Synthesis and Application of 1,2-Aminoalcohols with Neoisopulegol-Based Octahydrobenzofuran Core
Abstract
1. Introduction
2. Results
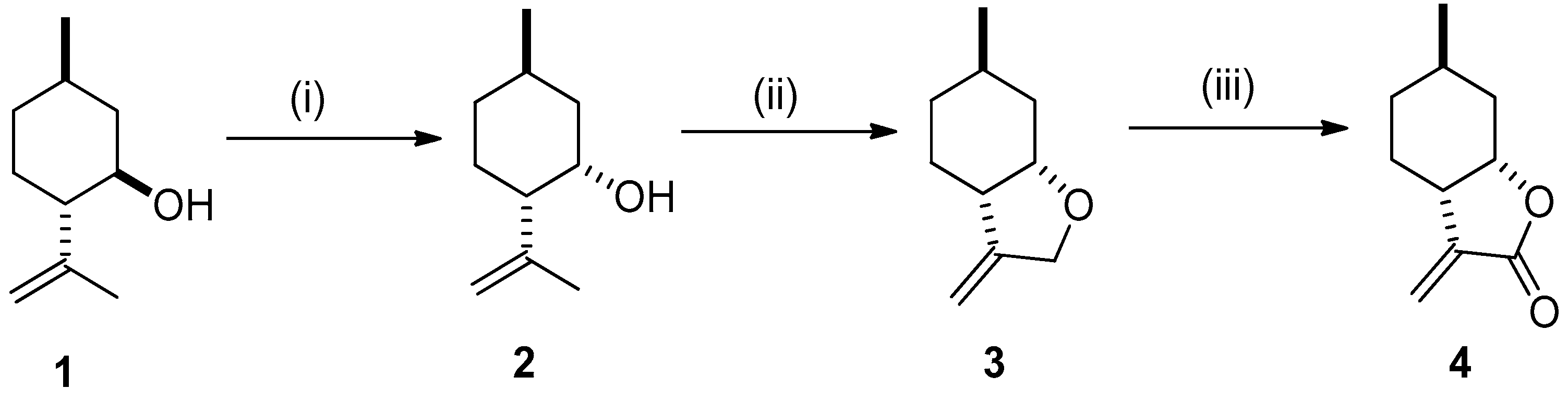
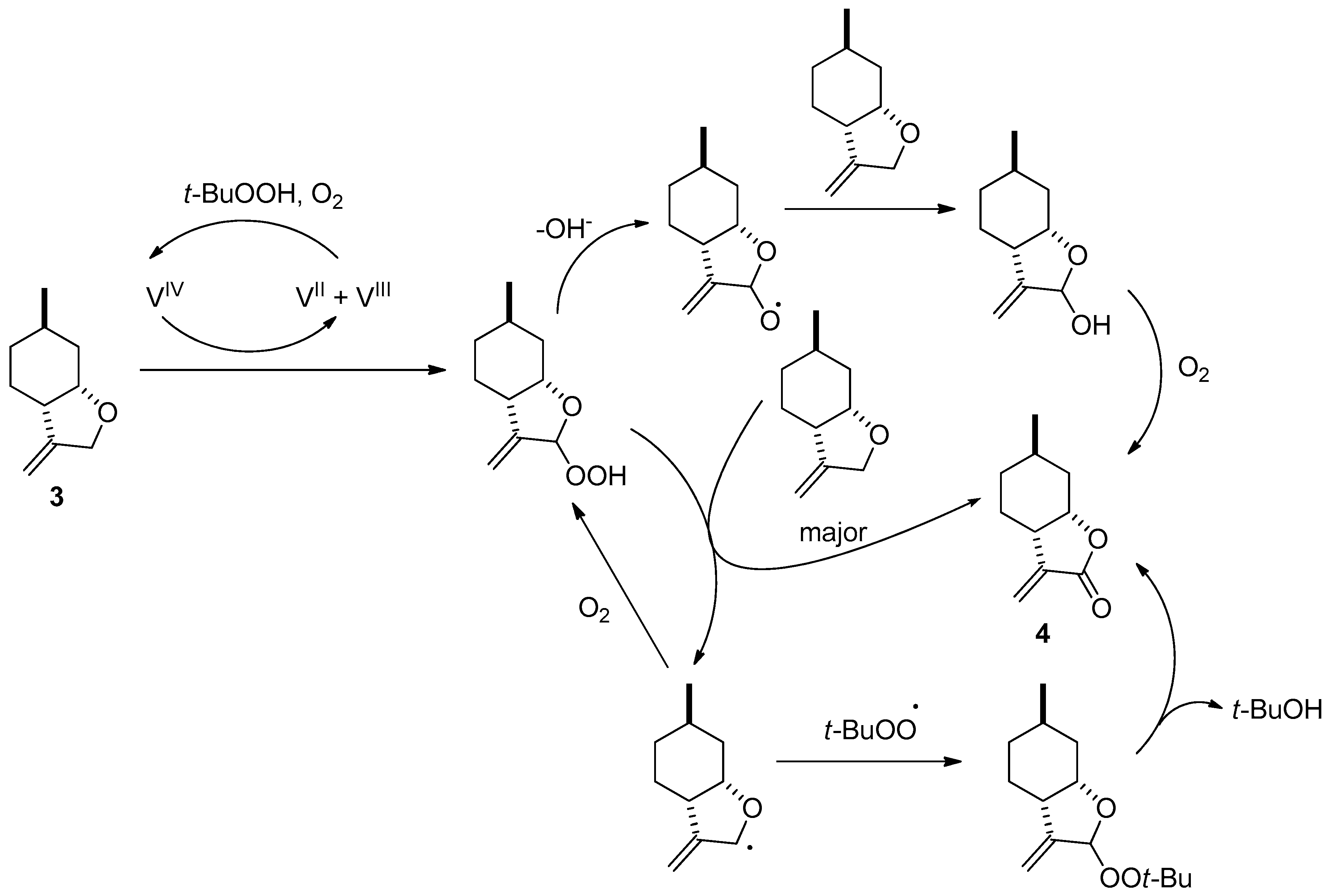
2.1. Synthesis of Key Intermediate 3
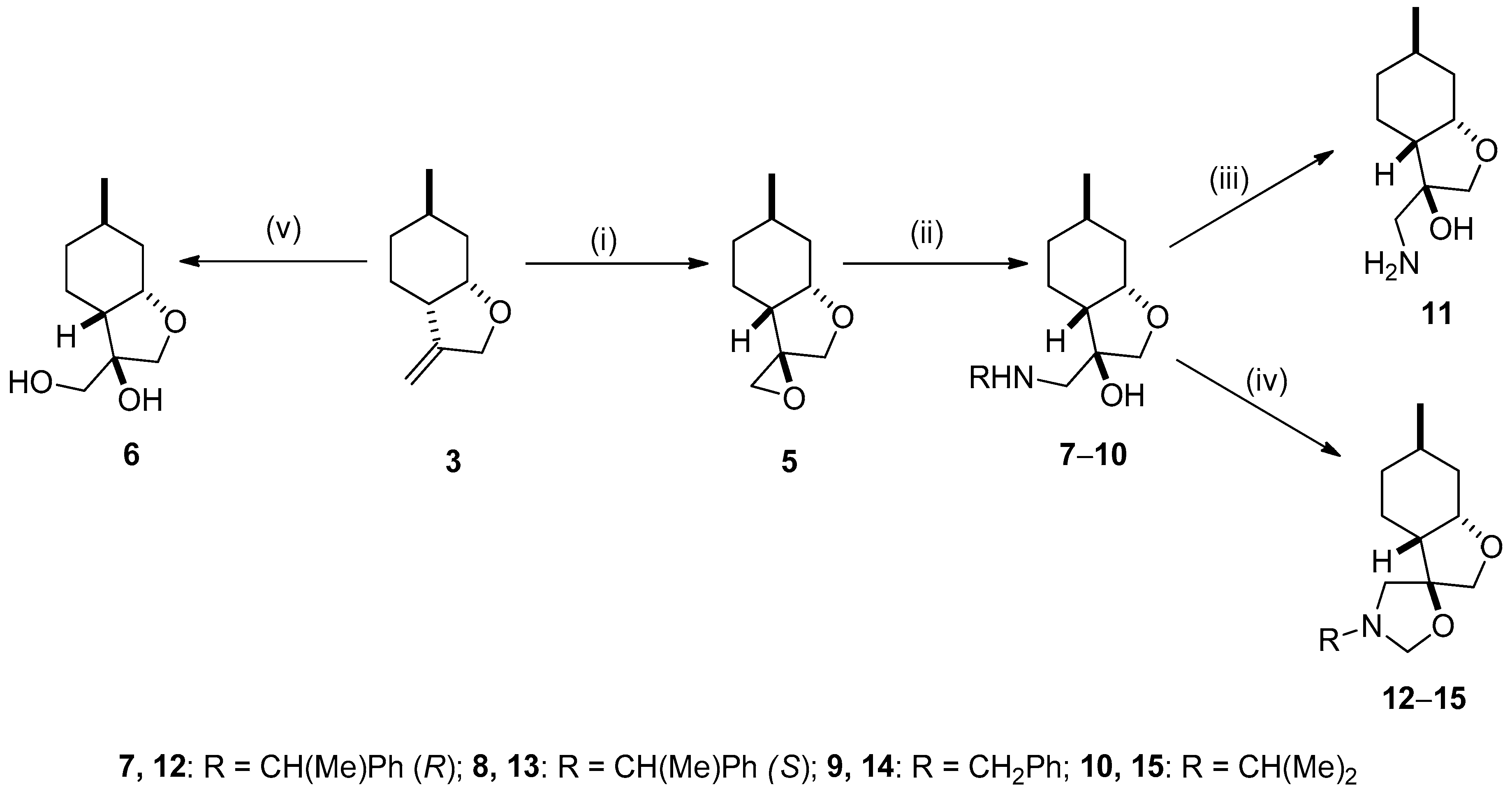
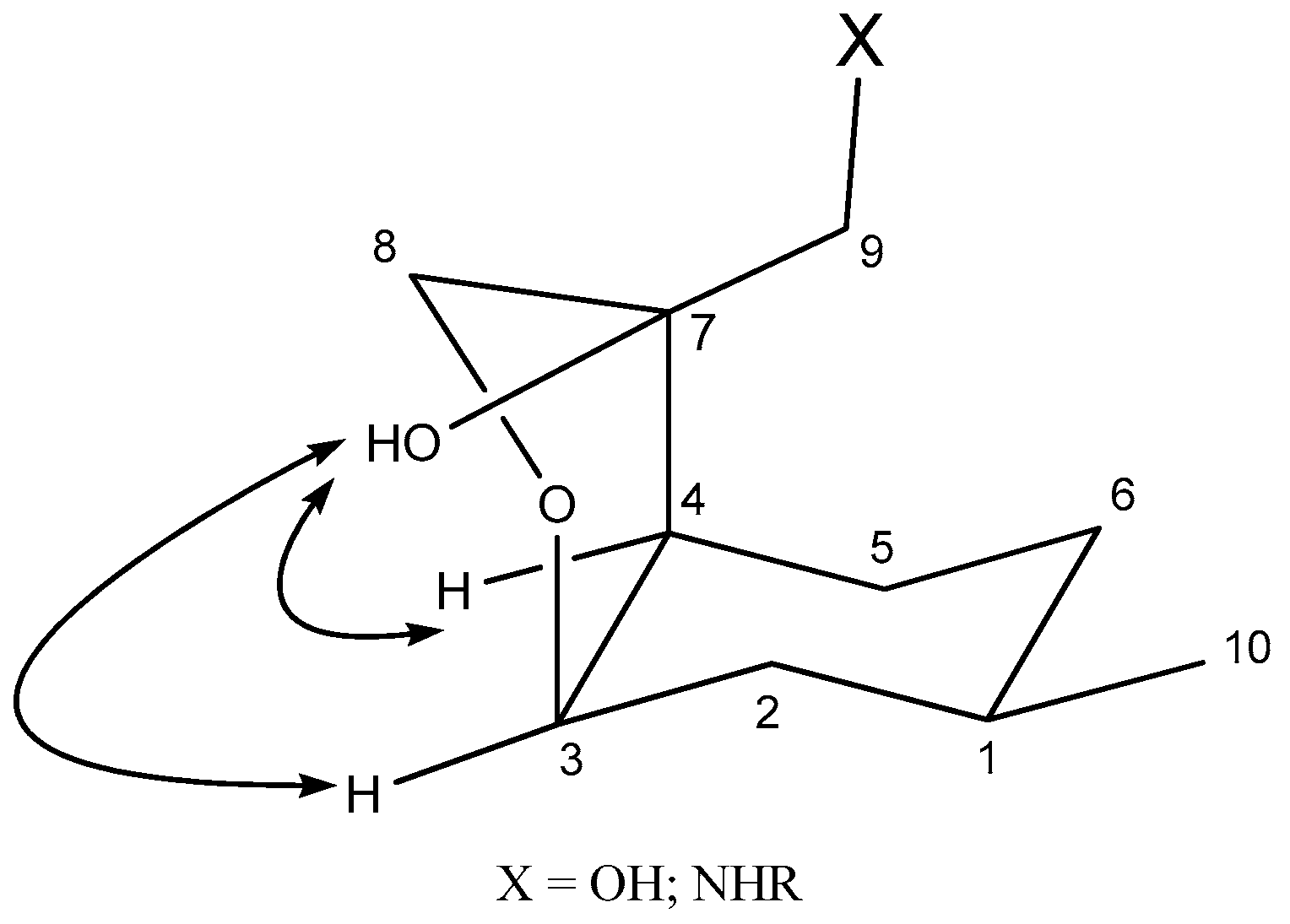
2.2. Synthesis of Ispulegol-Based 1,2-Aminoalcohols
2.3. Application of Aminoalcohol Derivatives as Chiral Ligands for Catalytic Addition of Diethylzinc to Benzaldehyde
2.4. Antimicrobial Effects
3. Materials and Methods
3.1. Materials and General Methods
3.2. (2′R,3aR,6R,7aS)-6-Methylhexahydro-2H-spiro[benzofuran-3,2′-oxirane] (5)
3.3. General Procedure for Ring-Opening of Epoxide with Primary Amines
3.3.1. (3R,3aR,6R,7aS)-6-Methyl-3-((((R)-1-phenylethyl)amino)methyl)octahydrobenzofuran-3-ol (7)
3.3.2. (3R,3aR,6R,7aS)-6-Methyl-3-((((S)-1-phenylethyl)amino)methyl)octahydrobenzofuran-3-ol (8)
3.3.3. (3R,3aR,6R,7aS)-3-((Benzylamino)methyl)-6-methyloctahydrobenzofuran-3-ol (9)
3.3.4. (3R,3aR,6R,7aS)-3-((Isopropylamino)methyl)-6-methyloctahydrobenzofuran-3-ol (10)
3.4. General Procedure for Ring Closure of Aminoalcohols 7–10 with Formaldehyde
3.4.1. (3R,3aR,6R,7aS)-6-Methyl-3′-((R)-1-phenylethyl)hexahydro-2H-spiro[benzofuran-3,5′-oxazolidine] (12)
3.4.2. (3R,3aR,6R,7aS)-6-Methyl-3′-((S)-1-phenylethyl)hexahydro-2H-spiro[benzofuran-3,5′-oxazolidine] (13)
3.4.3. (3R,3aR,6R,7aS)-3′-Benzyl-6-methylhexahydro-2H-spiro[benzofuran-3,5′-oxazolidine] (14)
3.4.4. (3R,3aR,6R,7aS)-3′-Isopropyl-6-methylhexahydro-2H-spiro[benzofuran-3,5′-oxazolidine] (15)
3.5. (3R,3aR,6R,7aS)-3-(Aminomethyl)-6-methyloctahydrobenzofuran-3-ol (11)
3.6. (3R,3aR,6R,7aS)-3-(Hydroxymethyl)-6-methyloctahydrobenzofuran-3-ol (6)
3.7. General Procedure for the Reaction of Benzaldehyde with Diethylzinc in the Presence of Chiral Catalysts
3.8. Antimicrobial Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Keay, B.A.; Hopkins, J.M.; Dibble, P.W. Furans and their benzo derivatives: Applications. In Comprehensive Heterocyclic Chemistry III, 1st ed.; Katritzky, A.R., Ramsden, C.A., Scriven, E.F.V., Taylor, R.J.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; Volume 5, pp. 571–623. [Google Scholar]
- Keay, B.A.; Dibble, P.W. Furans and their benzo derivatives: Applications. In Comprehensive Heterocyclic Chemistry II, 2nd ed.; Katritzky, A.R., Rees, C.V., Scriven, E.F.V., Eds.; Elsevier: Amsterdam, The Netherlands, 1996; Volume 2, pp. 395–436. [Google Scholar]
- Leibeling, M.; Koester, D.C.; Pawliczek, M.; Schild, S.C.; Werz, D.B. Domino access to highly substituted chromans and isochromans from carbohydrates. Nat. Chem. Biol. 2010, 6, 199–201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Negishi, E. Metal-promoted cyclization. 25. Palladium-catalyzed cascade carbometalation of alkynes and alkenes as an efficient route to cyclic and polycyclic structures. J. Am. Chem. Soc. 1989, 111, 3454–3456. [Google Scholar] [CrossRef]
- Huang, Q.; Larock, R.C. Synthesis of substituted Naphthalenes and Carbazoles by the Palladium-catalyzed annulation of internal alkynes. J. Org. Chem. 2003, 68, 7342–7349. [Google Scholar] [CrossRef] [PubMed]
- Manarin, F.; Roehrs, J.A.; Gay, R.M.; Brandão, R.; Menezes, P.H.; Nogueira, C.W.; Zeni, G. Electrophilic cyclization of 2-Chalcogenealkynylanisoles: Versatile access to 2-Chalcogen-benzo[b]furans. J. Org. Chem. 2009, 74, 2153–2162. [Google Scholar] [CrossRef] [PubMed]
- Isono, N.; Lautens, M. Rhodium(I)-catalyzed cyclization reaction of o-alkynyl Phenols and Anilines. Domino approach to 2,3-disubstituted Benzofurans and Indoles. Org. Lett. 2009, 11, 1329–1331. [Google Scholar] [CrossRef]
- Zhao, D.; Wu, N.; Zhang, S.; Xi, P.; Su, X.; Lan, J.; You, J. Synthesis of phenol, aromatic ether, and Benzofuran derivatives by Copper-catalyzed hydroxylation of aryl halides. Angew. Chem. Int. Ed. 2009, 48, 8729–8732. [Google Scholar] [CrossRef]
- Shen, Z.; Dong, V.M. Benzofurans prepared by C-H Bond functionalization with acylsilanes. Angew. Chem. Int. Ed. 2009, 48, 784–786. [Google Scholar] [CrossRef]
- Huang, X.-C.; Liu, Y.-L.; Liang, Y.; Pi, S.-F.; Wang, F.; Li, J.-H. Cycloaddition of arynes with Iodonium Ylides: A mild and general route for the synthesis of Benzofuran derivatives. Org. Lett. 2008, 10, 1525–1528. [Google Scholar] [CrossRef]
- Kokubo, K.; Harada, K.; Mochizuki, E.; Oshima, T. A new approach to benzofuran synthesis: Lewis acid mediated cycloaddition of benzoquinones with stilbene oxides. Tetrahedron Lett. 2010, 51, 955–958. [Google Scholar] [CrossRef]
- Baguley, P.A.; Jackson, L.V.; Walton, J.C. Preparation of 1-phenylcyclohexa-2,5-diene-1-carboxylates and their use in free-radical mediated syntheses. J. Chem. Soc. Perkin 1 2002, 304–309. [Google Scholar] [CrossRef]
- McCarroll, A.J.; Walton, J.C. Enhanced radical delivery from aldoxime esters for EPR and ring closure applications. Chem. Commun. 2000, 351–352. [Google Scholar] [CrossRef]
- Harvey, W.E.; Tarbell, D.S. The reaction of the magnesium salt of N-cyclohexylcyclohexylimine with epoxides. J. Org. Chem. 1967, 32, 1679–1681. [Google Scholar] [CrossRef]
- Coaviche-Yoval, A.; Andrade-Jorge, E.; Pérez-González, C.; Luna, H.; Tovar-Miranda, R.; Trujillo-Ferrara, J.G. Quantum reality in the selective reduction of a Benzofuran system. Molecules 2019, 24, 2061. [Google Scholar] [CrossRef] [PubMed]
- Yakura, T.; Yamada, S.; Shima, M.; Iwamoto, M.; Ikeda, M. Synthesis of Octahydrobenzo[b]furans using Tandem conjugate addition reactions initiated by oxygen nucleophile. Chem. Pharm. Bull. (Tokyo) 1998, 46, 744–748. [Google Scholar] [CrossRef][Green Version]
- Ferraz, H.M.C.; Longo, L.S. Bicyclic β-hydroxytetrahydrofurans as precursors of medium ring keto-lactones. J. Org. Chem. 2007, 72, 2945–2950. [Google Scholar] [CrossRef]
- Herrinton, P.H.; Hopkins, M.H.; Mishra, P.; Brown, M.J.; Overman, L.E. Ring-enlarging furan annulations. J. Org. Chem. 1987, 52, 3711–3712. [Google Scholar] [CrossRef]
- Groves, J.T. A stereochemical probe of the fate of carbon radicals oxidized by metals. Tetrahedron Lett. 1975, 16, 3113–3116. [Google Scholar] [CrossRef]
- Sohail, M.; Wang, Y.-F.; Wu, S.; Zeng, W.; Chen, F.-X. Asymmetric synthesis of octahydrobenzofuran core structure with three contiguous stereogenic centers and development of the absolute configurations. Synth. Commun. 2014, 44, 115–120. [Google Scholar] [CrossRef]
- Arimitsu, K.; Nomura, S.; Iwasaki, H.; Ozeki, M.; Yamashita, M. First total synthesis of (±)-adunctin B. Tetrahedron Lett. 2011, 52, 7046–7048. [Google Scholar] [CrossRef]
- Trost, B.M.; Shen, H.C.; Surivet, J.-P. An enantioselective biomimetic total synthesis of (–)-Siccanin. Angew. Chem. Int. Ed. 2003, 42, 3943–3947. [Google Scholar] [CrossRef]
- Bhagavathula, D.; Boddeti, G.; Reddy, V. A brief review on synthesis of β-amino alcohols by ring opening of epoxides. Res. Rev. J. Chem. 2017, 6, 27–46. [Google Scholar]
- Brik, A.; Wong, C.-H. HIV-1 protease: Mechanism and drug discovery. Org. Biomol. Chem. 2003, 1, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.K.; Bilcer, G.; Schiltz, G. Syntheses of FDA ppproved HIV protease inhibitors. Synthesis 2001, 2001, 2203–2229. [Google Scholar] [CrossRef] [PubMed]
- Andrews, K.T.; Fairlie, D.P.; Madala, P.K.; Ray, J.; Wyatt, D.M.; Hilton, P.M.; Melville, L.A.; Beattie, L.; Gardiner, D.L.; Reid, R.C.; et al. Potencies of human immunodeficiency virus protease inhibitors in vitro against Plasmodium falciparum and in vivo against murine malaria. Antimicrob. Agents Chemother. 2006, 50, 639–648. [Google Scholar] [CrossRef]
- Nöteberg, D.; Hamelink, E.; Hultén, J.; Wahlgren, M.; Vrang, L.; Samuelsson, B.; Hallberg, A. Design and synthesis of plasmepsin I and plasmepsin II inhibitors with activity in Plasmodium falciparum-infected cultured human erythrocytes. J. Med. Chem. 2003, 46, 734–746. [Google Scholar] [CrossRef]
- Parikh, S.; Gut, J.; Istvan, E.; Goldberg, D.E.; Havlir, D.V.; Rosenthal, P.J. Antimalarial cctivity of human immunodeficiency virus type 1 protease inhibitors. Antimicrob. Agents Chemother. 2005, 49, 2983–2985. [Google Scholar] [CrossRef]
- Savoia, D.; Allice, T.; Tovo, P.-A. Antileishmanial activity of HIV protease inhibitors. Int. J. Antimicrob. Agents 2005, 26, 92–94. [Google Scholar] [CrossRef]
- Conolly, M.E.; Kersting, F.; Dollery, C.T. The clinical pharmacology of beta-adrenoceptor-blocking drugs. Prog. Cardiovasc. Dis. 1976, 19, 203–234. [Google Scholar] [CrossRef]
- Shanks, R.G.; Wood, T.M.; Dornhorst, A.C.; Clark, M.L. Some pharmacological properties of a new adrenergic β-receptor antagonist. Nature 1966, 212, 88–90. [Google Scholar] [CrossRef]
- Zimmerman, T.J.; Boger, W.P. The beta-adrenergic blocking agents and the treatment of glaucoma. Surv. Ophthalmol. 1979, 23, 347–362. [Google Scholar] [CrossRef]
- Ager, D.J.; Prakash, I.; Schaad, D.R. 1,2-Amino alcohols and their heterocyclic derivatives as chiral auxiliaries in asymmetric synthesis. Chem. Rev. 1996, 96, 835–876. [Google Scholar] [CrossRef] [PubMed]
- Griesbeck, A.G.; Lex, J.; Saygin, K.M.; Steinwascher, J. Azidohydroperoxidation of pinenes: Stereoselectivity pattern and the first X-ray structure of a 2-azidohydroperoxide. Chem. Commun. 2000, 2205–2206. [Google Scholar] [CrossRef]
- Frensch, G.; Labes, R.; Wosch, C.L.; Munaretto, L.D.S.; Salomé, K.S.; Guerrero, P.G.; Marques, F.A. New chiral ligands derived from (+) and (–)-α-pinene for the enantioselective addition of diethylzinc to aldehydes. Tetrahedron Lett. 2016, 57, 420–422. [Google Scholar] [CrossRef]
- Hobuß, D.; Baro, A.; Laschat, S.; Frey, W. Catalytic enantioselective borane reduction of arylketones with pinene-derived amino alcohols. Tetrahedron 2008, 64, 1635–1640. [Google Scholar] [CrossRef]
- Masui, M.; Shioiri, T. A practical method for preparation of optically pure oxazaborolidines from α-Pinene. Tetrahedron 1995, 51, 8363–8370. [Google Scholar] [CrossRef]
- Boobalan, R.; Chang, Y.-M.; Chen, C.; Lee, G.-H. Copper complex of Pinene based Schiff base [CuSBADBH]2: Synthesis and its application in catalytic asymmetric nitroaldol (Henry) reaction. ChemistrySelect 2016, 1, 2028–2034. [Google Scholar] [CrossRef]
- Łączkowski, K.Z.; Kmieciak, A.; Kozakiewicz, A. Stereoselective synthesis of new monoterpene β-amino alcohols. Tetrahedron Asymmetry 2009, 20, 1487–1492. [Google Scholar] [CrossRef]
- Banina, O.A.; Sudarikov, D.V.; Nigmatov, A.G.; Frolova, L.L.; Slepukhin, P.A.; Zlotin, S.G.; Kutchin, A.V. Carane amino alcohols as organocatalysts in asymmetric aldol reaction of isatin with acetone. Russ. Chem. Bull. 2017, 66, 293–296. [Google Scholar] [CrossRef]
- Rafiński, Z.; Krzemiński, M.P. Synthesis of (–)-Verbenone-derived triazolium salts and their application in enantioselective intramolecular Stetter reaction. Catalysts 2019, 9, 117. [Google Scholar] [CrossRef]
- Frolova, L.L.; Sudarikov, D.V.; Alekseev, I.N.; Banina, O.A.; Slepukhin, P.A.; Kutchin, A.V. Synthesis of new enantiomerically pure β-amino alcohols of the pinane series. Russ. J. Org. Chem. 2017, 53, 335–343. [Google Scholar] [CrossRef]
- Dimitrov, V.; Dobrikov, G.; Genov, M. Chiral β- and γ-aminoalcohols derived from (+)-camphor and (–)-fenchone as catalysts for the enantioselective addition of diethylzinc to benzaldehyde. Tetrahedron Asymmetry 2001, 12, 1323–1329. [Google Scholar] [CrossRef]
- Rafiński, Z. Enantioselective benzoin condensation catalyzed by spirocyclic terpene-based N-heterocyclic carbenes. Tetrahedron 2016, 72, 1860–1867. [Google Scholar] [CrossRef]
- Rafiński, Z.; Kozakiewicz, A.; Rafińska, K. Highly efficient synthesis of spirocyclic (1R)-camphor-derived triazolium salts: Application in the catalytic asymmetric benzoin condensation. Tetrahedron 2014, 70, 5739–5745. [Google Scholar] [CrossRef]
- Wilkinson, H.S.; Grover, P.T.; Vandenbossche, C.P.; Bakale, R.P.; Bhongle, N.N.; Wald, S.A.; Senanayake, C.H. A new lithium alkoxide accelerated diastereoselective cyanation of ketones. Org. Lett. 2001, 3, 553–556. [Google Scholar] [CrossRef] [PubMed]
- Panev, S.; Linden, A.; Dimitrov, V. Chiral aminoalcohols with a menthane skeleton as catalysts for the enantioselective addition of diethylzinc to benzaldehyde. Tetrahedron Asymmetry 2001, 12, 1313–1321. [Google Scholar] [CrossRef]
- Friedrich, D.; Bohlmann, F. Total synthesis of various elemanolides. Tetrahedron 1988, 44, 1369–1392. [Google Scholar] [CrossRef]
- Rigamonti, M.G.; Gatti, F.G. Stereoselective synthesis of hernandulcin, peroxylippidulcine A, lippidulcines A, B and C and taste evaluation. Beilstein J. Org. Chem. 2015, 11, 2117–2124. [Google Scholar] [CrossRef]
- Moreira, J.A.; Corrêa, A.G. Enantioselective synthesis of three stereoisomers of 5,9-dimethylpentadecane, sex pheromone component of Leucoptera coffeella, from (–)-isopulegol. Tetrahedron Asymmetry 2003, 14, 3787–3795. [Google Scholar] [CrossRef]
- Nazimova, E.; Pavlova, A.; Mikhalchenko, O.; Il’ina, I.; Korchagina, D.; Tolstikova, T.; Volcho, K.; Salakhutdinov, N. Discovery of highly potent analgesic activity of isopulegol-derived (2R,4aR,7R,8aR)-4,7-dimethyl-2-(thiophen-2-yl)octahydro-2H-chromen-4-ol. Med. Chem. Res. 2016, 25, 1369–1383. [Google Scholar] [CrossRef]
- Engel, W. In vivo studies on the metabolism of the monoterpene Pulegone in humans using the metabolism of ingestion-correlated amounts (MICA) approach: Explanation for the toxicity differences between (S)-(–)- and (R)-(+)-Pulegone. J. Agric. Food Chem. 2003, 51, 6589–6597. [Google Scholar] [CrossRef]
- Bulliard, M.; Balme, G.; Gore, J. Fragmentation of isopulegol by a radical process. Tetrahedron Lett. 1989, 30, 2213–2216. [Google Scholar] [CrossRef]
- Hegde, S.G.; Beckwith, D.; Doti, R.; Wolinsky, J. Synthesis with hypochlorous acid. Conversion to pulegone and isopulegol to menthofuran. Preparation of 3,6-dimethyl-2,6-cycloheptadien-1-one from phorone. J. Org. Chem. 1985, 50, 894–896. [Google Scholar] [CrossRef]
- Brocksom, T.J.; dos Santos, R.B.; Varanda, N.A.; Brocksom, U. An efficient synthesis of monoterpene α-methylene-γ-butyrolactones. Synth. Commun. 1988, 18, 1403–1410. [Google Scholar] [CrossRef]
- Schlosser, M.; Kotthaus, M. Isopulegol as a model compound: Metalation and substitution of an allylic position in the presence of an unprotected hydroxy function. Eur. J. Org. Chem. 1999, 1999, 459–462. [Google Scholar] [CrossRef]
- Le, T.M.; Szilasi, T.; Volford, B.; Szekeres, A.; Fülöp, F.; Szakonyi, Z. Stereoselective synthesis and investigation of isopulegol-based chiral ligands. Int. J. Mol. Sci. 2019, 20, 4050. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, M.; Zhang, B.; Nie, R.; Huang, A.; Goh, T.W.; Volkov, A.; Zhang, Z.; Ren, Q.; Huang, W. Allylic oxidation of olefins with a manganese-based metal–organic framework. Green Chem. 2019, 21, 3629–3636. [Google Scholar] [CrossRef]
- Islam, S.M.; Roy, A.S.; Mondal, P.; Salam, N. Efficient allylic oxidation of olefins catalyzed by polymer supported metal Schiff base complexes with peroxides. J. Inorg. Organomet. Polym. Mater. 2012, 22, 717–730. [Google Scholar] [CrossRef]
- Jia, Y.X.; Wu, B.; Li, X.; Ren, S.K.; Tu, Y.Q.; Chan, A.S.C.; Kitching, W. Synthetic studies of the HIV-1 protease ihibitive didemnaketals: Stereocontrolled synthetic approach to the key mother spiroketals. Org. Lett. 2001, 3, 847–849. [Google Scholar] [CrossRef]
- Waddell, T.G.; Ross, P.A. Chemistry of 3,4-epoxy alcohols. Fragmentation reactions. J. Org. Chem. 1987, 52, 4802–4804. [Google Scholar] [CrossRef]
- Kim, J.H.; Lim, H.J.; Cheon, S.H. A facile synthesis of (6S,1′S)-(+)-hernandulcin and (6S,1′R )-(+)-epihernandulcin. Tetrahedron 2003, 59, 7501–7507. [Google Scholar] [CrossRef]
- Kim, J.H.; Lim, H.J.; Cheon, S.H. Synthesis of (+)-hernandulcin and (+)-epihernandulcin. Tetrahedron Lett. 2002, 43, 4721–4722. [Google Scholar] [CrossRef]
- Szakonyi, Z.; Csillag, K.; Fülöp, F. Stereoselective synthesis of carane-based aminodiols as chiral ligands for the catalytic addition of diethylzinc to aldehydes. Tetrahedron Asymmetry 2011, 22, 1021–1027. [Google Scholar] [CrossRef]
- Szakonyi, Z.; Csőr, Á.; Csámpai, A.; Fülöp, F. Stereoselective synthesis and modelling-driven optimisation of Carane-based aminodiols and 1,3-oxazines as catalysts for the enantioselective addition of diethylzinc to benzaldehyde. Chem. - Eur. J. 2016, 22, 7163–7173. [Google Scholar] [CrossRef] [PubMed]
- Shivani; Pujala, B.; Chakraborti, A.K. Zinc(II) perchlorate hexahydrate catalyzed opening of epoxide ring by amines: Applications to synthesis of (RS)/(R)-Propranolols and (RS)/(R)/(S)-Naftopidils. J. Org. Chem. 2007, 72, 3713–3722. [Google Scholar] [CrossRef] [PubMed]
- Bergmeier, S.C. The synthesis of vicinal amino alcohols. Tetrahedron 2000, 56, 2561–2576. [Google Scholar] [CrossRef]
- Gonda, T.; Szakonyi, Z.; Csámpai, A.; Haukka, M.; Fülöp, F. Stereoselective synthesis and application of tridentate aminodiols derived from (+)-pulegone. Tetrahedron Asymmetry 2016, 27, 480–486. [Google Scholar] [CrossRef]
- Morikawa, H.; Yamaguchi, J.; Sugimura, S.; Minamoto, M.; Gorou, Y.; Morinaga, H.; Motokucho, S. Systematic synthetic study of four diastereomerically distinct limonene-1,2-diols and their corresponding cyclic carbonates. Beilstein J. Org. Chem. 2019, 15, 130–136. [Google Scholar] [CrossRef]
- Tanaka, T.; Yasuda, Y.; Hayashi, M. New chiral Schiff base as a tridentate ligand for catalytic enantioselective addition of diethylzinc to aldehydes. J. Org. Chem. 2006, 71, 7091–7093. [Google Scholar] [CrossRef]
- Jimeno, C.; Pastó, M.; Riera, A.; Pericàs, M.A. Modular amino alcohol ligands containing bulky alkyl groups as chiral controllers for Et2Zn addition to aldehydes: Illustration of a design principle. J. Org. Chem. 2003, 68, 3130–3138. [Google Scholar] [CrossRef]
- Tashenov, Y.; Daniels, M.; Robeyns, K.; Van Meervelt, L.; Dehaen, W.; Suleimen, Y.; Szakonyi, Z. Stereoselective syntheses and application of chiral bi- and tridentate ligands derived from (+)-Sabinol. Molecules 2018, 23, 771. [Google Scholar] [CrossRef]
- Szakonyi, Z.; Hetényi, A.; Fülöp, F. Synthesis and application of monoterpene-based chiral aminodiols. Tetrahedron 2008, 64, 1034–1039. [Google Scholar] [CrossRef]
- Yendapally, R.; Lee, R.E. Design, synthesis, and evaluation of novel ethambutol analogues. Bioorg. Med. Chem. Lett. 2008, 18, 1607–1611. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cunico, W.; Gomes, C.R.B.; Ferreira, M.L.G.; Ferreira, T.G.; Cardinot, D.; de Souza, M.V.N.; Lourenço, M.C.S. Synthesis and anti-mycobacterial activity of novel amino alcohol derivatives. Eur. J. Med. Chem. 2011, 46, 974–978. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, M.P. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed.; Clinical and Laboratory: Wayne, PA, USA, 2018. [Google Scholar]
- Béni, Z.; Dékány, M.; Kovács, B.; Csupor-Löffler, B.; Zomborszki, Z.; Kerekes, E.; Szekeres, A.; Urbán, E.; Hohmann, J.; Ványolós, A. Bioactivity-guided isolation of antimicrobial and antioxidant metabolites from the mushroom Tapinella atrotomentosa. Molecules 2018, 23, 1082. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 3–15 are available from the authors. |
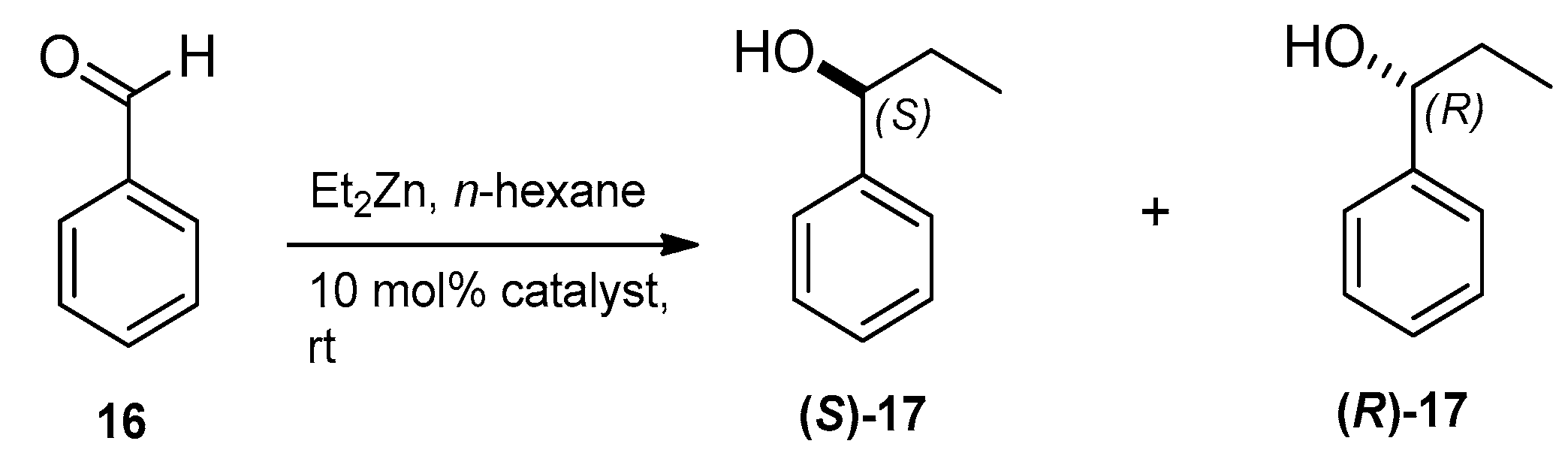
| Entry | Ligand | Yield a (%) | eeb (%) | Configuration of the Major Product c |
|---|---|---|---|---|
| 1 | 7 | 86 | 8 | (R) |
| 2 | 8 | 90 | 40 | (S) |
| 3 | 9 | 89 | 11 | (S) |
| 4 | 10 | 93 | 4 | (S) |
| 5 | 11 | 95 | 7 | (S) |
| 6 | 12 | 88 | 11 | (R) |
| 7 | 13 | 90 | 3 | (R) |
| 8 | 14 | 82 | 6 | (R) |
| 9 | 15 | 80 | 7 | (R) |
| Inhibitory effect (%) ± RSD (%) | |||||||
|---|---|---|---|---|---|---|---|
| Yeast | Gram-Negative | Gram-Positive | |||||
| Analogue | Conc. (µg/mL) | C. albicans | C. krusei | E. coli | P. aeruginosa | B. subtilis | S. aureus |
| 6 | 10 | − | 36.5 ± 8.43 | − | − | − | − |
| 100 | − | 58.4 ± 14.41 | − | − | 21.7 ± 6.05 | − | |
| 7 | 10 | − | − | 8.7 ± 3.15 | 7.5 ± 1.54 | − | − |
| 100 | − | − | 20.0 ± 2.81 | 8.7 ± 0.49 | − | 7.1 ± 4.3 | |
| 8 | 10 | − | − | − | − | 19.0 ± 2.61 | − |
| 100 | − | − | 17.1 ± 4.94 | 5.3 ± 4.31 | 31.9 ± 2.74 | − | |
| 9 | 10 | − | − | 16.7 ± 6.68 | 9.9 ± 1.8 | − | − |
| 100 | − | − | 21.0 ± 5.05 | 31.6 ± 1.73 | 9.8 ± 11.2 | 13.8 ± 1.73 | |
| 10 | 10 | − | − | 3.7 ± 1.68 | − | − | − |
| 100 | − | − | 4.3 ± 10.71 | 2.3 ± 5.93 | 10.5 ± 10.12 | − | |
| 11 | 10 | − | 3.7 ± 0.04 | − | − | − | − |
| 100 | − | 16.0 ± 14.5 | − | − | − | − | |
| 12 | 10 | − | − | 15.3 ± 4.35 | − | − | 9.2 ± 7.75 |
| 100 | − | − | 26.2 ± 4.06 | 1.8 ± 6.28 | − | 20.2 ± 8.92 | |
| 13 | 10 | − | − | 17.1 ± 8.19 | − | − | − |
| 100 | − | − | 27.7 ± 8.54 | 7.0 ± 4.62 | − | 3.9 ± 3.39 | |
| 14 | 10 | − | − | 14.6 ± 4.38 | 4.1 ± 7.10 | − | 12.6 ± 0.57 |
| 100 | − | − | 25.3 ± 2.99 | 16.8 ± 5.69 | − | 14.0 ± 3.68 | |
| 15 | 10 | − | − | 5.1 ± 7.92 | − | − | − |
| 100 | − | − | 14.8 ± 4.87 | − | 1.5 ± 11.4 | − | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bamou, F.Z.; Le, T.M.; Volford, B.; Szekeres, A.; Szakonyi, Z. Synthesis and Application of 1,2-Aminoalcohols with Neoisopulegol-Based Octahydrobenzofuran Core. Molecules 2020, 25, 21. https://doi.org/10.3390/molecules25010021
Bamou FZ, Le TM, Volford B, Szekeres A, Szakonyi Z. Synthesis and Application of 1,2-Aminoalcohols with Neoisopulegol-Based Octahydrobenzofuran Core. Molecules. 2020; 25(1):21. https://doi.org/10.3390/molecules25010021
Chicago/Turabian StyleBamou, Fatima Zahra, Tam Minh Le, Bettina Volford, András Szekeres, and Zsolt Szakonyi. 2020. "Synthesis and Application of 1,2-Aminoalcohols with Neoisopulegol-Based Octahydrobenzofuran Core" Molecules 25, no. 1: 21. https://doi.org/10.3390/molecules25010021
APA StyleBamou, F. Z., Le, T. M., Volford, B., Szekeres, A., & Szakonyi, Z. (2020). Synthesis and Application of 1,2-Aminoalcohols with Neoisopulegol-Based Octahydrobenzofuran Core. Molecules, 25(1), 21. https://doi.org/10.3390/molecules25010021









