The Link between Polyphenol Structure, Antioxidant Capacity and Shelf-Life Stability in the Presence of Fructose and Ascorbic Acid
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of Strawberry (Fragaria × ananassa ‘Nerina’) Phenolic Compounds
2.2. The Effect of Temperature on Polyphenol Stability and the Antioxidant Capacity
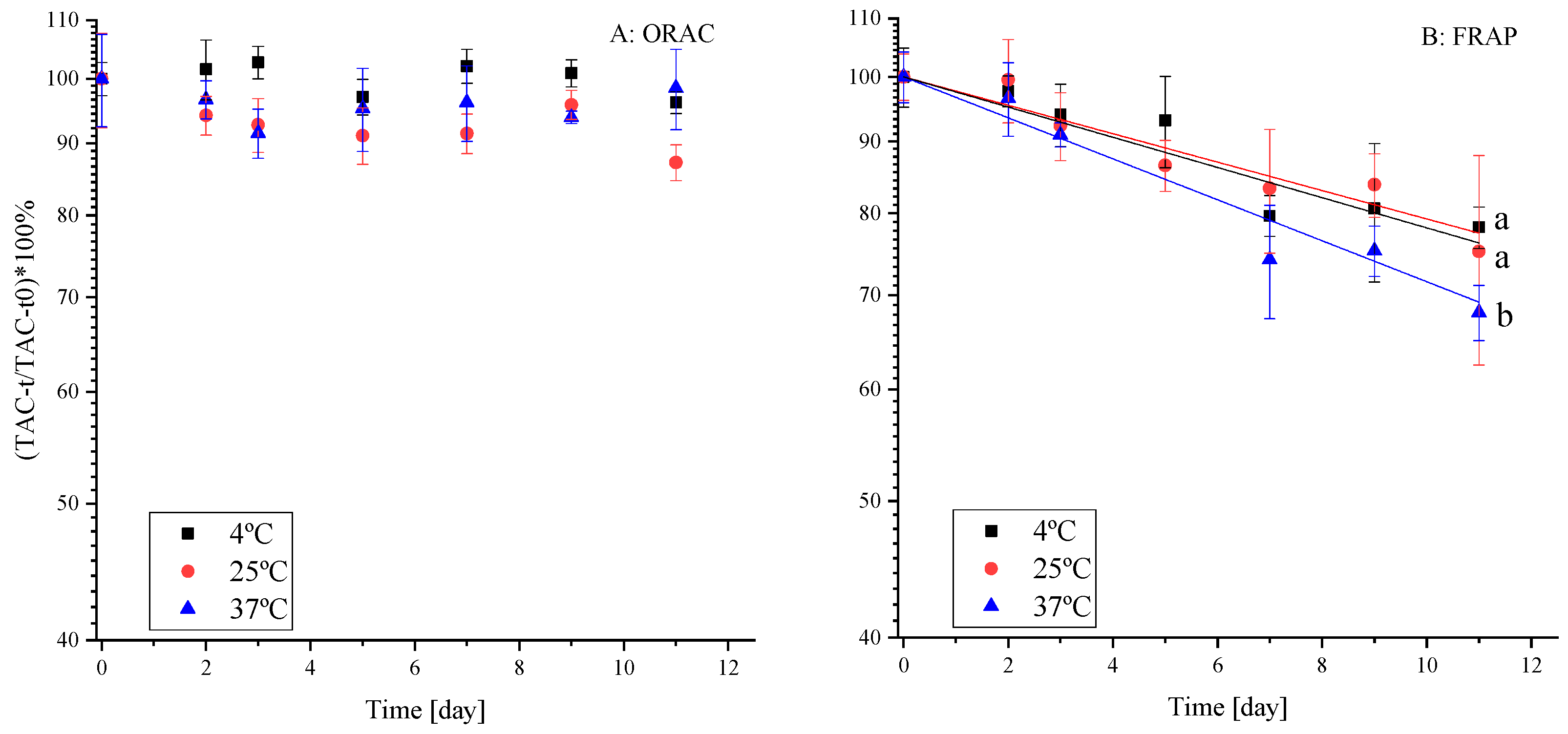
2.2.1. The Effect of Temperature on the Antioxidant Capacity
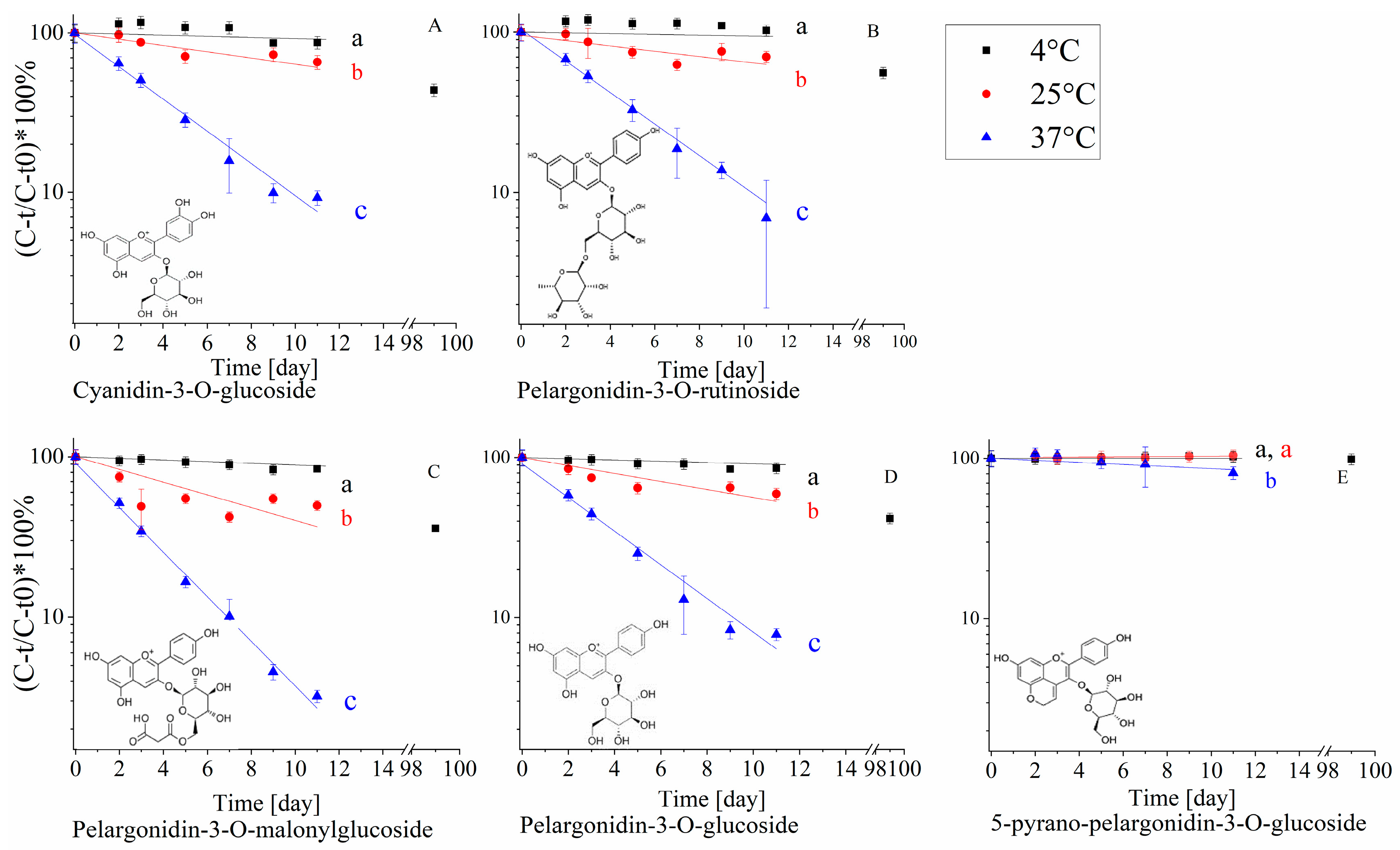
2.2.2. The Effect of Temperature on Polyphenol Stability in the Extract
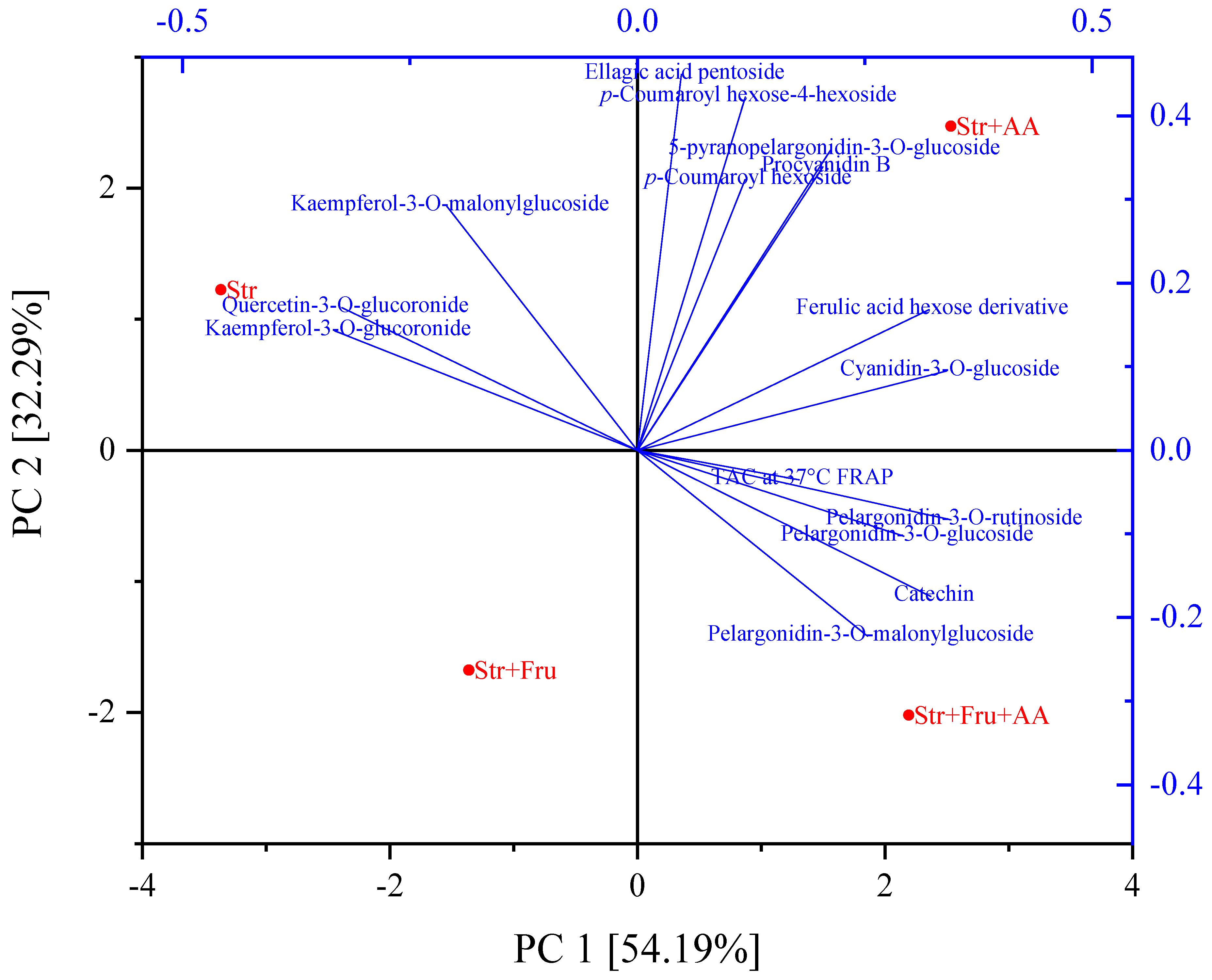
2.3. The Impact of Prevalent Food Components (Sugar and Ascorbic Acid) on Polyphenol Stability and Antioxidant Capacity
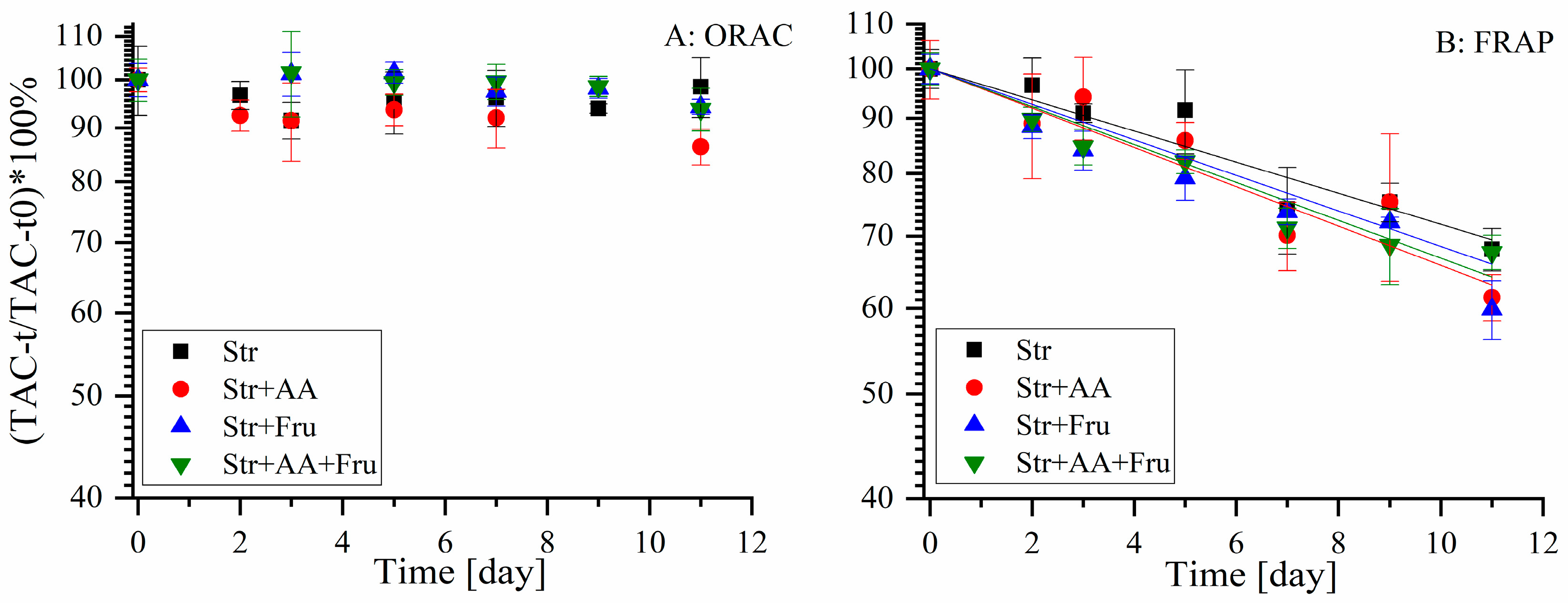
2.3.1. The Effect of Formulation with Fructose and Ascorbic Acid on the Antioxidant Capacity
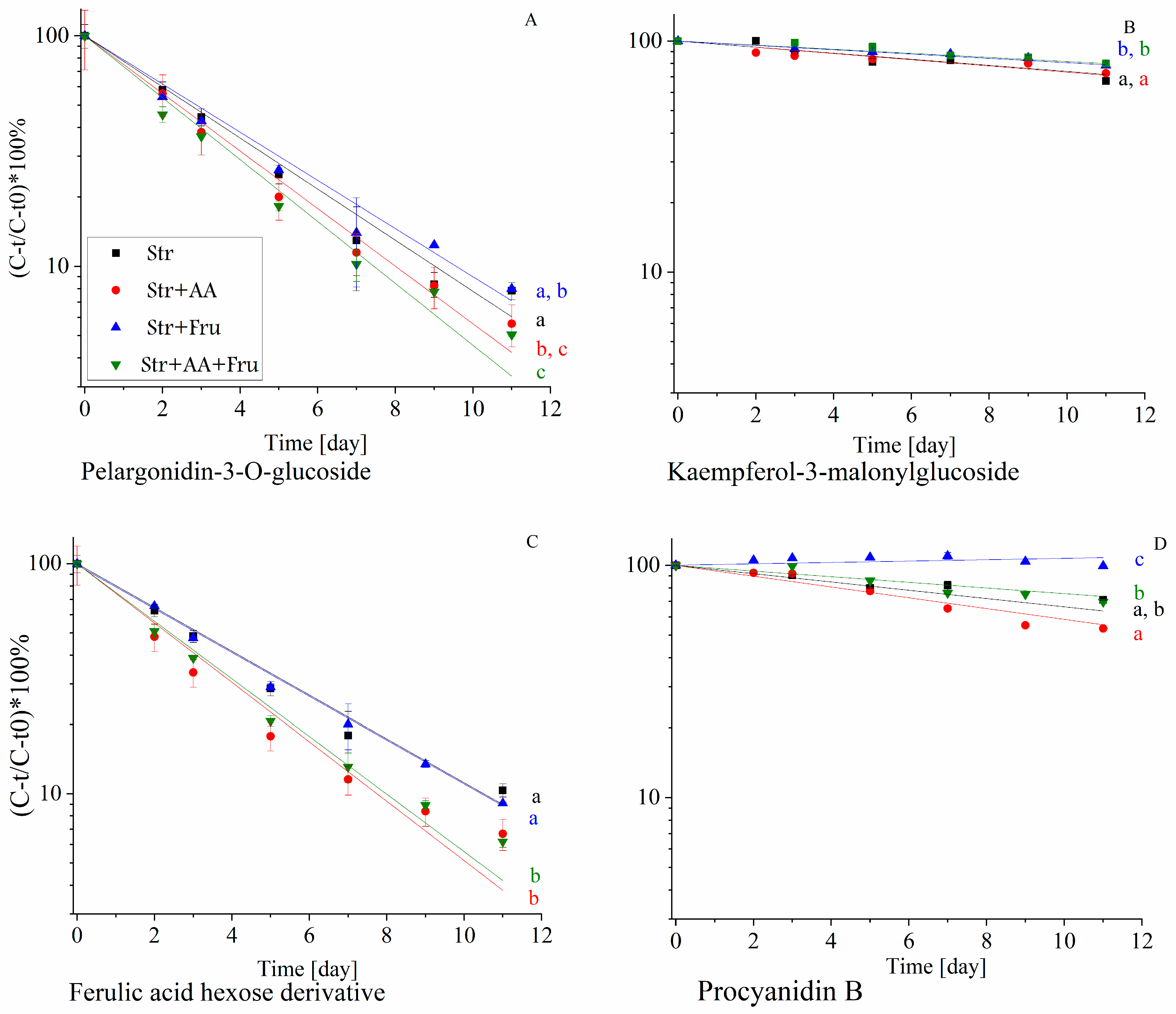
2.3.2. The Effect of Formulation with Fructose and Ascorbic Acid on Polyphenol Stability
2.4. Comparison of Multi-Component (Polyphenols Extract) and Mono-Component (Purified Commercial) Model System
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Strawberry Phenolic Compounds Extraction and Quantification
3.2.2. Characterization of Phenolic Compounds in the Extract
3.2.3. Antioxidant Capacity Measurement—ORAC
3.2.4. Antioxidant Capacity Measurement—FRAP
3.2.5. Shelf-Life Experiment: Sample Preparation
3.2.6. Sample Preparation for Co-Pigmentation during Shelf-Life Experiment
3.2.7. Enzymatic Activity in the Polyphenol Extract
Polyphenol Oxidase Activity
Peroxidase Activity
3.2.8. Determination of Metals Concentrations by Inductively Coupled Plasma Optical Emission Spectrometry (ICP)
3.2.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef] [PubMed]
- Rasouli, H.; Farzaei, M.H.; Khodarahmi, R. Polyphenols and their benefits: A review. Int. J. Food Prop. 2017, 20, 1700–1741. [Google Scholar] [CrossRef]
- Bravo, L. Polyphenols: Chemistry, Dietary Sources, Metabolism, and Nutritional Significance. Nutr. Rev. 2009, 56, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Cheynier, V.; Dueñas-Paton, M.; Salas, E.; Maury, C.; Souquet, J.-M.; Sarni-Manchado, P.; Fulcrand, H. Structure and Properties of Wine Pigments and Tannins. Am. J. Enol. Viticult. 2006, 57, 298–305. [Google Scholar]
- Buvé, C.; Kebede, B.T.; De Batselier, C.; Carrillo, C.; Pham, H.T.T.; Hendrickx, M.; Grauwet, T.; Van Loey, A. Kinetics of colour changes in pasteurised strawberry juice during storage. J. Food Eng. 2018, 216, 42–51. [Google Scholar] [CrossRef]
- Iacobucci, G.A.; Sweeny, J.G. The chemistry of anthocyanins, anthocyanidins and related flavylium salts. Tetrahedron 1983, 39, 3005–3038. [Google Scholar] [CrossRef]
- Ioannou, I.; Hafsa, I.; Hamdi, S.; Charbonnel, C.; Ghoul, M. Review of the effects of food processing and formulation on flavonol and anthocyanin behaviour. J. Food Eng. 2012, 111, 208–217. [Google Scholar] [CrossRef]
- Boussetta, N.; Vorobiev, E.; Deloison, V.; Pochez, F.; Falcimaigne-Cordin, A.; Lanoisellé, J.-L. Valorisation of grape pomace by the extraction of phenolic antioxidants: Application of high voltage electrical discharges. Food Chem. 2011, 128, 364–370. [Google Scholar] [CrossRef]
- Halake, K.; Birajdar, M.; Lee, J. Structural implications of polyphenolic antioxidants. J. Ind. Eng. Chem. 2016, 35, 1–7. [Google Scholar] [CrossRef]
- Levy, R.; Okun, Z.; Shpigelman, A. The influence of chemical structure and the presence of ascorbic acid on anthocyanins stability and spectral properties in purified model systems. Foods 2019, 8, 207. [Google Scholar] [CrossRef] [PubMed]
- Odriozola-Serrano, I.; Soliva-Fortuny, R.; Martín-Belloso, O. Phenolic acids, flavonoids, vitamin C and antioxidant capacity of strawberry juices processed by high-intensity pulsed electric fields or heat treatments. Eur. Food Res. Technol. 2008, 228, 239–248. [Google Scholar] [CrossRef]
- Banaszewski, K.; Edirisinghe, I.; Burton-Freeman, B. Detection and Quantitation of Polyphenolic Compounds in Strawberry Powder Using LC-MS/MS. Available online: https://www.researchgate.net/profile/Indika_Edirisinghe/publication/266573278_Detection_and_Quantitation_of_Polyphenolic_Compounds_in_Strawberry_Powder_Using_LC-MSMS/links/54cc55d00cf24601c08a75d2/Detection-and-Quantitation-of-Polyphenolic-Compounds-in-Strawberry-Powder-Using-LC-MS-MS.pdf (accessed on 6 January 2020).
- Kaur, H.; Kaur, G. A Critical Appraisal of Solubility Enhancement Techniques of Polyphenols. J. Pharm. 2014, 2014, 1–14. [Google Scholar] [CrossRef]
- Oliveira, A.; Almeida, D.P.F.; Pintado, M. Changes in Phenolic Compounds During Storage of Pasteurized Strawberry. Food Bioprocess Technol. 2014, 7, 1840–1846. [Google Scholar] [CrossRef]
- Garzon, G.A.; Wrolstad, R.E. Comparison of the Stability of Pelargonidin-based Anthocyanins in Strawberry Juice and Concentrate. J. Food Sci. 2002, 67, 1288–1299. [Google Scholar] [CrossRef]
- Zoric, Z.; Pedisic, S.; Elez Garofulic, I. Kinetics of the degradation of anthocyanins, phenolic acids and flavonols during heat treatments of freeze-dried sour cherry Marasca paste. Food Technol. Biotechnol. 2014, 52, 101–108. [Google Scholar]
- Teleszko, M.; Nowicka, P.; Wojdyło, A. Effect of cultivar and storage temperature on identification and stability of polyphenols in strawberry cloudy juices. J. Food Compos. Anal. 2016, 54, 10–19. [Google Scholar] [CrossRef]
- Kopjar, M.; Tiban, N.N.; Pilizota, V.; Babic, J. Stability of anthocyanins, phenols and free radical scavenging activity througgh sugar addition during frozen storage of blackberries. J. Food Process. Preserv. 2009, 33, 1–11. [Google Scholar] [CrossRef]
- Pérez-Ramírez, I.F.; Castaño-Tostado, E.; Ramírez-de León, J.A.; Rocha-Guzmán, N.E.; Reynoso-Camacho, R. Effect of stevia and citric acid on the stability of phenolic compounds and in vitro antioxidant and antidiabetic capacity of a roselle (Hibiscus sabdariffa L.) beverage. Food Chem. 2015, 172, 885–892. [Google Scholar] [CrossRef]
- Oszmiański, J.; Wojdyło, A.; Kolniak, J. Effect of L-ascorbic acid, sugar, pectin and freeze–thaw treatment on polyphenol content of frozen strawberries. LWT Food Sci. Technol. 2009, 42, 581–586. [Google Scholar] [CrossRef]
- Shkolnikov, H.; Belochvostov, V.; Okun, Z.; Shpigelman, A. The effect of pressure on the kinetics of polyphenolics degradation—Implications to hyperbaric storage using Epigallocatechin-gallate as a model. Innov. Food Sci. Emerg. Technol. 2019, 59, 102273. [Google Scholar] [CrossRef]
- Shpigelman, A.; Zisapel, A.; Cohen, Y.; Livney, Y.D. Mechanisms of saccharide protection against epigallocatechin-3-gallate deterioration in aqueous solutions. Food Chem. 2013, 139, 1105–1112. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-Y.; Zhu, Q.Y.; Wong, Y.F.; Zhang, Z.; Chung, H.Y. Stabilizing Effect of Ascorbic Acid on Green Tea Catechins. J. Agric. Food Chem. 1998, 46, 2512–2516. [Google Scholar] [CrossRef]
- Poei-Langston, M.S.; Wrolstad, R.E. Color Degradation in an ascorbic acid-anthocyanin-flavanol model system. J. Food Sci. 1981, 46, 1218–1236. [Google Scholar] [CrossRef]
- Özkan, M. Degradation of anthocyanins in sour cherry and pomegranate juices by hydrogen peroxide in the presence of added ascorbic acid. Food Chem. 2002, 78, 499–504. [Google Scholar] [CrossRef]
- Cavalcanti, R.N.; Santos, D.T.; Meireles, M.A.A. Non-thermal stabilization mechanisms of anthocyanins in model and food systems—An overview. Food Res. Int. 2011, 44, 499–509. [Google Scholar] [CrossRef]
- Dangles, O.; Brouillard, R. Polyphenol interactions. The copigmentation case: Thermodynamic data from temperature variation and relaxation kinetics. Medium effect. Can. J. Chem. 1992, 70, 2174–2189. [Google Scholar] [CrossRef]
- Törrönen, R.; Sarkkinen, E.; Tapola, N.; Hautaniemi, E.; Kilpi, K.; Niskanen, L. Berries modify the postprandial plasma glucose response to sucrose in healthy subjects. Br. J. Nutr. 2009, 103, 1. [Google Scholar] [CrossRef] [PubMed]
- Cook, N.C.; Samman, S. Flavonoids—Chemistry, metabolism, cardioprotective effects, and dietary sources. J. Nutr. Biochem. 1996, 7, 66–76. [Google Scholar] [CrossRef]
- Yao, L.H.; Jiang, Y.M.; Shi, J.; Tomas-Barberan, F.A.; Datta, N.; Singanusong, R.; Chen, S.S. Flavonoids in food and their health benefits. Plant Foods Hum. Nutr. 2004, 59, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Weinreb, O.; Mandel, S.; Amit, T.; Youdim, M.B.H. Neurological mechanisms of green tea polyphenols in Alzheimer’s and Parkinson’s diseases. J. Nutr. Biochem. 2004, 15, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Forbes-Hernandez, T.Y.; Gasparrini, M.; Afrin, S.; Bompadre, S.; Mezzetti, B.; Quiles, J.L.; Giampieri, F.; Battino, M. The Healthy Effects of Strawberry Polyphenols: Which Strategy behind Antioxidant Capacity? Crit. Rev. Food Sci. Nutr. 2016, 56, S46–S59. [Google Scholar] [CrossRef] [PubMed]
- Anyangwe, A.K. Nerina Strawberry: Recreating the Ancient Wild Strawberry; A Strawberry Cultivar Designed to Maximize Polyphenol Content and Anti-Oxidant Properties. Available online: https://vdocuments.site/nerina-strawberry-recreating-the-ancient-wild-iprona-ag-embarked-on-a-project.html (accessed on 6 January 2020).
- Terefe, N.S.; Yang, Y.H.; Knoerzer, K.; Buckow, R.; Versteeg, C. High pressure and thermal inactivation kinetics of polyphenol oxidase and peroxidase in strawberry puree. Innov. Food Sci. Emerg. Technol. 2010, 11, 52–60. [Google Scholar] [CrossRef]
- Quaranta, H.O.; Eterović, J.E.; Piccini, J.L. Essential elements in fresh and irradiated strawberries and strawberry marmalade. Int. J. Radiat. Appl. Instrum. 1986, 37, 633–634. [Google Scholar] [CrossRef]
- Sun, J.; Liu, X.; Yang, T.; Slovin, J.; Chen, P. Profiling polyphenols of two diploid strawberry (Fragaria vesca) inbred lines using UHPLC-HRMS. Food Chem. 2014, 146, 289–298. [Google Scholar] [CrossRef]
- Aaby, K.; Ekeberg, D.; Skrede, G. Characterization of Phenolic Compounds in Strawberry (Fragaria × ananassa) Fruits by Different HPLC Detectors and Contribution of Individual Compounds to Total Antioxidant Capacity. J. Agric. Food Chem. 2007, 55, 4395–4406. [Google Scholar] [CrossRef]
- Dudonnédudonn, S.; Vitrac, X.; Couti, P.; Woillez, M.; de Bélis, R.; Sen, L.; des Substances Végétales, E.; Biologique, A. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef]
- Munialo, C.D.; Naumovski, N.; Sergi, D.; Stewart, D.; Mellor, D.D. Critical evaluation of the extrapolation of data relative to antioxidant function from the laboratory and their implications on food production and human health: A review. Int. J. Food Sci. Technol. 2019, 54, 1448–1459. [Google Scholar] [CrossRef]
- Torres, B.; Tiwari, B.K.; Patras, A.; Cullen, P.J.; Brunton, N.; O’Donnell, C.P. Stability of anthocyanins and ascorbic acid of high pressure processed blood orange juice during storage. Innov. Food Sci. Emerg. Technol. 2011, 12, 93–97. [Google Scholar] [CrossRef]
- Ou, B.; Huang, D.; Hampsch-Woodill, M.; Flanagan, J.A.; Deemer, E.K. Analysis of antioxidant activities of common vegetables employing oxygen radical absorbance capacity (ORAC) and ferric reducing antioxidant power (FRAP) assays: A comparative study. J. Agric. Food Chem. 2002, 50, 3122–3128. [Google Scholar] [CrossRef]
- Chaovanalikit, A.; Wrolstad, R.E. Total anthocyanins and total phenolics of fresh and processed cherries and their antioxidant properties. J. Food Sci. 2004, 69, 67–72. [Google Scholar] [CrossRef]
- Fischer, U.A.; Carle, R.; Kammerer, D.R. Thermal stability of anthocyanins and colourless phenolics in pomegranate (Punica granatum L.) juices and model solutions. Food Chem. 2013, 138, 1800–1809. [Google Scholar] [CrossRef] [PubMed]
- Piljac-Žegarac, J.; Šamec, D. Antioxidant stability of small fruits in postharvest storage at room and refrigerator temperatures. Food Res. Int. 2011, 44, 345–350. [Google Scholar] [CrossRef]
- Kyi, T.M.; Daud, W.R.W.; Mohammad, A.B.; Wahid Samsudin, M.; Kadhum, A.A.H.; Talib, M.Z.M. The kinetics of polyphenol degradation during the drying of Malaysian cocoa beans. Int. J. Food Sci. Technol. 2005, 40, 323–331. [Google Scholar] [CrossRef]
- Patras, A.; Brunton, N.; O’Donnell, C.; Tiwari, B.K. Effect of thermal processing on anthocyanin stability in foods; mechanisms and kinetics of degradation. Trends Food Sci. Technol. 2010, 21, 3–11. [Google Scholar] [CrossRef]
- Rein, M. Copigmentation Reactions and Color Stability of Berry Anthocyanins. Available online: https://pdfs.semanticscholar.org/39b5/0e7a118ade7e5553f50e243443b18f6aa728.pdf (accessed on 6 January 2020).
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef]
- Wang, S.Y.; Lewers, K.S. Antioxidant Capacity and flavonoid content in wild strawberries. Am. Soc. Hortic. Sci. 2007, 132, 629–637. [Google Scholar] [CrossRef]
- Cao, S.; Liu, L.; Pan, S.; Lu, Q.I.; Xu, X. A comparison of two determination methods for studying degradation kinetics of the major anthocyanins from blood orange. J. Agric. Food Chem. 2009, 57, 245–249. [Google Scholar] [CrossRef]
- Rösch, D.; Bergmann, M.; Knorr, D.; Kroh, L.W. Structure−Antioxidant Efficiency Relationships of Phenolic Compounds and Their Contribution to the Antioxidant Activity of Sea Buckthorn Juice. J. Agric. Food Chem. 2003, 51, 4233–4239. [Google Scholar] [CrossRef]
- Aucamp, J.P.; Hara, Y.; Apostolides, Z. Simultaneous analysis of tea catechins, caffeine, gallic acid, theanine and ascorbic acid by micellar electrokinetic capillary chromatography. J. Chromatogr. A 2000, 876, 235–242. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Fleschhut, J.; Kratzer, F.; Rechkemmer, G.; Kulling, S.E. Stability and biotransformation of various dietary anthocyanins in vitro. Eur. J. Nutr. 2005, 45, 7–8. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.Y.; Hammerstone, J.F.; Lazarus, S.A.; Schmitz, H.H.; Keen, C.L. Stabilizing effect of ascorbic acid on flavan-3-ols and dimeric procyanidins from cocoa. J. Agric. Food Chem. 2003, 51, 828–833. [Google Scholar] [CrossRef]
- Maini, S.; Hodgson, H.L.; Krol, E.S. The UVA and Aqueous Stability of Flavonoids Is Dependent on B-Ring Substitution. J. Agric. Food Chem. 2012, 60, 6966–6976. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, M.; Murakami, K. Interaction of iron with polyphenolic compounds: Application to antioxidant characterization. Anal. Biochem. 1998, 257, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Eiro, M.J.; Heinonen, M. Anthocyanin color behavior and stability during storage: Effect of intermolecular copigmentation. J. Agric. Food Chem. 2002, 50, 7461–7466. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Ohara, N.; Tsukui, A. Stability of anthocyanins in various vegetables and fruits. Food Sci. Technol. Int. Tokyo 1996, 2, 30–33. [Google Scholar] [CrossRef][Green Version]
- Akagawa, M.; Shigemitsu, T.; Suyama, K. Production of hydrogen peroxide by polyphenols and polyphenols-rich beverages under quasi-physiological conditions. Biosci. Biotechnol. Biochem. 2003, 67, 2632–2640. [Google Scholar] [CrossRef]
- Anesini, C.; Ferraro, G.E.; Filip, R. Total Polyphenol Content and Antioxidant Capacity of Commercially Available Tea (Camellia sinensis) in Argentina. J. Agric. Food Chem. 2008, 56, 9225–9229. [Google Scholar] [CrossRef]
- Cano, M.P.; Hernandez, A.; Ancos, B. High Pressure and temperature effects on enzyme inactivation in strawberry and orange products. J. Food Sci. 1997, 62, 85–88. [Google Scholar] [CrossRef]
- Segovia-Bravo, K.A.; Guignon, B.; Bermejo-Prada, A.; Sanz, P.D.; Otero, L. Hyperbaric storage at room temperature for food preservation: A study in strawberry juice. Innov. Food Sci. Emerg. Technol. 2012, 15, 14–22. [Google Scholar] [CrossRef]
- Dyrby, M.; Westergaard, N.; Stapelfeldt, H. Light and heat sensitivity of red cabbage extract in soft drink model systems. Food Chem. 2001, 72, 431–437. [Google Scholar] [CrossRef]
- Moser, P.; Nicoletti Telis, V.R.; de Andrade Neves, N.; García-Romero, E.; Gomez-Aonso, S.; Hermosin-Gutierrez, I. Storage stability of phenolic compounds in powdered BRS Violeta grape juice microencapsulated with protein and maltodextrin blends. Food Chem. 2017, 214, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Kahn, V. Polyphenol oxidase activity and browning of three avocado varieties. J. Sci. Food Agric. 1975, 26, 1319–1324. [Google Scholar] [CrossRef]
- Civello, P.M.; Martinez, G.A.; Chves, A.R.; Anon, M.C. Peroxidase from Strawberry Fruit (Fragaria ananassa Duch.): Partial Purification and Determination of Some Properties. J. Agric. Food Chem. 1995, 43, 2596–2601. [Google Scholar] [CrossRef]
Sample Availability: Not available. |

| Peak | Compound Name | Retention Time [min] | Wavelength at λmax [nm] | Ion Mass [m/z] b,d |
|---|---|---|---|---|
| 1 | Ascorbic acid | 2.74 | 280 | 175, 115 [M − H]− |
| 2 | Citric acid | 3.11 | 280 | 191 [M − H]− |
| 3 | Gallotannin | 6.05 | 360 | 629, 601, 431, 287 [M − H]− |
| 4 | Hydroxybenzoyl-hexose | 7.93 | 280 | 299, 179, 137 [M − H]− |
| 5 | Hexosylhexose | 12.61 | 360 | 341, 179 [M − H]− |
| 6 | Procyanidin B a | 15.08 | 280 | 577, 425 [M − H]− |
| 7 | Catechin c | 17.56 | 280 | 289, 245, 203 [M − H]− |
| 8 | p-Coumaroyl hexose-4-hexoside | 18.26 | 360 | 487, 325, 163,145 [M − H]− 349 [M + Na]+ |
| 9 | p-Coumaroyl hexoside | 20.51 | 360 | 325, 265,145, 119 [M − H]− 675 [M + Na]+ |
| 10 | Cyanidin-3-O-glucoside | 27.43 | 520 | 449, 287 [M]+ |
| 11 | Pelargonidin-3-O-glucoside | 31.74 | 520 | 433, 271 [M]+ |
| 12 | Ferulic acid hexose derivative | 46.94 | 360 | 449, 431, 355, 329, 269, 193 [M − H]− 311, 293 [M + Na]+ |
| 13 | Pelargonidin-3-O-rutinoside | 36.52 | 520 | 579, 433, 271 [M]+ |
| 14 | 5-Pyranopelargonidin-3-O-glucoside | 38.84 | 520 | 501, 339,295 [M]+ |
| 15 | Galloyl-glucose | 41.08 | 280 | 331, 271 [M − H]− |
| 16 | Ellagic acid pentoside | 48.30 | 360 | 433, 185, 229, 301 [M − H]− |
| 17 | Quercetin-3-O-glucuronide c | 54.28 | 360 | 477, 301, 179, 151 [M − H]− 303 [M + H]+ |
| 18 | Pelargonidin-3-O-malonylglucoside | 54.95 | 520 | 519, 433, 271 [M]+ |
| 19 | Kaempferol-3-O-coumaroyl glucoside c | 58.75 | 360 | 593, 447, 285 [M − H]− 287 [M + H]+ |
| 20 | Kaempferol-3-O-glucuronide c | 58.97 | 360 | 461, 285 [M − H]− |
| 21 | Kaempferol-3-O-malonylglucoside | 60.98 | 360 | 533, 489, 285 [M − H]− |
| k [1/day] | Ea [kJ/mol] | ||||
|---|---|---|---|---|---|
| Storage Temperature | 4 °C | 25 °C | 37 °C | ||
| Compound Name | |||||
| Pelargonidin-3-O-glucoside | 0.0089 ± 0.0007 a, A | 0.05 ± 0.01 b, A | 0.274 ± 0.007 c, A | 71.5 ± 14.3 A | |
| Pelargonidin-3-O-rutinoside | 0.007 ± 0.001 a, B | 0.034 ± 0.007 b, B | 0.21 ± 0.01 c, B | 70.3 ± 17.0 A | |
| Cyanidin-3-O-glucoside | 0.010 ± 0.003 a, AB | 0.040 ± 0.008 b, A | 0.250 ± 0.005 c, AB | 66.7 ± 17.9 A | |
| 5-Pyranopelargonidin-3-O-glucoside | 0.0003 ± 0.00011a, C | 0.002 ± 0.009 a, C | 0.015 ± 0.004 b, C | 105.2 ± 8.8 B | |
| Pelargonidin-3-O-malonylglucoside | 0.0105 ± 0.0009 a, D | 0.07 ± 0.02 b, A | 0.35 ± 0.01 c, D | 73.6 ± 11.2 A | |
| Polyphenol | Strawberry Extract (k [1/day]) | Purified (k [1/day]) |
|---|---|---|
| Pelargonidin-3-O-glucoside | 0.27 ± 0.01 A | 0.0091 ± 0.0006 A |
| Cyanidin-3-O-glucoside | 0.25 ± 0.02 A, B | 0.019 ± 0.004 B |
| Pelargonidin-3-O-rutinoside | 0.22± 0.01 B | 0.009 ± 0.001 A |
| Kaempferol-3-O-glucuronide | 0.030 ± 0.008 C | 0.0015 ± 0.0005 C |
| Quercetin-3-O-glucuronide | 0.04 ± 0.02 C | 0.008 ± 0.001 A,B |
| Sample | k [1/day] |
|---|---|
| P3g in strawberry extract | 0.4 ± 0.2 A |
| P3g in purified system | 0.010 ± 0.001 B |
| Ferulic acid | 0.004 ± 0.002 C |
| P3g in P3G:ferulic acid 1:1 | 0.022 ± 0.002 D |
| P3g in p3g:ferulic acid 1:10 | 0.061 ± 0.003 E |
| Ferulic acid in p3g:ferulic acid 1:1 | 0.004 ±0.002 C |
| Ferulic acid in p3g:ferulic acid 1:10 | 0.009 ± 0.001 F |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanuka Katz, I.; Eran Nagar, E.; Okun, Z.; Shpigelman, A. The Link between Polyphenol Structure, Antioxidant Capacity and Shelf-Life Stability in the Presence of Fructose and Ascorbic Acid. Molecules 2020, 25, 225. https://doi.org/10.3390/molecules25010225
Hanuka Katz I, Eran Nagar E, Okun Z, Shpigelman A. The Link between Polyphenol Structure, Antioxidant Capacity and Shelf-Life Stability in the Presence of Fructose and Ascorbic Acid. Molecules. 2020; 25(1):225. https://doi.org/10.3390/molecules25010225
Chicago/Turabian StyleHanuka Katz, Inbal, Eden Eran Nagar, Zoya Okun, and Avi Shpigelman. 2020. "The Link between Polyphenol Structure, Antioxidant Capacity and Shelf-Life Stability in the Presence of Fructose and Ascorbic Acid" Molecules 25, no. 1: 225. https://doi.org/10.3390/molecules25010225
APA StyleHanuka Katz, I., Eran Nagar, E., Okun, Z., & Shpigelman, A. (2020). The Link between Polyphenol Structure, Antioxidant Capacity and Shelf-Life Stability in the Presence of Fructose and Ascorbic Acid. Molecules, 25(1), 225. https://doi.org/10.3390/molecules25010225






