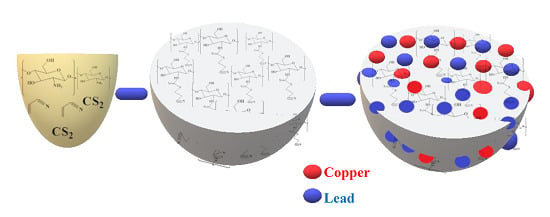
Chitosan-Based Bio-Composite Modified with Thiocarbamate Moiety for Decontamination of Cations from the Aqueous Media
Abstract
1. Introduction
2. Results and Discussion
2.1. Elemental Analysis
2.2. Infrared Spectroscopy
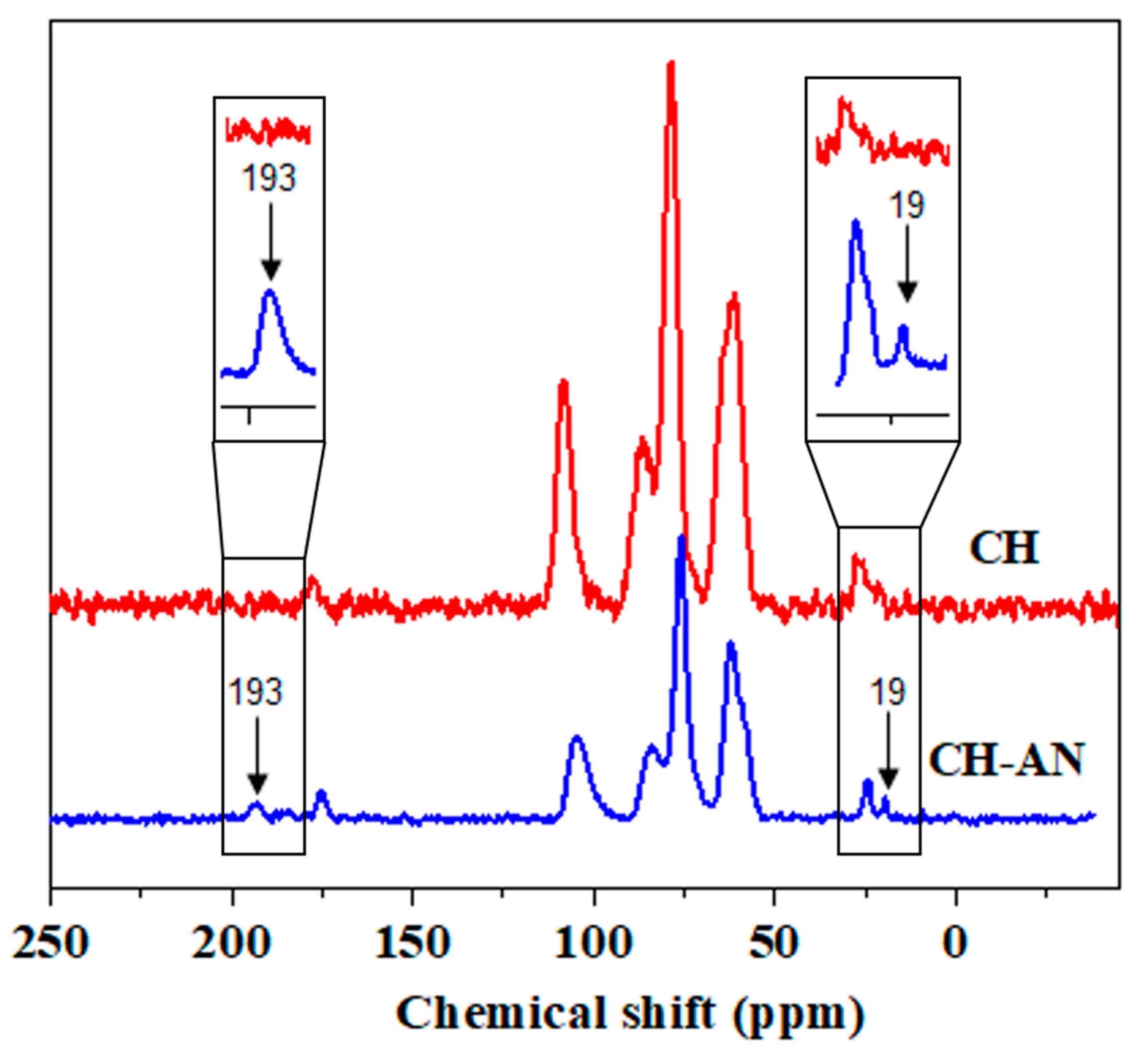
2.3. Nuclear Magnetic Resonance
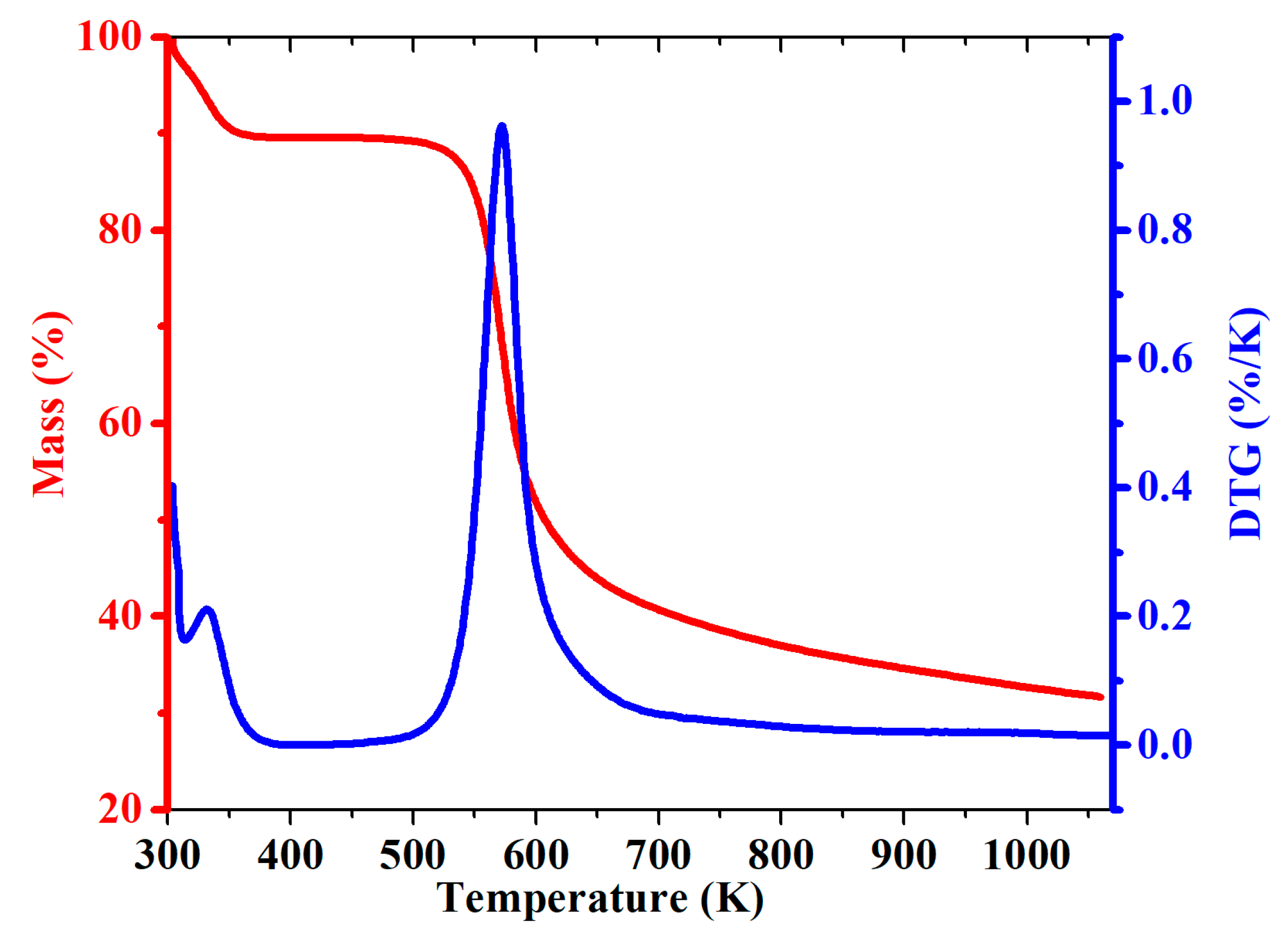
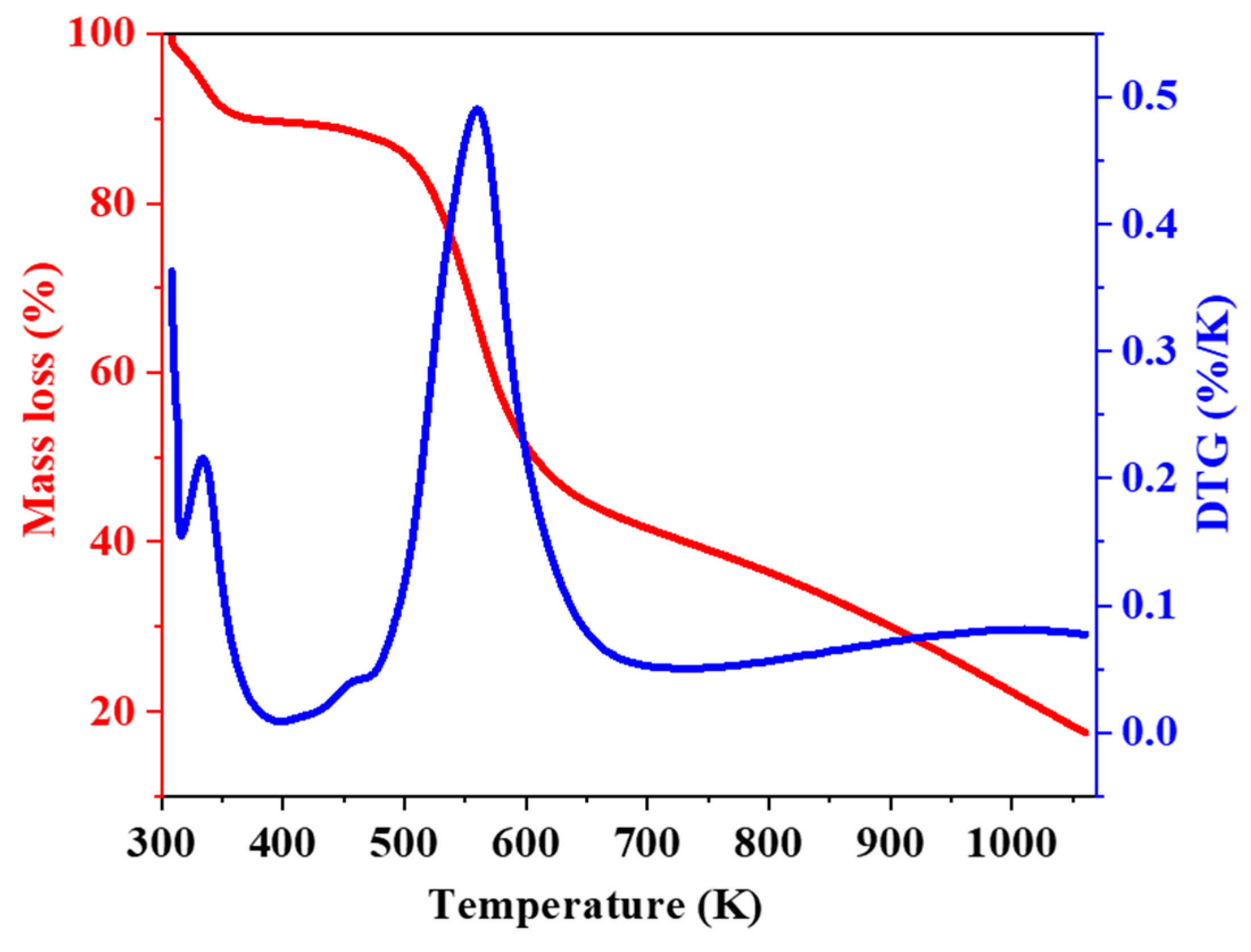
2.4. Thermogravimetry
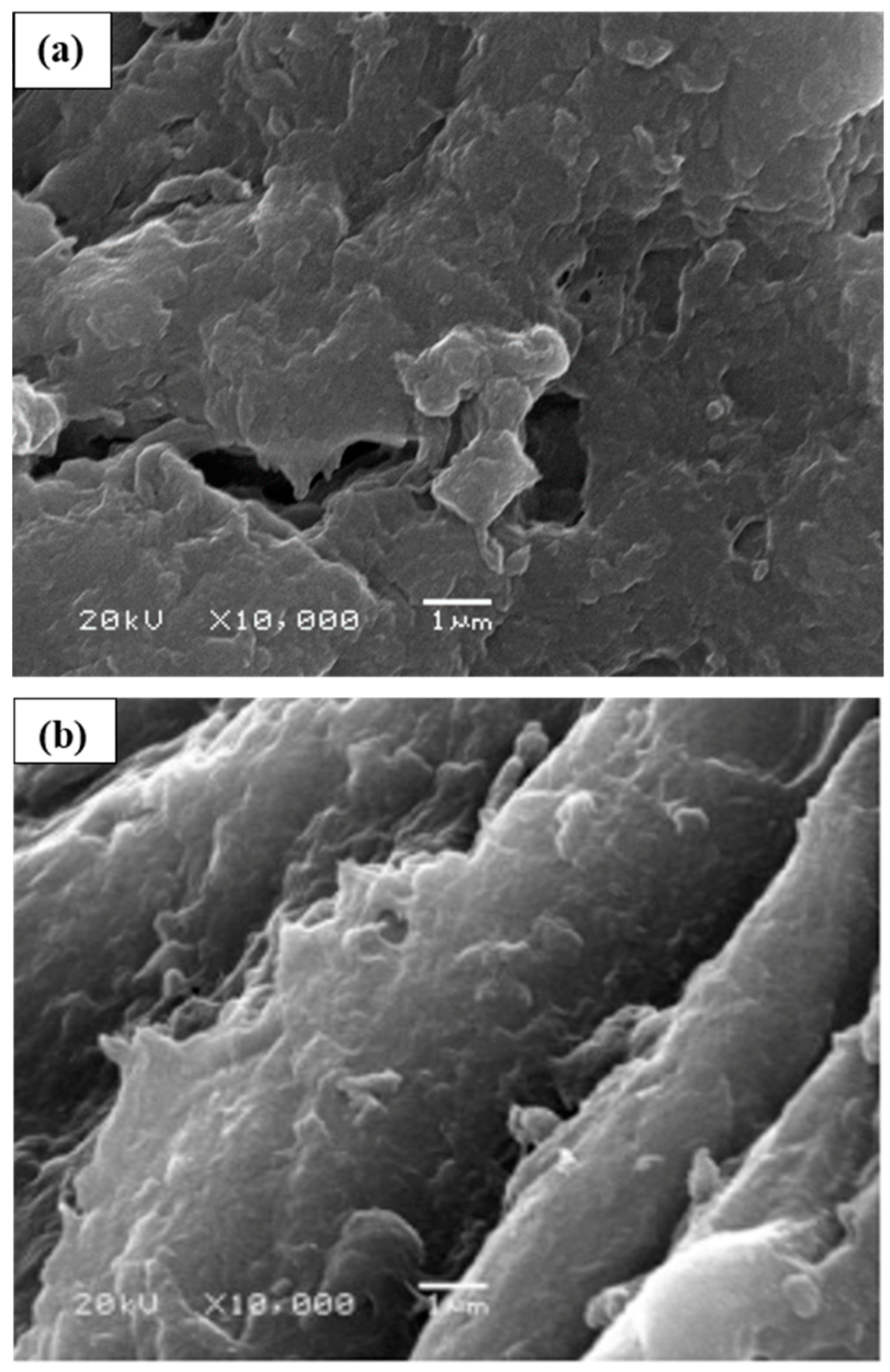
2.5. Scanning Electron Microscopy
2.6. Sorption Studies
3. Materials and Methods
3.1. Chemicals, Reagents, and Materials
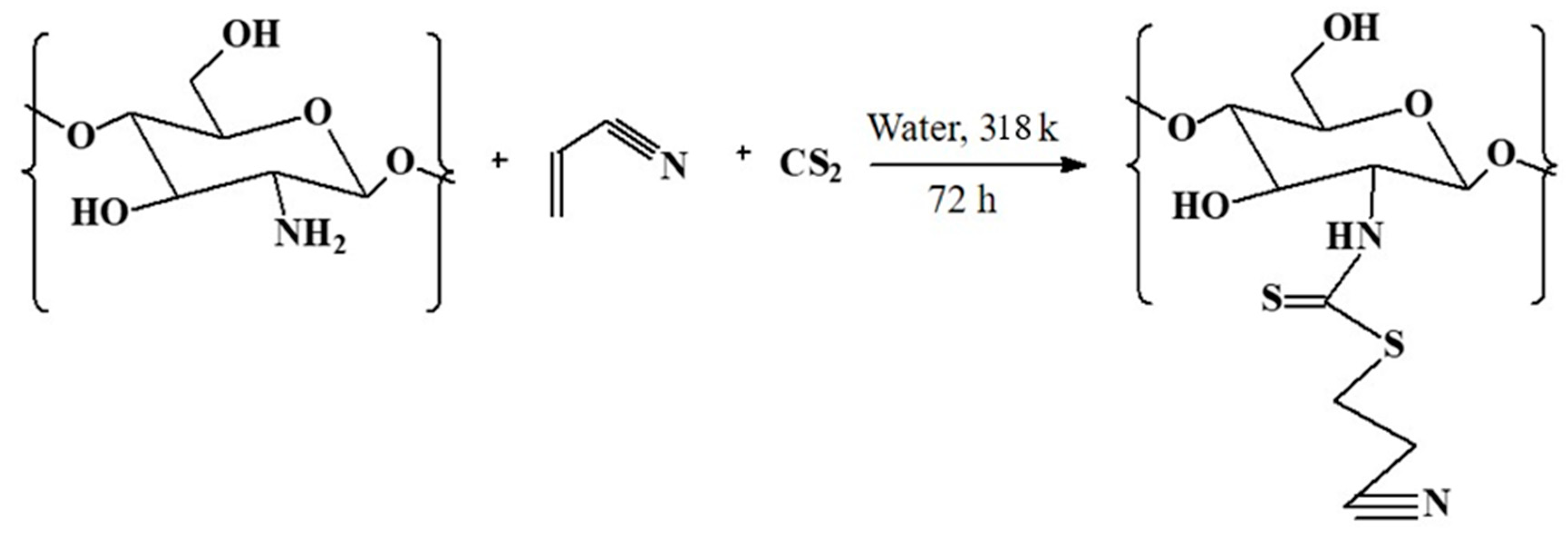
3.2. Synthesis and Modification of CH Bio-Composite
3.3. Characterization of CH-Based Bio-Composites
3.4. Sorption Potential of CH-AN Bio-Composite
3.5. Isotherm Models
3.5.1. Langmuir Isotherm
3.5.2. Freundlich Isotherm
3.5.3. Temkin Isotherm
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gusain, R.; Gupta, K.; Joshi, P.; Khatri, O.P. Adsorptive removal and photocatalytic degradation of organic pollutants using metal oxides and their composites: A comprehensive review. Adv. Colloid Interfaces Sci. 2019, 272, 102009. [Google Scholar] [CrossRef]
- Yang, X.; Wan, Y.; Zheng, Y.; He, F.; Yu, Z.; Huang, J.; Wang, H.; Ok, Y.S.; Jiang, Y.; Gao, B. Surface functional groups of carbon-based adsorbents and their roles in the removal of heavy metals from aqueous solutions: A critical review. Chem. Eng. J. 2019, 366, 608–621. [Google Scholar] [CrossRef]
- Hernandez-Vargas, G.; Sosa-Hernández, J.E.; Saldarriaga-Hernandez, S.; Villalba-Rodríguez, A.M.; Parra-Saldivar, R.; Iqbal, H.M.N. Electrochemical biosensors: A solution to pollution detection with reference to environmental contaminants. Biosensors 2018, 8, 29. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, T.; Nabeel, F.; Adeel, M.; Bilal, M.; Iqbal, H.M.N. “Turn-on” fluorescent sensor-based probing of toxic Hg (II) and Cu (II) with potential intracellular monitoring. Biocatal. Agric. Biotechnol. 2019, 17, 696–701. [Google Scholar] [CrossRef]
- Rasheed, T.; Li, C.; Bilal, M.; Yu, C.; Iqbal, H.M.N. Potentially toxic elements and environmentally-related pollutants recognition using colorimetric and ratiometric fluorescent probes. Sci. Total Environ. 2018, 640, 174–193. [Google Scholar] [CrossRef] [PubMed]
- Cabral Pinto, M.; Ferreira da Silva, E. Heavy Metals of Santiago Island (Cape Verde) alluvial deposits: Baseline value maps and human health risk assessment. Int. J. Environ. Res. Public Health 2019, 16, 2. [Google Scholar] [CrossRef]
- Bilal, M.; Rasheed, T.; Sosa-Hernández, J.E.; Raza, A.; Nabeel, F.; Iqbal, H.M.N. Biosorption: An interplay between marine algae and potentially toxic elements—A review. Mar. Drugs 2018, 16, 65. [Google Scholar] [CrossRef]
- Rasheed, T.; Bilal, M.; Nabeel, F.; Adeel, M.; Iqbal, H.M.N. Environmentally-related contaminants of high concern: Potential sources and analytical modalities for detection, quantification, and treatment. Environ. Int. 2019, 122, 52–66. [Google Scholar] [CrossRef]
- Kavitha, E.; Sowmya, A.; Prabhakar, S.; Jain, P.; Surya, R.; Rajesh, M.P. Removal and recovery of heavy metals through size enhanced ultrafiltration using chitosan derivatives and optimization with response surface modeling. Int. J. Biol. Macromol. 2019, 132, 278–288. [Google Scholar] [CrossRef]
- Tahira, I.; Aslam, Z.; Abbas, A.; Monim-ul-Mehboob, M.; Ali, S.; Asghar, A. Adsorptive removal of acidic dye onto grafted chitosan: A plausible grafting and adsorption mechanism. Int. J. Biol. Macromol. 2019, 136, 1209–1218. [Google Scholar] [CrossRef]
- Rasheed, T.; Nabeel, F.; Bilal, M.; Zhao, Y.; Adeel, M.; Iqbal, H.M.N. Aqueous monitoring of toxic mercury through a rhodamine-based fluorescent sensor. Mathemat. Biosci. Eng. MBE 2019, 16, 1861–1873. [Google Scholar] [CrossRef] [PubMed]
- Sophia, C.A.; Lima, E.C. Removal of emerging contaminants from the environment by adsorption. Ecotox. Environ. Saf. 2018, 150, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Kim, U.J.; Kimura, S.; Wada, M. Highly enhanced adsorption of Congo red onto dialdehyde cellulose cross-linked cellulose-chitosan foam. Carbohydr. Polym. 2019, 214, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Kausar, A.; Iqbal, M.; Javed, A.; Aftab, K.; Nazli, Z.; Bhatti, H.N.; Nouren, S. Dyes adsorption using clay and modified clay: A review. J. Mol. Liq. 2018, 256, 395–407. [Google Scholar] [CrossRef]
- Li, K.; Li, H.; Xiao, T.; Zhang, G.; Long, J.; Luo, D.; Zhang, H.; Xiong, J.; Wang, Q. Removal of thallium from wastewater by a combination of persulfate oxidation and iron coagulation. Process Saf. Environ. Protect. 2018, 119, 340–349. [Google Scholar] [CrossRef]
- Huang, J.; Huang, Z.L.; Zhou, J.X.; Li, C.Z.; Yang, Z.H.; Ruan, M.; Li, H.; Zhang, X.; Wu, Z.J.; Qin, X.L.; et al. Enhancement of heavy metals removal by microbial flocculants produced by Paenibacillus polymyxa combined with an insufficient hydroxide precipitation. Chem. Eng. J. 2019, 374, 880–894. [Google Scholar] [CrossRef]
- Wu, H.; Wang, W.; Huang, Y.; Han, G.; Yang, S.; Su, S.; Sana, H.; Peng, W.; Cao, Y.; Liu, J. Comprehensive evaluation on a prospective precipitation-flotation process for metal-ions removal from wastewater simulants. J. Haz. Mat. 2019, 371, 592–602. [Google Scholar] [CrossRef]
- Nguyen, V.K.; Lee, J. Effect of sulfur concentration on microbial removal of arsenic and heavy metals from mine tailings using mixed culture of Acidithiobacillus spp. J. Geochem. Explor. 2015, 148, 241–248. [Google Scholar] [CrossRef]
- Ou, B.; Wang, J.; Wu, Y.; Zhao, S.; Wang, Z. Efficient removal of Cr (VI) by magnetic and recyclable calcined CoFe-LDH/g-C3N4 via the synergy of adsorption and photocatalysis under visible light. Chem. Eng. J. 2020, 380, 122600. [Google Scholar] [CrossRef]
- Kongarapu, R.J.; Nayak, A.K.; Khobragade, M.U.; Pal, A. Surfactant bilayer on chitosan bead surface for enhanced Ni (II) adsorption. Sustain. Mat. Technol. 2018, 17, e00077. [Google Scholar] [CrossRef]
- Wang, H.; Luo, W.; Tian, Z.; Ouyang, C. First principles study of alkali and alkaline earth metal ions adsorption and diffusion on penta-graphene. Solid State Ion. 2019, 342, 115062. [Google Scholar] [CrossRef]
- Igiri, B.E.; Okoduwa, S.I.R.; Idoko, G.O.; Akabuogu, E.P.; Abraham OAdeyi, A.O.; Ejiogu, I.K. Toxicity and Bioremediation of Heavy Metals Contaminated Ecosystem from Tannery Wastewater: A Review. J. Toxicol. 2018, 2018, 2568038. [Google Scholar] [CrossRef] [PubMed]
- Kanamarlapudi, S.L.R.K.; Chintalpudi, V.K.; Muddada, S. Application of Biosorption for Removal of Heavy Metals from Wastewater. In Biosorption; Intech Open Publisher: London, UK, 2018; pp. 69–116. [Google Scholar]
- Tran, L.T.; Tran, H.V.; Le, T.D.; Bach, G.L.; Tran, L.D. Studying Ni(II) Adsorption of Magnetite/Graphene Oxide/Chitosan Nanocomposite. Adv. Polym. Technol. 2019, 2019, 8124351. [Google Scholar] [CrossRef]
- Zhang, Y.; Xue, Q.; Li, F. Removal of heavy metal ions from wastewater by capacitive deionization using polypyrrole/chitosan composite electrode. Adsorpt. Sci. Technol. 2019, 37, 205–216. [Google Scholar] [CrossRef]
- Khan, A.; Badshah, S.; Airoldi, C. Dithiocarbamated chitosan as a potent biopolymer for toxic cation remediation. Colloids Surf. B Biointerfaces 2011, 87, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Gopinathan, R.; Bhowal, A.; Garlapati, C. Adsorption Studies of Some Anionic Dyes Adsorbed by Chitosan and New Four-Parameter Adsorption Isotherm Model. J. Chem. Eng. Data 2019, 64, 2320–2328. [Google Scholar] [CrossRef]
- Luo, J.; Ritt, C.L.; Liu, X.; Pei, Y.; Crittenden, J.C.; Elimelech, M. Tuning Pb(II) Adsorption from Aqueous Solutions on Ultrathin Iron Oxychloride (FeOCl) Nanosheets. Environ. Sci. Technol. 2019, 53, 2075–2085. [Google Scholar] [CrossRef]
- Lu, F.; Dong, A.; Ding, G.; Xu, K.; Li, J.; You, L. Magnetic porous polymer composite for high performance adsorption of acid red 18 based on melamine resin and chitosan. J. Mol. Liq. 2019, 294, 111515. [Google Scholar] [CrossRef]
- Khan, A.; Badshah, S.; Airoldi, C. Biosorption of some toxic metal ions by chitosan modified with glycidylmethacrylate and diethylenetriamine. Chem. Eng. J. 2011, 171, 159–166. [Google Scholar] [CrossRef]
- Khan, A.; Badshah, S.; Airoldi, C. Environmentally benign modified biodegradable chitosan for cation removal. Polym. Bullet. 2015, 72, 353–370. [Google Scholar] [CrossRef]
- Khan, A.; Shah, S.J.; Mehmood, K.; Ali, N.; Khan, H. Synthesis of potent chitosan beads a suitable alternative for textile dye reduction in sunlight. J. Mat. Sci. Mat. Electron. 2019, 30, 406–414. [Google Scholar] [CrossRef]
- Begue, D.; Qiaon, G.G.; Wentrup, C. Nitrile Imines: Matrix Isolation, IR Spectra, Structures, and Rearrangement to Carbodiimides. J. Am. Chem. Soc. 2012, 134, 5339–5350. [Google Scholar] [CrossRef] [PubMed]
- Sousa, K.S.; Silva Filho, E.C.; Airoldi, C. Ethylenesulfide as a useful agent for incorporation into the biopolymer chitosan in a solvent-free reaction for use in cation removal. Carbohydr. Res. 2009, 344, 1716–1723. [Google Scholar] [CrossRef] [PubMed]
- Xue, D.; Li, T.; Chen, G.; Liu, Y.; Zhang, D.; Guo, Q.; Guo, J.; Yang, Y.; Sun, J.; Su, B.; et al. Sequential Recovery of Heavy and Noble Metals by Mussel-Inspired Polydopamine-Polyethyleneimine Conjugated Polyurethane Composite Bearing Dithiocarbamate Moieties. Polymers 2019, 11, 1125. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, J. Comparison of linearization methods for modeling the Langmuir adsorption isotherm. J. Mol. Liq. 2019, 294, 111850. [Google Scholar] [CrossRef]
- Jiang, X.; An, Q.D.; Xiao, Z.Y.; Zhai, S.R.; Shi, Z. Versatile core/shell-like alginate@polyethylenimine composites for efficient removal of multiple heavy metal ions (Pb2+, Cu2+, CrO42−): Batch and fixed-bed studies. Mat. Res. Bullet. 2019, 118, 110526. [Google Scholar] [CrossRef]
- Gustafsson, J.P.; Akram, M.; Tiberg, C. Predicting sulphate adsorption/desorption in forest soils: Evaluation of an extended Freundlich equation. Chemosphere 2015, 119, 83–89. [Google Scholar] [CrossRef]
- Araújo, C.S.T.; Almeida, I.L.S.; Rezende, H.C.; Marcionilio, S.M.L.O.; Léon, J.J.L.; Matos, T.N.D. Elucidation of mechanism involved in adsorption of Pb(II) onto lobeira fruit (Solanum lycocarpum) using Langmuir, Freundlich and Temkin isotherms. Microchem. J. 2018, 137, 348–354. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds/developed materials are available from the authors. |

| Sample | C/% | N/% | S/% | C/mmolg−1 | N/mmolg−1 | S/mmolg−1 | C/N |
|---|---|---|---|---|---|---|---|
| CH | 40.63 | 7.39 | − | 33.86 | 5.27 | − | 6.42 |
| CH-AN | 38.15 | 7.22 | 3.99 | 31.79 | 5.14 | 1.24 | 6.18 |
| Isotherm | Constant | Type I | Type II | Type III | Type IV |
|---|---|---|---|---|---|
| Cu2+ | Nf (mmol g−1) | 2.02 | 2.02 | 2.02 | 2.02 |
| Ns (mmol g−1) | 2.83 ± 0.02 | 2.82 ± 0.01 | 2.83 ± 0.02 | 2.84 ± 0.01 | |
| b (g mmol−1) | 0.30 ± 0.01 | 0.30 ± 0.01 | 0.30 ± 0.05 | 0.30 ± 0.01 | |
| R2 | 0.998 | 0.999 | 0.997 | 0.997 | |
| SE | 0.019 | 0.019 | 0.019 | 0.019 | |
| χ2 | 0.001 | 0.001 | 0.001 | 0.001 | |
| Pb2+ | Nf (mmol g−1) | 2.54 | 2.54 | 2.54 | 2.54 |
| Ns (mmol g−1) | 2.99 ± 0.03 | 2.99 ± 0.03 | 2.99 ± 0.03 | 2.99 ± 0.03 | |
| b (g mmol−1) | 0.31 ± 0.01 | 0.31 ± 0.01 | 0.31 ± 0.01 | 0.31 ± 0.01 | |
| R2 | 0.998 | 0.998 | 0.998 | 0.998 | |
| SE | 0.032 | 0.032 | 0.032 | 0.032 | |
| χ2 | 0.005 | 0.005 | 0.005 | 0.005 |
| Isotherm | Constant | Cu(II) | Pb(II) |
|---|---|---|---|
| Freundlich | Kf (mmol g−1) | 0.61 ± 0.01 | 0.91 ± 0.02 |
| n | 1.61 ± 0.03 | 2.56 ± 0.02 | |
| R2 | 0.975 | 0.954 | |
| SE | 0.21 | 0.42 | |
| χ2 | 3.55 | 0.928 | |
| Temkin | KT (mmol dm−3) | 3.55 ± 0.03 | 2.96 ± 0.04 |
| b (kJ mol−1) | 1.46 ± 0.02 | 1.64 ± 0.02 | |
| R2 | 0.987 | 0.984 | |
| SE | 0.03 | 0.05 | |
| χ2 | 0.09 | 0.021 |
| Isotherm | Constant | Cu(II) | Pb(II) |
|---|---|---|---|
| Langmuir | Nf (mmol g−1) | 2.02 | 2.54 |
| Ns (mmol g−1) | 2.87 ± 0.03 | 3.02 ± 0.03 | |
| b (g mmol−1) | 0.29 ± 0.01 | 0.30 ± 0.01 | |
| R2 | 0.999 | 0.996 | |
| χ2 | 0.002 | 0.001 | |
| Freundlich | Kf (mmol g−1) | 0.70 ± 0.04 | 0.98 ± 0.06 |
| n | 1.89 ± 0.12 | 2.87 ± 0.22 | |
| R2 | 0.982 | 0.948 | |
| χ2 | 0.007 | 0.017 | |
| Temkin | KT (mmol dm−3) | 3.52 ± 0.32 | 2.96 ± 0.32 |
| b (kJ mol−1) | 1.68 ± 0.06 | 1.50 ± 0.05 | |
| R2 | 0.988 | 0.986 | |
| χ2 | 0.005 | 0.004 |
| Isotherm | Nonlinear Form | Linear Form | Plot |
|---|---|---|---|
| Langmuir type 1 | |||
| Langmuir type 2 | − | ||
| Langmuir type 3 | |||
| Langmuir type 4 | |||
| Freundlich | |||
| Temkin | |||
| Standard Error | |||
| Chi-square |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, N.; Khan, A.; Bilal, M.; Malik, S.; Badshah, S.; Iqbal, H.M.N. Chitosan-Based Bio-Composite Modified with Thiocarbamate Moiety for Decontamination of Cations from the Aqueous Media. Molecules 2020, 25, 226. https://doi.org/10.3390/molecules25010226
Ali N, Khan A, Bilal M, Malik S, Badshah S, Iqbal HMN. Chitosan-Based Bio-Composite Modified with Thiocarbamate Moiety for Decontamination of Cations from the Aqueous Media. Molecules. 2020; 25(1):226. https://doi.org/10.3390/molecules25010226
Chicago/Turabian StyleAli, Nisar, Adnan Khan, Muhammad Bilal, Sumeet Malik, Syed Badshah, and Hafiz M. N. Iqbal. 2020. "Chitosan-Based Bio-Composite Modified with Thiocarbamate Moiety for Decontamination of Cations from the Aqueous Media" Molecules 25, no. 1: 226. https://doi.org/10.3390/molecules25010226
APA StyleAli, N., Khan, A., Bilal, M., Malik, S., Badshah, S., & Iqbal, H. M. N. (2020). Chitosan-Based Bio-Composite Modified with Thiocarbamate Moiety for Decontamination of Cations from the Aqueous Media. Molecules, 25(1), 226. https://doi.org/10.3390/molecules25010226