The N’-Substituted Derivatives of 5-Chloro-3-Methylisothiazole-4-Carboxylic Acid Hydrazide with Antiproliferative Activity
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. General Information
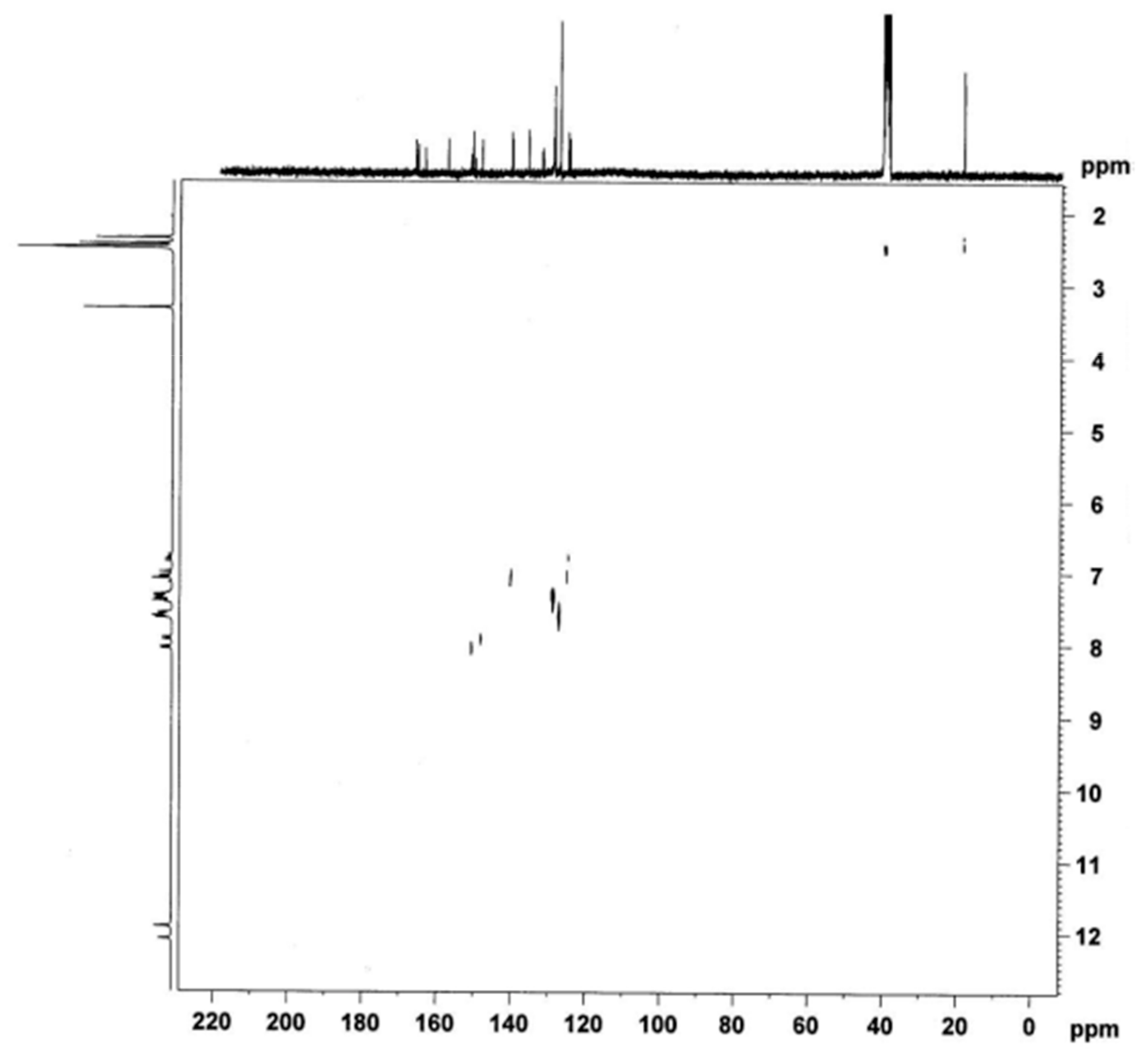
4.2. Procedures for the Synthesis All the New Compounds and Their Spectroscopic Data (IR, 1H-NMR, 13C-NMR, 2D 1H-13C NMR, ESI-MS)
4.2.1. 5-Chloro-3-Methylisothiazole-4-Carbohydrazide 2
4.2.2. 5-Chloro-N’-[(1E,2E)-3-phenylprop-2-en-1-ylidene]-3-methylisothiazole-4-carbohydrazide 3
4.2.3. 5-Chloro-N’-[(E)-(3-chlorophenyl)methylidene]-3-methylisothiazole-4-carbohydrazide 4
4.2.4. 5-Chloro-N’-[(E)-(3-nitrophenyl)methylidene]-3-methylisothiazole-4-carbohydrazide 5
4.2.5. 5-Chloro-N’-[(E)-(4-ethylphenyl)methylidene]-3-methylisothiazole-4-carbohydrazide 6
4.2.6. 5-Chloro-N’-[(E)-(3-methoxyphenyl)methylidene]-3-methylisothiazole-4-carbohydrazide 7
4.2.7. 5-Chloro-N’-[(E)-phenylmethylidene]-3-methylisothiazole-4-carbohydrazide 8
4.2.8. 5-Chloro-N’-[(E)-(2,4-dimethylphenyl)methylidene]-3-methylisothiazole-4-carbohydrazide 9
4.2.9. 5-Chloro-N’-[(E)-(2-methylphenyl)methylidene]-3-methylisothiazole-4-carbohydrazide 10
4.2.10. 5-Chloro-N’-[(E)-(2-chlorophenyl)methylidene]-3-methylisothiazole-4-carbohydrazide 11
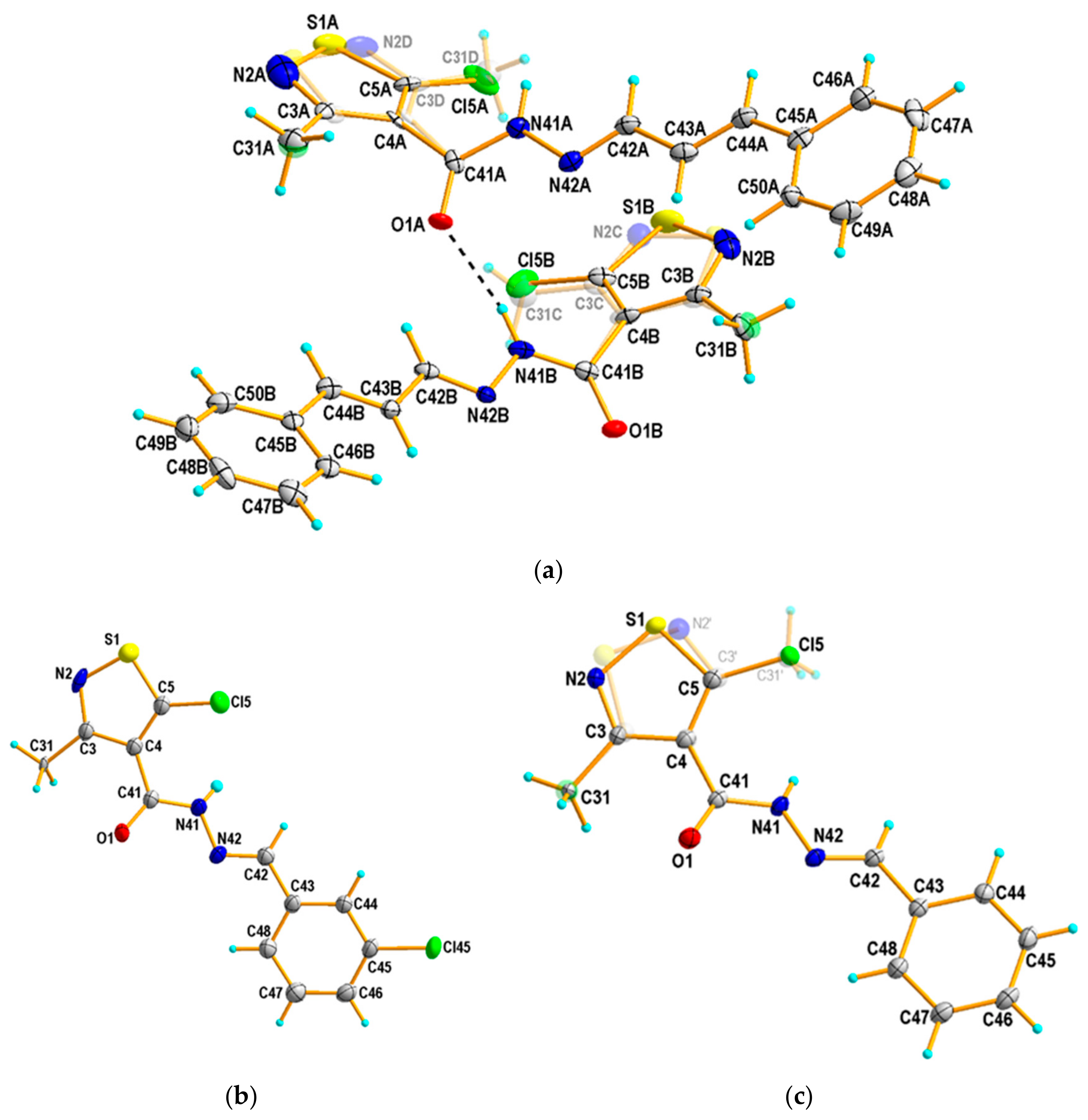
4.3. Single Crystal X-ray Structure Determination of 3, 4 and 8
4.4. Pharmacology
4.4.1. Cell Culture
4.4.2. Cells Preparing for Antiproliferative Assays
4.4.3. Cytotoxicity Assay In Vitro
MTT Assay (for MV4-11 Cell Line)
Sulforhodamine B Assay (for Other Cell Lines)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Si, W.; Shen, J.; Zheng, H.; Fan, W. The role and mechanisms of action of microRNAs in cancer drug resistance. Clin. Epigenet. 2019, 11, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Pusuluri, A.; Krishnan, V.; Wu, D.; Wyatt Shields, C.W.; Wang, L.W.; Mitragotri, S. Role of synergy and immunostimulation in design of chemotherapy combinations: An analysis of doxorubicin and camptothecin. Bioeng. Transl. Med. 2019, 4, 10129–10140. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Chen, Z.; Sub, Q.; Ye, S.; Yuan, H.; Kuai, M.; Lv, M.; Tu, Z.; Yang, X.; Liue, R.; et al. Dual Inhibitors of RAF-MEK-ERK and PI3K-PDK1-AKT pathways: Design, Synthesis and Preliminary Anticancer Activity Studies of 3-Substituted-5-(phenylamino) indolone Derivatives. Bioorgan. Med. Chem. 2019, 27, 944–954. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, M.; Sallam, H.A.; Shaban, S.S.; Abdel-Wahab, S.S.; Amr, A.E.; Azab, M.E.; Nossier, E.S.; Al-OmaR, M.A. Design, Synthesis, and Molecular Docking Study of Novel Heterocycles Incorporating 1,3,4-Thiadiazole Moiety as Potential Antimicrobial and Anticancer Agents. Molecules 2019, 24, 1066. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, N.M.Y.; Serya, R.A.T.; Tolba, M.F.; Ahmed, M.; Barakat, K.; Ella, K.D.A.A.E.; Abouzid, K.A.M. Design, synthesis, biological evaluation and dynamics simulation of indazole derivatives with antiangiogenic and antiproliferative anticancer activity. Bioorgan. Chem. 2019, 82, 340–359. [Google Scholar] [CrossRef] [PubMed]
- Tahlan, S.; Kumar, S.; Ramasamy, K.; Lim, S.M.; Shah, S.A.A.A.; Mani, V.; Pathania, R.; Narasimhan, B. In-silico molecular design of heterocyclic benzimidazole scafolds as prospective anticancer agents. BMC Chem. 2019, 13, 90. [Google Scholar] [CrossRef] [PubMed]
- Beebe, J.S.; Jani, J.P.; Knauth, E.; Goodwin, P.; Higdon, C.; Rossi, A.M.; Emerson, E.; Finkelstein, M.; Floyd, E.; Harriman, S.; et al. Pharmacological Characterization of CP-547,632, a Novel Vascular Endothelial Growth Factor Receptor-2 Tyrosine Kinase Inhibitor for Cancer Therapy. Cancer Res. 2003, 63, 7301–7309. [Google Scholar]
- Sridevi, G.; Arul Antony, S.; Angayarkani, R. Schiff Base Metal Complexes as Anticancer Agents. Asian J. Chem. 2019, 31, 493–504. [Google Scholar] [CrossRef]
- Cohen, R.B.; Langer, C.J.; Simon, G.R.; Eisenberg, P.D.; Hainsworth, J.D.; Madajewicz, S.; Cosgriff, T.M.; Pierce, K.; Xu, H.; Liau, K.; et al. A phase I/randomized phase II, non-comparative, multicenter, open label trial of CP-547,632 in combination with paclitaxel and carboplatin or paclitaxel and carboplatin alone as first-line treatment for advanced non-small cell lung cancer (NSCLC). Cancer Chemother. Pharmacol. 2007, 60, 81–89. [Google Scholar] [CrossRef]
- Sierko, E.; Wojtukiewicz, M.Z. Podstawy terapii antyangiogennej u chorych na nowotwory. Nowotw. J. Oncol. 2008, 58, 349–357. [Google Scholar]
- Abdellaoui, H.E.; Varaprasad, C.V.N.S.; Barawkar, D.; Chakravarty, S.; Maderna, A.; Tam, R.; Chen, H.; Allan, M.; Wu, J.Z.; Appleby, T.; et al. Identification of isothiazole-4-carboxamidines derivatives as a novel class of allosteric MEK1 inhibitors. Bioorgan. Med. Chem. Lett. 2006, 16, 5561–5566. [Google Scholar] [CrossRef] [PubMed]
- Melagraki, G.; Afantitis, A.; Sarimveis, H.; Igglessi-Markopoulou, O.; Koutentis, P.A.; Kollias, G. In Silico Exploration for Identifying Structure–Activity Relationship of MEK Inhibition and Oral Bioavailability for Isothiazole Derivatives. Chem. Biol. Drug Des. 2010, 76, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Reddy, B.M.; Tanneeru, K.; Angamba Meetei, P.; Guruprasad, L. 3D-QSAR and Molecular Docking Studies on Substituted Isothiazole Analogs as Inhibitors Against MEK-1 Kinase. Chem. Biol. Drug Des. 2012, 79, 84–91. [Google Scholar] [CrossRef] [PubMed]
- AhmadPasha, F.; Muddassar, M.; JooCho, S. Molecular Docking and 3D QSAR Studies of Chk2 Inhibitors. Chem. Biol. Drug Des. 2009, 73, 292–300. [Google Scholar] [CrossRef]
- Velic, D.; Couturier, A.M.; Tedim Ferreira, M.; Rodrigue, A.; Poirier, G.G.; Fleury, F.; Masson, J. DNA Damage Signalling and Repair Inhibitors: The Long-Sought-After Achilles’ Heel of Cancer. Biomolecules 2015, 5, 3204–3259. [Google Scholar] [CrossRef] [PubMed]
- Larson, G.; Yan, S.; Chen, H.; Rong, F.; Hong, Z.; Wu, J.Z. Identification of novel, selective and potent Chk2 inhibitors. Bioorgan. Med. Chem. Lett. 2007, 17, 172–175. [Google Scholar] [CrossRef]
- Rao Ambati, S.; Gudala, S.; Sharma, A.; Penta, S.; Loka Reddy, V.; Bomma, Y.; RaoJanapala, V.; Pola, S. Facile Synthesis of Novel 3-(4-phenylisothiazol-5-yl)-2H-chromen-2-one Derivatives as Potential Anticancer Agents. J. Heterocycl. Chem. 2017, 54, 2333–2341. [Google Scholar] [CrossRef]
- Coffey, K.; Blackburn, T.J.; Cook, S.; Golding, B.T.; Griffin, R.J.; Hardcastle, I.R.; Hewitt, L.; Huberman, K.; McNeill, H.V.; Newell, D.R.; et al. Characterisation of a Tip60 Specific Inhibitor, NU9056, in Prostate Cancer. PLoS ONE 2012, 7, e45539. [Google Scholar] [CrossRef]
- Kuczyński, L.; Kuriata, M.; Ciupka, B. Synthesis of new 4 and 5 disubstituted isothiazoles. Pol. J. Pharmacol. Pharm. 1984, 36, 485–491. [Google Scholar]
- Machoń, Z. Synthesis and cytostatic properties of some isothiazole derivatives. Diss. Pharm. Pharmacol. 1969, 21, 135–144. [Google Scholar]
- Demirbas, N.; Karaoglu, S.A.; Demirbas, A.; Sancak, K. Synthesis and antimicrobial activities of some new 1-(5-phenylamino-[1,3,4]thiadiazol-2-yl)methyl-5-oxo-[1,2,4]triazole and 1-(4-phenyl-5-thioxo-[1,2,4]triazol-3-yl)methyl-5-oxo-[1,2,4]triazole derivatives. Eur. J. Med. Chem. 2004, 39, 793–804. [Google Scholar] [CrossRef] [PubMed]
- Bayrak, H.; Demirbas, A.; Karaoglu, S.I.A.; Demirbas, N. Synthesis of some new 1,2,4-triazoles, their Mannich and Schiff bases and evaluation of their antimicrobial activities. Eur. J. Med. Chem. 2009, 44, 1057–1066. [Google Scholar] [CrossRef] [PubMed]
- Vicini, P.; Incerti, M.; La Colla, P.; Loddo, R. Anti-HIV evaluation of benzo[d]isothiazole hydrazones. Eur. J. Med. Chem. 2009, 44, 1801–1807. [Google Scholar] [CrossRef] [PubMed]
- Vicini, P.; Incerti, M.; Doytchinova, I.A.; La Colla, P.; Busonera, B.; Loddo, R. Synthesis and antiproliferative activity of benzo[d]isothiazole hydrazones. Eur. J. Med. Chem. 2006, 41, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Dongare, S.B.; Bandgar, B.P.; Bhale, P.S.; Shringare, S.N.; Chavan, H.V. Design, Synthesis, and Spectroscopic Study of 7-Azaindolyl Hydrazones with Anti-Breast Cancer Activity. Croat. Chem. Acta 2019, 92, 1–9. [Google Scholar] [CrossRef]
- Mamolo, M.G.; Falagiani, V.; Zampieri, D.; Vio, L.; Banfi, E. Synthesis and antimycobacterial activity of [5-(pyridin-2-yl)-1,3,4-thiadiazol-2-ylthio]acetic acid arylidene-hydrazide derivatives. Farmaco 2001, 56, 587–592. [Google Scholar] [CrossRef]
- Wyrzykiewicz, E.; Prukah, D. New Isomeric N-substituted Hydrazones of 2-, 3- and 4-Pyridinecarboxaldehydes. J. Heterocycl. Chem. 1998, 35, 381–387. [Google Scholar] [CrossRef]
- Galic, N.; Peric, B.; Kojic-Prodic, B.; Cimerman, Z. Structural and spectroscopic characteristics of aroylhydrazones derived from nicotinic acid hydrazide. J. Mol. Stuct. 2001, 559, 187–194. [Google Scholar] [CrossRef]
- Jęśkowiak, I.; Mączyński, M.; Trynda, J.; Wietrzyk, J.; Ryng, S. The 5-hydrazino-3-methylisothiazole-4-carboxylic acid, its new 5-substituted derivatives and their antiproliferative activity. Bioorgan. Chem. 2019, 91, 103082–103091. [Google Scholar] [CrossRef]
- Shahnawaz Khan, M.; Parveen Siddiqui, S.; Tarannum, N. A systematic review on the synthesis and biological activity of hydrazide derivatives. Hygeia 2017, 9, 61–79. [Google Scholar] [CrossRef]
- Sreenivasulu, R.; Reddy, K.T.; Sujitha, P.; Kumar, C.G.; Raju, R.R. Synthesis, antiproliferative and apoptosis induction potential activities of novel Bis(indolyl)hydrazide-hydrazone derivatives. Bioorgan. Med. Chem. 2019, 27, 1043–1055. [Google Scholar] [CrossRef] [PubMed]
- Bingul, M.; Gardner, C.R.; Kumar, N.; Black, D.; Bingul, M.; Tan, O.; Gardner, C.R.; Sutton, K.S.; Arndt, M.G.; Marshall, M.G.; et al. Synthesis, Characterization and Anti-Cancer Activity of Hydrazide Derivatives Incorporating a Quinoline Moiety. Molecules 2016, 21, 916. [Google Scholar] [CrossRef] [PubMed]
- Agilent Technologies; Rigaku Oxford Diffraction. CrysAlisPro; Agilent Technologies: Yarnton, UK, 2012; Rigaku Oxford Diffraction: Yarnton, UK, 2018. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A: Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C: Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Brandenburg, K. Diamond, Version 3.2i; Crystal Impact GbR: Bonn, Germany, 2012. [Google Scholar]
- Gliszczyńska, A.; Niezgoda, N.; Gładkowski, W.; Czarnecka, M.; Świtalska, M.; Wietrzyk, J. Synthesis and Biological Evaluation of Novel Phosphatidylcholine Analogues Containing Monoterpene Acids as Potent Antiproliferative Agents. PLoS ONE 2016, 11, e0157278. [Google Scholar] [CrossRef]
- Harker, W.G.; Slade, D.L.; Dalton, W.S.; Meltzer, P.S.; Trent, J.M. Multidrug resistance in mitoxantrone-selected HL-60 leukemia cells in the absence of P-glycoprotein overexpression. Cancer Res. 1989, 49, 4542–4549. [Google Scholar]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef]
- Wakelee, H.A.; Schiller, J.H. Targeting Angiogenesis with Vascular Endothelial Growth Factor Receptor Small-Molecule Inhibitors: Novel Agents with Potential in Lung Cancer. Clin. Lung Cancer 2005, 7, S31–S38. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

| MV4-11 | |||||
|---|---|---|---|---|---|
| Name of Compound | IC50 ± SD | Name of Compound | IC50±SD | ||
| [µg/mL] | μM | [µg/mL] | μΜ | ||
| Cisplatin | 0.38 ± 0.14 | 1.28 ± 0.45 | 6 | 21.3 ± 8.2 | 69.4 ± 26.8 |
| 1 | n.a. | 7 | 22.4 ± 8.6 | 72.3 ± 27.8 | |
| 2 | n.a. [45%] * | 8 | 36.6 ± 11.6 | 131.2 ± 41.7 | |
| 3 | 4.3 ± 1.9 | 14 ± 6.4 | 9 | n.a. [42.5%] * | |
| 4 | 15.2 ± 2.4 | 48.4 ± 7.8 | 10 | n.a. [47%] * | |
| 5 | 18.7 ± 1.7 | 57.7 ± 5.3 | 11 | n.a | |
| DMSO (0.5%) | 9% ** | ||||
| Name of Compound | IC50 ± SD | RI | |||||||
|---|---|---|---|---|---|---|---|---|---|
| MCF-7 | MCF-10A | LoVo | LoVoDX | ||||||
| [µg/mL] | μM | [µg/mL] | μM | [µg/mL] | μM | [µg/mL] | μM | ||
| Cisplatin | 1.56 ± 0.3 | 5.2 ± 1.0 | 2.9 ± 0.4 | 9.7 ± 1.4 | 1.7 ± 0.8 | 5.6 ± 2.6 | 0.84 ± 0.17 | 2.8 ± 0.6 | 0.49 |
| 3 | 12 ± 1.9 | 39.3 ± 6.3 | 20.9 ± 2.2 | 68.6 ± 7.3 | 7.64 ± 1.7 | 25 ± 5.5 | 10.4 ± 0.7 | 34.2 ± 2.3 | 1.37 |
| 4 | 15.2 ± 1.8 | 48.6 ± 5.7 | 24.6 ± 2.2 | 78.5 ± 7.1 | 18.6 ± 1.1 | 59.4 ± 3.4 | 15 ± 1.1 | 47.9 ± 3.7 | 0.81 |
| 5 | 20.1 ± 2.6 | 65.1 ± 8.4 | 60.8 ± 5.1 | 196.6 ± 16.6 | 22.7 ± 0.3 | 73.4 ± 0.8 | 29.2 ± 5.3 | 94.6 ± 17.3 | 1.29 |
| 6 | 13.9 ± 1.9 | 42.9 ± 5.9 | 40.6 ± 3.7 | 125.4 ± 11.3 | 31.7 ± 6.7 | 97.7 ± 20.8 | 28.8 ± 2.7 | 88.8 ± 8.4 | 0.91 |
| 7 | 17.8 ± 3.1 | 58 ± 10.2 | 56 ± 14.1 | 182.5 ± 48.9 | 27.8 ± 10.4 | 90.5 ± 34 | 20 ± 1.1 | 65 ± 3.5 | 0.72 |
| DMSO (0.5%) | 16% ** | 5.3% ** | 10% ** | 2.7% ** | - | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jęśkowiak, I.; Ryng, S.; Świtalska, M.; Wietrzyk, J.; Bryndal, I.; Lis, T.; Mączyński, M. The N’-Substituted Derivatives of 5-Chloro-3-Methylisothiazole-4-Carboxylic Acid Hydrazide with Antiproliferative Activity. Molecules 2020, 25, 88. https://doi.org/10.3390/molecules25010088
Jęśkowiak I, Ryng S, Świtalska M, Wietrzyk J, Bryndal I, Lis T, Mączyński M. The N’-Substituted Derivatives of 5-Chloro-3-Methylisothiazole-4-Carboxylic Acid Hydrazide with Antiproliferative Activity. Molecules. 2020; 25(1):88. https://doi.org/10.3390/molecules25010088
Chicago/Turabian StyleJęśkowiak, Izabela, Stanisław Ryng, Marta Świtalska, Joanna Wietrzyk, Iwona Bryndal, Tadeusz Lis, and Marcin Mączyński. 2020. "The N’-Substituted Derivatives of 5-Chloro-3-Methylisothiazole-4-Carboxylic Acid Hydrazide with Antiproliferative Activity" Molecules 25, no. 1: 88. https://doi.org/10.3390/molecules25010088
APA StyleJęśkowiak, I., Ryng, S., Świtalska, M., Wietrzyk, J., Bryndal, I., Lis, T., & Mączyński, M. (2020). The N’-Substituted Derivatives of 5-Chloro-3-Methylisothiazole-4-Carboxylic Acid Hydrazide with Antiproliferative Activity. Molecules, 25(1), 88. https://doi.org/10.3390/molecules25010088





