The Effect of (1S,2R-((3-bromophenethyl)amino)-N-(4-chloro-2-trifluoromethylphenyl) cyclohexane-1-sulfonamide) on Botrytis cinerea through the Membrane Damage Mechanism
Abstract
1. Introduction
2. Results
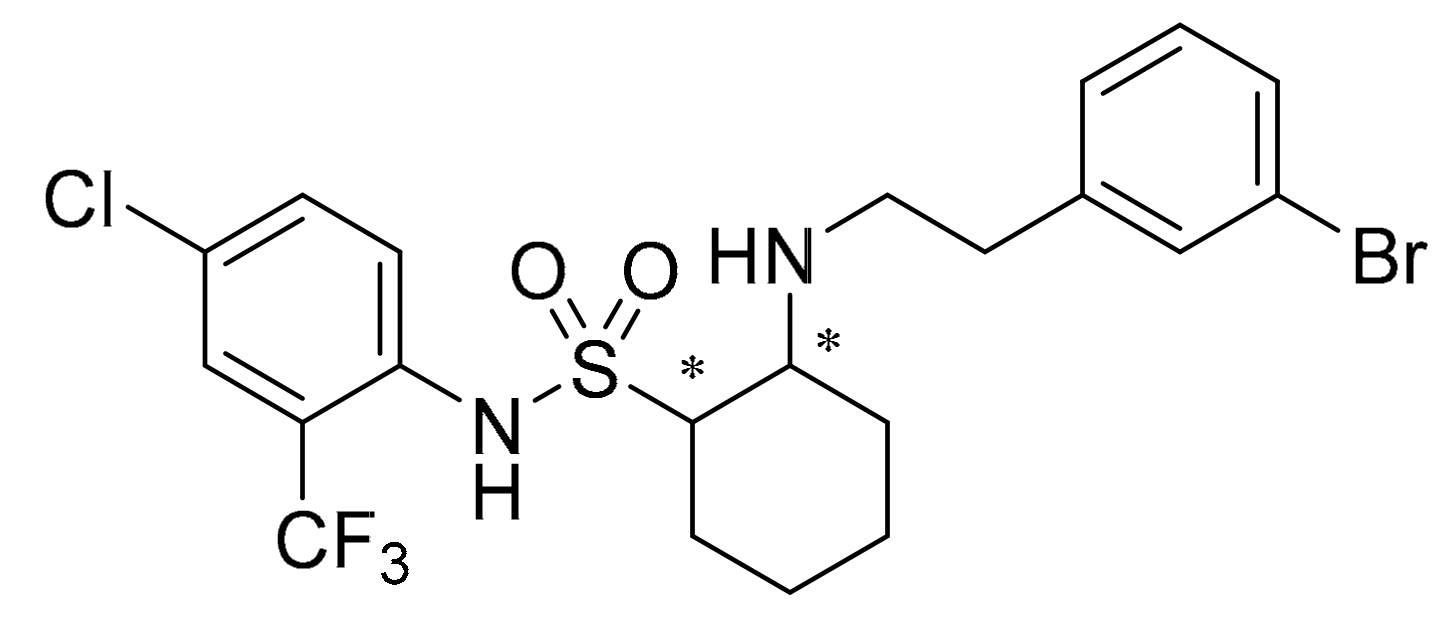
2.1. Relative Electric Conductivity of B. Cinerea
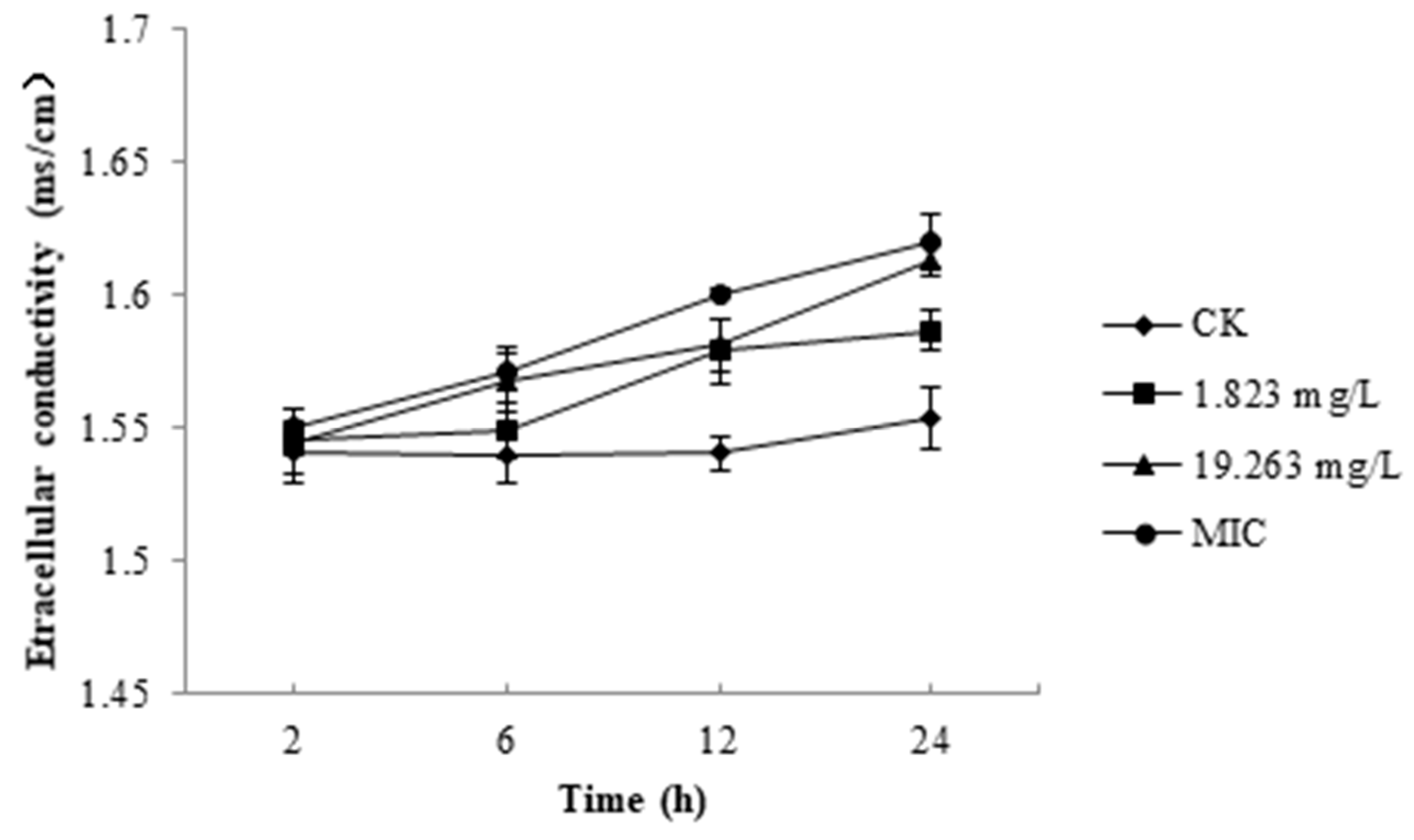
2.2. Nucleic Acid of B. Cinerea
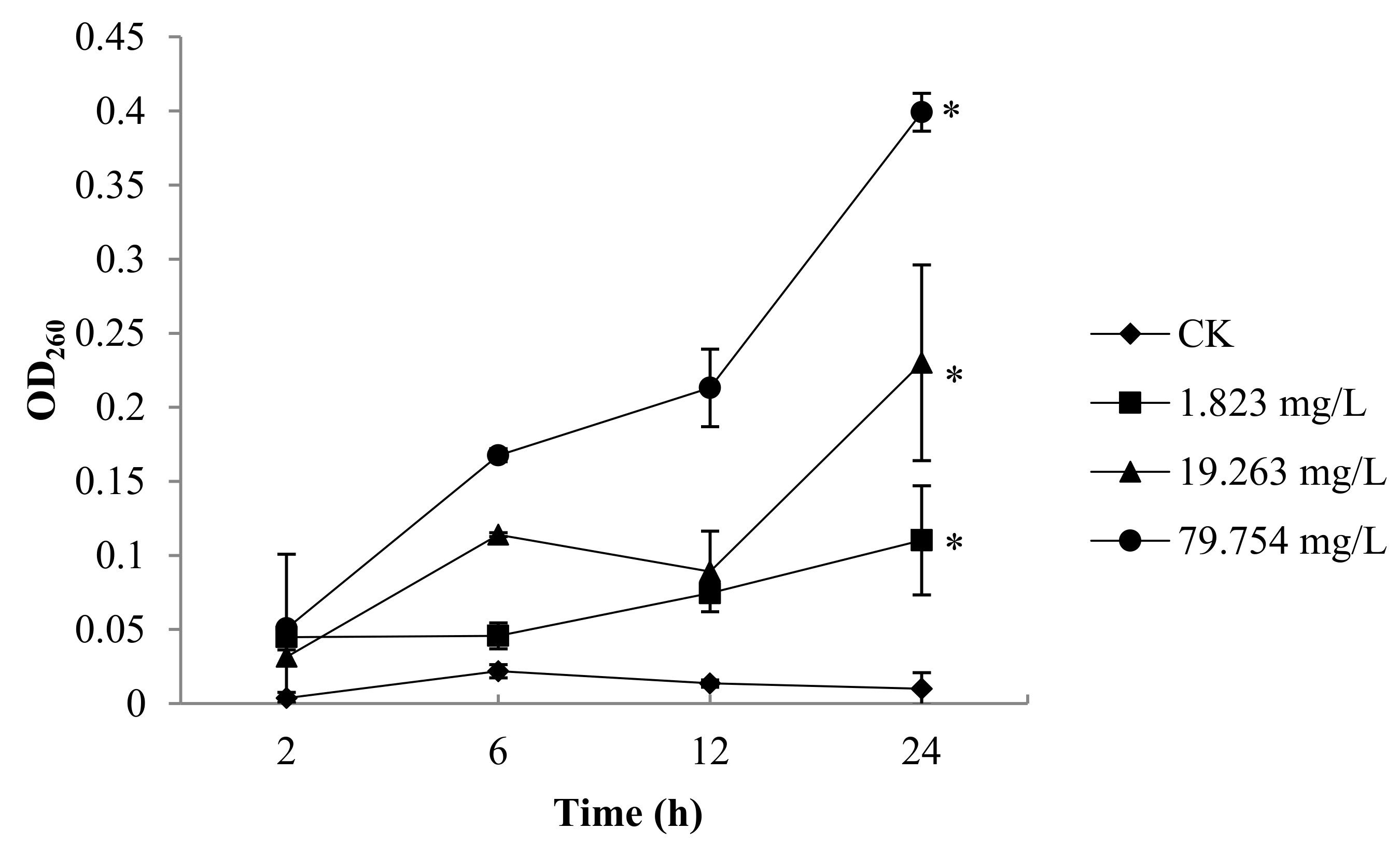
2.3. Malondialdehyde (MDA) Contents of B. Cinerea
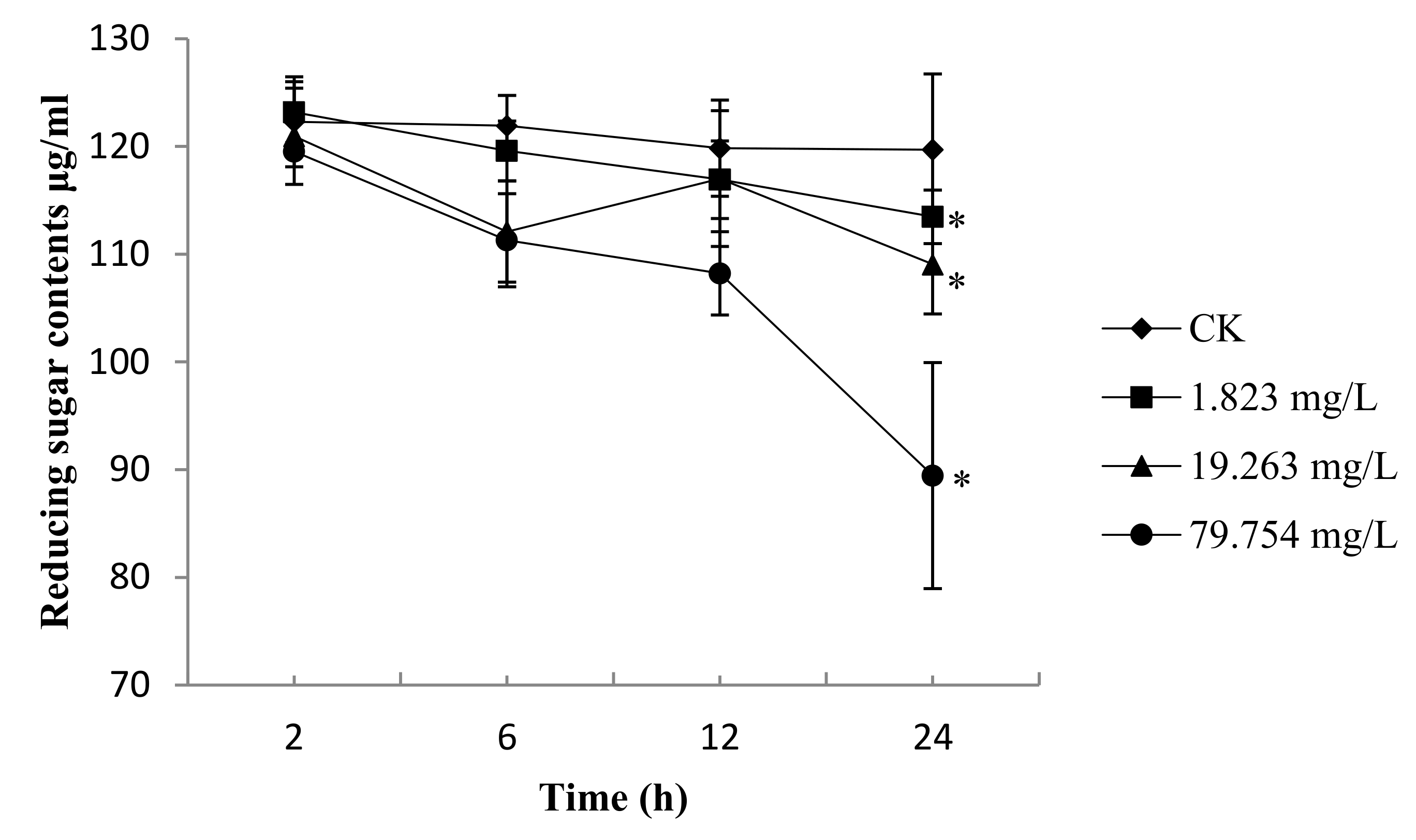
2.4. Reducing Sugar Content of B. Cinerea
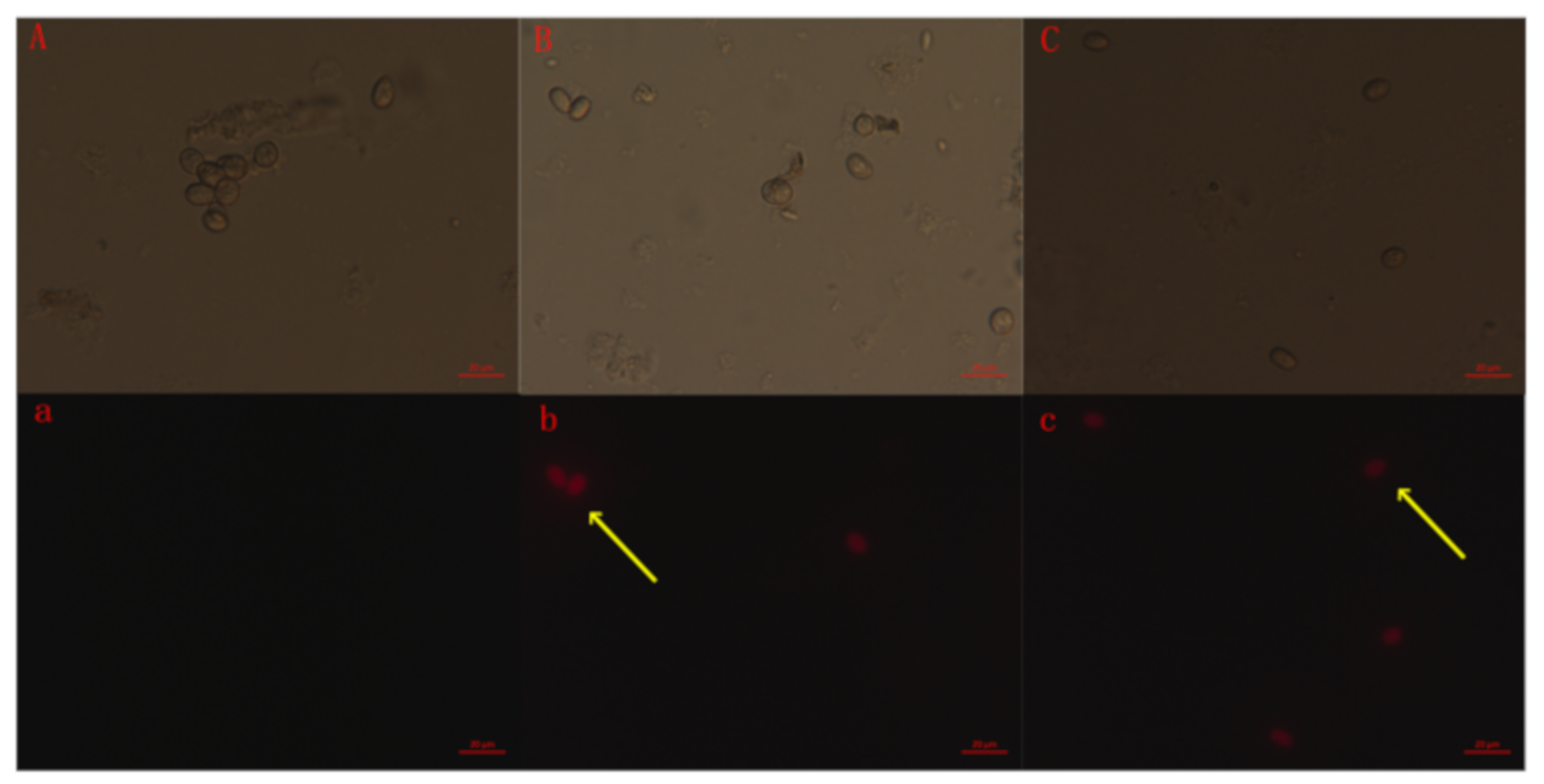
2.5. Membrane Integrity of the B. Cinerea Spore
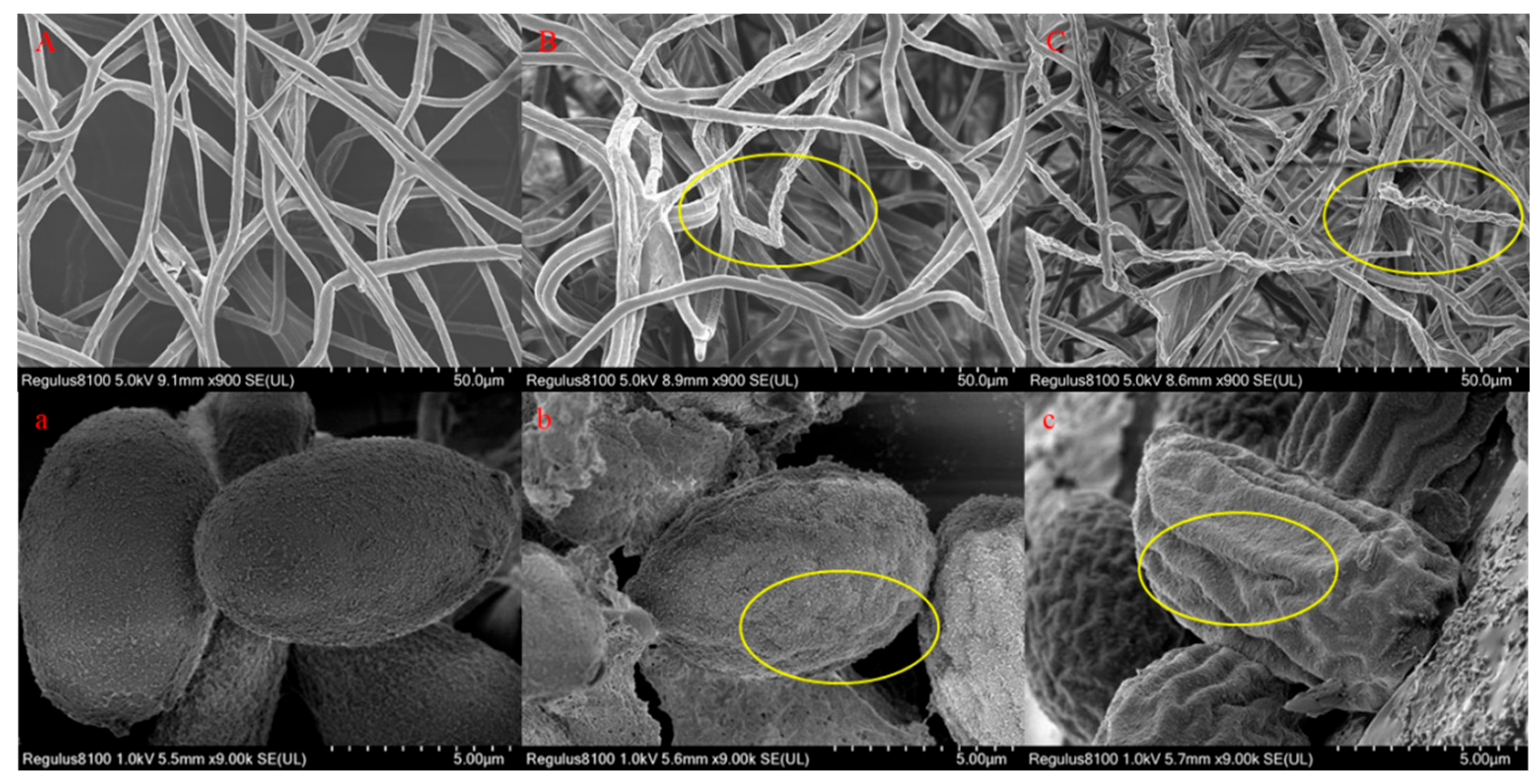
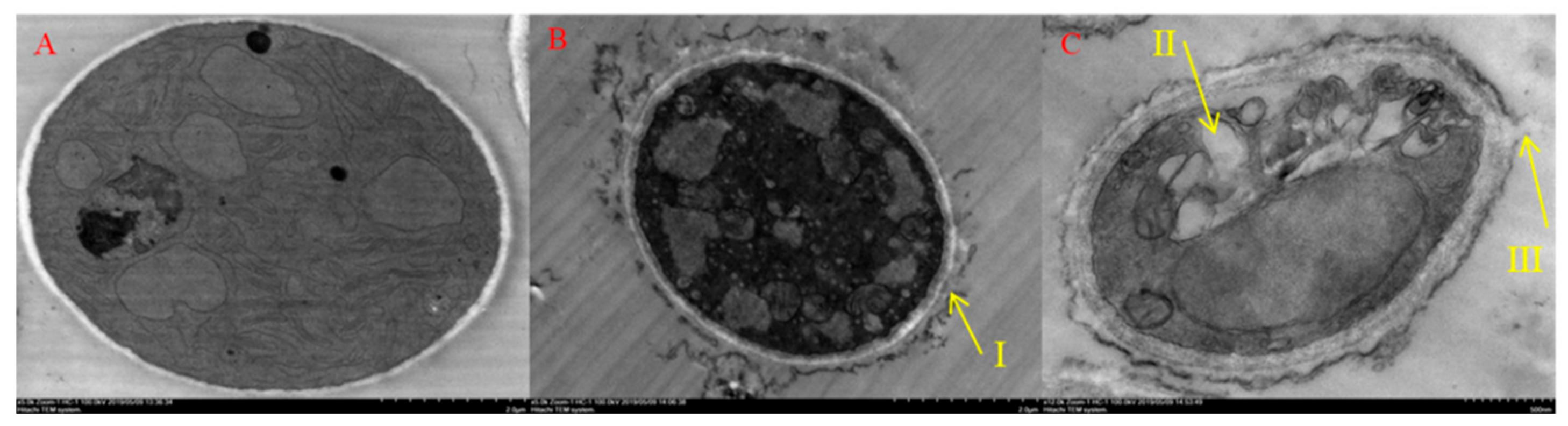
2.6. Morphology and Ultrastructure of B. Cinerea
3. Discussion
4. Materials and Methods
4.1. Collection of Isolate and Chemicals
4.2. Measurement of Relative Electric Conductivity
4.3. Measurement of Nucleic Acids
4.4. Lipid Peroxidation
4.5. Measurement of Reducing Sugar
4.6. Fluorescence Microscopy Observations
4.7. Scanning Electron Microscopy (SEM) Observations
4.8. Transmission Electron Microscopy (TEM) Observations
4.9. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chang, K.F.; Hwang, S.F.; Ahmed, H.U.; Fu, H. First report of Phytophthora sansomeana causing root rot in field pea in Alberta, Canada. Crop. Prot. 2017, 101, 1–4. [Google Scholar] [CrossRef]
- Ma, L.J.; Does, H.C.V.D.; Borkovich, K.A.; Coleman, J.J.; Daboussi, M.J.; Pietro, A.D.; Dufresne, M.; Freitag, M.; Grabherr, M.; Henrissat, B.; et al. Comparative genomics reveals mobile pathogenicity chromosomes in Fusarium. Nature 2010, 464, 367–373. [Google Scholar] [CrossRef]
- Gurr, S.; Samalova, M.; Fisher, M. The rise and rise of emerging infectious fungi challenges food security and ecosystem health. Fungal. Biol. Rev. 2011, 25, 0–188. [Google Scholar] [CrossRef]
- Dean, R.; Van Kan, J.A.L.; Pretorius, Z.A.; Hammond-kosack, K.E.; Pietro, A.D.; Spanu, R.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The top 10 fungal pathogens in molecular plant pathology. Mol. Plant. Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ouyang, C.; Wang, Q.; Li, Y.; Yan, D.; Yang, D. Effects of oil extracts of Eupatorium adenophorum on phytophthora capsici, and other plant pathogenic fungi in vitro. Pestic. Biochem. Phys. 2017, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Baptista, F.J.; Bailey, B.J.; Meneses, J.F. Effect of nocturnal ventilation on the occurrence of Botrytis cinerea in Mediterranean unheated tomato greenhouses. Crop. Prot. 2012, 32, 144–149. [Google Scholar] [CrossRef]
- Wilcox, W.F.; Seem, R.C. Relationship between strawberry gray mold incidence, environmental variables, and fungicide applications during different periods of the fruiting season. Phytopathology. 1993, 84, 264–270. [Google Scholar] [CrossRef]
- Shao, W.Y.; Ren, W.C.; Zhang, Y.; Hou, Y.P.; Duan, Y.B.; Wang, J.X.; Zhou, M.G.; Chen, C.J. Baseline sensitivity of natural populations and characterization of resistant strains of Botrytis cinerea to fluazinam. Australas. Plant. Path. 2015, 44, 375–383. [Google Scholar] [CrossRef]
- Williamson, B.; Tudzynski, B.; Tudzynski, P.; Van Kan, J.A.L. Botrytis cinerea: The cause of grey mould disease. Mol. Plant. Pathol. 2007, 8, 561–580. [Google Scholar] [CrossRef]
- Leroux, P.; Chapeland, F.; Desbrosses, D.; Gredt, M. Patterns of cross-resistance to fungicides in Botryotinia fuckeliana (Botrytis cinerea) isolates from French vineyards. Crop. Pro. 1999, 18, 687–697. [Google Scholar] [CrossRef]
- Fernández-Ortuño, D.; Grabke, A.; Bryson, P.K.; Amiri, A.; Peres, N.A.; Schnabel, G. Fungicide Resistance Profiles in Botrytis cinerea from Strawberry Fields of Seven Southern U.S. States. Plant. Dis. 2014, 98, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Yin, D.; Chen, X.; Hamada, M.S.; Yu, M.; Yin, Y.; Ma, Z. Multiple resistance to QoIs and other classes of fungicides in Botrytis cinerea populations from strawberry in Zhejiang Province, China. Eur. J. Plant. Pathol. 2015, 141, 169–177. [Google Scholar] [CrossRef]
- Liu, S.M.; Che, Z.P.; Chen, G.Q. Multiple-fungicide resistance to carbendazim, diethofencarb, procymidone, and pyrimethanil in field isolates of Botrytis cinerea from tomato in Henan Province, China. Crop. Prot. 2016, 84, 56–61. [Google Scholar] [CrossRef]
- Leroux, P.; Fritz, R.; Debieu, D.; Albertini, C.; Lanen, C.; Bach, J.; Gredt, M.; Chapeland, F. Mechanisms of resistance to fungicides in field strains of Botrytis cinerea. Pest. Manag. Sci. 2002, 58, 876–888. [Google Scholar] [CrossRef] [PubMed]
- Elad, Y.; Yunis, H.; Katan, T. Multiple fungicide resistance to benzimidazoles, dicarboximides and diethofencarb in field isolates of Botrytis cinerea in Israel. Plant. Pathol. 1992, 41, 41–46. [Google Scholar] [CrossRef]
- Myresiotis, C.K.; Karaoglanidiset, G.S.; Tzavella-Klonari, K. Resistance of Botrytis cinerea isolates from vegetable crops to Anilinopyrimidine, Phenylpyrrole, Hydroxyanilide, Benzimidazole, and Dicarboximide Fungicides. Plant. Dis. 2007, 91, 407–413. [Google Scholar] [CrossRef]
- SkoLd, O. Sulfonamide resistance: Mechanisms and trends. Drug. Resist. Update. 2000, 3, 155–160. [Google Scholar] [CrossRef]
- Jabusch, T.W.; Tjerdema, R.S. Partitioning of Penoxsulam, a New Sulfonamide Herbicide. J. Agr. Food. Chem. 2005, 53, 7179–7183. [Google Scholar] [CrossRef]
- Tanaka, S.; Kochi, S.I.; Kunita, H.; Ito, S.; Kameya-lwakiet, M. Biological mode of Action of the fungicide, flusulfamide, against Plasmodiophora brassicae (clubroot). Eur. J. Plant. Pathol. 1999, 105, 577–584. [Google Scholar] [CrossRef]
- Yang, J.C.; Guan, A.Y.; Yang, F.; Liu, C.L. Progress of Fungicides in China and Abroad. Mod Agrochem. 2015, 14, 1–9. [Google Scholar]
- Mitani, S.; Araki, S.; Yamaguchi, S.; Takii, Y.; Ohshima, T.; Matsuo, N. Antifungal Activity of the Novel Fungicide Cyazofamid against Phytophthora infestans and Other Plant Pathogenic Fungi in Vitro. Pestic. Biochem. Phys. 2001, 70, 92–99. [Google Scholar] [CrossRef]
- Wang, M.L.; Rui, P.; Liu, C.X.; Du, Y.; Qin, P.W.; Qi, Z.Q.; Ji, M.S.; Li, X.H.; Cui, Z.N. Design, Synthesis and Fungicidal Activity of 2-Substituted Phenyl-2-oxo-, 2-Hydroxy-and 2-Acyloxyethylsulfonamides. Molecules 2017, 22, 738. [Google Scholar] [CrossRef] [PubMed]
- Walpole, C.; Wei, Z.; Liu, Z.; Tremblay, M.; Desfosses, H.; Milburn, C. 5-sulfonamide benzimidazoles: A class of cannabinoid receptors agonists with potent in vivo Antinociception activity. Lett. Drug. Des. Discov. 2010, 7, 208–213. [Google Scholar]
- Keche, A.P.; Hatnapure, G.D.; Tale, R.H.; Rodge, A.H.; Birajdar, S.S.; Kamble, V.M. A novel pyrimidine derivatives with aryl urea, thiourea and sulfonamide moieties: Synthesis, anti-inflammatory and antimicrobial evaluation. Bioorg. Med. Chem. Lett. 2012, 22, 3445–3448. [Google Scholar] [CrossRef] [PubMed]
- Li, X.H.; Pan, Q.; Cui, Z.N.; Ji, M.S.; Qi, Z.Q. Synthesis and fungicidal activity of N-(2,4,5-trichlorophenyl)-2-oxoand 2-hydroxycycloalkylsulfonamides. Lett. Drug. Des. Discov. 2013, 10, 353–359. [Google Scholar] [CrossRef]
- Wang, M.Y.; Li, Z.M. Synthesis and Bioactivities of Novel 2-methoxycarbonyl-5-arylazomethine Benzenesulfonamide. Chin. J. Synth. Chem. 2012, 20, 65–68. [Google Scholar]
- Zhang, H.B.; Zhang, J.J.; Yan, X.J.; Liang, X.M.; Wang, D.Q. A Novel Fungicide Chesulfamide. Agrochemicals 2012, 51, 287–288. [Google Scholar]
- Li, X.H.; Ji, M.S.; Qi, Z.Q.; Li, X.W.; Shen, Y.X.; Gu, Z.M.; Zhang, Y.; Wei, S.H.; Wang, Y.Z.; Wang, D.Q. Synthesis of 2-amino-6-oxocyclohexenyl-sulfonamides and their activity against Botrytis cinerea. Pest. Manag. Sci. 2011, 67, 986–992. [Google Scholar] [CrossRef]
- Cai, N.; Liu, C.X.; Feng, Z.H.; Li, X.H.; Qi, Z.Q.; Ji, M.S.; Qin, P.W.; Ahmed, W.; Cui, Z.N. Design, Synthesis, and SAR of Novel 2-GlycinamideCyclohexyl Sulfonamide Derivatives against Botrytis cinerea. Molecules 2018, 23, 740. [Google Scholar] [CrossRef]
- Cai, N.; He, L.; Wang, K.; Feng, Z.H.; Cui, Z.N.; Ji, M.S.; Qi, Z.Q.; Qin, P.W.; Li, X.H. Novel sulfonamides against Botrytis cinerea with no positive cross-resistance to commercial fungicides: Design, synthesis and SAR study. Bioorg. Med. Chem. Lett. 2019. [Google Scholar] [CrossRef]
- Chen, Z.; Xu, W.M.; Liu, K.M.; Yang, S.; Fan, H.T.; Bhadury, P.S.; Hu, D.Y.; Zhang, Y.P. Synthesis and Antiviral Activity of 5-(4-Chlorophenyl)-1,3,4-Thiadiazole Sulfonamides. Molecules 2010, 15, 9046–9056. [Google Scholar] [CrossRef] [PubMed]
- Basanagouda, M.; Shivashankar, K.; Kulkarni, M.V.; Rasal, V.P.; Patel, H.; Mutha, S.S.; Mohite, A.A. Synthesis and antimicrobial studies on novel sulfonamides containing4-azidomethyl coumarin. Eur. J. Med. Chem. 2010, 45, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Cox, S.D.; Mann, C.M.; Markham, J.L.; Gustafson, J.E.; Warmington, J.R.; Wyllie, S.G. Determining the Antimicrobial Actions of Tea Tree Oil. Molecules 2001, 6, 87–91. [Google Scholar] [CrossRef]
- Trumpower, B.L.; Gennis, R.B. Energy Transduction by Cytochrome Complexes in Mitochondrial and Bacterial Respiration: The Enzymology of Coupling Electron Transfer Reactions to Transmembrane Proton Translocation. Annu. Rev. Biochem. 1994, 63, 675–716. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.J.; Li, Z.B.; Wang, J.J.; Weng, Q.F.; Chen, S.H.; Hu, M.Y. Antifungal Activity of Isoliquiritin and Its Inhibitory Effect against Peronophythora litchi Chen through a Membrane Damage Mechanism. Molecules 2016, 21, 237. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Zhang, L.P. Effects of Heavy Metals on Membrane Lipid Peroxidation and Proline and Soluble Sugar Contents in Barley Seedlings. J. Agro-Environ Sci 2006, 25, 857–860. [Google Scholar]
- Choi, G.J.; Lee, H.J.; Cho, K.Y. Lipid Peroxidation and Membrane Disruption by Vinclozolin in Dicarboximide-Susceptible and -Resistant Isolates of Botrytis cinerea. Pestic. Biochem. Phys. 1996, 55, 29–39. [Google Scholar] [CrossRef]
- Ma, D.Y.; Ji, D.C.; Zhang, Z.Q.; Li, B.Q.; Qin, G.Z.; Xu, Y.; Chen, T.; Tian, S.Q. Efficacy of rapamycin in modulating autophagic activity of Botrytis cinerea for controlling gray mold. Postharvest. Biol. Tec. 2019, 150, 158–165. [Google Scholar] [CrossRef]
- Mach, R.L.; Peterbauer, C.K.; Payer, K.; Jaksits, S.; Woo, S.L.; Zeilinger, S. Expression of Two Major Chitinase Genes of Trichoderma atroviride (T. harzianum P1) Is Triggered by Different Regulatory Signals. Appl. Environ. Microbiol 1999, 65, 1858–1863. [Google Scholar] [CrossRef]
- Gao, T.; Zhou, H.; Zhou, W.; Hu, L.B.; Chen, J.; Shi, Z.Q. The Fungicidal Activity of Thymol against Fusarium graminearum via Inducing Lipid Peroxidation and Disrupting Ergosterol Biosynthesis. Molecules 2016, 21, 770. [Google Scholar] [CrossRef]
- Qi, Z.Q.; Sun, Q.B.; Li, X.H.; Gu, Z.M.; Li, X.W.; Ji, M.S. Inhibitory effect of N- (2,4,5- trichlorophenyl)- 2-oxocyclohexylsulfonamide against Botrytis cinerea. Chinese J. Pestic. Sci. 2014, 16, 523–528. [Google Scholar]
- Lewis, J.A.; Papavizas, G.C. Permeability Changes in Hyphae of Rhizoctonia solani Induced by Germling Preparations of Trichoderma and Gliocladium. Phytopathology. 1987, 77, 699–703. [Google Scholar] [CrossRef]
- Cai, J.H.; Chen, J.; Lu, G.B.; Zhao, Y.M.; Tian, S.P.; Qin, G.Z. Control of brown rot on jujube and peach fruits by trisodium phosphate. Postharvest Biol. Tec. 2015, 99, 93–98. [Google Scholar] [CrossRef]
- Wang, C.M.; Zhang, J.; Chen, H.; Shi, Z.Q.; Fan, Y.J. Lipid Peroxidation and Membrane Disruption by Natural Compound Eugenol in Mycelia of Botrytis cinerea. Chinese J. Pestic. Sci. 2009, 11, 104–108. [Google Scholar]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Biochem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Qin, G.; Zong, Y.; Chen, Q.; Hua, D.; Tian, S. Inhibitory effect of boron against Botrytis cinerea on table grapes and its possible mechanisms of action. Int. J. Food. Microbiol. 2010, 138, 145–150. [Google Scholar] [CrossRef]
- Wang, B.; Liu, F.; Li, Q.; Xu, S.; Zhao, X.Z.; Xue, P.L.; Feng, X. Antifungal activity of zedoary turmeric oil against Phytophthora capsici through damaging cell membrane. Pestic. Biochem. Phys. 2019, 159, 59–67. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, J.; Wang, K.; Feng, T.; Zhang, H.; Li, X.; Qi, Z. The Effect of (1S,2R-((3-bromophenethyl)amino)-N-(4-chloro-2-trifluoromethylphenyl) cyclohexane-1-sulfonamide) on Botrytis cinerea through the Membrane Damage Mechanism. Molecules 2020, 25, 94. https://doi.org/10.3390/molecules25010094
Peng J, Wang K, Feng T, Zhang H, Li X, Qi Z. The Effect of (1S,2R-((3-bromophenethyl)amino)-N-(4-chloro-2-trifluoromethylphenyl) cyclohexane-1-sulfonamide) on Botrytis cinerea through the Membrane Damage Mechanism. Molecules. 2020; 25(1):94. https://doi.org/10.3390/molecules25010094
Chicago/Turabian StylePeng, Jingnan, Kai Wang, Tingyue Feng, Huazhong Zhang, Xinghai Li, and Zhiqiu Qi. 2020. "The Effect of (1S,2R-((3-bromophenethyl)amino)-N-(4-chloro-2-trifluoromethylphenyl) cyclohexane-1-sulfonamide) on Botrytis cinerea through the Membrane Damage Mechanism" Molecules 25, no. 1: 94. https://doi.org/10.3390/molecules25010094
APA StylePeng, J., Wang, K., Feng, T., Zhang, H., Li, X., & Qi, Z. (2020). The Effect of (1S,2R-((3-bromophenethyl)amino)-N-(4-chloro-2-trifluoromethylphenyl) cyclohexane-1-sulfonamide) on Botrytis cinerea through the Membrane Damage Mechanism. Molecules, 25(1), 94. https://doi.org/10.3390/molecules25010094




