Improvement of Oxazolone-Induced Ulcerative Colitis in Rats Using Andrographolide
Abstract
1. Introduction
2. Results
2.1. Effect of AND on DAI Score and Inflammatory Markers
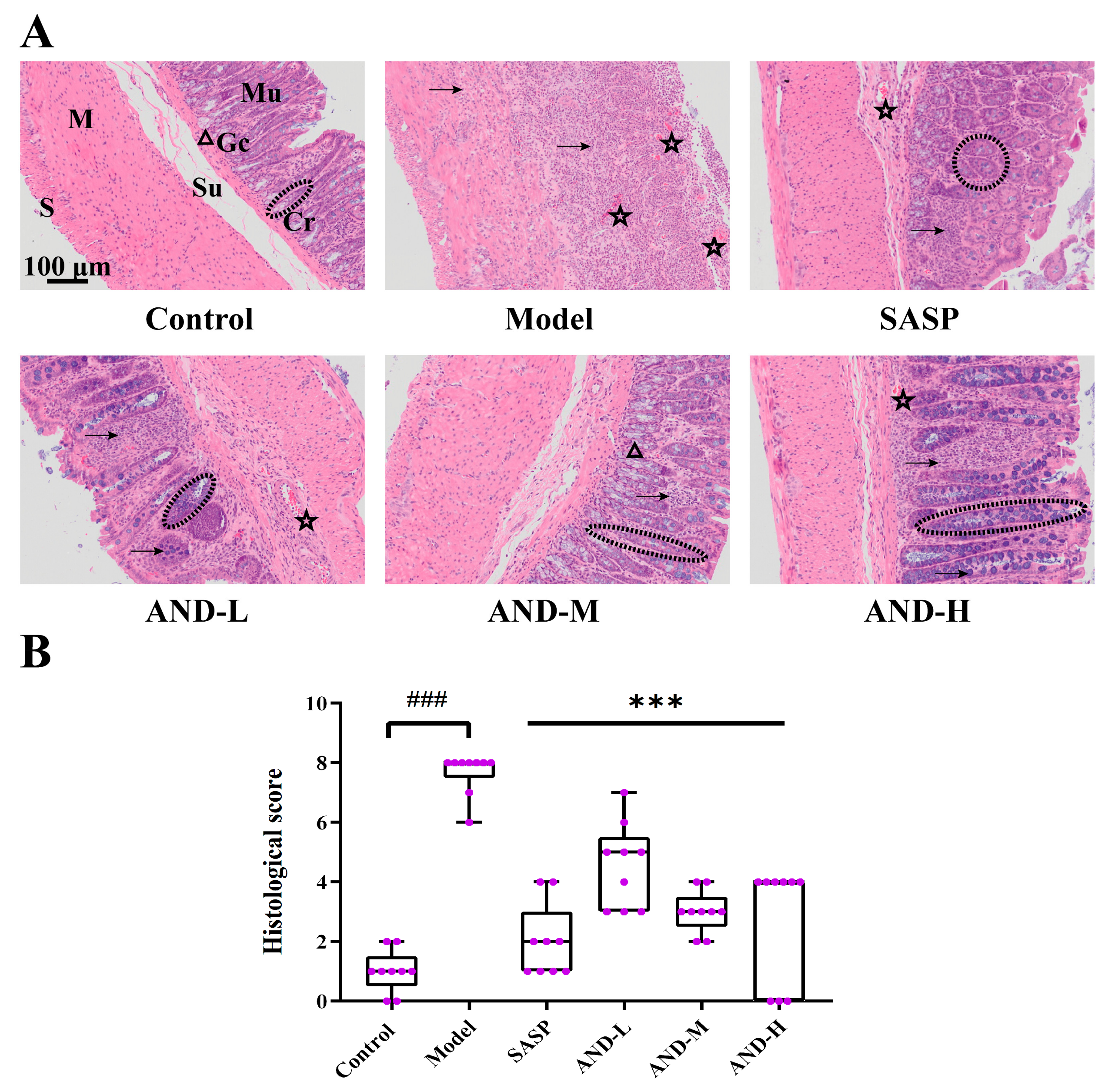
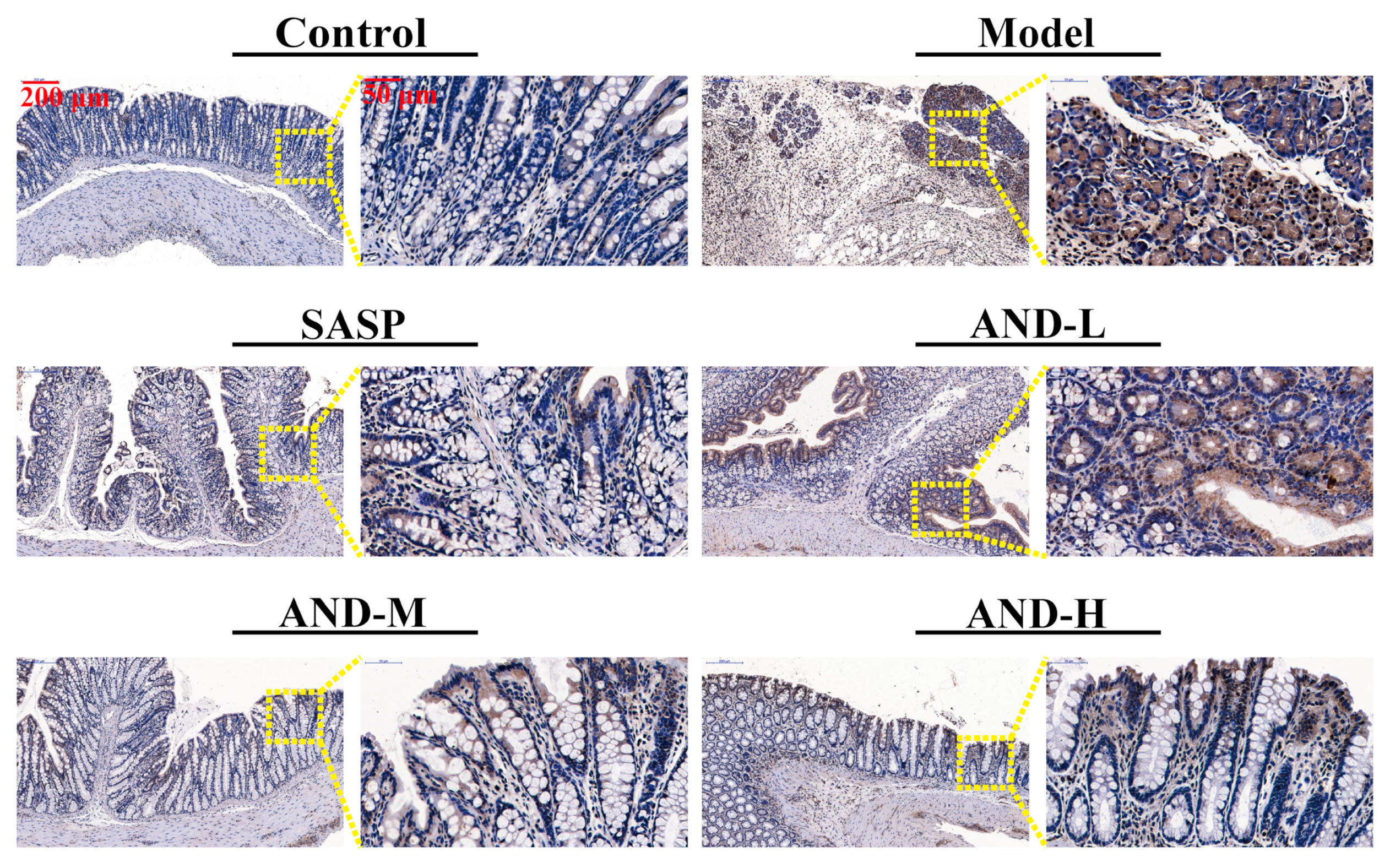
2.2. Hematoxylin and Eosin (H&E) Staining and Immunohistochemical Analysis
2.3. MPO Content and Inflammatory Cytokine Expression
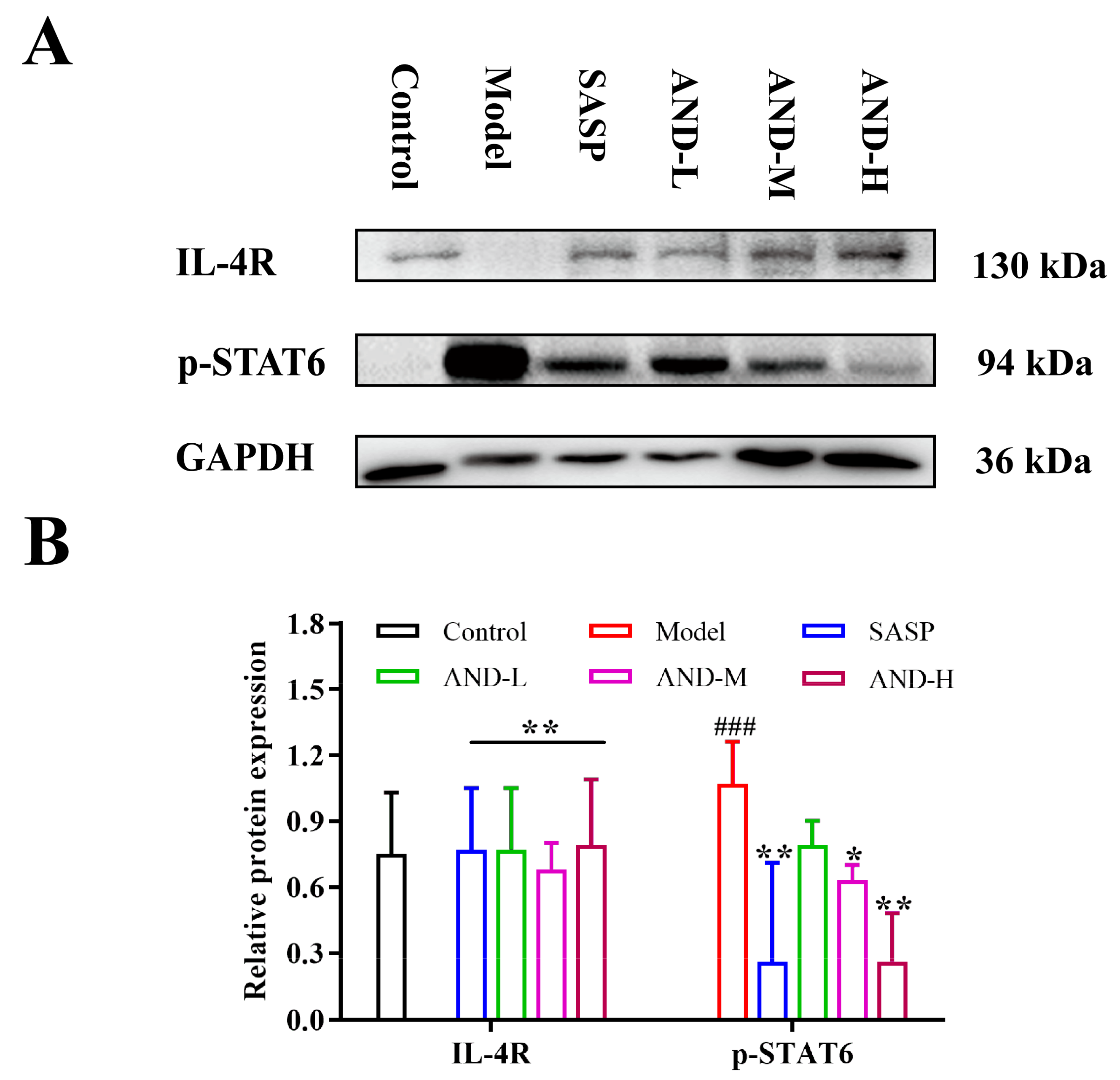
2.4. The Effect of IL-4 Receptor (IL-4R)/STAT6 Signal Pathway in OXZ-Induced UC In Vivo
3. Discussion
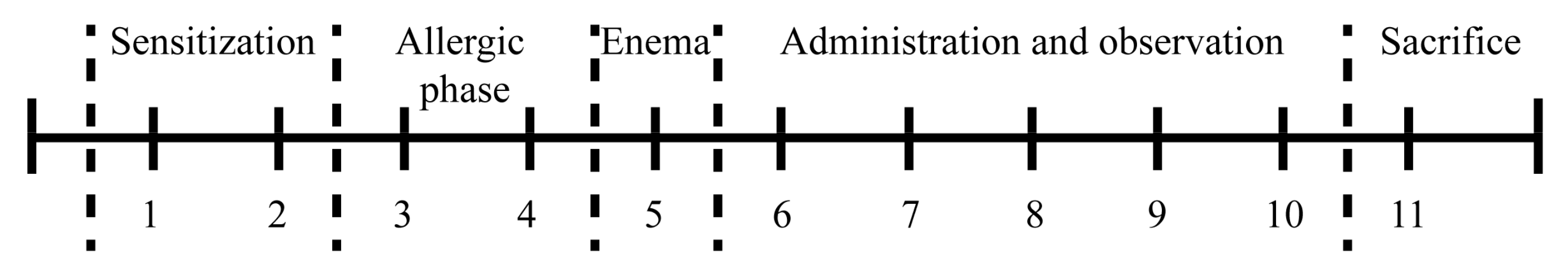
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Animals
4.3. Induction and Assessment of OXZ-Induced Colitis
4.4. MPO, TNF-α, IL-4, and IL-13 Assay
4.5. Western Blot Analysis
4.6. Data Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Aggarwal, A.; Sabol, T.; Vaziri, H. Update on the Use of Biologic Therapy in Ulcerative Colitis. Curr. Treat. Options Gastroenterol. 2017, 15, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Chambrun, G.P.; Tassy, B.; Kollen, L.; Dufour, G.; Valats, J.C.; Bismuth, M.; Funakoshi, N.; Panaro, F.; Blanc, P. Refractory ulcerative proctitis: How to treat it? Best Pract. Res. Clin. Gastroenterol. 2018, 32, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Recio, M.C.; Andujar, I.; Rios, J.L. Anti-inflammatory agents from plants: Progress and potential. Curr. Med. Chem. 2012, 19, 2088–2103. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.J.; Bian, Z.X.; Qiu, H.C.; Wang, Y.T.; Wang, Y. Biological and clinical implications of herbal medicine and natural products for the treatment of inflammatory bowel disease. Ann. N. Y. Acad. Sci. 2017, 1401, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Shapira, S.; Leshno, A.; Katz, D.; Maharshak, N.; Hevroni, G.; Maayan, J.D.; Kraus, S.; Galazan, L.; Aroch, I.; Kazanov, D.; et al. Of mice and men: A novel dietary supplement for the treatment of ulcerative colitis. Ther. Adv. Gastroenterol. 2018, 11, 1–10. [Google Scholar] [CrossRef]
- An Editorial Committee of the Administration Bureau of Traditional Chinese Medicine. Chinese Materia Media (Zhonghua Bencao); Shanghai Science & Technology Press: Shanghai, China, 1999. [Google Scholar]
- Tang, T.; Targan, S.R.; Li, Z.S.; Xu, C.; Byers, V.S.; Sandborn, W.J. Randomized clinical trial: Herbal extract HMPL-004 in active ulcerative colitis—A double-blind comparison with sustained release mesalazine. Aliment. Pharmacol. Ther. 2011, 33, 194–202. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Targan, S.R.; Byers, V.S.; Rutty, D.A.; Mu, H.; Zhang, X.; Tang, T. Andrographis paniculata extract (HMPL-004) for active ulcerative colitis. Am. J. Gastroenterol. 2013, 108, 90–98. [Google Scholar] [CrossRef]
- Michelsen, K.S.; Wong, M.H.; Ko, B.; Thomas, L.S.; Dhall, D.; Targan, S.R. HMPL-004 (Andrographis paniculata extract) prevents development of murine colitis by inhibiting T-cell proliferation and TH1/TH17 responses. Inflamm. Bowel Dis. 2012, 19, 151–164. [Google Scholar] [CrossRef]
- Zhu, Q.; Zheng, P.F.; Chen, X.Y.; Zhou, F.; He, Q.N.; Yang, Y.F. Andrographolide presents therapeutic effect on ulcerative colitis through the inhibition of IL-23/IL-17 axis. Am. J. Transl. Res. 2018, 10, 465–473. [Google Scholar]
- Zhu, Q.; Zheng, P.F.; Zhou, J.Y.; Chen, X.Y.; Feng, Y.L.; Wang, W.F.; Zhou, F.; He, Q.N. Andrographolide affects Th1/Th2/Th17 responses of peripheral blood mononuclear cells from ulcerative colitis patients. Mol. Med. Rep. 2018, 18, 622–626. [Google Scholar] [CrossRef]
- Gao, Z.F.; Yu, C.C.; Liang, H.Y.; Wang, X.K.; Liu, Y.; Li, X.; Ji, K.; Xu, H.; Yang, M.Y.; Liu, K.; et al. Andrographolide derivative CX-10 ameliorates dextran sulphate sodium-induced ulcerative colitis in mice: Involvement of NF-κB and MAPK signaling pathways. Int. Immunopharmacol. 2018, 57, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Weigmann, B.; Neurath, M.F. Oxazolone-induced colitis as a model of Th2 immune responses in the intestinal mucosa. Methods Mol. Biol. 2016, 1422, 253–261. [Google Scholar] [PubMed]
- Randhawa, P.K.; Singh, K.; Singh, N.; Jaggi, A.S. A review on chemical-induced inflammatory bowel disease models in rodents. Korean J. Physiol. Pharmacol. 2014, 18, 279–288. [Google Scholar] [CrossRef]
- Kasaian, M.T.; Page, K.M.; Fish, S.; Brennan, A.; Cook, T.A.; Moreira, K.; Zhang, M.; Jesson, M.; Marquette, K.; Agostinelli, R.; et al. Therapeutic activity of an interleukin-4/interleukin-13 dual antagonist on oxazolone-induced colitis in mice. Immunology 2014, 143, 416–427. [Google Scholar] [CrossRef] [PubMed]
- Berin, M.C.; Yang, P.C.; Ciok, L.; Waserman, S.; Perdue, M.H. Role for IL-4 in macromolecular transport across human intestinal epithelium. Am. J. Physiol. 1999, 276, C1046–C1052. [Google Scholar] [CrossRef]
- Heller, F.; Fromm, A.; Gitter, A.H.; Mankertz, J.; Schulzke, J.D. Epithelial apoptosis is a prominent feature of the epithelial barrier disturbance in intestinal inflammation: Effect of pro-inflammatory interleukin-13 on epithelial cell function. Mucosal Immunol. 2008, 1, S58–S61. [Google Scholar] [CrossRef]
- Heller, F.; Fuss, I.J.; Nieuwenhuis, E.E.; Blumberg, R.S.; Strober, W. Oxazolone colitis, a Th2 colitis model resembling ulcerative colitis, is mediated by IL-13-producing NK-T cells. Immunity 2002, 17, 629–638. [Google Scholar] [CrossRef]
- Weigmann, B.; Lehr, H.A.; Yancopoulos, G.; Valenzuela, D.; Murphy, A.; Stevens, S.; Schmidt, J.; Galle, P.R.; Rose-John, S.; Neurath, M.F. The transcription factor NFATc2 controls IL-6-dependent T cell activation in experimental colitis. J. Exp. Med. 2008, 205, 2099–2110. [Google Scholar] [CrossRef]
- Rosen, M.J.; Frey, M.R.; Washington, K.M.; Chaturvedi, R.; Kuhnhein, L.A.; Matta, P.; Revetta, F.L.; Wilson, K.T.; Polk, B.D. STAT6 activation in ulcerative colitis: A new target for prevention ofIL-13-induced colon epithelial cell dysfunction. Inflamm. Bowel Dis. 2011, 17, 2224–2234. [Google Scholar] [CrossRef]
- Rosen, M.J.; Chaturvedi, R.; Washington, M.K.; Kuhnhein, L.A.; Moore, P.D.; Coggeshall, S.S.; McDonough, E.M.; Weitkamp, J.H.; Singh, A.B.; Coburn, L.A.; et al. STAT6 deficiency ameliorates severity of oxazolone colitis by decreasing expression of claudin-2 and Th2-inducing cytokines. J. Immunol. 2013, 190, 1849–1858. [Google Scholar] [CrossRef]
- Axelsson, L.G.; Landström, E.; Bylund-Fellenius, A.C. Experimental colitis induced by dextran sulphate sodium in mice: Beneficial effects of sulphasalazine and olsalazine. Aliment. Pharmacol. Ther. 1998, 12, 925–934. [Google Scholar] [CrossRef] [PubMed]
- Sun, A.N.; Ren, G.Y.; Deng, C.; Zhang, J.J.; Luo, X.P.; Wu, X.J.; Mani, S.; Dou, W.; Wang, Z.T. C-glycosyl flavonoid orientin improves chemically induced inflammatory bowel disease in mice. J. Funct. Foods 2016, 21, 418–430. [Google Scholar] [CrossRef]
- Dames, P.; Bergann, T.; Fromm, A.; Bücker, R.; Barmeyer, C.; Krug, S.M.; Fromm, M.; Schulzke, J.D. Interleukin-13 affects the epithelial sodium channel in the intestine by coordinated modulation of STAT6 and p38 MAPK activity. J. Physiol. 2015, 593, 5269–5282. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.L.; Zhang, S.; He, W.X.; Lu, J.L.; Xu, Y.J.; Yang, J.Y.; Liu, D. Baicalin may alleviate inflammatory infiltration in dextran sodium sulfate-induced chronic ulcerative colitis via inhibiting IL-33 expression. Life Sci. 2017, 186, 125–132. [Google Scholar] [CrossRef]
- Yang, M.; Wang, J.; Yang, C.X.; Han, H.X.; Rong, W.F.; Zhang, G.H. Oral administration of curcumin attenuates visceral hyperalgesia through inhibiting phosphorylation of TRPV1 in rat model of ulcerative colitis. Mol. Pain 2017, 13, 1–11. [Google Scholar] [CrossRef]
- Zhang, L.C.; Wang, Y.; Tong, L.C.; Sun, S.; Liu, W.Y.; Zhang, S.; Wang, R.M.; Wang, Z.B.; Li, L. Berberine alleviates dextran sodium sulfate-induced colitis by improving intestinal barrier function and reducing inflammation and oxidative stress. Exp. Ther. Med. 2017, 13, 3374–3382. [Google Scholar] [CrossRef]
- Chen, Q.Y.; Duan, X.Y.; Fan, H.; Xu, M.; Tang, Q.; Zhang, L.J.; Shou, Z.X.; Liu, X.X.; Zuo, D.M.; Yang, J.; et al. Oxymatrine protects against DSS-induced colitis via inhibiting the PI3K/AKT signaling pathway. Int. Immunopharmacol. 2017, 53, 149–157. [Google Scholar] [CrossRef]
- Lv, J.; Zhang, Y.H.; Tian, Z.Q.; Liu, F.; Shi, Y.; Liu, Y.; Xia, P.Y. Astragalus polysaccharides protect against dextran sulfate sodium-induced colitis by inhibiting NF-κB activation. Int. J. Biol. Macromol. 2017, 98, 723–729. [Google Scholar] [CrossRef]
- Parian, A.; Limketkai, B.N. Dietary supplement therapies for inflammatory bowel disease: Crohn’s disease and ulcerative colitis. Curr. Pharm. Des. 2016, 22, 180–188. [Google Scholar] [CrossRef]
- Parian, A.M.; Limketkai, B.N.; Shah, N.D.; Mullin, G.E. Nutraceutical Supplements for Inflammatory Bowel Disease. Nutr. Clin. Pract. 2015, 30, 551–558. [Google Scholar] [CrossRef]
- Martin, D.A.; Bolling, B.W. A review of the efficacy of dietary polyphenols in experimental models of inflammatory bowel diseases. Food Funct. 2015, 6, 1773–1786. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Gu, P.Q.; Shen, H. Gallic acid improved inflammation via NF-κB pathway in TNBS-induced ulcerative colitis. Int. Immunopharmacol. 2019, 67, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, M.T.; Vezza, T.; Patricia, D.E.; Utrilla, P.; Villamiel, M.; Moreno, F.J. Anti-inflammatory bowel effect of industrial orange by-products in DSS-treated mice. Food Funct. 2018, 9, 4888–4896. [Google Scholar] [CrossRef] [PubMed]
- Shirazi, K.M.; Nikniaz, Z.; Shirazi, A.M.; Rohani, M. Vitamin A supplementation decreases disease activity index in patients with ulcerative colitis: A randomized controlled clinical trial. Complement. Ther. Med. 2018, 41, 215–219. [Google Scholar]
- Lucena, A.M.M.; Souza, C.R.M.; Jales, J.T.; Guedes, P.M.M.; Miranda, G.E.C.; Moura, A.M.A.; Araújo-Júnior, J.X.; Nascimento, G.J.; Scortecci, K.C.; Santos, B.V.O.; et al. The bisindole alkaloid caulerpin, from seaweeds of the genus Caulerpa, attenuated colon damage in murine colitis model. Mar. Drugs 2018, 16, 318. [Google Scholar] [CrossRef]
- Zbakh, H.; Talero, E.; Avila, J.; Alcaide, A.; Reyes, C.; Zubía, E.; Motilva, V. The algal meroterpene 11-Hydroxy-1’-O-Methylamentadione ameliorates dextran sulfate sodium-induced colitis in mice. Mar. Drugs 2016, 14, 149. [Google Scholar] [CrossRef]
- Yamada, S.; Koyama, T.; Noguchi, H.; Ueda, Y.; Kitsuyama, R.; Shimizu, H.; Tanimoto, A.; Wang, K.Y.; Nawata, A.; Nakayama, T.; et al. Marine hydroquinone zonarol prevents inflammation and apoptosis in dextran sulfate sodium-induced mice ulcerative colitis. PLoS ONE 2014, 9, e113509. [Google Scholar] [CrossRef]
- Ferrándiz, M.L.; Sanz, M.J.; Bustos, G.; Payá, M.; Alcaraz, M.J.; Rosa, S.D. Avarol and avarone, two new anti-inflammatory agents of marine origin. Eur. J. Pharmacol. 1994, 253, 75–82. [Google Scholar] [CrossRef]
- Pejin, B.; Iodice, C.; Tommonaro, G.; Rosa, S.D. Synthesis and Biological Activities of Thio-avarol Derivatives. J. Nat. Prod. 2008, 71, 1850–1853. [Google Scholar] [CrossRef]
- Tommonaro, G.; Nuria, G.F.; Vitale, R.M.; Pejin, B.; Iodice, C.; Canadas, S.; José, M.C.; María, J.O.G. Avarol derivatives as competitive AChE inhibitors, non hepatotoxic and neuroprotective agents for Alzheimer’s disease. Eur. J. Med. Chem. 2016, 122, 326–338. [Google Scholar] [CrossRef]
- American Gastroenterological Association. Management of Mild-to-Moderate Ulcerative Colitis: Patient Guide. Gastroenterology 2019, 156, 766. [Google Scholar] [CrossRef] [PubMed]
- Naganuma, M.; Iwao, Y.; Ogata, H.; Inoue, N.; Funakoshi, S.; Yamamoto, S.; Nakamura, Y.; Ishii, H.; Hibi, T. Measurement of colonic mucosal concentrations of 5-aminosalicylic acid is useful for estimating its therapeutic efficacy in distal ulcerative colitis: Comparison of orally administered mesalamine and sulfasalazine. Inflamm. Bowel Dis. 2001, 7, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Ferry, G.D.; Kirschner, B.S.; Grand, R.J.; Issenman, R.M.; Griffiths, A.M.; Vanderhoof, J.A.; Fiedorek, S.C.; Winter, H.S.; Hassall, E.G.; Watkins, J.B. Olsalazine versus sulfasalazine in mild to moderate childhood ulcerative colitis: Results of the paediatric gastroenterology collaborative research group clinical trial. J. Pediatr. Gastroenterol. Nutr. 1993, 17, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.F.; Zhou, J.T.; Qu, C.; Dou, Y.X.; Huang, Q.H.; Lin, Z.X.; Xian, Y.F.; Xie, J.H.; Xie, Y.L.; Lai, X.P.; et al. Anti-inflammatory effects of Brucea javanica oil emulsion by suppressing NF-κB activation on dextran sulfate sodium-induced ulcerative colitis in mice. J. Ethnopharmacol. 2017, 198, 389–398. [Google Scholar] [CrossRef]
- Mi, H.; Liu, F.B.; Li, H.W.; Hou, J.T.; Li, P.W. Anti-inflammatory effect of Chang-An-Shuan on TNBS-induced experimental colitis in rats. BMC Complement. Altern. Med. 2017, 17, 315. [Google Scholar] [CrossRef]
- Gao, W.Y.; Wang, C.H.; Yu, L.; Sheng, T.J.; Wu, Z.L.; Wang, X.Q.; Zhang, D.Q.; Lin, Y.F.; Gong, Y. Chlorogenic acid attenuates dextran sodium sulfate-induced ulcerative colitis in mice through MAPK/ERK/JNK pathway. Biomed. Res. Int. 2019, 2019. [Google Scholar] [CrossRef]
- Shin, M.R.; Kim, K.J.; Kim, S.H.; Kim, S.J.; Seo, B.I.; An, H.J.; Roh, S.S. Comparative evaluation between sulfasalazine alone and in combination with herbal medicine on DSS-induced ulcerative colitis mice. Biomed. Res. Int. 2017, 674265. [Google Scholar] [CrossRef]
- Yoshino, T.; Sono, M.; Yazumi, S. Usefulness of sulfasalazine for patients with refractory-ulcerative colitis. BMJ Open Gastroenterol. 2016, 3, e000103. [Google Scholar] [CrossRef]
- Handa, S.S.; Sharma, A. Hepatoprotective activity of andrographolide from Andrographis paniculata against carbontetrachloride. Indian J. Med. Res. 1990, 92, 276–283. [Google Scholar]
- Al Batran, R.; Al-Bayaty, F.; Al-Obaidi, M.M.J.; Abdulla, M.A. Acute toxicity and the effect of andrographolide on porphyromonas gingivalis-induced hyperlipidemia in rats. Biomed. Res. Int. 2013, 2013. [Google Scholar] [CrossRef]
- Majumder, K.; Fukuda, T.; Zhang, H.; Sakurai, T.; Taniguchi, Y.; Watanabe, H.; Mitsuzumi, H.; Matsui, T.; Mine, Y. Intervention of isomaltodextrin mitigates intestinal inflammation in a dextran sodium sulfate-induced mouse model of colitis via inhibition of toll-like receptor-4. J. Agric. Food Chem. 2017, 65, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Dou, W.; Zhang, E.Y.; Sun, A.N.; Ding, L.L.; Wei, X.H.; Chou, G.X.; Mani, S.; Wang, Z.T. Paeoniflorin abrogates DSS-induced colitis via a TLR4-dependent pathway. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 306, G27–G36. [Google Scholar] [CrossRef] [PubMed]
- Ozsoy, Z.; Ozsoy, S.; Gevrek, F.; Demir, E.; Benli, I.; Daldal, E.; Yenidogan, E. Effect of bevacizumab on acetic acid-induced ulcerative colitis in rats. J. Surg. Res. 2017, 216, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qin, O.Y.; Luo, W.J. Oxazolone-induced murine model of ulcerative colitis. Chin. J. Dig. Dis. 2004, 5, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Boirivant, M.; Fuss, I.J.; Chu, A.; Strober, W. Oxazolone colitis: A murine model of T helper cell type 2 colitis treatable with antibodies to interleukin 4. J. Exp. Med. 1998, 188, 1929–1939. [Google Scholar] [CrossRef] [PubMed]
- Heller, F.; Florian, P.; Bojarski, C.; Richter, J.; Christ, M.; Hillenbrand, B.; Mankertz, J.; Gitter, A.H.; Bürgel, N.; Fromm, M.; et al. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology 2005, 129, 550–564. [Google Scholar] [CrossRef] [PubMed]
- Koller, F.L.; Hwang, D.G.; Dozier, E.A.; Fingleton, B. Epithelial interleukin-4 receptor expression promotes colon tumor growth. Carcinogenesis 2010, 31, 1010–1017. [Google Scholar] [CrossRef]
- Wang, X.W.; Yang, J.H.; Cao, Q.; Tang, J.M. Therapeutic efficacy and mechanism of water-soluble extracts of Banxiaxiexin decoction on BALB/c mice with oxazolone-induced colitis. Exp. Ther. Med. 2014, 8, 1201–1204. [Google Scholar] [CrossRef]
- Zheng, L.; Zhang, Y.L.; Dai, Y.C.; Chen, X.; Chen, D.L.; Dai, Y.T.; Tang, Z.P. Jianpi Qingchang decoction alleviates ulcerative colitis by inhibiting nuclear factor-κB activation. World J. Gastroenterol. 2017, 23, 1180–1188. [Google Scholar] [CrossRef]
Sample Availability: Sample of compound andrographolide is available from the authors. |
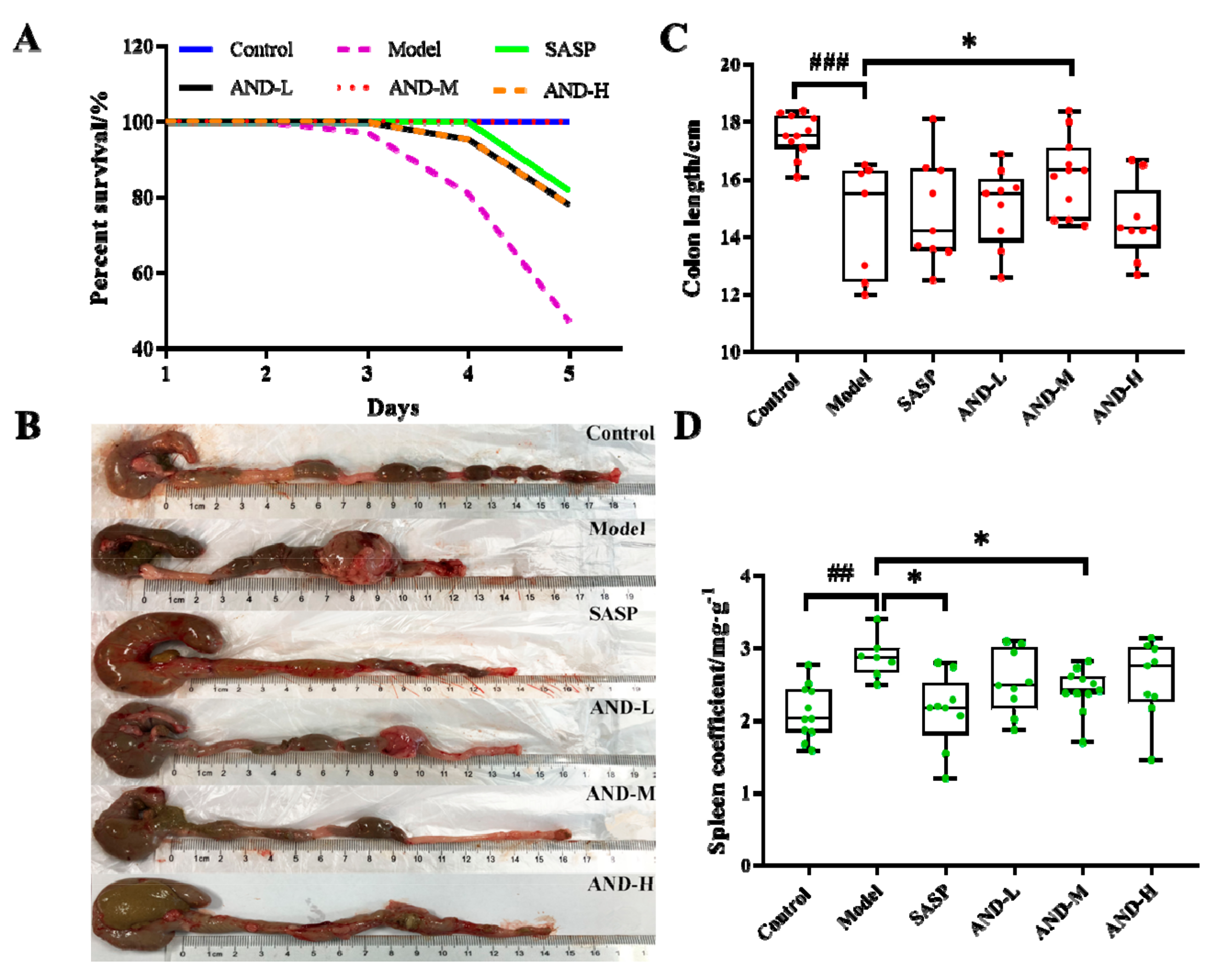
| Control | Model | SASP | AND-L | AND-M | AND-H | |
|---|---|---|---|---|---|---|
| 1 | 0.00 (0.67, 0.00) | 2.00 (2.67, 0.67) ### | 2.00 (2.67, 1.33) | 2.00 (2.67, 0.67) | 2.00 (2.67, 0.67) | 1.33 (2.67, 0.67) |
| 2 | 0.00 (0.67, 0.00) | 1.67 (2.67, 0.67) ### | 1.33 (2.00, 0.00) * | 1.33 (2.00, 0.00) | 1.00 (2.00, 0.00) ** | 1.00 (2.33, 0.67) * |
| 3 | 0.33 (0.67, 0.00) | 1.50 (2.33, 0.67) ### | 0.67 (2.33, 0.00) * | 1.33 (1.67, 0.00) | 0.67 (1.67, 0.00) ** | 1.00 (1.67, 0.00) * |
| 4 | 0.00 (0.67, 0.00) | 1.33 (1.67, 0.67) ### | 0.67 (2.33, 0.00) * | 1.17 (1.67, 0.00) | 0.67 (1.00, 0.00) * | 0.67 (1.67, 0.00) * |
| 5 | 0.00 (0.67, 0.00) | 0.67 (1.33, 0.67) ### | 0.67 (0.67, 0.00) * | 0.67 (2.33, 0.00) | 0.67 (1.00, 0.00) * | 0.00 (1.00, 0.00) * |
| Groups | Control | Model | SASP | AND-L | AND-M | AND-H |
|---|---|---|---|---|---|---|
| Density | 3.03 ± 0.96 | 46.35 ± 11.54 ### | 7.71 ± 3.41 *** | 33.44 ± 2.35 * | 12.51 ± 1.75 *** | 14.47 ± 3.88 *** |
| Groups | MPO | TNF-α | IL-4 | IL-13 |
|---|---|---|---|---|
| Control | 43.40 ± 6.47 | 80.29 ± 15.46 | 31.00 ± 5.51 | 10.79 ± 1.38 |
| Model | 54.14 ± 6.92 ### | 105.49 ± 12.49 ### | 37.07 ± 4.72 ## | 12.80 ± 1.46 ## |
| SASP | 40.09 ± 4.81 *** | 86.95 ± 10.98 ** | 32.50 ± 2.53 * | 10.61 ± 1.39 ** |
| AND-L | 47.32 ± 3.83 * | 99.83 ± 11.09 | 33.70 ± 3.28 | 12.26 ± 1.29 |
| AND-M | 44.49 ± 6.23 ** | 91.29 ± 9.95 * | 30.59 ± 4.39 ** | 10.80 ± 1.49 ** |
| AND-H | 44.70 ± 6.13 ** | 91.11 ± 6.63 * | 30.54 ± 2.69 ** | 10.13 ± 0.91 *** |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Cao, N.; Wang, Y.; Wang, Y.; Wu, C.; Cheng, X.; Wang, C. Improvement of Oxazolone-Induced Ulcerative Colitis in Rats Using Andrographolide. Molecules 2020, 25, 76. https://doi.org/10.3390/molecules25010076
Zhang L, Cao N, Wang Y, Wang Y, Wu C, Cheng X, Wang C. Improvement of Oxazolone-Induced Ulcerative Colitis in Rats Using Andrographolide. Molecules. 2020; 25(1):76. https://doi.org/10.3390/molecules25010076
Chicago/Turabian StyleZhang, Liuhong, Ning Cao, Yuwen Wang, Youxu Wang, Chao Wu, Xuemei Cheng, and Changhong Wang. 2020. "Improvement of Oxazolone-Induced Ulcerative Colitis in Rats Using Andrographolide" Molecules 25, no. 1: 76. https://doi.org/10.3390/molecules25010076
APA StyleZhang, L., Cao, N., Wang, Y., Wang, Y., Wu, C., Cheng, X., & Wang, C. (2020). Improvement of Oxazolone-Induced Ulcerative Colitis in Rats Using Andrographolide. Molecules, 25(1), 76. https://doi.org/10.3390/molecules25010076





