Choline Salicylate Analysis: Chemical Stability and Degradation Product Identification
Abstract
1. Introduction
2. Results and Discussion
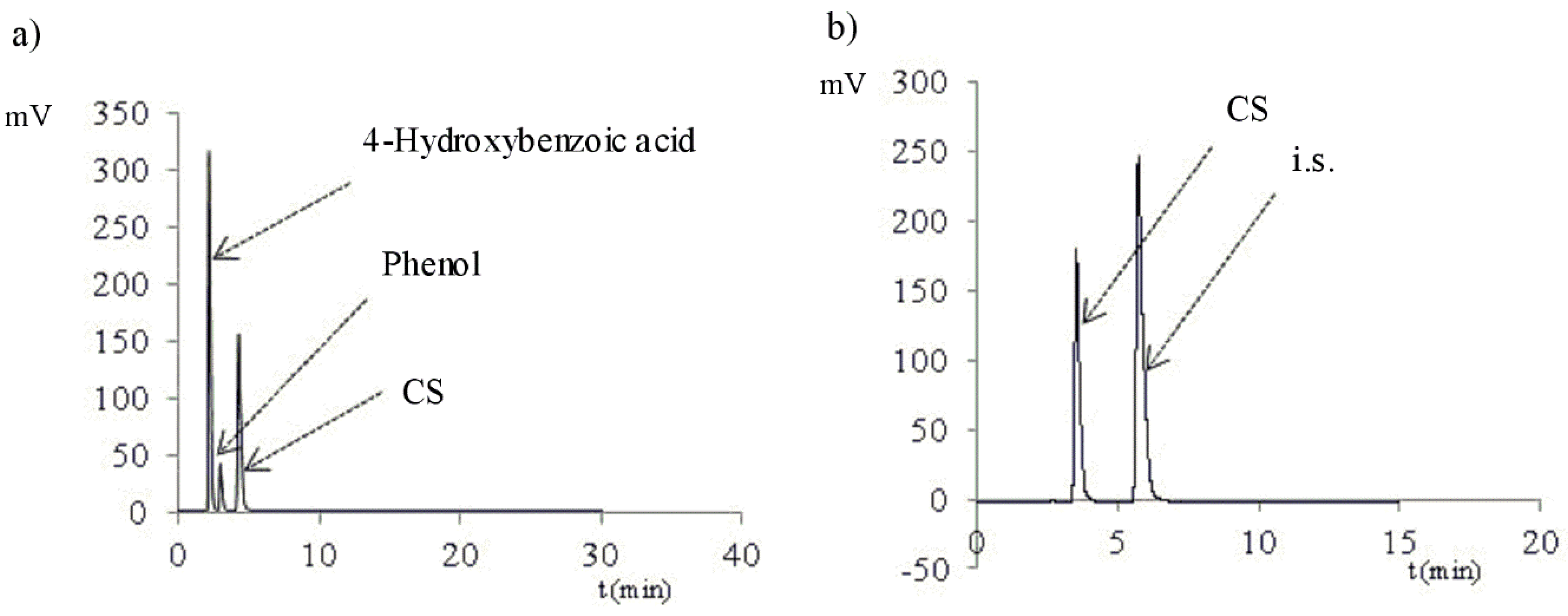
2.1. Method Validation
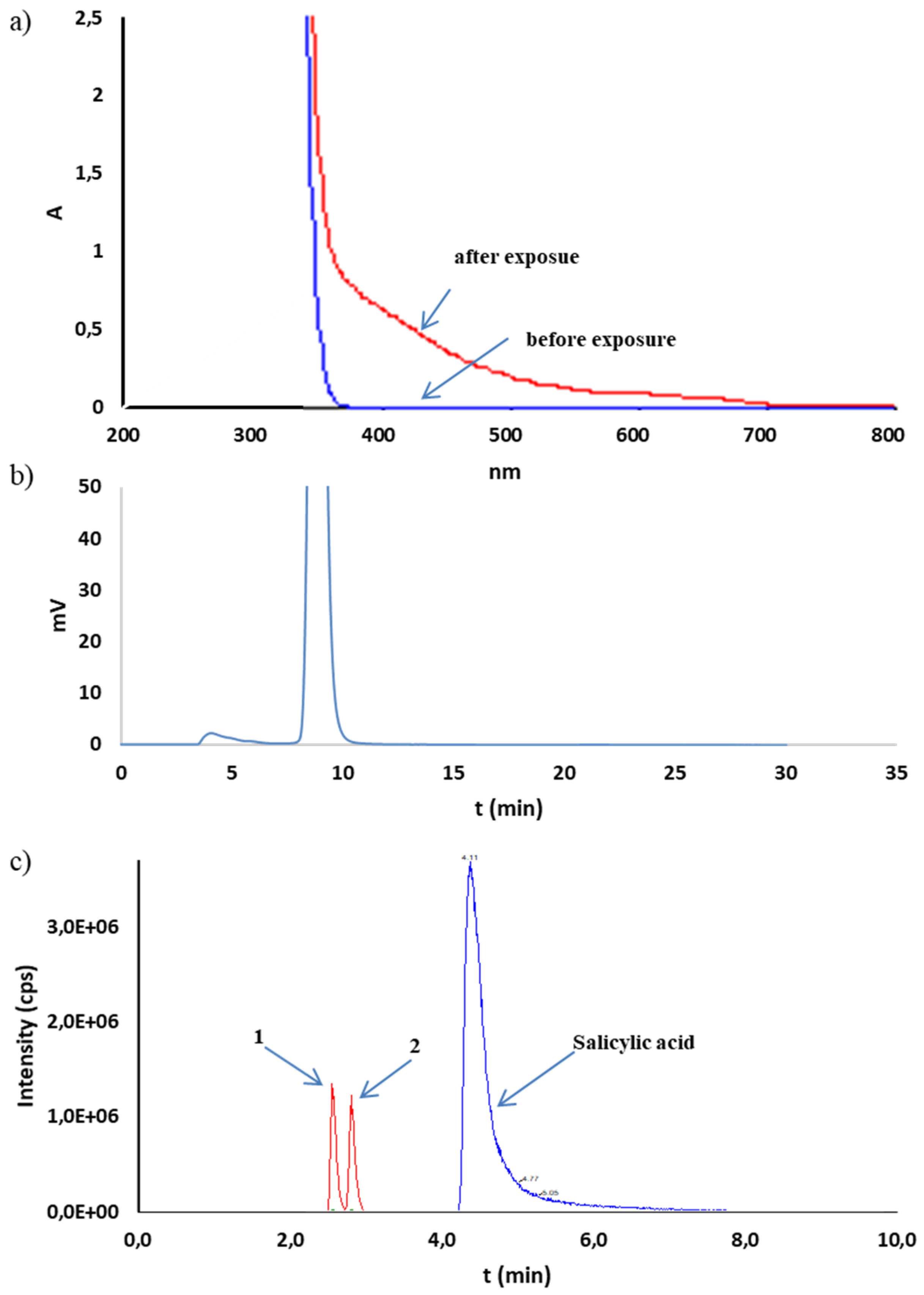
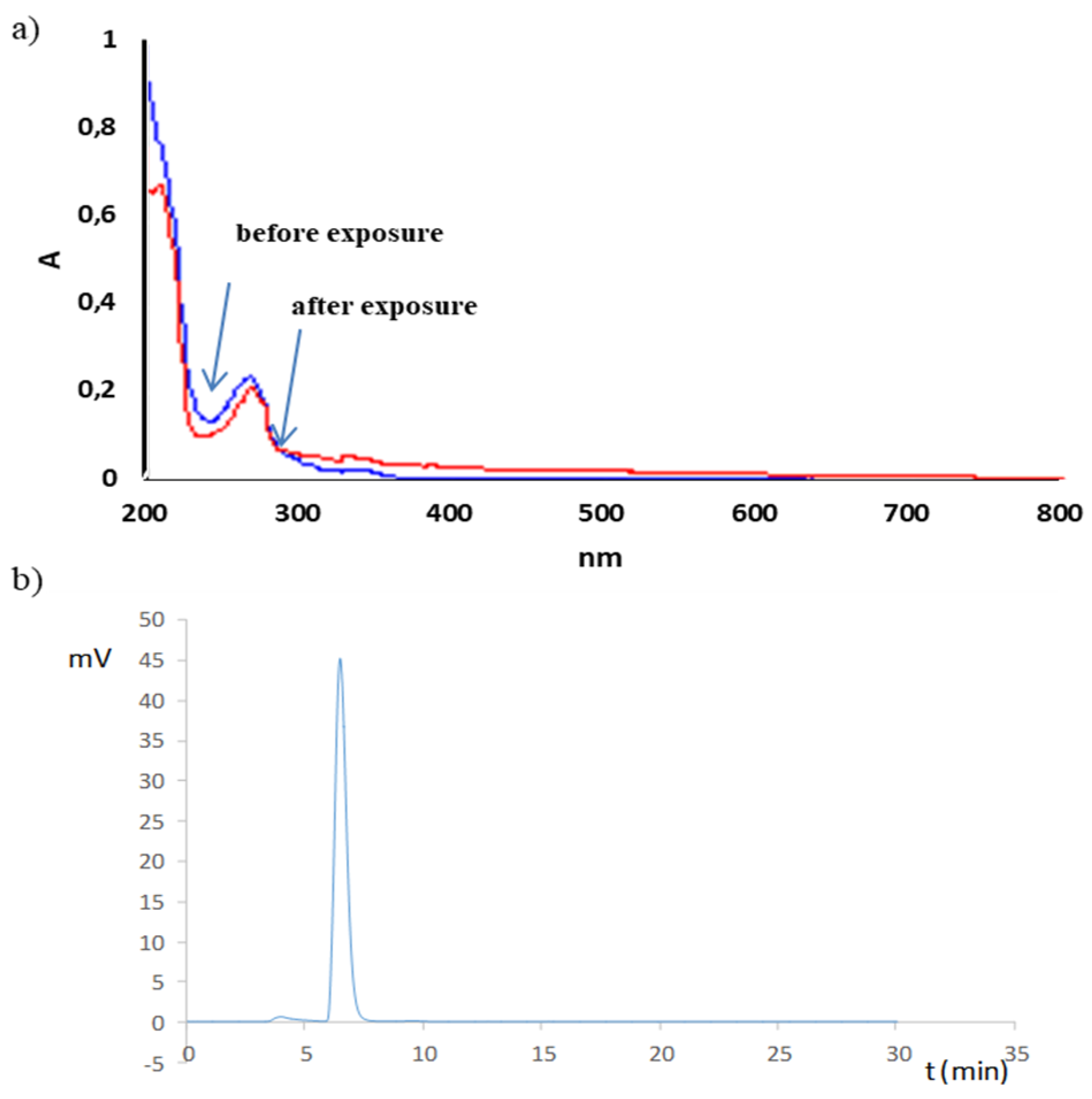
2.2. Stress Test
3. Materials and Methods
3.1. Apparatus and Chromatographic Conditions
3.1.1. HPLC-UV
3.1.2. HPLC-MS/MS
3.1.3. Other Equipment
3.2. Validation of the HPLC Method
3.2.1. Selectivity
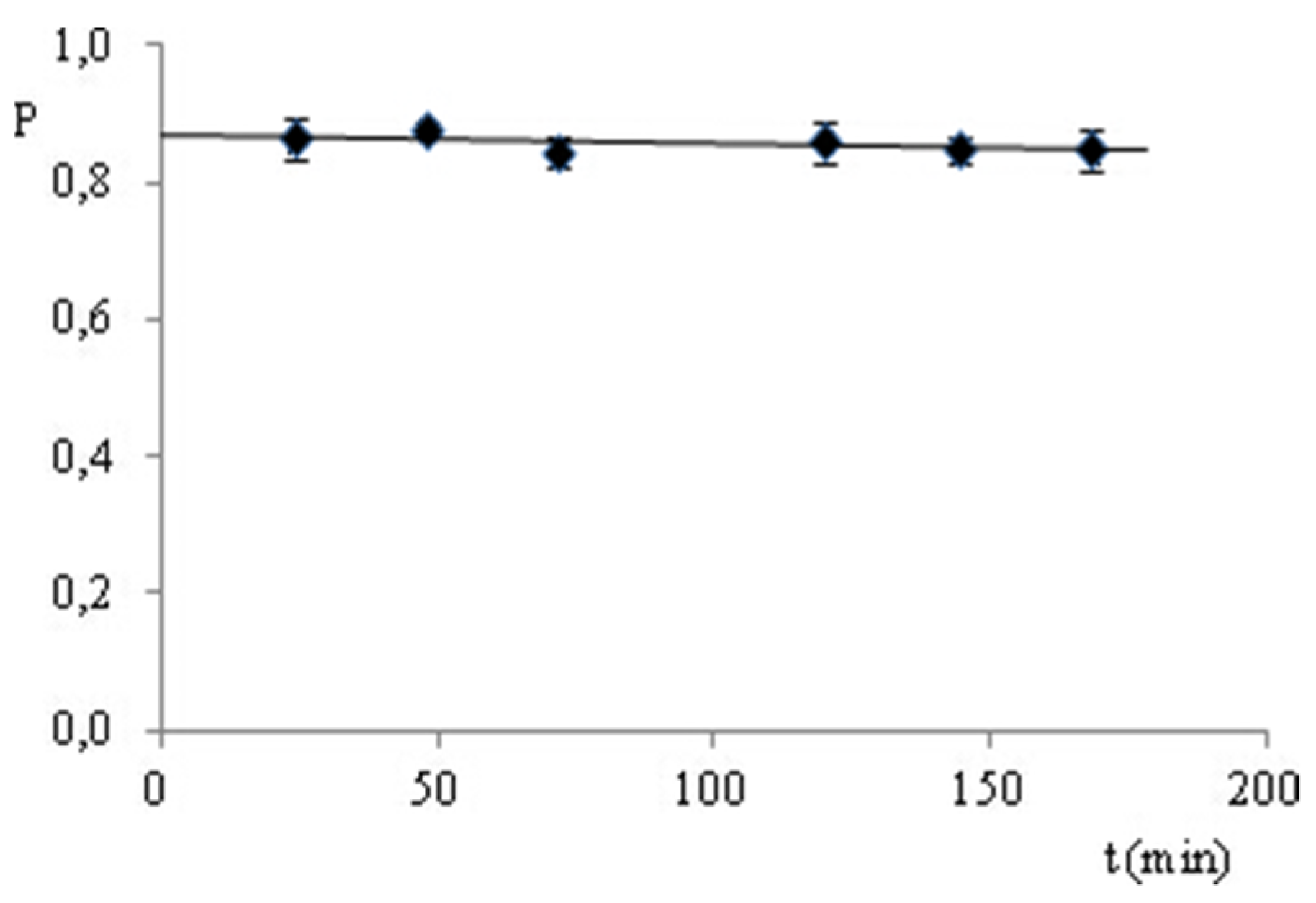
3.2.2. Stability of the Analyte
3.2.3. Calibration Curve
3.2.4. Linearity
2.2.5. Precision, Accuracy, and Reproducibility
3.3. Validation of the UV Method
3.3.1. Calibration Curve
3.3.2. Linearity
3.3.3. Precision, Accuracy, and Reproducibility
3.4. Comparison of HPLC and UV Methods
3.5. Stress Test
3.5.1. Strong Acid, Strong Base Degradation, and Neutral Hydrolysis
3.5.2. Oxidative Degradation
3.5.3. Photostability
3.5.4. CS Stability Under Sterilization Conditions
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kobylańska, M.; Limanowska-Shaw, H. Topical application of choline salicylate drugs in dental practice (in Polish). Stom. Współcz. 2000, 7, 41–44. [Google Scholar]
- Amangeldykyzy, S.; Nurlybek, A.N.; Nurlan, A.N.; Juszkiewicz, K.T.; Polski, A.; Seisembay, U.B.; Umirzakovna, U.K.; Mukasheva, A.M.; Poleszak, E. The effect of a combined choline salicylate and cetalkonium chloride gel on particular strains of Pseudomonas aeruginosa, Staphylococcus spp. and Streptococcus spp. Curr. Issues Pharm. Med. Sci. 2015, 28, 77–80. [Google Scholar] [CrossRef]
- Razyozzhaey, A.A.; Starodumva, T.A.; Nemstsyeridze, E. The experience with topical application of non-steroidal anti-inflammatory agents for the teatment of otitis media (in Russian). Vestn. Otorinolaringol. 2012, 2, 66–68. [Google Scholar]
- Dummer, D.S.; Sutherland, I.A.; Murray, J.A. A single-blind, randomized study to compare the efficacy of two ear drop preparations (“Audax” and “Cerumol”) in the softening of ear wax. C. Curr. Med. Res. Opin. 1992, 13, 26–30. [Google Scholar] [CrossRef] [PubMed]
- British Pharmacopoeia; London Her Majesty’s Stationary Office: London, UK, 1988; pp. 137, 669.
- Kumar, M.; Khar, R.K.; Agarwal, S.P.; Ahuja, A. Formulation of choline salicylate as Lozenge tablet for improved delivery to oral cavity. Ind. J. Pharm. Sci. 2000, 3, 233–235. [Google Scholar]
- Gupta, S.K.; Joshi, S.; Tandon, R.; Mathur, P. Topical aspirin provides protection against galactosemic cataract. Ind. J. Opthalmol. 1997, 45, 221–225. [Google Scholar]
- Flach, A.J. Topical nonsteroidal antiinflammatory drugs in ophthalmology. Int. Ophthalmol. Clin. 2002, 42, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Flach, A.J.; Jampol, L.M. Nonsteroidal anti-inflammatory drugs in ophthalmology. Surv. Ophthalmol. 2010, 55, 108–133. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, M.; Dhake, A.S.; Sharma, S.K.; Majumdar, D.K. Topical ocular delivery of NSAIDs. AAPS J. 2008, 10, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Kähler, C.M.; Kieselbach, G.F.; Reinisch, N.; Troger, J.; Gättinger, W.; Wiedermann, C.J. Fibroblast growth-promoting activity in proliferative vitreoretinopathy: Antagonism by acetylsalicylic acid. Eur. J. Pharmacol. 1994, 262, 261–269. [Google Scholar]
- Voigt, M.; Kralinger, M.; Kieselbach, G.; Chapon, P.; Anagnoste, S.; Hayden, B.; Parel, J.M. Ocular aspirin distribution: A comparison of intravenous, topical, and coulomb-controlled iontophoresis administration. Inv. Ophthalmol. Vis. Sci. 2002, 43, 3299–3306. [Google Scholar]
- Wroblewska, K.; Kucinska, K.; Murias, M.; Lulek, J. Characterization of new eye drops with choline salicylate and assessment of their irritancy by in vitro short time exposure. Saudi Pharm. J. 2014, 23, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Oman, T.K.; Stewart, M.C.; Burns, A.; Lang, T.F. Topical choline salicylate implicated in Reye’s syndrome. Br. Med. J. 2008, 336, 1376. [Google Scholar] [CrossRef]
- Wilson, P.H.R.; Mason, C. The trouble with teething–misdiagnosis and misue of a topical medicament. Int. J. Paediatr. Dent. 2002, 12, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.C.; Laxaman, H.; Mead, R.; Cameron, E.A.B. Oral gel choline salicylate induced refractory gastric ulceration. J. R. Soc. Med. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Aparitio, S.; Atilhan, M. A computational study on choline benzoate and choline salicylate ionic liquids in the pure state and after CO2 adsorption. J. Phys. Chem. B 2012, 116, 9171–9185. [Google Scholar] [CrossRef] [PubMed]
- Robert, H.; Broh-Kahn, E.J. Choline salicylate composition and methods of use. U.S. Patent 3069321, 18 December 1962. [Google Scholar]
- Budavari, S.; O’Neil, M.; Smith, A.; Heckelman, P.; Obenchain, J. The Merck Index: An. encyklopedia of chemicals, drugs and biological, 12th ed.; Merck Research Laboratories, Ed.; MERCK & CO. Inc.: Whitehouse Station, NJ, USA, 1996. [Google Scholar]
- Stability Testing of New Drug Substances and Products Q1A(R2). Available online: http://www.ich.org/products/guidelines/quality/article/quality-guidelines.html (accessed on 6 August 2019).
- Kumari, K.P.; Sankar, G.; Sowjanya, P.; Madhubabu, S. Stability indicating RP-HPLC method development and validation of salicylic acid in choline magnesium trisalicilate (Trisilate) tablets. J. Pharma. Care Health Syst. 2014, 1, 120. [Google Scholar] [CrossRef]
- Vione, D.; Picatonotto, T.; Carlotti, M.E. Photodegradation of phenol and salicylic acid by coated rutile-based pigments: A new approach for the assessment of sunscreen treatment efficiency. J. Cosmet. Sci. 2003, 54, 513–524. [Google Scholar] [PubMed]
- Adán, C.; Coronado, J.M.; Bellod, R.; Soria, J.; Yamaoka, H. Photochemical and photocatalytic degradation of salicylic acid with hydrogen peroxide over TiO2/SiO2 fibres. Appl. Catal. A Gen. 2006, 303, 199–206. [Google Scholar] [CrossRef]
- European Pharmacopoeia, 8th ed.; Council of Europe: Strasbourg, France, 2017; p. 3198.
Sample Availability: Not available. |




| Compound | Retention Time, min | Compound | Retention Time, min |
|---|---|---|---|
| 4-Hydroksybenzoic acid | 4.51 | 2,3-Dihydroxybenzoic acid | 5.48 |
| Pyrocatechol | 5.02 | Phenol | 6.24 |
| 2,5-Dihydroxybenzoic acid | 5.08 | Choline salicylate | 8.85 |
| Compound | Molecular Weight, Da | Precursor Ion, m/z | MRM Transition, m/z | Retention Time, min | DP, V | EP, V | CE, V | CXP, V |
|---|---|---|---|---|---|---|---|---|
| 2,3-Dihydroxybenzoic acid | 154.1 | 152.9 | 152.9→108.9 | 2.47 | −30 | −8 | −22 | −15 |
| 152.9→80.9 | −30 | −15 | ||||||
| 2,5-Dihydroxybenzoic acid | 152.9→108.9 | 2.21 | −22 | −15 | ||||
| 152.9→80.9 | −30 | −15 | ||||||
| Salicylic acid | 138.1 | 136.9 | 136.9→92.9 | 4.13 | −30 | −8 | −25 | −15 |
| 136.9→65.0 | −45 | −15 | ||||||
| Pyrocatechol | 110.0 | 108.9 | 108.9→90.9 | 2.15 | −70 | −8 | −27 | −15 |
| 108.9→80.9 | −24 | −15 | ||||||
| Phenol | 94.1 | 92.9 | 92.9→74.9 | - | −80 | −8 | −30 | −12 |
| 92.9→65.0 | −28 | −10 |
| Calibration Curves | ||||||
| Method | Range, µg/mL | n | Statistical Parameters | LOD, µg/mL | LOQ, µg/mL | |
| y = ax + b | y = ax | |||||
| HPLC | 3.94–119.10 | 9 | a = (1.10 ± 0.01) 10−2 | a = (1.10 ± 0.01) 10−2 | 1.16 | 3.51 |
| b = (5.75 ± 9.13) 10−3 | r = 0.9999 | |||||
| r = 0.9999 | ||||||
| tb = 1.486 < t0.05 (7) = 2.365 | ||||||
| UV | 2.52–80.56 | 14 | a = (1.34 ± 0.01) 10−2 | a = (1.34 ± 0.01) 10−2 | 0.43 | 1.30 |
| b = (−3.74 ± 3.82) 10−3 | r = 0.9999 | |||||
| r = 0.9999 | ||||||
| tb = −2.130 < t0.05 (12) = 2.179 | ||||||
| Linearity | ||||||
| Method | Range, % | n | Statistical Parameters | |||
| y = ax + b | y = ax | |||||
| HPLC | 1.00–3.01 | 5 | a = 0.436 ± 0.032 | a = 0.444 ± 0.024 | ||
| b = (1.82 ± 6.91) 102 | r = 0.9992 | |||||
| r = 0.9992 | ||||||
| tb = 0.837 < t0.05 (3) = 3.182 | ||||||
| UV | 1.00–3.01 | 5 | a = 0.267 ± 0.010 | a = (1.34 ± 0.01) 10−2 | ||
| b = (6.16 ± 22.41) 10−3 | r = 0.9998 | |||||
| r = 0.9998 | ||||||
| tb = 0.874 < t0.05 (3) = 3.182 | ||||||
| Precision and Accuracy | |||||||
|---|---|---|---|---|---|---|---|
| Method | Declared Content, % | Amount Found, % (Recovery, %) | Repeatability | ||||
| Inter-Day | RSD, % | Intra-Day | RSD, % | RSDr, % | |||
| HPLC | 1.00 | 1.01 ± 0.01 (101.0 ± 1.4) | 1.33 | 1.01 ± 0.02 (101.0 ± 2.0) | 1.86 | 0.40 | |
| 2.01 | 2.01 ± 0.03 (100.2 ± 1.3) | 1.26 | 2.00 ± 0.03 (99.6 ± 1.6) | 1.56 | 0.53 | ||
| 3.01 | 3.02 ± 0.02 (100.4 ± 0.9) | 0.82 | 3.05 ± 0.02 (101.5 ± 0.8) | 0.73 | 0.25 | ||
| UV | 1.00 | 1.00 ± 0.01 (101.0 ± 1.4) | 1.26 | 0.99 ± 0.01 (101.0 ± 1.4) | 1.20 | 0.31 | |
| 2.01 | 2.04 ± 0.01 (100.2 ± 1.3) | 0.43 | 2.03 ± 0.01 (100.2 ± 1.3) | 0.55 | 0.19 | ||
| 3.01 | 3.02 ± 0.02 (100.4 ± 0.9) | 0.67 | 2.99 ± 0.01 (100.4 ± 0.9) | 0.32 | 0.11 | ||
| Comparative Analysis of HPLC and UV Method | |||||||
| Method | x ± Δx, % | SD, % | SDx, % | RSD, % | F-Snedecor Test | t-Student Test | |
| HPLC | 2.01 ± 0.03 | 2.54 · 10−2 | 1.04 · 10−2 | 1.26 | F = 0.52 | t = 0.371 | |
| UV | 2.02 ± 0.02 | 1.82 · 10−2 | 7.45 · 10−3 | 0.90 | F0.05(5;5) = 5.05 | t0.05(10) = 2.228 | |
| Medium | x ± Δx, % | SD, % | SDx, % | RSD, % | HPLC tr, min (Compound or Part in %) |
|---|---|---|---|---|---|
| Hydrolysis in a Neutral Environment | |||||
| H2O 90 °C, 36 h | 95.8 ± 5.7 | 2.29 | 1.32 | 2.39 | HPLC-UV: no additional peaks |
| H2O 90 °C, 2 days | 102.7 ± 10.0 | 4.01 | 2.32 | 3.91 | HPLC-UV: no additional peaks |
| H2O 90 °C, 4 days | 99.3 ± 18.7 | 7.51 | 4.34 | 7.57 | HPLC-UV: no additional peaks |
| H2O 90 °C, 10 days | 103.7 ± 6.7 | 2.70 | 1.56 | 2.60 | HPLC-UV: no additional peaks |
| Hydrolysis in an Acidic Environment | |||||
| HCl 0.1 M 90 °C, 24 h | 99.5 ± 3.1 | 1.27 | 0.732 | 1.27 | HPLC-UV: no additional peaks |
| HCl 1 M 90 °C, 24 h | 98.7 ± 5.4 | 2.20 | 1.27 | 2.22 | HPLC-UV: 3.24 (0.18), 6.19 (0.08), 15.24 (0.08) |
| Hydrolysis in an Alkaline Environment | |||||
| NaOH 0.1 M 90 °C, 24 h | 102.0 ± 1.9 | 0.779 | 0.450 | 0.76 | HPLC-UV: no additional peaks |
| NaOH 1 M 90 °C, 24 h | 63.3 ± 2.0 | 0.807 | 0.466 | 1.28 | HPLC-UV: 3.28 (20.5), 4.40 (7.3), 6.20 (1.4) 7.10 (1.4), 16.10 (1.4) HPLC-MS/MS * |
| Decomposition in an Oxidizing Environment | |||||
| 3% H2O2 room temp., 6 h | 97.2 ± 1.2 | 0.490 | 0.283 | 0.50 | HPLC-UV: no additional peaks |
| 3% H2O2 room temp., 24 h | 102.3 ± 3.0 | 1.19 | 0.685 | 1.17 | HPLC-UV: no additional peaks |
| 10% H2O2 room temp., 24 h | 92.9 ± 0.3 | 0.124 | 0.0715 | 0.13 | HPLC-UV: 6.21 (0.2), 15.24 (0.8) HPLC-MS/MS: 2.21 (2,5-dihydroxybenzoic acid) 2.47 (2,3-dihydroxybenzoic acid) |
| Photostability in Solution (1.2 · 106 lux·h) | |||||
| protected | 100.0 ± 9.3 | 3.73 | 2.15 | 3.73 | HPLC-UV: no additional peaks |
| not protected | 93.4 ± 1.9 | 0.769 | 0.444 | 0.82 | HPLC-MS/MS: 2.21 (2,5-dihydroxybenzoic acid) 2.47 (2,3-dihydroxybenzoic acid) |
| Photostability in Solution (6.0 · 106 lux·h) | |||||
| protected | 99.6 ± 2.5 | 1.02 | 0.590 | 1.02 | HPLC-UV: no additional peaks |
| not protected | 90.2 ± 0.3 | 0.137 | 0.0791 | 0.15 | HPLC-MS/MS: 2.21 (2,5-dihydroxybenzoic acid) 2.47 (2,3-dihydroxybenzoic acid) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wróblewska, K.B.; Plewa, S.; Dereziński, P.; Muszalska-Kolos, I. Choline Salicylate Analysis: Chemical Stability and Degradation Product Identification. Molecules 2020, 25, 51. https://doi.org/10.3390/molecules25010051
Wróblewska KB, Plewa S, Dereziński P, Muszalska-Kolos I. Choline Salicylate Analysis: Chemical Stability and Degradation Product Identification. Molecules. 2020; 25(1):51. https://doi.org/10.3390/molecules25010051
Chicago/Turabian StyleWróblewska, Katarzyna B., Szymon Plewa, Paweł Dereziński, and Izabela Muszalska-Kolos. 2020. "Choline Salicylate Analysis: Chemical Stability and Degradation Product Identification" Molecules 25, no. 1: 51. https://doi.org/10.3390/molecules25010051
APA StyleWróblewska, K. B., Plewa, S., Dereziński, P., & Muszalska-Kolos, I. (2020). Choline Salicylate Analysis: Chemical Stability and Degradation Product Identification. Molecules, 25(1), 51. https://doi.org/10.3390/molecules25010051





