A Method to Study the Distribution Patterns for Metabolites in Xylem and Phloem of Spatholobi Caulis
Abstract
1. Introduction
2. Results
2.1. Optimization of Extraction Conditions
2.2. Optimization of UFLC Conditions
2.3. Optimization of MS Conditions
2.4. Method Validation
2.4.1. Linearity, Limit of Detection and Limit of Quantification
2.4.2. Precision, Repeatability, Stability, Accuracy and Matrix Effect
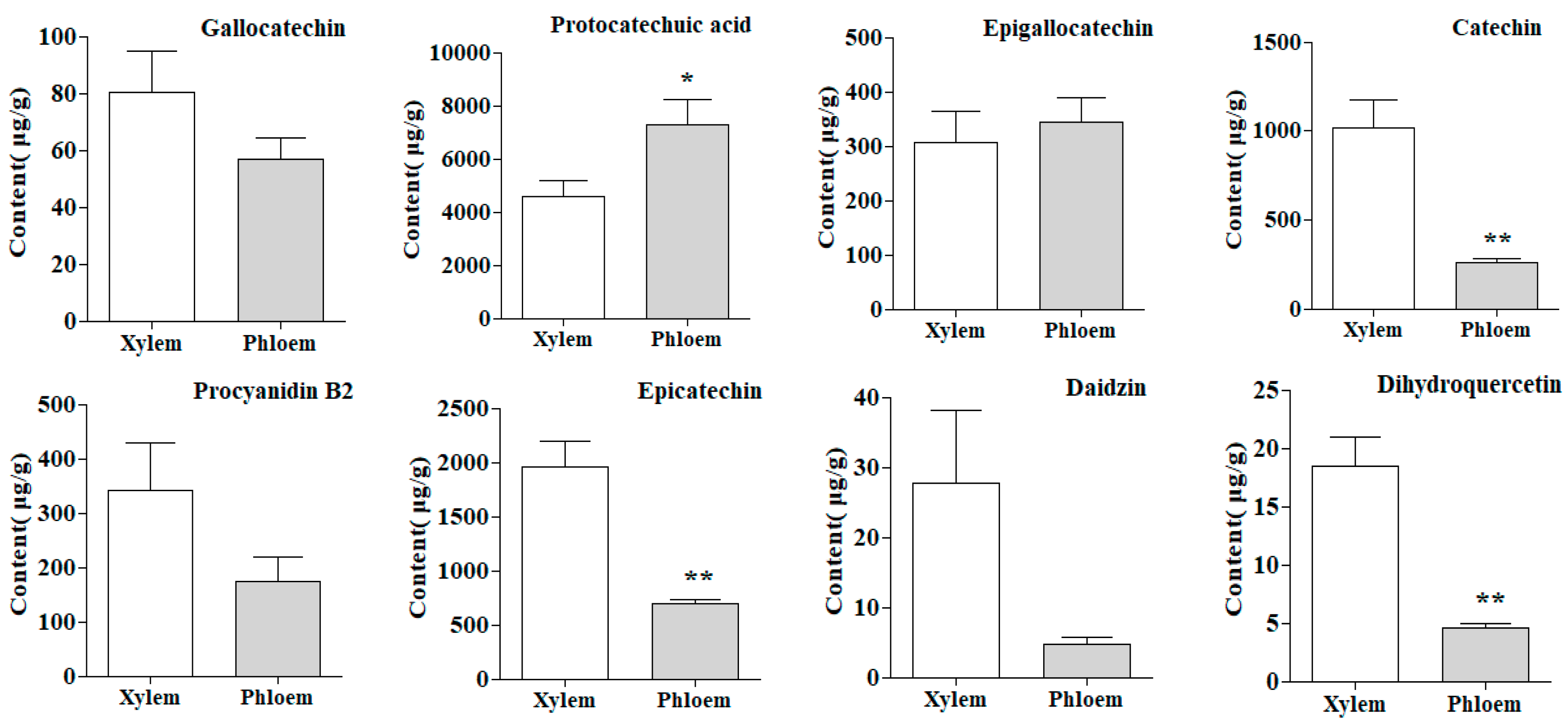
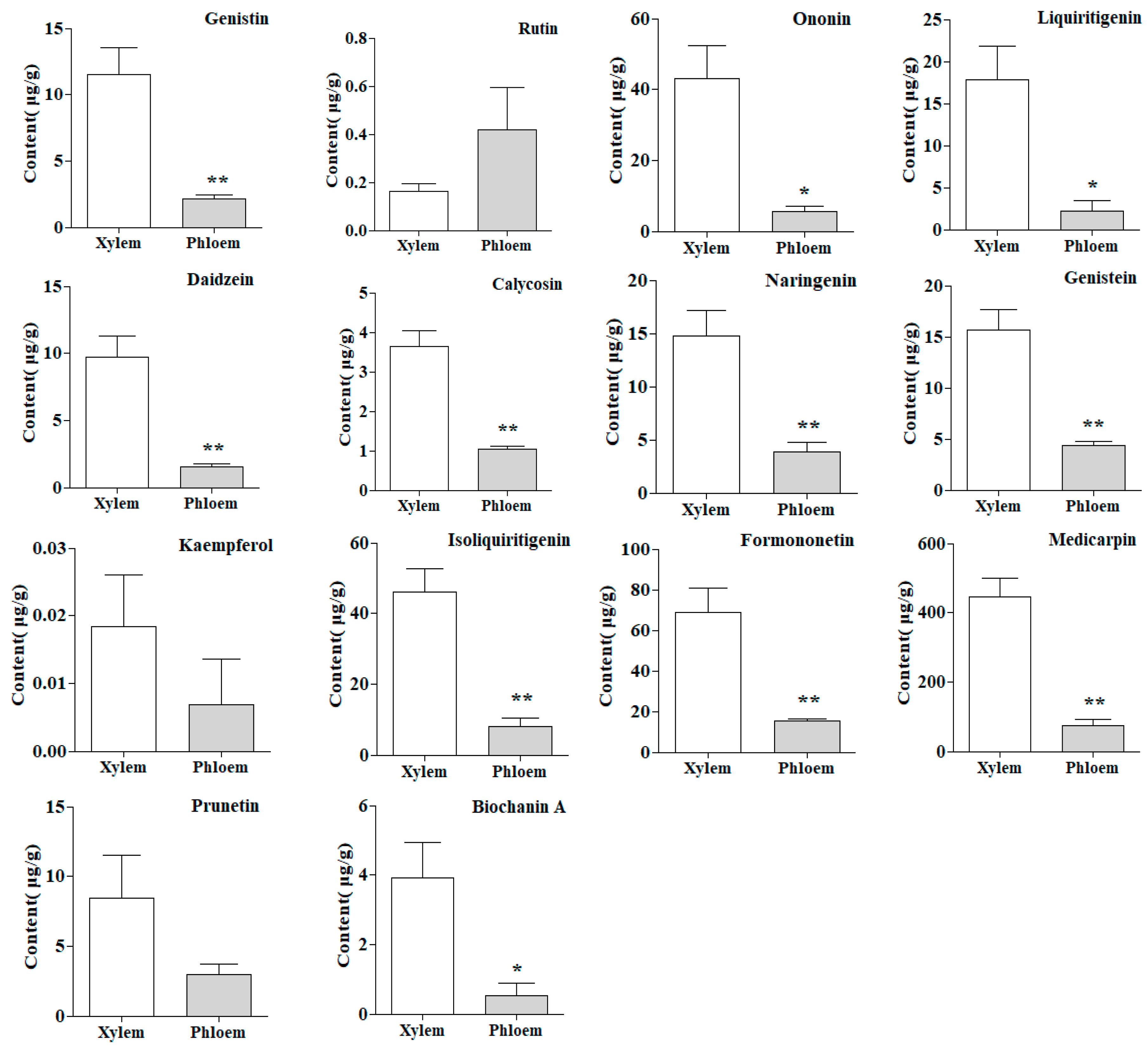
2.5. Quantitative Analysis of Samples
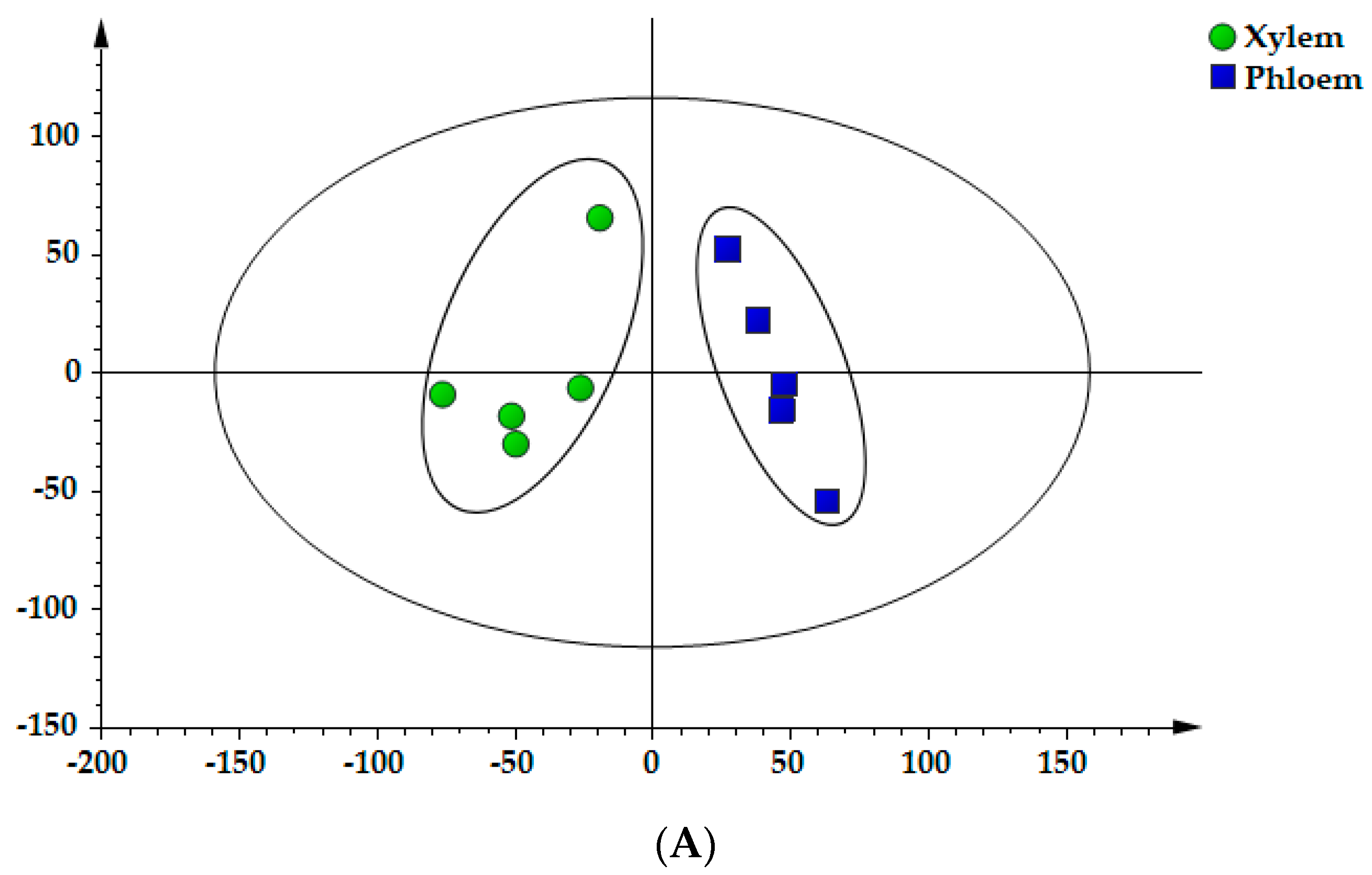
2.6. PCA of Samples
2.7. OPLS–DA of Samples
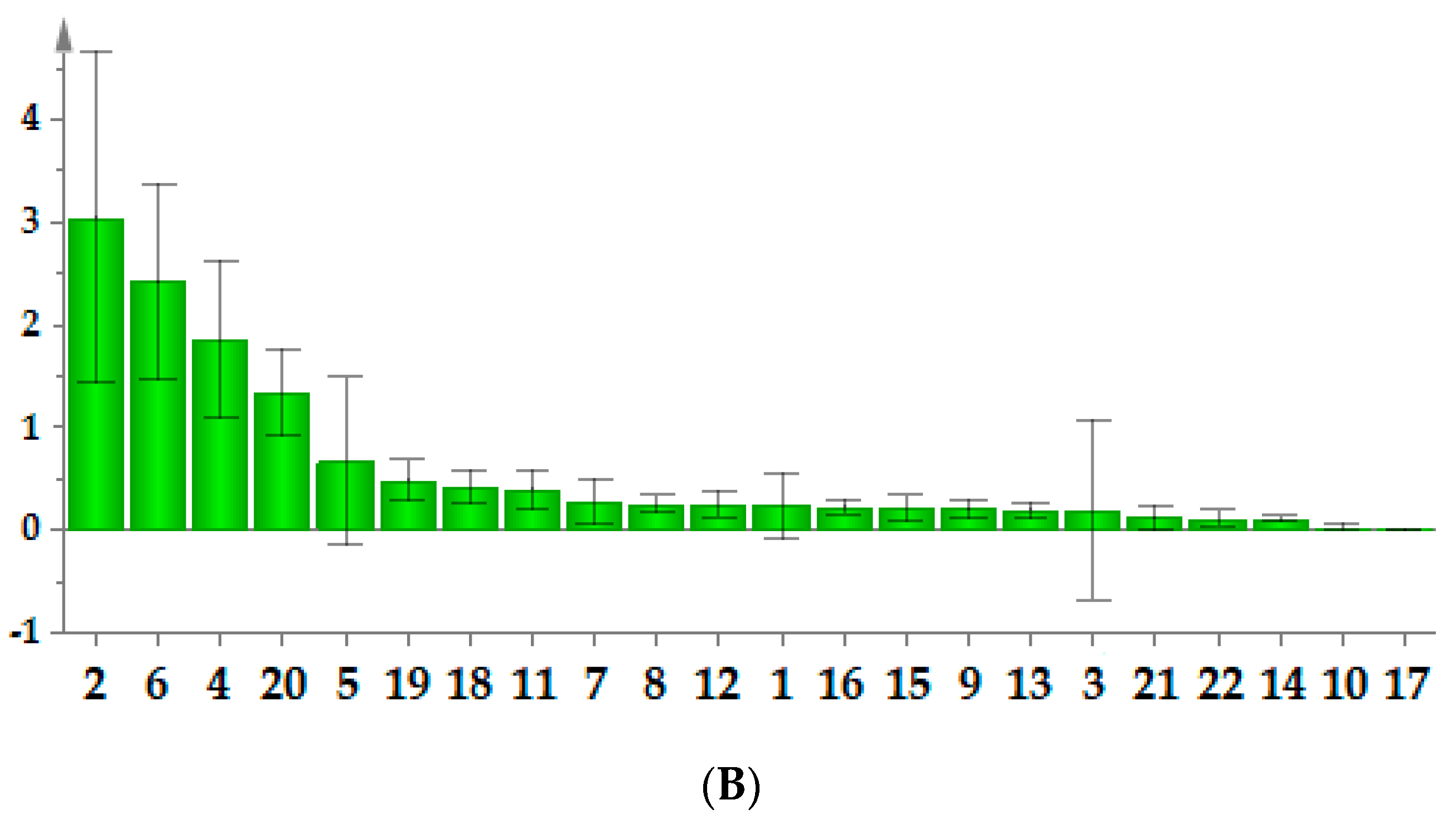
2.8. T–Test of Samples
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Chemicals and Reagents
4.3. Preparation of Standard Solutions
4.4. Preparation of Sample Solutions
4.5. Chromatographic and Mass Spectrometric Conditions
4.6. Validation of the Method
4.7. Multivariate Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chinese Pharmacopoeia Committee. Pharmacopoeia of the People’s Republic of China. Part I; Chemical Industry Press: Beijing, China, 2015; p. 194. [Google Scholar]
- Fu, Y.; Cheng, Y.; Chen, J.P.; Wang, D.M. Advances in Studies on Chemical Constituents in Spatholobi Caulis and their Pharmacological Activities. Chin. Tradit. Herbal. Drugs. 2011, 42, 1229–1234. [Google Scholar]
- Chen, X.; Li, Q.; Kan, X.X.; Wang, Y.J.; Li, Y.J.; Yang, Q.; Xiao, H.B.; Chen, Y.; Weng, X.G.; Cai, W.Y.; et al. Extract of Caulis Spatholobi, a Novel Blocker Targeting Tumor Cell-Induced Platelet Aggregation, Inhibits Breast Cancer Metastasis. Oncol. Rep. 2016, 36, 3215–3224. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Liu, J.L.; Chen, J.; Zhu, D.M.; Zhou, H.J.; Wang, X.M. A Study on Anticancer Activity of Caulis Spatholobi Extract on Human Osteosarcoma Saos-2 cells. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.N.; Tang, Q.L.; Luo, L.; Gao, Z.Y.; Zhang, S.Z. A Preliminary Study on the Anticancer Efficacy of Caulis Spatholobi Compound 1802. Pak. J. Pharm. Sci. 2018, 31, 1145–1150. [Google Scholar] [PubMed]
- Qin, S.S.; Zhu, Y.X.; Wei, K.H.; Li, M.J.; Miao, J.H.; Zhang, Z.Y. Study on Herbal Textual Evolution and Flavonoids and their Pharmacological of Spatholobi Caulis. China J. Chin. Mater. Med. 2018, 43, 2216–2223. [Google Scholar]
- Huang, S.Y.; Luo, J.H.; Zhang, L.D.; Meng, C.Y.; Feng, L.; He, Q.Z. Extraction of Total Flavanone from Spatholobus suberctus Dunn and its Effects on Scavenging of Free Radicals. Lishizhen Med. Mater. Med. Res. 2007, 9, 2337–2338. [Google Scholar]
- Liang, N.; Wei, S.J.; Lin, Q.Y. Study on the Blood Enriching Effect and Mechanism of Spatholobus Suberect Total Flavonoids. Lishizhen Med. Mater. Med. Res. 2009, 20, 362–363. [Google Scholar]
- Zhai, M.; Liu, J.M.; An, R.; Wu, F.L.; Xu, H.H. Content Determination of Protocatechuic Acid in Spatholobus suberctus Dunn. Tradit. Chin. Drug. Res. Clin. Pharmacol. 2009, 20, 462–465. [Google Scholar]
- State Administration of Traditional Chinese Medicine. Chinese Materia Medica. Part 4; Shanghai Science and Technology Press: Shanghai, China, 1999; p. 656. [Google Scholar]
- Yao, R.L.; Rong, Y.W. Identification Technology of Traditional Chinese Medicine, 3rd ed.; China Medical Science and Technology Press: Beijing, China, 2017; p. 159. [Google Scholar]
- Wu, W.Y.; Guo, D.A. Strategies for Elaboration of Comprehensive Quality Standard System on Traditional Chinese Medicine. China J. Chin. Mater. Med. 2014, 39, 351–356. [Google Scholar]
- Lu, X.L.; Pan, X.J.; Deng, M.M.; Wu, J.X.; Zhao, J.H. TLC Identification and Determination of Catechin and Epicatechin in Spatholobi Caulis Extract. Chin. J. Exp. Tradit. Med. Form. 2018, 24, 88–92. [Google Scholar]
- Liang, Y.S.; An, R.; Liu, J.M.; Huang, Y.Y. Determination of Genistein and Formononetin in Spatholobi Caulis from Different Habitats. Lishizhen Med. Mater. Med. Res. 2013, 24, 1655–1657. [Google Scholar]
- Huang, C.H. Determination of Dihydroxy Benzoic Acid in Suberect Spatholobus of Different Place of Production with HPLC Method. Guid. J. Tradit. Chin. Med. Pharm. 2009, 15, 83–84. [Google Scholar]
- Zhang, Y.; Guo, L.; Duan, L.; Dong, X.; Zhou, P.; Liu, E.H.; Li, P. Simultaneous Determination of 16 Phenolic Constituents in Spatholobi Caulis by High Performance Liquid Chromatography/Electrosprayionization Triple Quadrupole Mass Spectrometry. J. Pharm. Biomed. Anal. 2015, 102, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.X.; Xu, J.; Zhang, S.J.; Wang, R.J.; Huang, Q.; Chen, H.B.; Dong, X.P.; Zhao, Z.Z. Qualitatively and Quantitatively Comparing Secondary Metabolites in Three Medicinal Parts Derived from Poria cocos (Schw.) Wolf using UHPLC-QTOF-MS/MS-Based Chemical Profiling. J. Pharm. Biomed. Anal. 2018, 150, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.X.; Li, J.H.; Zhang, X.; Pei, J.; Huang, L.F. An Integrated LC-MS-Based Strategy for the Quality Assessment and Discrimination of three Panax Species. Molecules 2018, 23, 2988–3008. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Tang, Z.S.; Song, Z.X.; Liu, Y.R.; Hu, J.H.; Shi, X.B.; Sun, C.; Jiang, D.H.; Li, X.H. Quantitative Determination of Nine Furanocoumarins for Quality Evaluation of Angelica dahurica from Different Habitats. China J. Chin. Mater. Med. 2019, 44, 3002–3009. [Google Scholar]
- Chen, S.Y.; Shi, J.J.; Zou, L.S.; Liu, X.H.; Tang, R.M.; Ma, J.M.; Wang, C.C.; Tan, M.X.; Chen, J.L. Quality Evaluation of Wild and Cultivated Schisandrae chinensis Fructus Based on Simultaneous Determination of Multiple Bioactive Constituents Combined with Multivariate Statistical Analysis. Molecules 2019, 24, 1335–1350. [Google Scholar] [CrossRef]
- Tan, M.X.; Chen, J.L.; Wang, C.C.; Zou, L.S.; Chen, S.Y.; Shi, J.J.; Mei, Y.Q.; Wei, L.F.; Liu, X.H. Quality Evaluation of Ophiopogonis Radix from two Different Producing Areas. Molecules 2019, 24, 3220–3233. [Google Scholar] [CrossRef]
- Senizza, B.; Rocchetti, G.; Ghisoni, S.; Busconi, M.; De Los Mozos Pascual, M.; Fernandez, J.A.; Lucini, L.; Trevisan, M. Identification of Phenolic Markers for Saffron Authenticity and Origin: An Untargeted Metabolomics Approach. Food. Res. Int. 2019, 126, 108584–108590. [Google Scholar] [CrossRef]
- Peng, H.S.; Zhang, H.T.; Peng, D.Y.; Cheng, M.E.; Zha, L.P.; Huang, L.Q. Evolution and Characteristics of System, Assessing Quality by Distinguishing Features of Traditional Chinese Medicinal Materials, of Dao-Di Herbs of Astragali Radix. China J. Chin. Mater. Med. 2017, 42, 1646–1651. [Google Scholar]
- Wang, P.; Jiang, X.H.; Wang, L. Assessment of Matrix Effect in Quantitative Bioanalytical Methods Based on LC-MSn. Chin. New Drugs J. 2011, 20, 1953–1956. [Google Scholar]
- Wang, L.Q.; He, L.M.; Zeng, Z.L.; Chen, J.X. Progress in Matrix Effect of Veterinary Drug Residues Analysis by High-Performance Liquid Chromatography Tandem Mass Spectrometry. J. Mass. Spectrom. 2011, 32, 321–332. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |
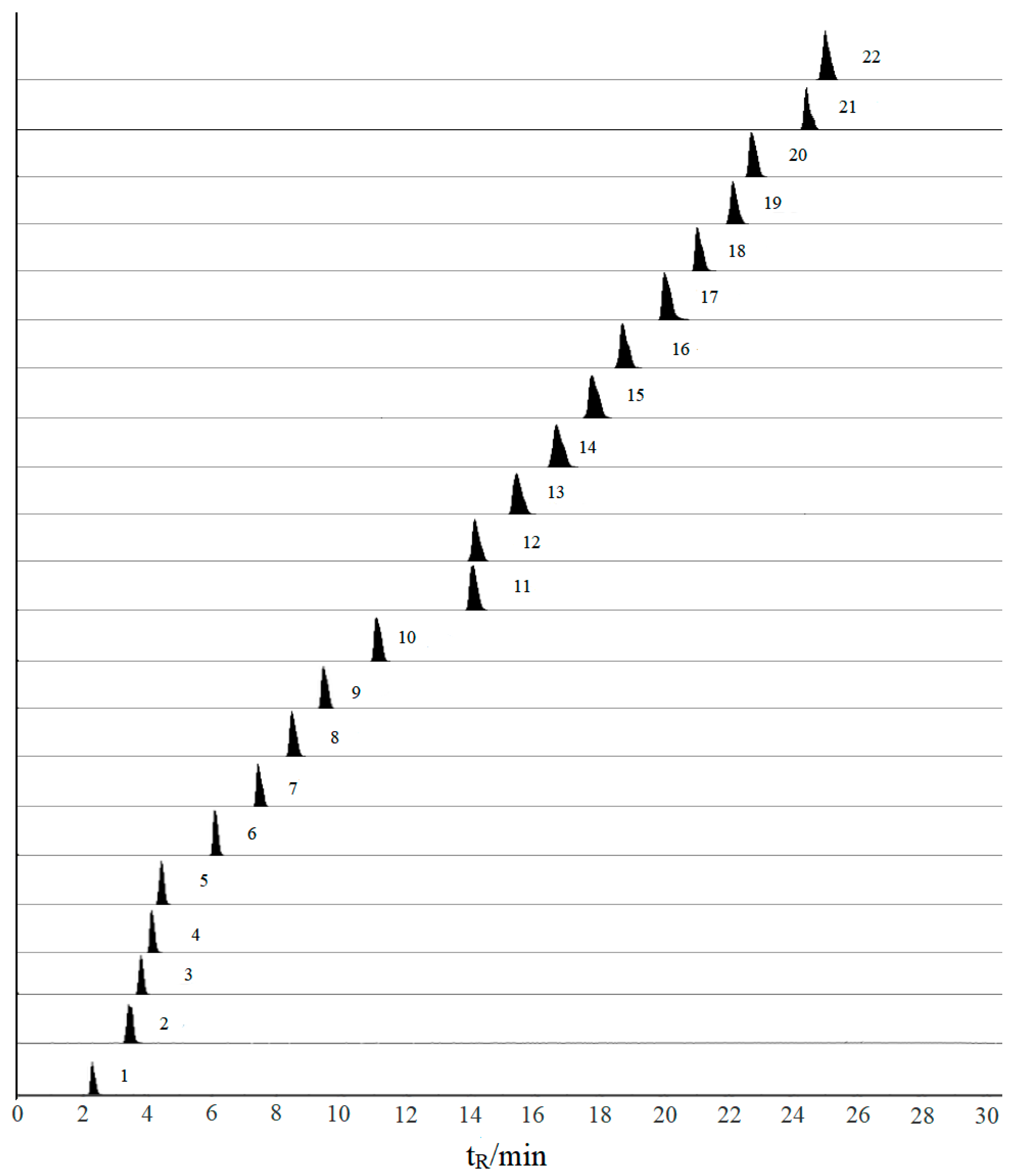
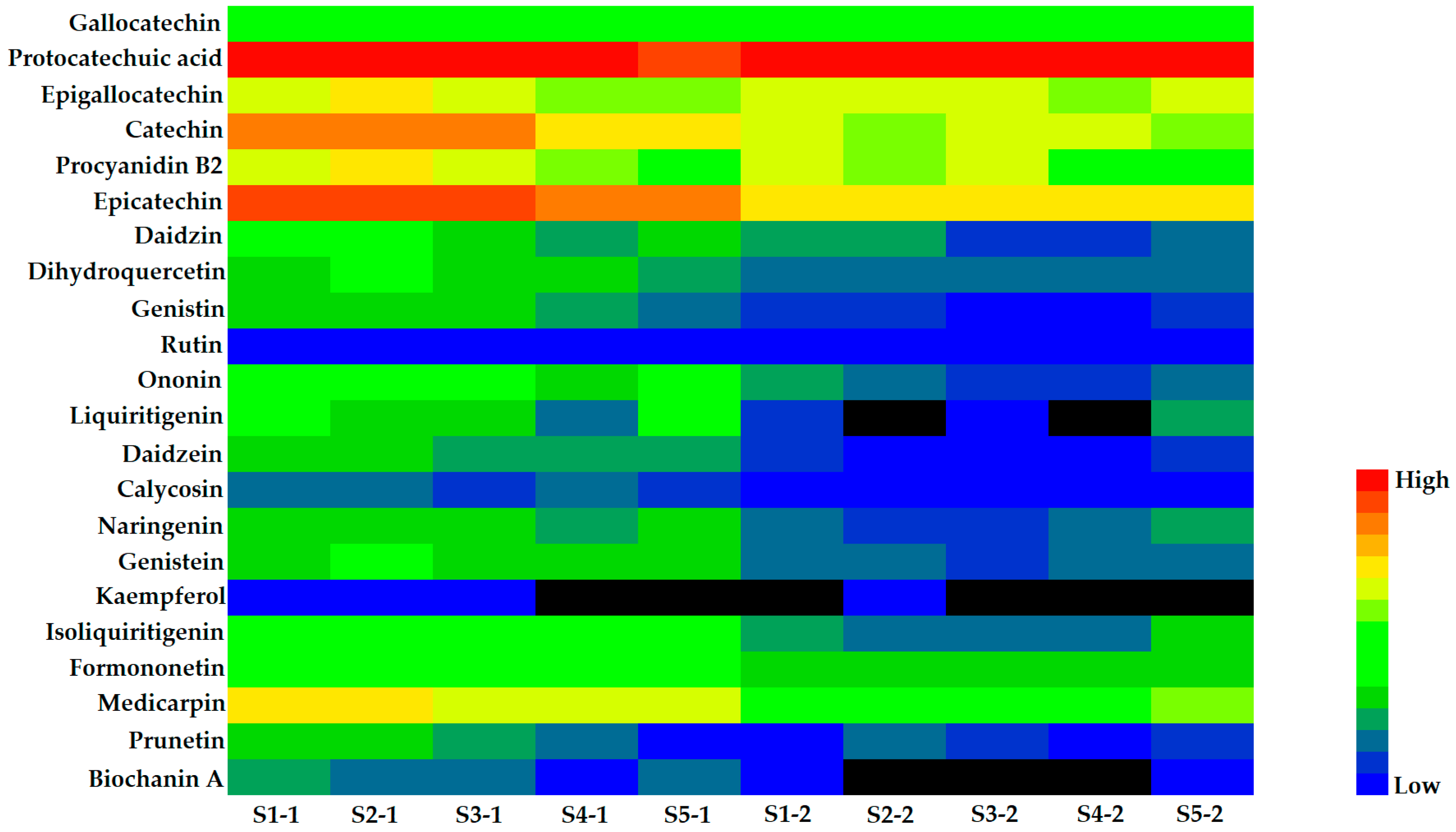
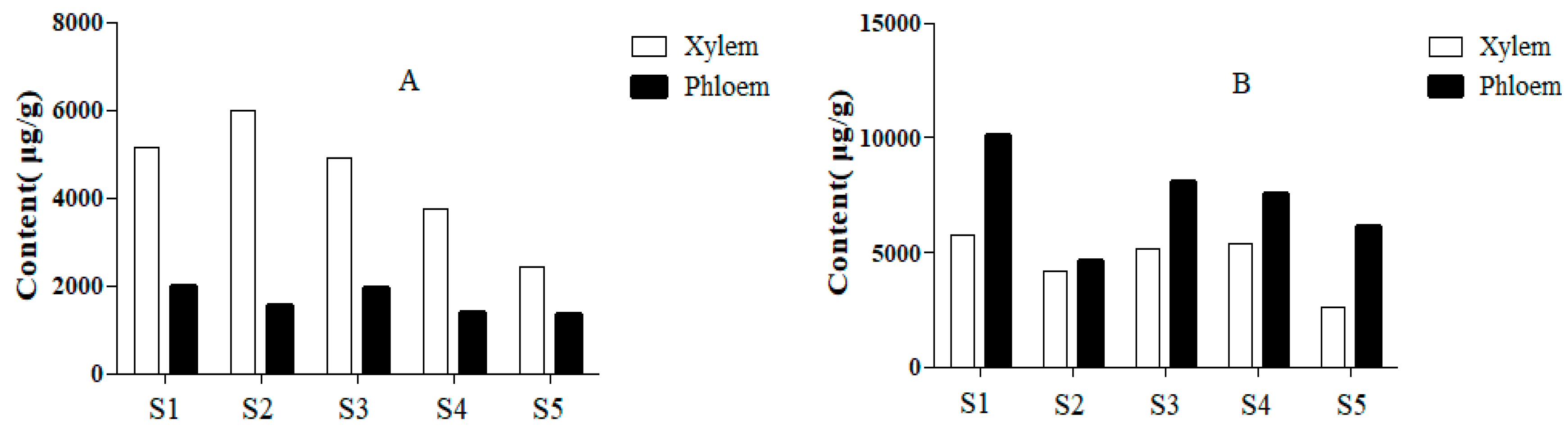
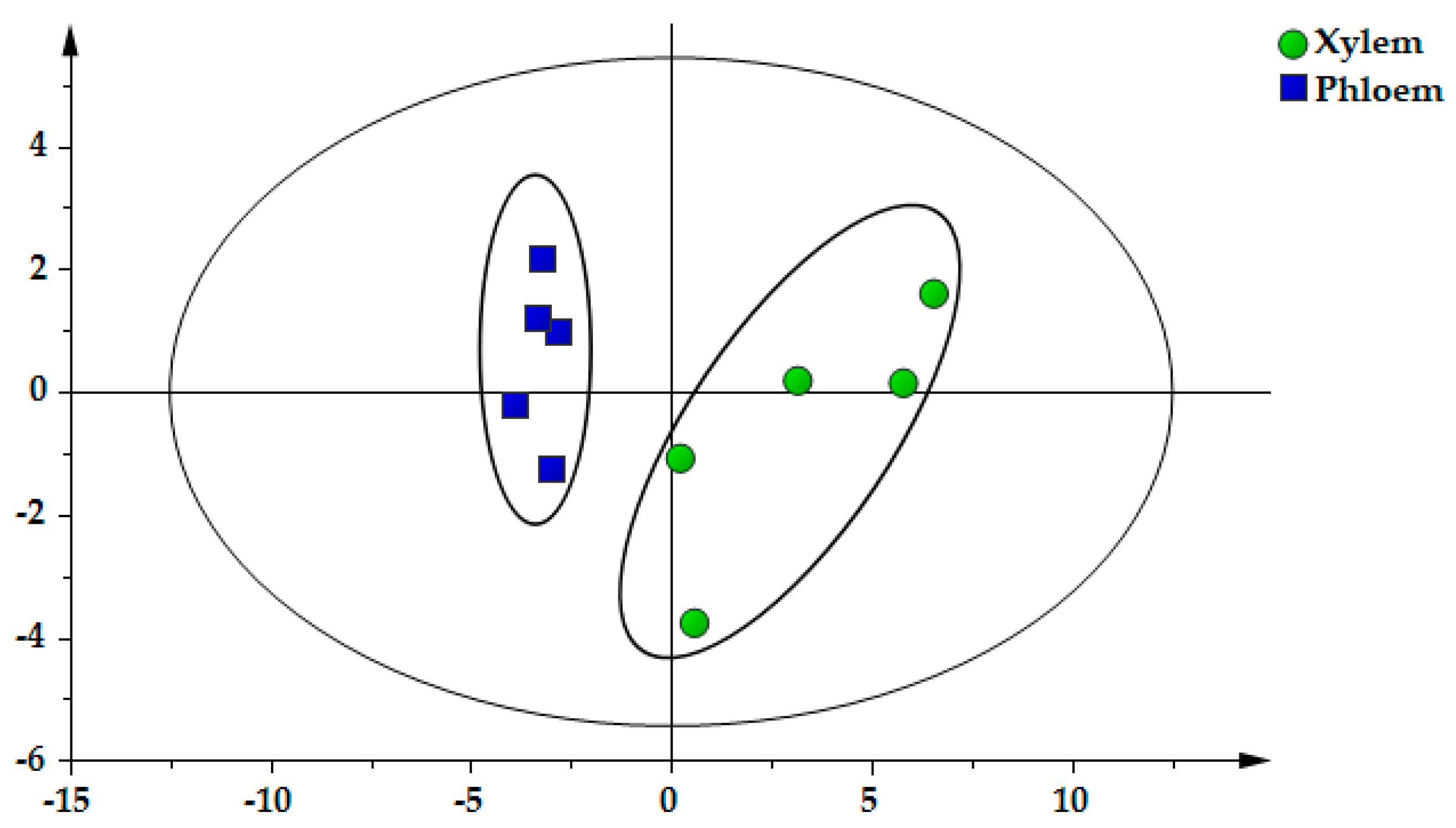

| No. | Compounds | tR (min) | Precursor Ion (m/z) | Product Ion (m/z) | DP (V) | CE (eV) |
|---|---|---|---|---|---|---|
| 1 | Gallocatechin | 2.30 | 307.10 | 139.00 | 100 | 13 |
| 2 | Protocatechuic acid | 3.39 | 152.90 | 109.00 | −85 | −18 |
| 3 | Epigallocatechin | 3.69 | 307.10 | 139.00 | 100 | 13 |
| 4 | Catechin | 4.06 | 291.10 | 139.00 | 90 | 13 |
| 5 | Procyanidin B2 | 4.34 | 579.20 | 291.10 | 120 | 13 |
| 6 | Epicatechin | 5.93 | 291.10 | 139.00 | 90 | 13 |
| 7 | Daidzin | 7.24 | 417.70 | 256.60 | 32 | 23 |
| 8 | Dihydroquercetin | 8.24 | 305.10 | 153.10 | 105 | 15 |
| 9 | Genistin | 9.20 | 431.30 | 268.00 | −105 | −39 |
| 10 | Rutin | 10.74 | 610.93 | 302.94 | 30 | 18 |
| 11 | Ononin | 13.63 | 431.10 | 269.10 | 100 | 10 |
| 12 | Liquiritigenin | 13.75 | 257.10 | 137.10 | 125 | 15 |
| 13 | Daidzein | 14.94 | 255.10 | 199.10 | 155 | 29 |
| 14 | Calycosin | 16.24 | 285.10 | 270.00 | 135 | 25 |
| 15 | Naringenin | 17.23 | 273.00 | 153.00 | 120 | 25 |
| 16 | Genistein | 18.16 | 271.10 | 91.00 | 155 | 35 |
| 17 | Kaempferol | 19.56 | 257.00 | 137.10 | −120 | −36 |
| 18 | Isoliquiritigenin | 20.38 | 285.00 | 116.90 | 115 | 15 |
| 19 | Formononetin | 21.54 | 269.10 | 197.10 | 135 | 35 |
| 20 | Medicarpin | 22.02 | 271.47 | 137.08 | 116 | 19 |
| 21 | Prunetin | 23.79 | 285.10 | 192.00 | 165 | 30 |
| 22 | Biochanin A | 24.36 | 285.10 | 192.00 | 165 | 30 |
| No. | Compounds | Regression Equation | r2 | Liner Range (ng/mL) | LOD (ng/mL) | LOQ (ng/mL) | Precision RSD (%) | Repeat-Ability RSD (%) (n = 6) | Stability RSD (%)(n = 6) | Recovery (%) (n = 9) | Matrix Effect | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intra–Day (n = 6) | Inter–Day (n = 9) | Low | Medium | High | |||||||||||||
| Mean | RSD (%) | Mean | RSD (%) | Mean | RSD (%) | ||||||||||||
| 1 | Gallocatechin | Y = 4000X − 102,000 | 0.9995 | 40.00–4000 | 2.03 | 6.75 | 3.46 | 3.47 | 4.42 | 3.71 | 99.37 | 0.97 | 100.80 | 1.18 | 98.17 | 3.26 | 1.03 |
| 2 | Protocatechuic acid | Y = 63.3X + 233,000 | 0.9994 | 58.59–600,000 | 7.99 | 26.63 | 2.75 | 3.81 | 1.65 | 3.08 | 98.29 | 3.05 | 100.96 | 4.57 | 98.36 | 2.96 | 0.98 |
| 3 | Epigallocatechin | Y = 2650X − 348,000 | 0.9999 | 164.82–16,500 | 4.31 | 14.36 | 0.93 | 2.79 | 3.71 | 3.35 | 98.51 | 3.14 | 97.90 | 1.41 | 98.21 | 1.08 | 0.95 |
| 4 | Catechin | Y = 1990X + 330,000 | 0.9993 | 9.53–19,100 | 0.70 | 2.32 | 3.29 | 3.65 | 1.57 | 1.84 | 100.71 | 4.28 | 100.17 | 2.81 | 99.43 | 2.77 | 1.06 |
| 5 | Procyanidin B2 | Y = 506X + 21,000 | 0.9999 | 10.87–10,900 | 2.04 | 6.79 | 2.71 | 4.30 | 2.66 | 2.34 | 102.31 | 2.90 | 99.45 | 4.62 | 101.36 | 4.81 | 1.02 |
| 6 | Epicatechin | Y = 1900X + 428,000 | 0.9996 | 3.13–31,300 | 0.82 | 2.74 | 2.43 | 3.09 | 2.46 | 4.24 | 100.16 | 2.46 | 102.87 | 0.96 | 100.22 | 1.57 | 1.05 |
| 7 | Daidzin | Y = 221X − 236 | 0.9997 | 5.63–1408 | 0.94 | 3.13 | 3.48 | 3.76 | 2.03 | 2.68 | 100.27 | 0.76 | 100.30 | 2.90 | 99.24 | 2.44 | 1.00 |
| 8 | Dihydroquercetin | Y = 494X + 2340 | 0.9997 | 10.20–511 | 1.02 | 3.40 | 4.19 | 2.22 | 3.03 | 2.44 | 101.22 | 2.85 | 103.93 | 1.13 | 97.99 | 2.02 | 1.04 |
| 9 | Genistin | Y = 658X − 6 | 0.9997 | 1.26–630 | 0.27 | 0.90 | 2.68 | 1.86 | 1.69 | 0.73 | 103.83 | 0.95 | 101.18 | 4.41 | 100.77 | 4.94 | 0.96 |
| 10 | Rutin | Y = 5300X + 3540 | 0.9990 | 0.37–36.90 | 0.09 | 0.28 | 3.29 | 3.55 | 1.08 | 4.16 | 100.73 | 1.56 | 102.37 | 2.45 | 99.52 | 1.32 | 0.99 |
| 11 | Ononin | Y = 7620X + 77,700 | 0.9995 | 3.78–3785 | 0.79 | 2.63 | 2.55 | 4.16 | 1.99 | 1.90 | 101.99 | 2.02 | 101.28 | 1.17 | 99.93 | 4.46 | 1.04 |
| 12 | Liquiritigenin | Y = 1420X + 18,500 | 0.9991 | 14.52–1450 | 2.59 | 8.65 | 3.31 | 2.95 | 3.67 | 4.11 | 96.69 | 2.08 | 102.00 | 1.95 | 100.32 | 2.28 | 1.04 |
| 13 | Daidzein | Y = 8280X + 11,500 | 0.9997 | 1.54–772 | 0.3 | 1.00 | 4.20 | 3.53 | 4.23 | 2.60 | 99.38 | 4.19 | 98.76 | 2.81 | 101.80 | 2.16 | 1.02 |
| 14 | Calycosin | Y = 12,700X − 15,000 | 0.9995 | 2.44–244 | 0.17 | 0.58 | 1.08 | 3.62 | 2.77 | 3.52 | 95.88 | 1.20 | 97.98 | 3.01 | 100.71 | 3.87 | 0.94 |
| 15 | Naringenin | Y = 6160X + 91,100 | 0.9996 | 6.87–1370 | 1.04 | 3.47 | 1.91 | 3.86 | 3.39 | 1.16 | 96.91 | 2.78 | 99.66 | 4.24 | 99.96 | 4.26 | 0.99 |
| 16 | Genistein | Y = 1280X + 6000 | 0.9996 | 15.30–611 | 1.87 | 6.24 | 2.84 | 3.38 | 0.76 | 2.99 | 102.17 | 1.92 | 103.48 | 1.20 | 102.50 | 1.50 | 0.97 |
| 17 | Kaempferol | Y = 1630X + 194 | 0.9994 | 0.28–11.10 | 0.07 | 0.22 | 1.99 | 2.02 | 2.83 | 2.34 | 103.68 | 0.91 | 103.21 | 1.38 | 102.22 | 1.49 | 1.02 |
| 18 | Isoliquiritigenin | Y = 961X + 15,700 | 0.9998 | 31.59–1260 | 4.12 | 13.73 | 3.02 | 3.55 | 1.27 | 1.09 | 103.35 | 1.01 | 101.06 | 3.15 | 101.91 | 2.11 | 1.00 |
| 19 | Formononetin | Y = 2530X + 71,300 | 0.9992 | 9.51–3800 | 1.95 | 6.51 | 1.46 | 3.62 | 1.01 | 1.39 | 103.22 | 2.04 | 102.84 | 1.06 | 99.17 | 3.65 | 0.93 |
| 20 | Medicarpin | Y = 2030X + 76,100 | 0.9995 | 19.29–19,292 | 3.33 | 11.09 | 1.03 | 1.88 | 3.72 | 3.78 | 101.02 | 4.78 | 97.78 | 3.97 | 101.09 | 3.15 | 0.98 |
| 21 | Prunetin | Y = 10.9X + 229 | 0.9990 | 5.80–232 | 1.39 | 4.64 | 2.23 | 1.75 | 2.23 | 2.70 | 97.95 | 3.19 | 102.44 | 3.10 | 101.39 | 4.73 | 1.00 |
| 22 | Biochanin A | Y = 21.3X + 131 | 0.9995 | 8.83–177 | 0.76 | 2.52 | 3.54 | 2.60 | 2.39 | 2.15 | 101.03 | 0.99 | 100.58 | 2.61 | 102.47 | 2.22 | 1.08 |
| No. | Compounds | Xylem | Phloem | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| S1–1 | S2–1 | S3–1 | S4–1 | S5–1 | S1–2 | S2–2 | S3–2 | S4–2 | S5–2 | ||
| 1 | Gallocatechin | 96.8 | 121.06 | 88.06 | 52.32 | 43.80 | 63.53 | 70.29 | 72.01 | 39.30 | 39.32 |
| 2 | Protocatechuic acid | 5745.66 | 4166.29 | 5161.65 | 5352.32 | 2586.10 | 10118.61 | 4607.29 | 8048.11 | 7515.76 | 6127.87 |
| 3 | Epigallocatechin | 369.74 | 488.65 | 296.56 | 216.59 | 159.92 | 450.73 | 377.96 | 416.70 | 225.61 | 253.64 |
| 4 | Catechin | 1214.57 | 1300.13 | 1244.85 | 853.02 | 483.92 | 293.38 | 210.51 | 306.38 | 270.38 | 217.70 |
| 5 | Procyanidin B2 | 399.01 | 559.15 | 456.37 | 229.12 | 64.43 | 258.62 | 176.45 | 284.25 | 94.48 | 55.76 |
| 6 | Epicatechin | 2124.84 | 2519.83 | 2177.69 | 1883.30 | 1088.00 | 766.72 | 619.46 | 808.65 | 677.54 | 583.03 |
| 7 | Daidzin | 35.90 | 63.91 | 20.34 | 7.12 | 11.74 | 7.53 | 7.21 | 2.32 | 2.39 | 3.91 |
| 8 | Dihydroquercetin | 17.34 | 26.25 | 20.78 | 16.62 | 11.45 | 4.42 | 3.59 | 4.02 | 5.40 | 5.34 |
| 9 | Genistin | 14.58 | 15.81 | 12.80 | 9.88 | 4.53 | 3.18 | 2.22 | 1.73 | 1.78 | 1.92 |
| 10 | Rutin | 0.26 | 0.15 | 0.20 | 0.08 | 0.12 | 1.01 | 0.28 | 0.06 | 0.62 | 0.13 |
| 11 | Ononin | 69.19 | 60.14 | 38.62 | 21.43 | 26.28 | 11.47 | 4.92 | 2.93 | 3.22 | 5.27 |
| 12 | Liquiritigenin | 25.18 | 21.02 | 14.12 | 3.74 | 25.11 | 2.17 | – | 1.43 | – | 7.15 |
| 13 | Daidzein | 12.06 | 14.72 | 7.63 | 8.11 | 6.29 | 2.05 | 1.46 | 0.80 | 1.29 | 2.12 |
| 14 | Calycosin | 3.76 | 5.06 | 3.21 | 3.57 | 2.61 | 0.89 | 0.98 | 0.94 | 1.05 | 1.32 |
| 15 | Naringenin | 19.95 | 11.96 | 14.42 | 7.24 | 20.11 | 4.20 | 1.95 | 2.22 | 3.72 | 7.00 |
| 16 | Genistein | 19.14 | 21.64 | 11.95 | 12.89 | 12.73 | 4.12 | 3.99 | 3.31 | 4.36 | 6.01 |
| 17 | Kaempferol | 0.03 | 0.03 | 0.03 | – | – | – | 0.03 | – | – | – |
| 18 | Isoliquiritigenin | 59.97 | 51.23 | 41.14 | 22.93 | 55.29 | 8.77 | 4.64 | 4.08 | 5.54 | 17.00 |
| 19 | Formononetin | 92.83 | 84.15 | 82.57 | 28.73 | 56.87 | 15.52 | 16.63 | 12.08 | 14.50 | 18.50 |
| 20 | Medicarpin | 557.83 | 597.30 | 371.66 | 353.01 | 352.41 | 93.27 | 58.31 | 38.50 | 45.52 | 136.22 |
| 21 | Prunetin | 18.08 | 12.95 | 6.29 | 3.63 | 1.18 | 1.79 | 5.61 | 3.25 | 1.38 | 3.02 |
| 22 | Biochanin A | 7.51 | 3.90 | 3.49 | 1.17 | 3.59 | 1.69 | – | – | – | 0.97 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mei, Y.; Wei, L.; Chai, C.; Zou, L.; Liu, X.; Chen, J.; Tan, M.; Wang, C.; Cai, Z.; Zhang, F.; et al. A Method to Study the Distribution Patterns for Metabolites in Xylem and Phloem of Spatholobi Caulis. Molecules 2020, 25, 167. https://doi.org/10.3390/molecules25010167
Mei Y, Wei L, Chai C, Zou L, Liu X, Chen J, Tan M, Wang C, Cai Z, Zhang F, et al. A Method to Study the Distribution Patterns for Metabolites in Xylem and Phloem of Spatholobi Caulis. Molecules. 2020; 25(1):167. https://doi.org/10.3390/molecules25010167
Chicago/Turabian StyleMei, Yuqi, Lifang Wei, Chuan Chai, Lisi Zou, Xunhong Liu, Jiali Chen, Mengxia Tan, Chengcheng Wang, Zhichen Cai, Furong Zhang, and et al. 2020. "A Method to Study the Distribution Patterns for Metabolites in Xylem and Phloem of Spatholobi Caulis" Molecules 25, no. 1: 167. https://doi.org/10.3390/molecules25010167
APA StyleMei, Y., Wei, L., Chai, C., Zou, L., Liu, X., Chen, J., Tan, M., Wang, C., Cai, Z., Zhang, F., & Yin, S. (2020). A Method to Study the Distribution Patterns for Metabolites in Xylem and Phloem of Spatholobi Caulis. Molecules, 25(1), 167. https://doi.org/10.3390/molecules25010167






