIV | 72 | 74 | 49 |
| (S) | (R) | (S)–(R) | ||||
|---|---|---|---|---|---|---|
| Plain DFT | <∙>b | s(<∙>b) | <∙>b | s(<∙>b) | ∆<∙>b | s(∆<∙>b) |
| RMSE | 0.0190 | 0.0017 | 0.0190 | 0.0017 | 0.00004 | 0.00243 |
| MMAR | 1477 | 9 | 1475 | 8 | 2 | 12 |
| COSI | −0.9979 | 0.0004 | 0.9980 | 0.0004 | −1.9959 | 0.0005 |
| MA-DFT | <∙>b | s(<∙>b) | <∙>b | s(<∙>b) | ∆<∙>b | s(∆<∙>b) |
| RMSE | 2.21 | 0.10 | 2.01 | 0.11 | 0.21 | 0.14 |
| MMAR | 1.08 | 0.01 | 0.99 | 0.01 | 0.097 | 0.019 |
| COSI | −0.15 | 0.05 | 0.15 | 0.05 | −0.31 | 0.07 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monaco, G.; Tiffner, M.; Mola, A.D.; Herrebout, W.; Waser, M.; Massa, A. Chiral Phase Transfer Catalysis in the Asymmetric Synthesis of a 3,3-Disubstituted Isoindolinone and Determination of Its Absolute Configuration by VCD Spectroscopy. Molecules 2020, 25, 2272. https://doi.org/10.3390/molecules25102272
Monaco G, Tiffner M, Mola AD, Herrebout W, Waser M, Massa A. Chiral Phase Transfer Catalysis in the Asymmetric Synthesis of a 3,3-Disubstituted Isoindolinone and Determination of Its Absolute Configuration by VCD Spectroscopy. Molecules. 2020; 25(10):2272. https://doi.org/10.3390/molecules25102272
Chicago/Turabian StyleMonaco, Guglielmo, Maximilian Tiffner, Antonia Di Mola, Wouter Herrebout, Mario Waser, and Antonio Massa. 2020. "Chiral Phase Transfer Catalysis in the Asymmetric Synthesis of a 3,3-Disubstituted Isoindolinone and Determination of Its Absolute Configuration by VCD Spectroscopy" Molecules 25, no. 10: 2272. https://doi.org/10.3390/molecules25102272
APA StyleMonaco, G., Tiffner, M., Mola, A. D., Herrebout, W., Waser, M., & Massa, A. (2020). Chiral Phase Transfer Catalysis in the Asymmetric Synthesis of a 3,3-Disubstituted Isoindolinone and Determination of Its Absolute Configuration by VCD Spectroscopy. Molecules, 25(10), 2272. https://doi.org/10.3390/molecules25102272