Fe-Loaded MOF-545(Fe): Peroxidase-Like Activity for Dye Degradation Dyes and High Adsorption for the Removal of Dyes from Wastewater
Abstract
1. Introduction
2. Results and Discussion
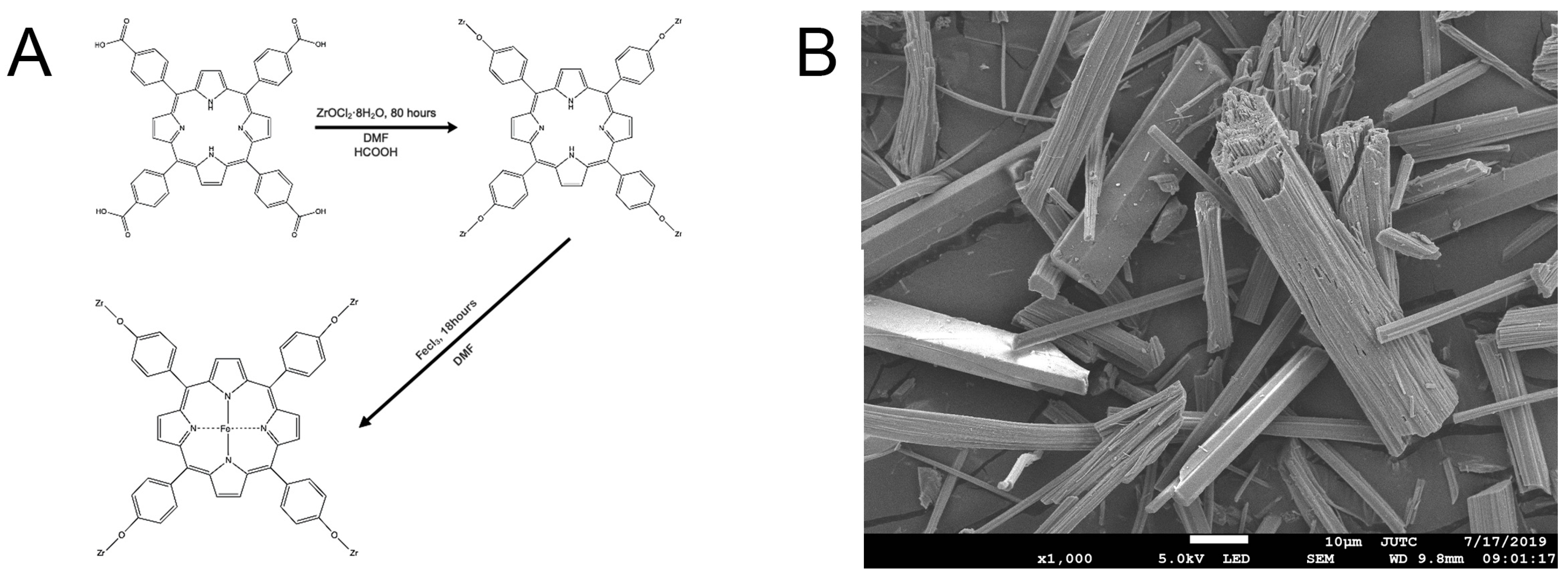
2.1. Sample Characterization of Fe-Loaded MOF-545(Fe)
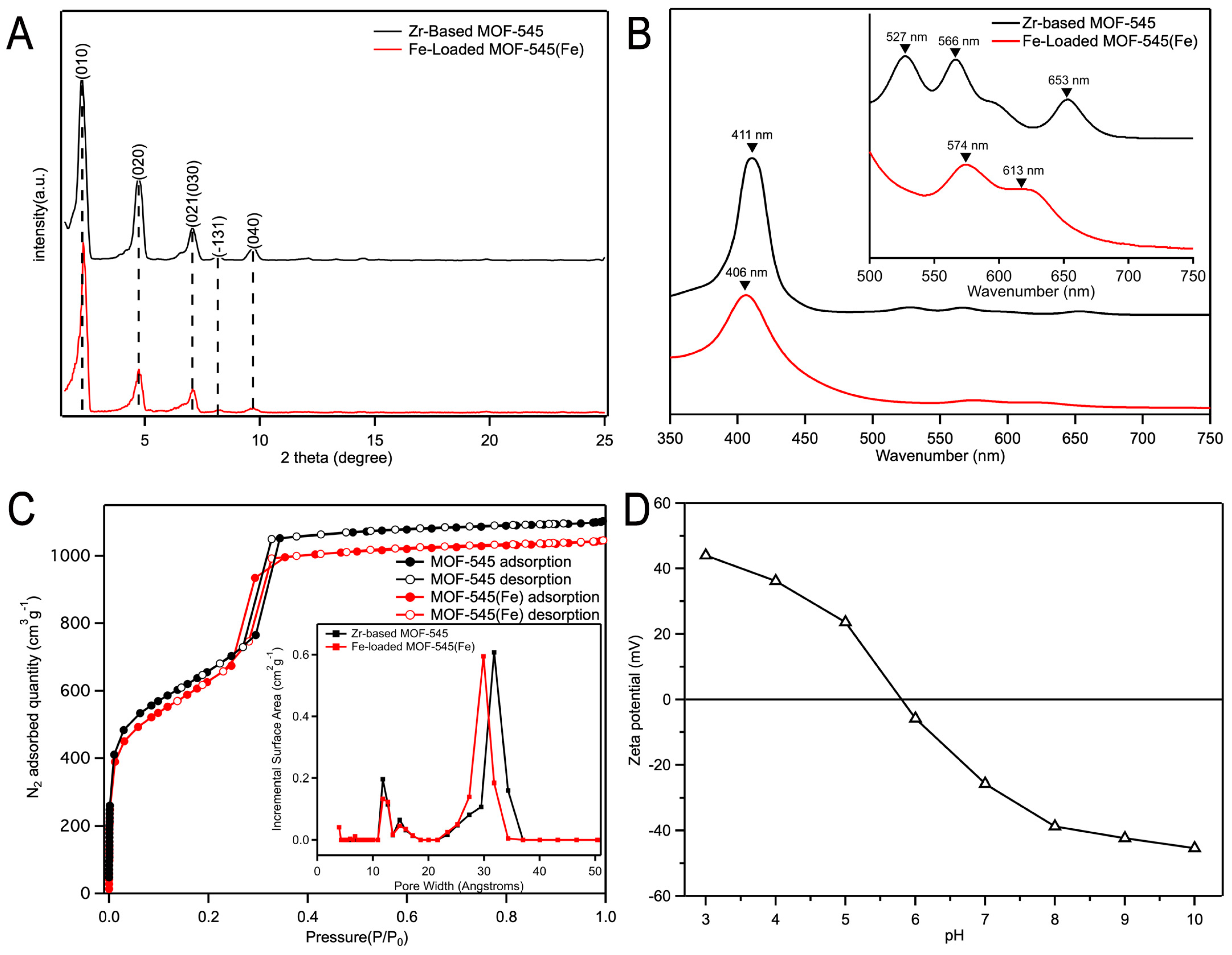
2.1.1. Sample Characterization by SEM, XRD, and UV-Vis Absorption Spectra
2.1.2. N2 Adsorption/Desorption Isotherms
2.1.3. Zeta Potential Analysis of Fe-Loaded MOF-545(Fe)
2.2. The Enzyme-Like Activities of the Fe-Loaded MOF-545(Fe)
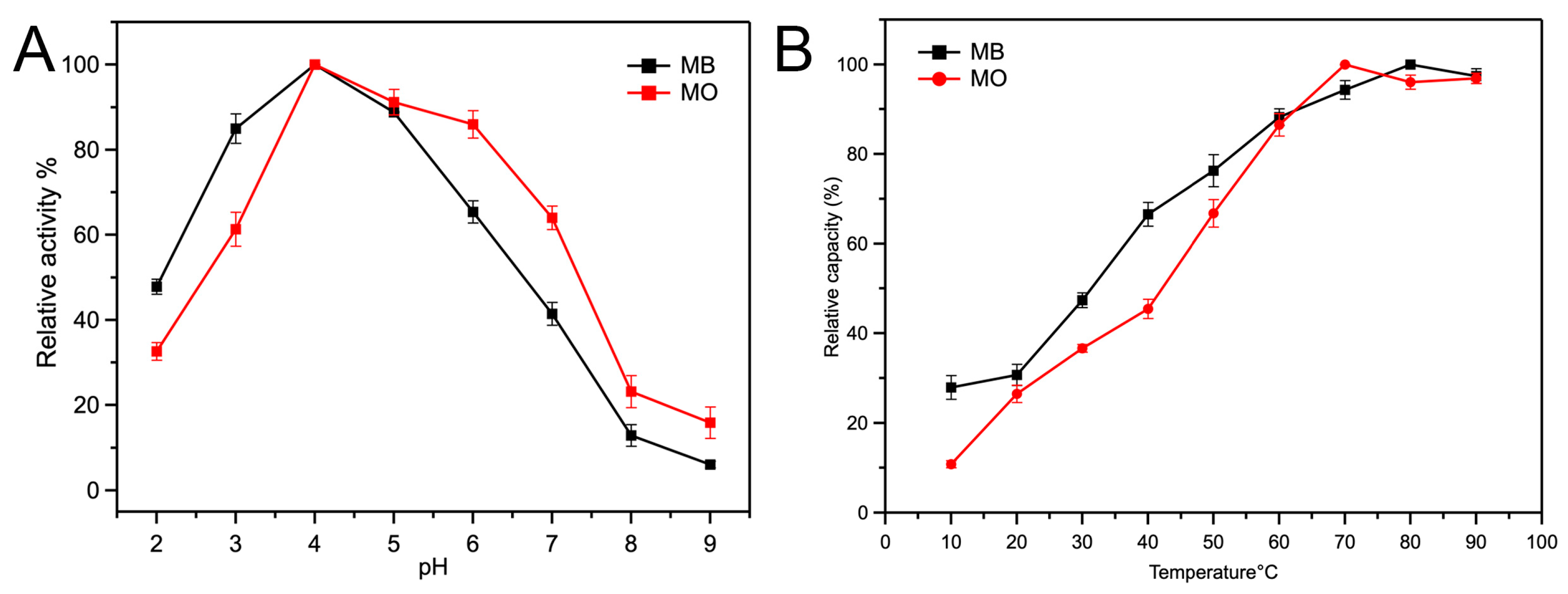
2.2.1. Intrinsic Peroxidase Activity of Fe-Loaded MOF-545(Fe)
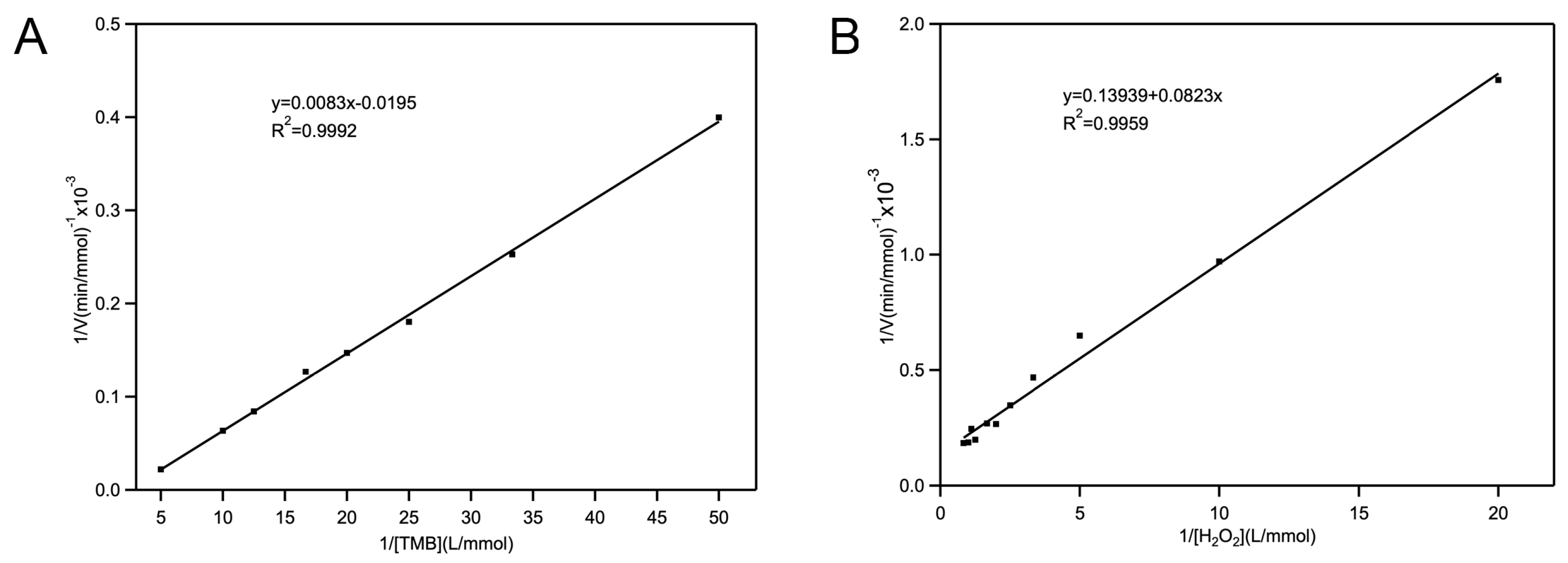
2.2.2. Kinetic Studies of Fe-Loaded MOF-545(Fe) POD-Like Activity
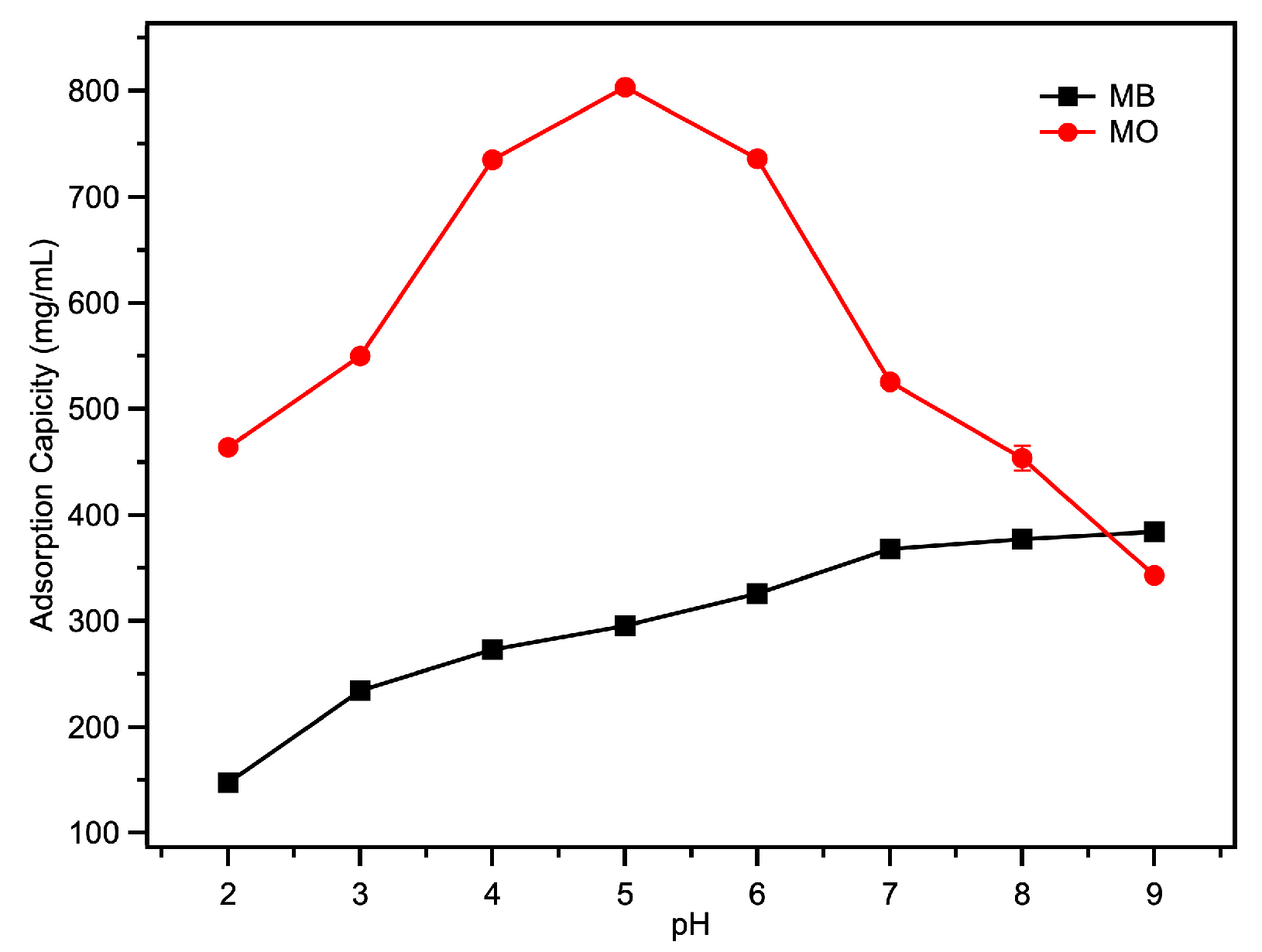
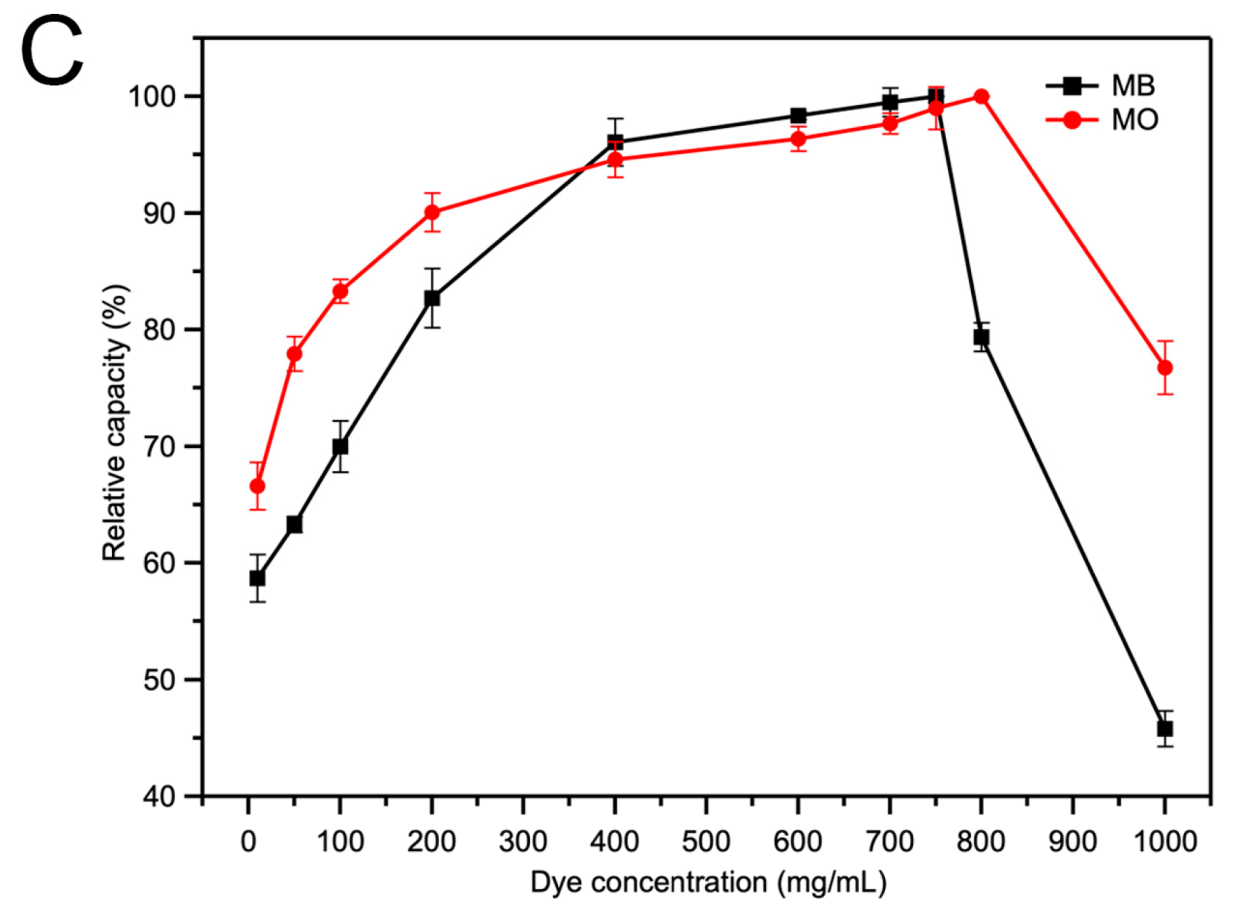
2.3. The Adsorption of Acid/Basic Dyes
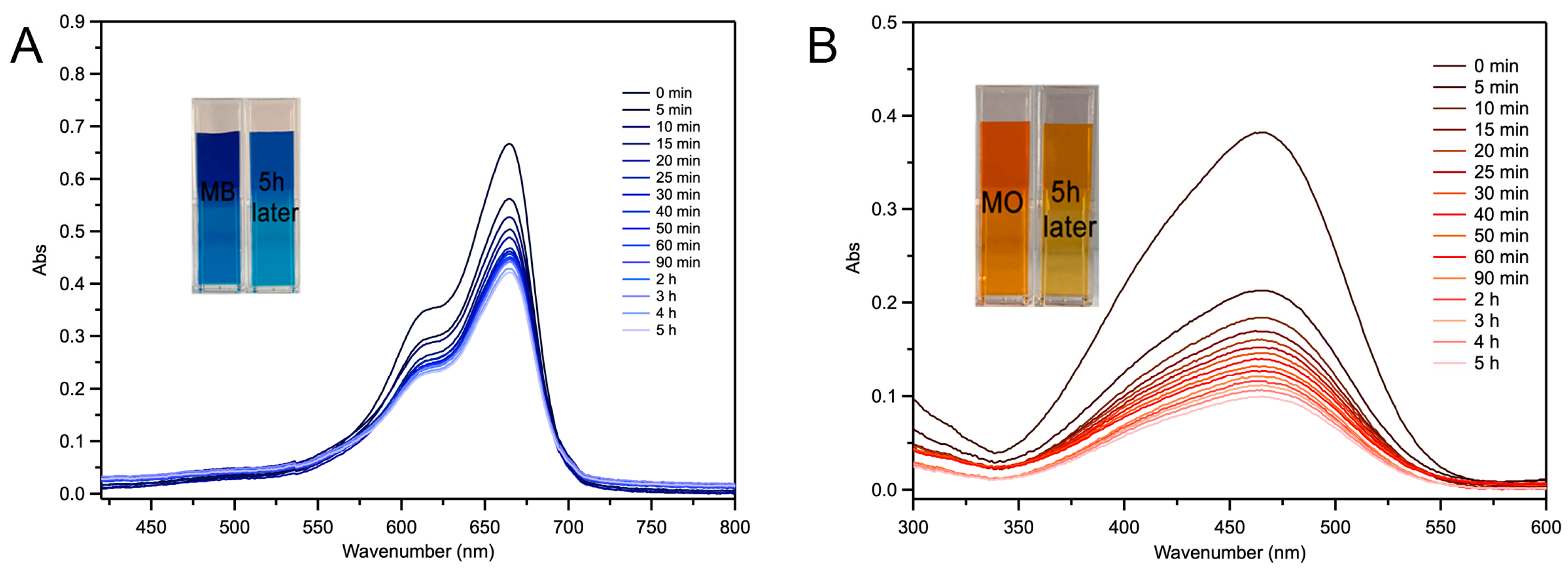
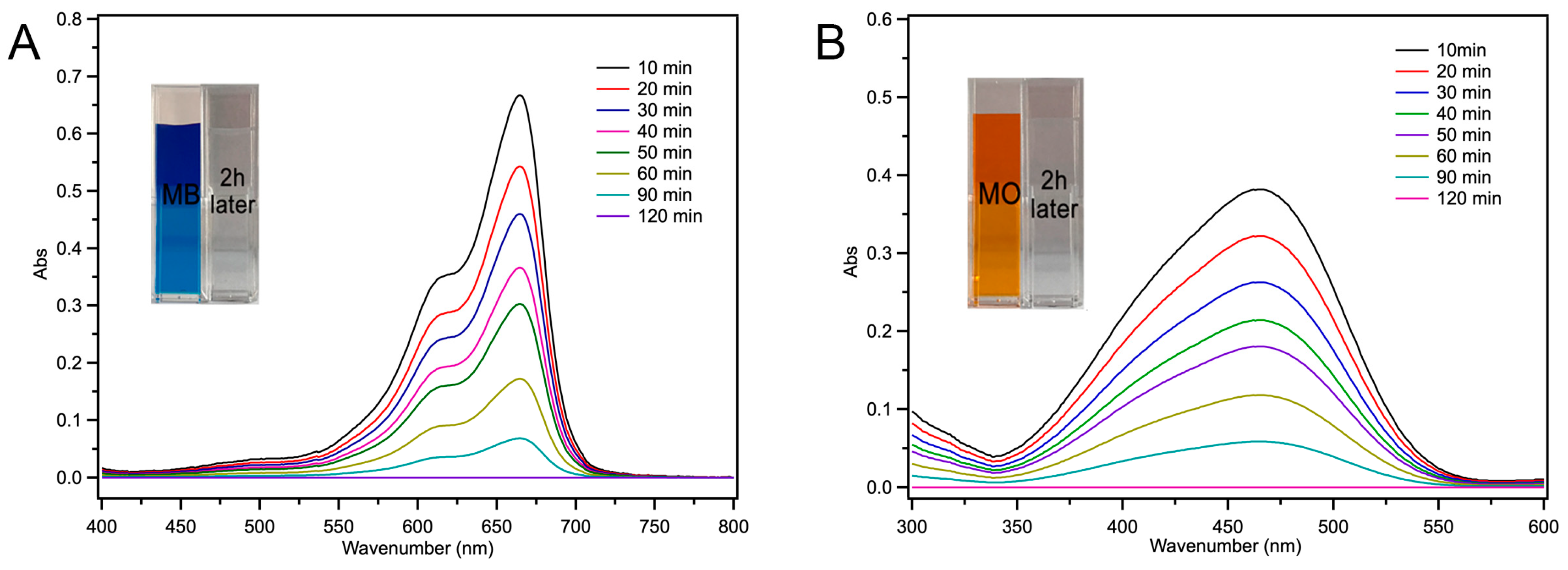
2.4. Fe-Loaded MOF-545(Fe) Degradation of the Acid/Basic Dyes
3. Materials and Methods
3.1. Materials
3.2. Preparation of Fe-Loaded MOF-545(Fe)
3.3. Characterization of Fe-Loaded MOF-545(Fe)
3.4. POD-Like Activity of Fe-Loaded MOF-545(Fe)
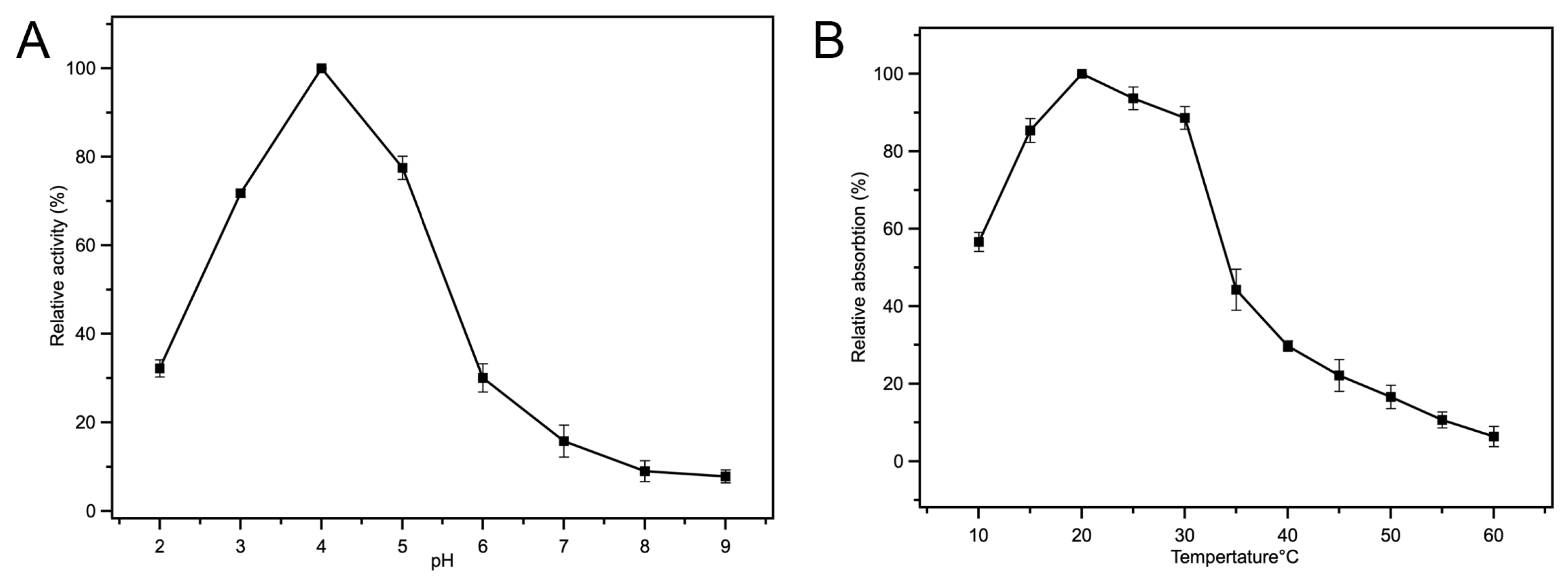
3.4.1. Influence of Reaction pH
3.4.2. Influence of Incubation Temperature
3.4.3. Steady-State Kinetics Parameters Analysis
3.5. Adsorption of Dyes from Water without H2O2
3.6. Degradation of Dyes from Water with H2O2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ambashta, R.D.; Sillanpää, M. Water purification using magnetic assistance: A review. J. Hazard. Mater. 2010, 180, 38–49. [Google Scholar] [CrossRef]
- Padhi, B.S. Pollution due to synthetic dyes toxicity & carcinogenicity studies and remediation. Int. J. Environ. Sci. 2012, 3, 940–955. [Google Scholar]
- Sen, T.K.; Afroze, S.; Ang, H.M. Equilibrium, kinetics and mechanism of removal of methylene blue from aqueous solution by adsorption onto pine cone biomass of pinus radiata. Water Air Soil Pollut. 2011, 218, 499–515. [Google Scholar] [CrossRef]
- Yagub, M.T.; Sen, T.K.; Ang, H.M. Equilibrium, kinetics, and thermodynamics of methylene blue adsorption by pine tree leaves. Water Air Soil Pollut. 2012, 223, 5267–5282. [Google Scholar] [CrossRef]
- Yagub, M.T.; Sen, T.K.; Afroze, S.; Ang, H.M. Dye and its removal from aqueous solution by adsorption: A review. Adv. Colloid Interface Sci. 2014, 209, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Sarker, M.; Shin, S.; Jeong, J.H.; Jhung, S.H. Mesoporous metal-organic framework PCN-222 (Fe): Promising adsorbent for removal of big anionic and cationic dyes from water. Chem. Eng. J. 2019, 371, 252–259. [Google Scholar] [CrossRef]
- Parasuraman, D.; Serpe, M.J. Poly(n-isopropylacrylamide) microgels for organic dye removal from water. ACS Appl. Mater. Interfaces 2011, 3, 2732–2737. [Google Scholar] [CrossRef]
- Gupta, V.K.; Saleh, T.A. Sorption of pollutants by porous carbon, carbon nanotubes and fullerene—An overview. Environ. Sci. Pollut. Res. Int. 2013, 20, 2828–2843. [Google Scholar] [CrossRef]
- Raman, C.D.; Kanmani, S. Textile dye degradation using nano zero valent iron: A review. J. Environ. Manag. 2016, 177, 341–355. [Google Scholar] [CrossRef]
- Rafatullah, M.; Sulaiman, O.; Hashim, R.; Ahmad, A. Adsorption of methylene blue on low-cost adsorbents: A review. J. Hazard. Mater. 2010, 177, 70–80. [Google Scholar] [CrossRef]
- Mezohegyi, G.; van der Zee, F.P.; Font, J.; Fortuny, A.; Fabregat, A. Towards advanced aqueous dye removal processes: A short review on the versatile role of activated carbon. J. Environ. Manag. 2012, 102, 148–164. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, X.; Lee, D.J.; Tay, J.H.; Zhang, Y.; Wan, C.L.; Chen, X.F. Recent advances on biosorption by aerobic granular sludge. J. Hazard. Mater. 2018, 357, 253–270. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, L.; Long, X.; Zhang, X.; Pan, B.; Qian, J. Multi-functional magnetic water purifier for disinfection and removal of dyes and metal ions with superior reusability. J. Hazard. Mater. 2018, 347, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Gomez, J.L.; Bodalo, A.; Gomez, E.; Bastida, J.; Hidalgo, A.M.; Gomez, M. Immobilization of peroxidases on glass beads: An improved alternative for phenol removal. Enzym. Microb. Technol. 2006, 39, 1016–1022. [Google Scholar] [CrossRef]
- Mohideen, M.I.H.; Pillai, R.S.; Adil, K.; Bhatt, P.M.; Belmabkhout, Y.; Shkurenko, A.; Maurin, G.; Eddaoudi, M. A fine-tuned mof for gas and vapor separation: A multipurpose adsorbent for acid gas removal, dehydration, and btx sieving. Chem 2017, 3, 822–833. [Google Scholar] [CrossRef]
- AbdulHalim, R.G.; Bhatt, P.M.; Belmabkhout, Y.; Shkurenko, A.; Adil, K.; Barbour, L.J.; Eddaoudi, M. A fine-tuned metal–organic framework for autonomous indoor moisture control. J. Am. Chem. Soc. 2017, 139, 10715–10722. [Google Scholar] [CrossRef]
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; Van Duyne, R.P.; Hupp, J.T. Metal–organic framework materials as chemical sensors. Chem. Rev. 2011, 112, 1105–1125. [Google Scholar] [CrossRef]
- Tchalala, M.R.; Bhatt, P.M.; Chappanda, K.N.; Tavares, S.R.; Adil, K.; Belmabkhout, Y.; Shkurenko, A.; Cadiau, A.; Heymans, N.; De Weireld, G. Fluorinated mof platform for selective removal and sensing of SO2 from flue gas and air. Nat. Commun. 2019, 10, 1328–1338. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, Y.; Liu, H.; Liu, Z. Fabrication of porous metal–organic frameworks via a mixed-ligand strategy for highly selective and efficient dye adsorption in aqueous solution. Crystengcomm 2015, 17, 6037–6043. [Google Scholar] [CrossRef]
- Hahm, H.; Kim, S.; Ha, H.; Jung, S.; Kim, Y.; Yoon, M.; Kim, M. Charged functional group effects on a metal–organic framework for selective organic dye adsorptions. CrystEngComm 2015, 17, 8418–8422. [Google Scholar] [CrossRef]
- Qi, Z.-P.; Yang, J.-M.; Kang, Y.-S.; Guo, F.; Sun, W.-Y. Facile water-stability evaluation of metal–organic frameworks and the property of selective removal of dyes from aqueous solution. Dalton Trans. 2016, 45, 8753–8759. [Google Scholar] [CrossRef]
- Morris, W.; Volosskiy, B.; Demir, S.; Gándara, F.; McGrier, P.L.; Furukawa, H.; Cascio, D.; Stoddart, J.F.; Yaghi, O.M. Synthesis, structure, and metalation of two new highly porous zirconium metal–organic frameworks. Inorg. Chem. 2012, 51, 6443–6445. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Gu, Z.Y.; Li, J.R.; Jiang, H.L.; Wei, Z.; Zhou, H.C. Zirconium-metalloporphyrin PCN-222: Mesoporous metal–organic frameworks with ultrahigh stability as biomimetic catalysts. Angew. Chem. Int. Ed. 2012, 51, 10307–10310. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Cao, X.; Zhang, C.; Yu, Q.; Zhao, Z.; Niu, X.; Sun, X.; Liu, Y.; Ma, L.; Li, Z. Enhanced adsorptive removal of anionic and cationic dyes from single or mixed dye solutions using MOF PCN-222. Rsc Adv. 2017, 7, 16273–16281. [Google Scholar] [CrossRef]
- Kalsoom, U.; Bhatti, H.N.; Asgher, M. Characterization of plant peroxidases and their potential for degradation of dyes: A review. Appl. Biochem. Biotechnol. 2015, 176, 1529–1550. [Google Scholar] [CrossRef] [PubMed]
- Mielgo, I.; López, C.; Moreira, M.T.; Feijoo, G.; Lema, J.M. Oxidative degradation of azo dyes by manganese peroxidase under optimized conditions. Biotechnol. Prog. 2003, 19, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, L.; Zeng, T.; Shang, Q.; Zhou, H.; Pan, Z.; Cheng, Q. Two pure MOF-photocatalysts readily prepared for the degradation of methylene blue dye under visible light. Dalton Trans. 2018, 47, 4251–4258. [Google Scholar] [CrossRef]
- Zong, Z.-A.; Fan, C.-B.; Zhang, X.; Meng, X.-M.; Jin, F.; Fan, Y.-H. Four Co (ii) coordination polymers based on 4,4′-(1h-1,2,4-triazol-1-yl) methylenebis (benzoic acid): Syntheses, structural diversity, magnetic properties, dye adsorption and photocatalytic properties. CrystEngComm 2019, 21, 673–686. [Google Scholar] [CrossRef]
- Othmani, A.; Kesraoui, A.; Boada, R.; Seffen, M.; Valiente, M. Textile wastewater purification using an elaborated biosorbent hybrid material (luffa–cylindrica–zinc oxide) assisted by alternating current. Water 2019, 11, 1326. [Google Scholar] [CrossRef]
- Das, T.R.; Sharma, P.K. Bimetal oxide decorated graphene oxide (Gd2O3/Bi2O3@ GO) nanocomposite as an excellent adsorbent in the removal of methyl orange dye. Mater. Sci. Semicond. Process. 2020, 105, 104721. [Google Scholar] [CrossRef]
- Tokalioglu, S.; Yavuz, E.; Demir, S.; Patat, S. Zirconium-based highly porous metal-organic framework (MOF-545) as an efficient adsorbent for vortex assisted-solid phase extraction of lead from cereal, beverage and water samples. Food Chem. 2017, 237, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Yang, L.; Zhang, H.; Chang, K.; Zhao, G.; Kako, T.; Ye, J. Implantation of iron (iii) in porphyrinic metal organic frameworks for highly improved photocatalytic performance. Appl. Catal. B 2018, 224, 60–68. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, X.; Biswas, S.; Xie, Y.; Wang, Y.; Hu, X. Integrating polythiophene derivates to PCN-222 (Fe) for electrocatalytic sensing of l-dopa. Biosens. Bioelectron. 2019, 111470. [Google Scholar] [CrossRef] [PubMed]
- Yao, B.; Peng, C.; Zhang, W.; Zhang, Q.; Niu, J.; Zhao, J. A novel Fe (iii) porphyrin-conjugated TiO2 visible-light photocatalyst. Appl. Catal. B 2015, 174, 77–84. [Google Scholar] [CrossRef]
- Tompsett, G.A.; Krogh, L.; Griffin, D.W.; Conner, W.C. Hysteresis and scanning behavior of mesoporous molecular sieves. Langmuir 2005, 21, 8214–8225. [Google Scholar] [CrossRef]
- Ma, J.; Yu, F.; Zhou, L.; Jin, L.; Yang, M.; Luan, J.; Tang, Y.; Fan, H.; Yuan, Z.; Chen, J. Enhanced adsorptive removal of methyl orange and methylene blue from aqueous solution by alkali-activated multiwalled carbon nanotubes. ACS Appl. Mater. Interfaces 2012, 4, 5749–5760. [Google Scholar] [CrossRef]
- Gao, L.Z.; Zhuang, J.; Nie, L.; Zhang, J.B.; Zhang, Y.; Gu, N.; Wang, T.H.; Feng, J.; Yang, D.L.; Perrett, S.; et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef]
- Shi, W.; E., S.; Wang, M.M.; Li, T.Z.; Yang, T.; Liu, S.R.; Chen, M.L.; Wang, J.H. Facile synthesis of metal-organic framework-derived SiW12@Co3O4 and its peroxidase-like activity in colorimetric assay. Analyst 2019, 144, 5455–5461. [Google Scholar] [CrossRef]
- Chen, J.Y.; Shu, Y.; Li, H.L.; Xu, Q.; Hu, X.Y. Nickel metal-organic framework 2d nanosheets with enhanced peroxidase nanozyme activity for colorimetric detection of H2O2. Talanta 2018, 189, 254–261. [Google Scholar] [CrossRef]
- Chen, X.M.; Tian, X.T.; Su, B.Y.; Huang, Z.Y.; Chen, X.; Oyama, M. Au nanoparticles on citrate-functionalized graphene nanosheets with a high peroxidase-like performance. Dalton Trans. 2014, 43, 7449–7454. [Google Scholar] [CrossRef]
- Wu, N.; Wang, Y.T.; Wang, X.Y.; Guo, F.N.; Wen, H.; Yang, T.; Wang, J.H. Enhanced peroxidase-like activity of aunps loaded graphitic carbon nitride nanosheets for colorimetric biosensing. Anal. Chim. Acta 2019, 1091, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Bhunia, A.; Durani, S.; Wangikar, P.P. Horseradish peroxidase catalyzed degradation of industrially important dyes. Biotechnol. Bioeng. 2001, 72, 562–567. [Google Scholar] [CrossRef]
- Everse, J.; Grisham, M.B.; Everse, K.E. Peroxidases in Chemistry and Biology; CRC Press: Boca Raton, FL, USA, 1990; Volume 1. [Google Scholar]
- Veitch, N.C. Horseradish peroxidase: A modern view of a classic enzyme. Phytochemistry 2004, 65, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, F.; Liu, C.; Wang, Z.; Kang, L.; Huang, Y.; Dong, K.; Ren, J.; Qu, X. Nanozyme decorated metal–organic frameworks for enhanced photodynamic therapy. ACS Nano 2018, 12, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Attar, F.; Shahpar, M.G.; Rasti, B.; Sharifi, M.; Saboury, A.A.; Rezayat, S.M.; Falahati, M. Nanozymes with intrinsic peroxidase-like activities. J. Mol. Liq. 2019, 278, 130–144. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, G.; Yang, J.; Yuan, A.; Shen, X. Peroxidase-like catalytic activity of Ag3PO4 nanocrystals prepared by a colloidal route. PLoS ONE 2014, 9, e109158. [Google Scholar] [CrossRef]
- Wei, H.; Wang, E. Fe3O4 magnetic nanoparticles as peroxidase mimetics and their applications in H2O2 and glucose detection. Anal. Chem. 2008, 80, 2250–2254. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
| Km (mM) | Vmax (10−8M·S−1) | Reference | |||
|---|---|---|---|---|---|
| TMB | H2O2 | TMB | H2O2 | ||
| HRP | 0.434 | 3.70 | 10.0 | 8.71 | [37] |
| SiW12@Co3O4 | 0.023 | 167.8 | 5.3 | 167.8 | [38] |
| Nickel metal-organic framework | 0.365 | 2.49 | 6.53 | 8.98 | [39] |
| Au NPs/PVP–GNs | 2.63 | 104 | 13.04 | 11.98 | [40] |
| AuNPs@g-C3N4 | 0.097 | 12.3 | 1.52 | 9.0 | [41] |
| Fe-loaded MOF-545(Fe) | 2.34 | 1.69 | 85.4 | 12 | This work |
| Adsorbents | Adsorption Capacity (mg g−1) | Reference | |
|---|---|---|---|
| MB | MO | ||
| Fe-loaded MOF-545(Fe) | 382.35 | 803.664 | This work |
| PCN-222(MOF-545) | 906 | 589 | [24] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Li, H.; Li, C.; Li, Z. Fe-Loaded MOF-545(Fe): Peroxidase-Like Activity for Dye Degradation Dyes and High Adsorption for the Removal of Dyes from Wastewater. Molecules 2020, 25, 168. https://doi.org/10.3390/molecules25010168
Zhang C, Li H, Li C, Li Z. Fe-Loaded MOF-545(Fe): Peroxidase-Like Activity for Dye Degradation Dyes and High Adsorption for the Removal of Dyes from Wastewater. Molecules. 2020; 25(1):168. https://doi.org/10.3390/molecules25010168
Chicago/Turabian StyleZhang, Chuang, Haichao Li, Chen Li, and Zhengqiang Li. 2020. "Fe-Loaded MOF-545(Fe): Peroxidase-Like Activity for Dye Degradation Dyes and High Adsorption for the Removal of Dyes from Wastewater" Molecules 25, no. 1: 168. https://doi.org/10.3390/molecules25010168
APA StyleZhang, C., Li, H., Li, C., & Li, Z. (2020). Fe-Loaded MOF-545(Fe): Peroxidase-Like Activity for Dye Degradation Dyes and High Adsorption for the Removal of Dyes from Wastewater. Molecules, 25(1), 168. https://doi.org/10.3390/molecules25010168






