Development of a Direct Competitive ELISA Kit for Detecting Deoxynivalenol Contamination in Wheat
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Preparation of the Antigen and Anti-DON Monoclonal Antibody (mAb)
2.3. Development of the DON dcELISA Kit
2.4. Components of the ELISA Kit
2.5. Establishment of the Kit Standard Curve
2.6. Pretreatment of Samples
2.7. Operating Procedure of the Kit
- (1)
- Addition of anti-DON mAb: anti-DON mAb at a working concentration was added (50 μL/well), set as the negative and blank control, incubated for 15 min at 37 °C, and then washed.
- (2)
- Addition of HRP-DON and the sample to be tested: HRP-DON at a working concentration was added (50 μL/well), the sample to be tested was added at the same volume, the plate was incubated for 25 min at 37 °C, and then washed.
- (3)
- Coloration: the TMB-containing color substrate solution (50 μL/well) was added, and the plate was placed in the dark for 5 min at room temperature (RT).
- (4)
- Termination: a 2 mol/L H2SO4 termination solution was added (50 μL/well).
- (5)
- Measurement with the microplate reader: the absorbance was measured at 450 nm, and the inhibition rate was calculated.
2.8. Characteristics of the DON dcELISA Kit
2.8.1. Sensitivity Determination
2.8.2. Accuracy and Precision Determination
2.8.3. Specificity Determination
2.8.4. Stability Determination
2.8.5. Matrix Effect Determination
2.9. Confirmation of the DON dcELISA Kit with HPLC
3. Results
3.1. Development of the DON dcELISA Kit
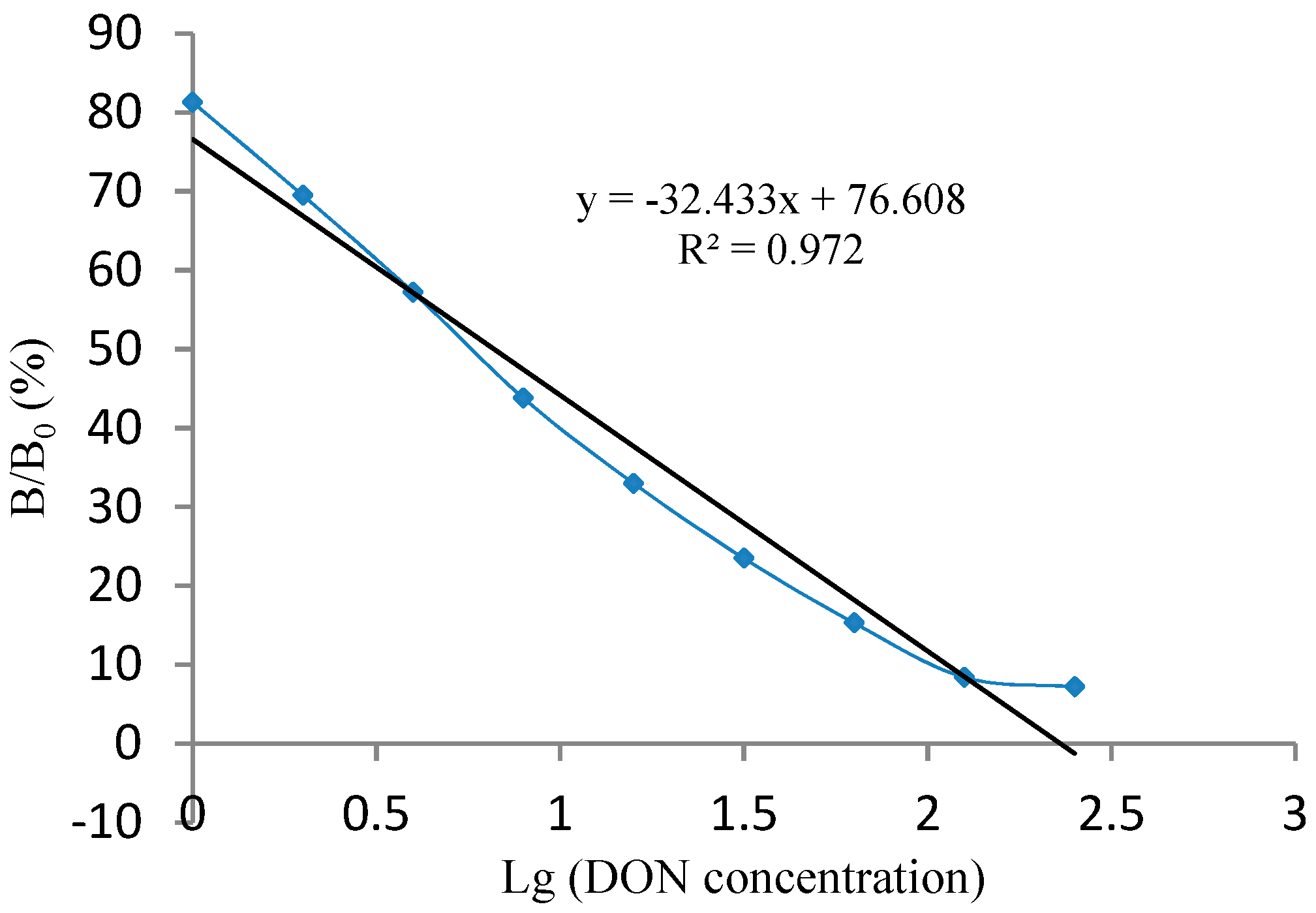
3.2. Generating and Fitting the Standard Curve of the Kit
3.3. Performance Measurements of the Kit
3.3.1. Sensitivity Determination
3.3.2. Accuracy and Precision Measurement
3.3.3. Specificity Determination
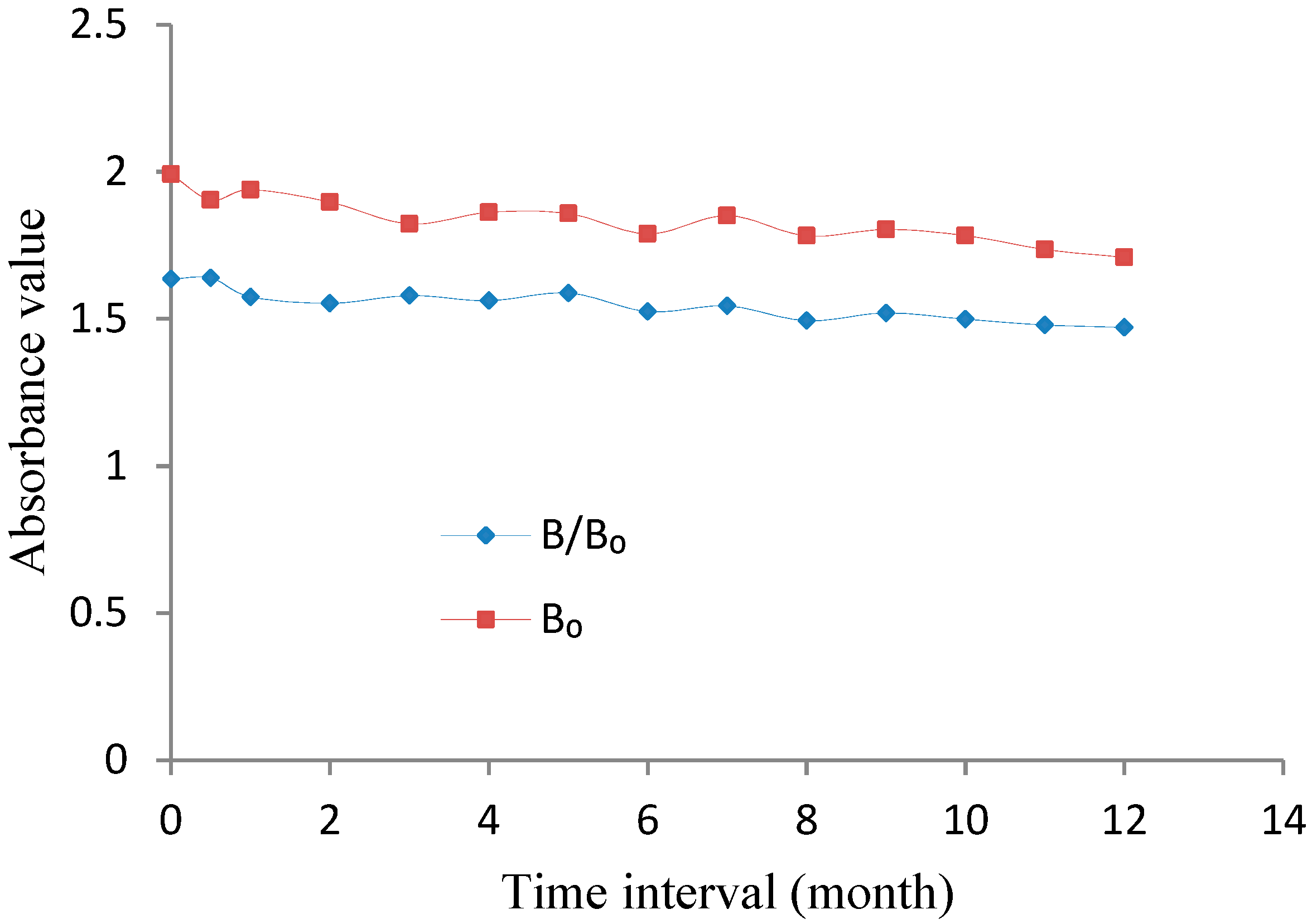
3.3.4. Stability Determination
3.3.5. Matrix Effect Determination
3.4. Confirmation of the DON dcELISA Kit with HPLC
4. Discussions
4.1. Pretreatment of Biotoxin Samples
4.2. Determination of dcELISA Kit Performance
4.3. Comparison of the Results of the dcELISA Kit and HPLC
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zuo, H.G.; Zhu, J.X.; Shi, L.; Zhan, C.R.; Guo, P.; Wang, Y.; Zhang, Y.M.; Liu, J.P. Development of a novel immunoaffinity column for the determination of deoxynivalenol and its acetylated derivatives in cereals. Food Anal. Method. 2018, 11, 2252–2260. [Google Scholar] [CrossRef]
- Jiang, D.; Chen, J.; Li, F.; Li, W.; Wang, X. Deoxynivalenol and its acetyl derivatives in bread and biscuits in Shandong province in China. Food Addit. Contam. B 2017, 11, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Dezhao, K.; Xiaoling, W.; Yue, L.; Liqiang, L.; Shanshan, S.; Qiankun, Z.; Hua, K.; Chuanlai, X. Ultrasensitive and eco-friendly immunoassays based monoclonal antibody for detection of deoxynivalenol in cereal and feed samples. Food Chem. 2019, 270, 130–137. [Google Scholar] [CrossRef]
- Ennouari, A.; Sanchis, V.; Marín, S.; Rahouti, M.; Zinedine, A. Occurrence of deoxynivalenol in durum wheat from morocco. Food Control 2013, 32, 115–118. [Google Scholar] [CrossRef]
- Pestka, J.J.; Smolinski, A.T. Deoxynivalenol: Toxicology and potential effects on humans. J. Toxicol. Environ. Heal B 2005, 8, 39–69. [Google Scholar] [CrossRef]
- Ji, F.; Li, H.; Xu, J.; Shi, J. Enzyme-linked immunosorbent-assay for deoxynivalenol (DON). Toxins 2011, 3, 968–978. [Google Scholar] [CrossRef]
- Liao, Y.; Peng, Z.; Chen, L.; Nüssler, A.K.; Liu, L.; Yang, W. Deoxynivalenol, gut microbiota and immunotoxicity: A potential approach? Food Chem. Toxicol. 2018, 112, 342–354. [Google Scholar] [CrossRef]
- Young, J.C.; Fulcher, R.G.; Hayhoe, J.H.; Scott, P.M.; Dexter, J.E. Effect of milling and baking on deoxynivalenol (vomitoxin) content of eastern canadian wheats. J. Agric. Food Chem. 1984, 32, 659–664. [Google Scholar] [CrossRef]
- Tima, H.; Berkics, A.; Hannig, Z.; Ittzés, A.; Kecskésné Nagy, E.; Mohácsi-Farkas, C.; Kiskó, G. Deoxynivalenol in wheat, maize, wheat flour and pasta: Surveys in hungary in 2008–2015. Food Addit. Contam. B 2017, 11, 37–42. [Google Scholar] [CrossRef]
- Arrúa Alvarenga, A.A.; Moura Mendes Arrua, J.; Cazal Martínez, C.C.; Arrúa Alvarenga, P.D.; Fernández Ríos, D.; Pérez Estigarribia, P.E.; Mohan Kohli, M. Deoxynivalenol screening in wheat-derived products in Gran Asunción, Paraguay. J. Food Saf. 2018, 39, 51–56. [Google Scholar] [CrossRef]
- Iqbal, S.Z.; Alim, M.; Jinap, S.; Arino, A. Regulations for Food Toxins. In Food Safety; Springer International Publishing: Basel, Switzerland, 2016. [Google Scholar] [CrossRef]
- Li, M.; Sun, M.; Hong, X.; Duan, J.; Du, D. Survey of Deoxynivalenol Contamination in Agricultural Products in the Chinese Market Using An ELISA Kit. Toxins 2019, 11, 6. [Google Scholar] [CrossRef] [PubMed]
- China Food and Drug Administration. China National Standard No. GB2761-2017; Ministry of Health of P. R. China: Beijing, China, 2017.
- Maragos, C.M.; Mccormick, S.P. Monoclonal antibodies for the mycotoxins deoxynivalenol and 3-acetyl-deoxynivalenol. Food Agric. Immunol. 2000, 12, 181–192. [Google Scholar] [CrossRef]
- Wang, X.Y. Determination of deoxynivalenol in maize by using gas chromatography-electron capture detector. J. Changzhi Univ. 2006, 23, 7–10. [Google Scholar]
- Righetti, L.; Galaverna, G.; Dall’Asta, C. Group detection of don and its modified forms by an elisa kit. Food Addit. Contam. A 2016, 34, 248–254. [Google Scholar] [CrossRef]
- Wang, Z.P.; Wang, D.L.; Feng, Z.S.; Yang, J.; Wang, X.J.; Chen, X. Detection of deoxynivalenol in malting barley by immunoaffinity column clear up and high performance liquid chromatography. Food Ferment. Ind. 2008, 34, 137–139. [Google Scholar] [CrossRef]
- Dos Santos, J.S.; Takabayashi, C.R.; Ono, E.Y.S.; Itano, E.N.; Mallmann, C.A.; Kawamura, O.; Hirooka, E.Y. Immunoassay based on monoclonal antibodies versus lc-ms: Deoxynivalenol in wheat and flour in southern brazil. Food Addit. Contam. A 2011, 28, 1083–1090. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.S.; Zhou, Y.J.; Zhou, M.G. A sensitive chemiluminescence enzyme immunoassay for the determination of deoxynivalenol in wheat samples. Anal. Methods UK 2015, 7, 2196–2202. [Google Scholar] [CrossRef]
- Li, C.L.; Wen, K. A universal multi-wavelength fluorescence polarization immunoassay for multiplexed detection of mycotoxins in maize. Biosens. Bioelectron. 2016, 79, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Gao, L.; Zhou, B.; Zhu, L.; Zhang, Y.; Huang, B. Simultaneous detection of deoxynivalenol and zearalenone by dual-label time-resolved fluorescence immunoassay. J. Sci. Food Agric. 2011, 91, 193–197. [Google Scholar] [CrossRef]
- Yang, X.; Huang, Z.B.; He, Q.H.; Deng, S.Z.; Li, L.S.; Li, Y.P. Development of an immunochromatographic strip test for the rapid detection of deoxynivalenol in wheat and maize. Food Chem. 2010, 119, 834–839. [Google Scholar]
- Burmistrova, N.A.; Rusanova, T.Y.; Yurasov, N.A.; Goryacheva, I.Y.; De Saeger, S. Multi-detection of mycotoxins by membrane based flow-through immunoassay. Food Control 2014, 46, 462–469. [Google Scholar] [CrossRef]
- Kadota, T.; Takezawa, Y.; Hirano, S.; Tajima, O.; Maragos, C.M.; Nakajima, T.; Tanaka, T.; Kamata, Y.; Yoshiko, S.K. Rapid detection of nivalenol and deoxynivalenol in wheat using surface plasmon resonance immunoassay. Anal. Chim. Acta 2010, 673, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Li, H.; Li, C.L.; Zhang, S.X.; Shen, J.Z.; Wang, Z.H. Gold nanoparticles-based lateral flow immunoassay with silver staining for simultaneous detection of fumonisin B1 and deoxynivalenol. Food Control 2015, 54, 347–352. [Google Scholar] [CrossRef]
- Qiu, Y.L.; He, Q.H.; Xu, Y.; Bhunia, A.K.; Tu, Z.; Chen, B.; Liu, Y.Y. Deoxynivalenol-mimic nanobody isolated from a naïve phage display nanobody library and its application in immunoassay. Anal. Chim. Acta 2015, 887, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shi, W.; Shen, J.; Zhang, S.; Cheng, L.; Wang, Z. Development of a rapid competitive indirect elisa procedure for the determination of deoxynivalenol in cereals. Food Agric. Immunol. 2012, 23, 41–49. [Google Scholar] [CrossRef]
- Jia-Jia, T.; Xiao-Bing, L.I.; Guo-Wen, L.; Tao, K.; Dong-Na, L.I.; Liang, Z. Production of two anti-cadmium monoclonal antibodies by hock immunization. Chin. Vet. Sci. 2011, 41, 936–940. [Google Scholar]
- Zeng, K.; Zou, Y.; Liu, J.; Wei, W.; Zhang, M.; Zhou, J.; Zhang, Z.; Gai, Z.K. Enzyme-linked immunosorbent assay for triclocarban in aquatic environments. Water Sci. Technol. A J. Inter. Associ. Water Pollut. Res. 2015, 72, 1682–1691. [Google Scholar] [CrossRef]
- Kuang, H.; Xing, C.; Hao, C.; Liu, L.; Wang, L.; Xu, C. Rapid and highly sensitive detection of lead ions in drinking water based on a strip immunosensor. Sensors 2013, 13, 4214–4224. [Google Scholar] [CrossRef]
- Xi, J.; Shi, Q. Development of an indirect competitive elisa kit for the detection of soybean allergenic protein gly m bd 28k. Food Anal. Method 2016, 9, 2998–3005. [Google Scholar] [CrossRef]
- Hayashi, Y.; Matsuda, R.; Ito, K.; Nishimura, W.; Imai, K.; Maeda, M. Detection limit estimated from slope of calibration curve: An application to competitive elisa. Anal. Sci. 2005, 21, 167–169. [Google Scholar] [CrossRef]
- Li, H. The Artificial Antigen Synthesis and Detection of Deoxynivalenol Using Indirect Competitive ELISA. Ph.D. Thesis, Nanjing Agricultural University, Nanjing, China, 2003. [Google Scholar]
- Sakamoto, S.; Nagamitsu, R.; Yusakul, G.; Miyamoto, T.; Tanaka, H.; Morimoto, S. Ultrasensitive immunoassay for monocrotaline using monoclonal antibody produced by N, N’ -carbonyldiimidazole mediated hapten-carrier protein conjugates. Talanta 2017, 168, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Niessen, L.; BoHm-Schrami, M.; Vogel, H.; Donhauser, S. Deoxynivalenol in commercial beer – screening for the toxin with an indirect competitive elisa. Mycotoxin Res. 1993, 9, 99–109. [Google Scholar] [CrossRef] [PubMed]
- China Food and Drug Administration. China National Standard No. GB5009.111-2016; Ministry of Health of P. R. China: Beijing, China, 2016.
- Yang, D.N.; Wang, H.F. ELISA and HPLC for detection of DON in wheat. J. Anhui Agric. Sci. 2013, 41, 1269–1270. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |
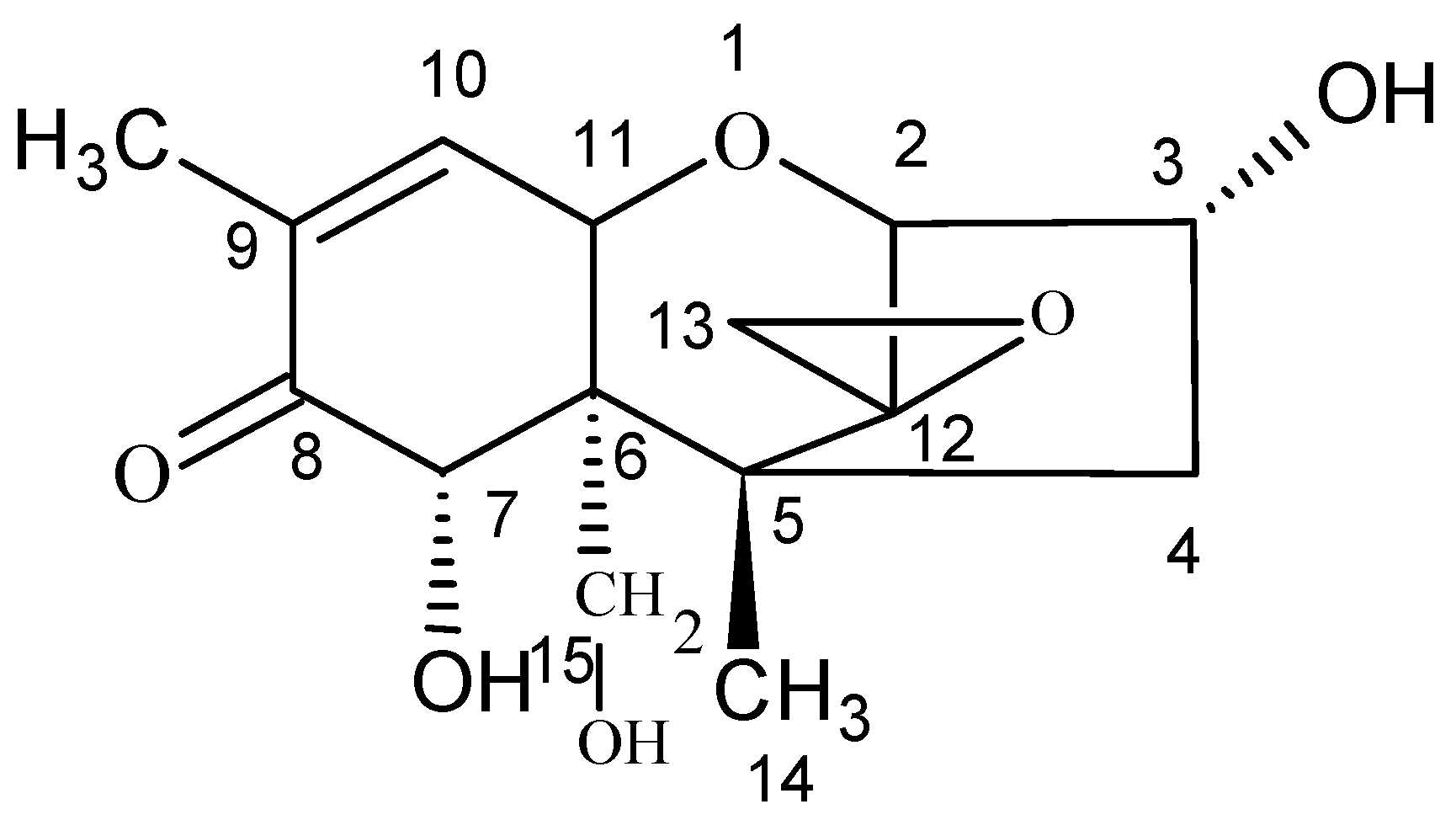

| Number | Composition | Quantity | Unit | Parameters |
|---|---|---|---|---|
| 1 | ELISA microplates | 1 | board | coated 96-well transparent microplates |
| 2 | Mab solution | 1 | tube | 6 mL (concentration of 1:6400 diluted in PBS) |
| 3 | DON standard | 1 | tube | 1 mg/mL |
| 4 | HRP-DON | 1 | tube | 3 mL (concentration of 1:800 diluted in PBS) |
| 5 | 1 × Working buffer | 1 | bottle | 50 mL (5% methanol, 0.5 mol/L Na+, pH 7.4 in PBS) |
| 6 | 10 × Washing buffer | 1 | bottle | 50 mL (10 × PBST, pH 7.4) |
| 7 | Color substrate buffer | 1 | bottle | 15 mL (0.4 mmol/L TMB and 3 mmol/L H2O2 diluted in citrate buffer, pH 5.0) |
| 8 | Stop buffer | 1 | bottle | 10 mL (2 mol/L H2SO4 diluted in H2O) |
| Factor | Parameter | Factor | Parameter |
|---|---|---|---|
| RaMIgG | 10 ng/mL | Methanol (v/v, %) | 5 |
| anti-DON mAb | 1:6400 (1.56 ng/mL) | Na+ (mol/L) | 0.5 |
| HRP-DON | 1:800 (28.5 ng/mL) | pH | 7.4 |
| Samples | Spiked (ng/g) | Mean Recovery ± SD (%) | RSD (%) | |
|---|---|---|---|---|
| 200 | 89.2 ± 6.2 | 7 | ||
| Wheat bran | 500 | 88.1 ± 5.7 | 6.5 | |
| 1000 | 79.4 ± 7.5 | 9.4 | ||
| 200 | 77.1 ± 9.2 | 11.9 | ||
| Wheat flour | 500 | 81.7 ± 5.6 | 6.8 | |
| 1000 | 96.5 ± 4.1 | 4.2 | ||
| 200 | 104.4 ± 5.8 | 5.5 | ||
| Corn meal | 500 | 96.4 ± 6.3 | 6.5 | |
| 1000 | 107.0 ± 7.6 | 7.1 | ||
| 200 | 103.7 ± 4.6 | 4.4 | ||
| Corn | 500 | 95.0 ± 5.3 | 5.6 | |
| 1000 | 98.4 ± 7.3 | 7.4 | ||
| Compounds | IC50 (ng/mL) | Cross-Reactivity (%) |
|---|---|---|
| DON | 6.61 | 100 |
| 3-Ac-DON | 142.1 | 4.7 |
| 15-Ac-DON | >5 × 103 | <0.2 |
| DON-3-G | >1 × 104 | <0.1 |
| NIV | >1 × 104 | <0.1 |
| Fusarenon-X | >1 × 104 | <0.1 |
| T-2 toxin | >1 × 104 | <0.1 |
| ZEN | >1 × 104 | <0.1 |
| AFB1 | >1 × 104 | <0.1 |
| Province | dcELISA Kit | HPLC | ||||
|---|---|---|---|---|---|---|
| Range (ng/g) | Average Value ± SD% | RSD (%) | Range (ng/g) | Average Value ± SD% | RSD (%) | |
| Henan | 309.5–1243.8 | 933.4 ± 324.1 | 34.7 | 277.1–1231.4 | 918.1 ± 304.7 | 33.2 |
| Anhui | 378.9–1230.7 | 810.5 ± 276.3 | 34.1 | 350.5–1218.4 | 796.6 ± 268.5 | 33.7 |
| Hebei | 696.4–1087.9 | 853 ± 119.4 | 14 | 681.9–1023.1 | 834.3 ± 103.1 | 12.4 |
| Shandong | 741.9–1258.4 | 1020.3 ± 168.2 | 16.5 | 752.8–1235.6 | 1049.1 ± 158.2 | 15.1 |
| Jiangsu | 591.2–891.2 | 713.4 ± 93 | 13 | 583.5–870.3 | 703.9 ± 92.4 | 13.1 |
| Gansu | 254.7–1080.5 | 580.5 ± 254.3 | 43.8 | 236.1–991.6 | 560.4 ± 243.3 | 43.4 |
| Province | Samples (Wheat) | dcELISA Kit | HPLC |
|---|---|---|---|
| 1 | + | + | |
| 2 | + | + | |
| Henan | 3 | - | - |
| 4 | + | + | |
| 5 | + | - | |
| 1 | + | + | |
| 2 | - | + | |
| Anhui | 3 | + | - |
| 4 | + | + | |
| 5 | + | + | |
| 1 | + | + | |
| 2 | + | + | |
| Hebei | 3 | - | - |
| 4 | + | + | |
| 5 | - | + | |
| 1 | - | - | |
| 2 | + | + | |
| Shandong | 3 | + | + |
| 4 | + | + | |
| 5 | - | - | |
| 1 | + | + | |
| 2 | + | + | |
| Jiangsu | 3 | - | - |
| 4 | - | - | |
| 5 | + | + | |
| 1 | + | - | |
| 2 | + | + | |
| Gansu | 3 | + | + |
| 4 | + | + | |
| 5 | + | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, L.; Li, Y.-T.; Jiang, J.-Q.; Li, R.-F.; Fan, G.-Y.; Lv, J.-M.; Zhou, Y.; Zhang, W.-J.; Wang, Z.-L. Development of a Direct Competitive ELISA Kit for Detecting Deoxynivalenol Contamination in Wheat. Molecules 2020, 25, 50. https://doi.org/10.3390/molecules25010050
Han L, Li Y-T, Jiang J-Q, Li R-F, Fan G-Y, Lv J-M, Zhou Y, Zhang W-J, Wang Z-L. Development of a Direct Competitive ELISA Kit for Detecting Deoxynivalenol Contamination in Wheat. Molecules. 2020; 25(1):50. https://doi.org/10.3390/molecules25010050
Chicago/Turabian StyleHan, Li, Yue-Tao Li, Jin-Qing Jiang, Ren-Feng Li, Guo-Ying Fan, Jun-Mei Lv, Ye Zhou, Wen-Ju Zhang, and Zi-Liang Wang. 2020. "Development of a Direct Competitive ELISA Kit for Detecting Deoxynivalenol Contamination in Wheat" Molecules 25, no. 1: 50. https://doi.org/10.3390/molecules25010050
APA StyleHan, L., Li, Y.-T., Jiang, J.-Q., Li, R.-F., Fan, G.-Y., Lv, J.-M., Zhou, Y., Zhang, W.-J., & Wang, Z.-L. (2020). Development of a Direct Competitive ELISA Kit for Detecting Deoxynivalenol Contamination in Wheat. Molecules, 25(1), 50. https://doi.org/10.3390/molecules25010050





