Comparison of Anthraquinones, Iridoid Glycosides and Triterpenoids in Morinda officinalis and Morinda citrifolia Using UPLC/Q-TOF-MS and Multivariate Statistical Analysis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Identifying the Chemical Constituents of Morinda officinalis (M. officinalis) and Morinda citrifolia (M. citrifolia) Based on UPLC/Q-TOF-MS/MS
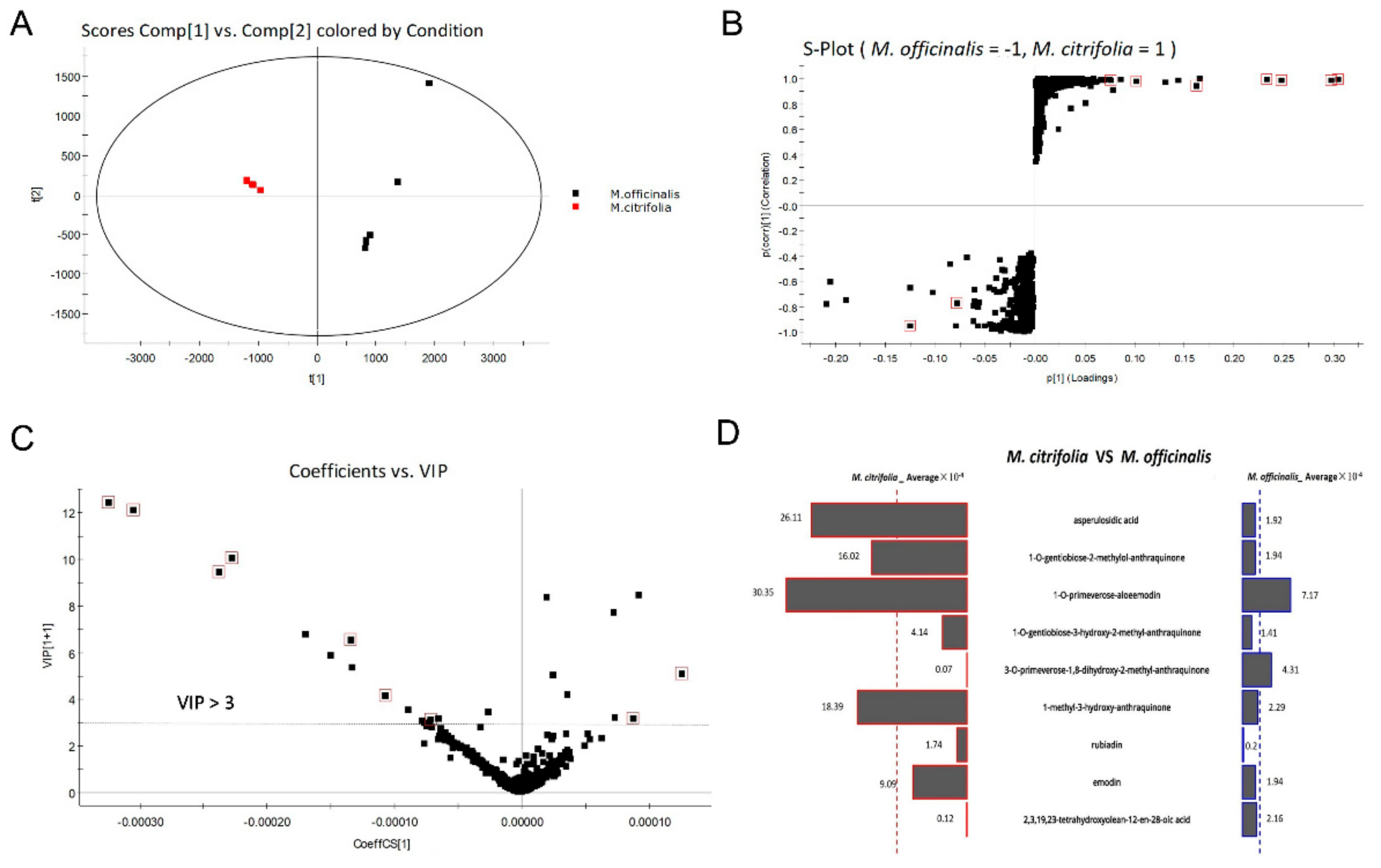
2.2. Tentative Identification of Potential Marker Compounds from M. officinalis and M. citrifolia
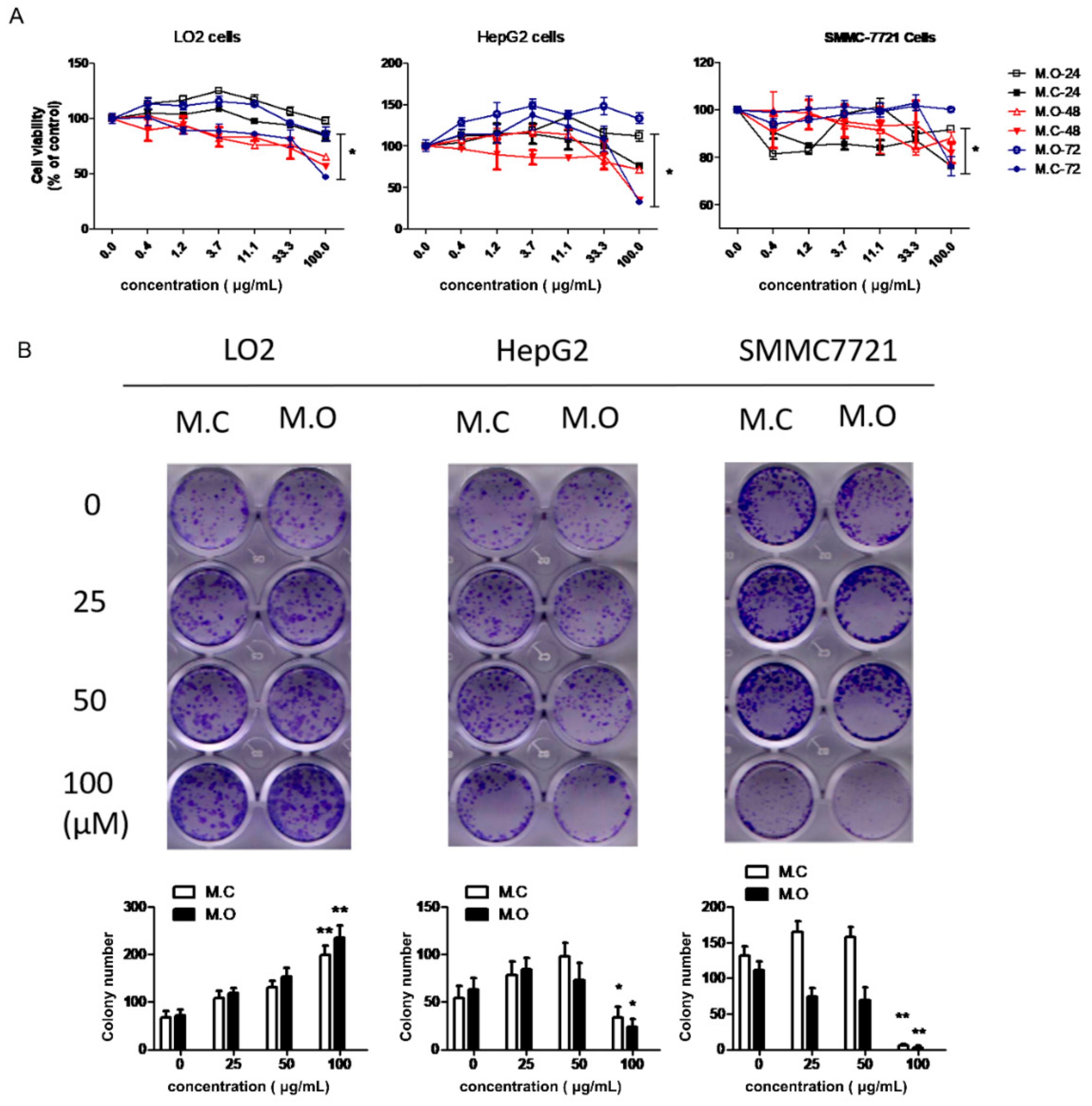
2.3. The Cytotoxicity of the Methanol Extracts of M. officinalis and M. citrifolia Roots
3. Materials and Methods
3.1. Instruments and Materials
3.2. Plant Materials
3.3. Sample Preparation
3.4. UPLC/Q-TOF-MS Detection Conditions
3.4.1. Chromatographic Conditions
3.4.2. Mass Spectometry Conditions
3.5. Mass Data Processing and Analysis
3.6. Chemical Composition and Biomarker Identification
3.7. Cell Viability Assay
3.8. Cell Colony Formation Assay
3.9. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yoshikawa, M.; Yamaguchi, S.; Nishisaka, H.; Yamahara, J.; Murakami, N. Chemical constituents of Chinese natural medicine, Morindae Radix, the dried roots of Morinda officinalis How: Structures of morindolide and morofficinaloside. Chem. Pharm. Bull. 1995, 43, 1462–1465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.-H.; Xin, H.-L.; Xu, Y.-M.; Shen, Y.; He, Y.-Q.; Hsien-Yeh, H.; Lin, B.; Song, H.-T.; Liu, J.; Yang, H.-Y. Morinda officinalis How—A comprehensive review of traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 2017, 213, 230–255. [Google Scholar] [PubMed]
- Xia, T.; Dong, X.; Lin, L.; Jiang, Y.; Ma, X.; Xin, H.; Zhang, Q.; Qin, L. Metabolomics profiling provides valuable insights into the underlying mechanisms of Morinda officinalis on protecting glucocorticoid-induced osteoporosis. J. Pharm. Biomed. Anal. 2019, 166, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.T.; Park, H.J.; Nam, J.H.; Park, Y.M.; Won, J.H.; Choi, J.; Choe, B.K.; Lee, K.T. In-vitro and in-vivo anti-inflammatory and antinociceptive effects of the methanol extract of the roots of Morinda officinalis. J. Pharm. Pharmacol. 2010, 57, 607–615. [Google Scholar] [CrossRef]
- Soon, Y.; Tan, B.K.H. Evaluation of the hypoglycaemic antioxidant activities of Morinda officinalis in streptozotocin induced diabetic rats. Singap. Med. J. 2002, 43, 77–85. [Google Scholar]
- Zhang, H.-L.; Li, J.; Li, G.; Wang, D.-M.; Zhu, L.-P.; Yang, D.-P. Structural characterization and anti-fatigue activity of polysaccharides from the roots of Morinda officinalis. Int. J. Biol. Macromol. 2009, 44, 257–261. [Google Scholar] [CrossRef]
- Potterat, O.; Hamburger, M. Morinda citrifolia (Noni) fruit-phytochemistry, pharmacology, safety. Planta Med. 2007, 73, 191–199. [Google Scholar] [CrossRef] [Green Version]
- Nayak, B.S.; Marshall, J.R.; Isitor, G.; Adogwa, A. Hypoglycemic and hepatoprotective activity of fermented fruit juice of Morinda citrifolia (Noni) in diabetic rats. Evid. Based Complement. Altern. Med. 2011, 2011, 875293. [Google Scholar] [CrossRef] [Green Version]
- Chan-Blanco, Y.; Vaillant, F.; Perez, A.M.; Reynes, M.; Brillouet, J.-M.; Brat, P. The noni fruit (Morinda citrifolia L.): A review of agricultural research, nutritional and therapeutic properties. J. Food Compos. Anal. 2006, 19, 645–654. [Google Scholar] [CrossRef]
- Assi, R.A.; Darwis, Y.; Abdulbaqi, I.M.; Vuanghao, L.; Laghari, M. Morinda citrifolia (Noni): A comprehensive review on its industrial uses, pharmacological activities, and clinical trials. Arab. J. Chem. 2017, 10, 691–707. [Google Scholar] [CrossRef]
- Krishnakumar, N.; Latha, P.; Suja, S.; Rajasekharan, S. A review on the ethnomedicinal, therapeutic and nutraceutical importance of “Noni” (Morinda citrifolia L.). IJMPNP 2015, 1, 1–14. [Google Scholar]
- Deng, S.; West, B.J.; Palu, A.K.; Zhou, B.-N.; Jensen, C.J. Noni as an anxiolytic and sedative: A mechanism involving its gamma-aminobutyric acidergic effects. Phytomedicine (Jena) 2007, 14, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Palu, A.K.; Kim, A.H.; West, B.J.; Deng, S.; Jensen, J.; White, L. The effects of Morinda citrifolia L. (noni) on the immune system: Its molecular mechanisms of action. J. Ethnopharmacol. 2008, 115, 502–506. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shi, J.; Wei, X.F.; Ren, X.H.; Li, X.W. Effect of Morinda officinalis on Kidney-Yang Deficiency Infertility Rats. J. Chin. Med. Mater. 2017, 40, 1826–1832. [Google Scholar]
- Kamiya, K.; Tanaka, Y.; Endang, H.; Umar, M.; Satake, T. Chemical constituents of Morinda citrifolia fruits inhibit copper-induced low-density lipoprotein oxidation. J. Agric. Food Chem. 2004, 52, 5843–5848. [Google Scholar] [CrossRef]
- Xu, Y.; Cai, H.; Cao, G.; Duan, Y.; Pei, K.; Tu, S.; Zhou, J.; Xie, L.; Sun, D.; Zhao, J. Profiling and analysis of multiple constituents in Baizhu Shaoyao San before and after processing by stir-frying using UHPLC/Q-TOF-MS/MS coupled with multivariate statistical analysis. J. Chromatogr. B 2018, 1083, 110–123. [Google Scholar] [CrossRef]
- Aly, A.H.; Edrada-Ebel, R.; Wray, V.; Müller, W.E.; Kozytska, S.; Hentschel, U.; Proksch, P.; Ebel, R. Bioactive metabolites from the endophytic fungus Ampelomyces sp. isolated from the medicinal plant Urospermum picroides. Phytochemistry 2008, 69, 1716–1725. [Google Scholar] [CrossRef]
- Lu, J.Q.; Sun, M.Q.; Zhang, H.G. Mechanisms of catalpol and jasminoidin by electrospray ionization mass spectrometry. Chin. Tradit. Herb. Drugs 2008, 7, 1011–1014. [Google Scholar]
- Zhao, X.; Wei, J.; Yang, M. Simultaneous analysis of iridoid glycosides and anthraquinones in Morinda officinalis using UPLC-QqQ-MS/MS and UPLC-Q/TOF-MSE. Molecules 2018, 23, 1070. [Google Scholar] [CrossRef] [Green Version]
- Zhai, H.-J.; Yu, J.-H.; Zhang, Q.; Liu, H.-S.; Zhang, J.-S.; Song, X.-Q.; Zhang, Y.; Zhang, H. Cytotoxic and antibacterial triterpenoids from the roots of Morinda officinalis var. officinalis. Fitoterapia 2019, 133, 56–61. [Google Scholar] [CrossRef]
- Lu, X.; Zou, W.; Dong, Y.; Zhou, D.; Tong, X.; Dong, Z.; Liang, G.; Tang, L.; Liu, M. Anti-renal fibrosis effect of asperulosidic acid via TGF-β1/smad2/smad3 and NF-κB signaling pathways in a rat model of unilateral ureteral obstruction. Phytomedicine 2019, 53, 274–285. [Google Scholar]
- He, J.; Xianyuan, L.; Ting, W.; Yaqian, D.; Zheng, C.; Lan, T.; Menghua, L. Asperuloside and Asperulosidic Acid Exert an Anti-Inflammatory Effect via Suppression of the NF-κB and MAPK Signaling Pathways in LPS-Induced RAW 264.7 Macrophages. Int. J. Mol. Sci. 2018, 19, 2027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, A.C. Anticancer Activity of Morinda citrifolia (Noni) Fruit: A Review. Phytother. Res. 2012, 26, 1427–1440. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.L.; Goh, Y.M.; Noordin, M.M.; Rahman, H.S.; Othman, H.H.; Bakar, N.A.A.; Mohamed, S. Morinda citrifolia edible leaf extract enhanced immune response against lung cancer. Food Funct. 2016, 7, 741–751. [Google Scholar] [CrossRef]
- Huang, C.; Wei, Y.-X.; Shen, M.-C.; Tu, Y.-H.; Wang, C.-C.; Huang, H.-C. Chrysin, Abundant in Morinda citrifolia Fruit Water–EtOAc Extracts, Combined with Apigenin Synergistically Induced Apoptosis and Inhibited Migration in Human Breast and Liver Cancer Cells. J. Agric. Food Chem. 2016, 64, 4235–4245. [Google Scholar] [CrossRef]
- Lin, H.; Zhu, H.; Tan, J.; Wang, C.; Dong, Q.; Wu, F.; Wang, H.; Liu, J.; Li, P.; Liu, J. Comprehensive investigation on metabolites of Wild-Simulated American Ginseng Root based on Ultra-high Performance Liquid Chromatography-Quadrupole Time-Of-Flight Mass Spectrometry. J. Agric. Food Chem. 2019, 67, 5801–5819. [Google Scholar] [CrossRef]
- Gu, R.; Rybalov, L.; Negrin, A.; Morcol, T.; Long, W.; Myers, A.K.; Isaac, G.; Yuk, J.; Kennelly, E.J.; Long, C. Metabolic Profiling of Different Parts of Acer truncatum from the Mongolian Plateau Using UPLC-QTOF-MS with Comparative Bioactivity Assays. J. Agric. Food Chem. 2019, 67, 1585–1597. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds of Morinda officinalis and Morinda citrifolia are available from the authors. |
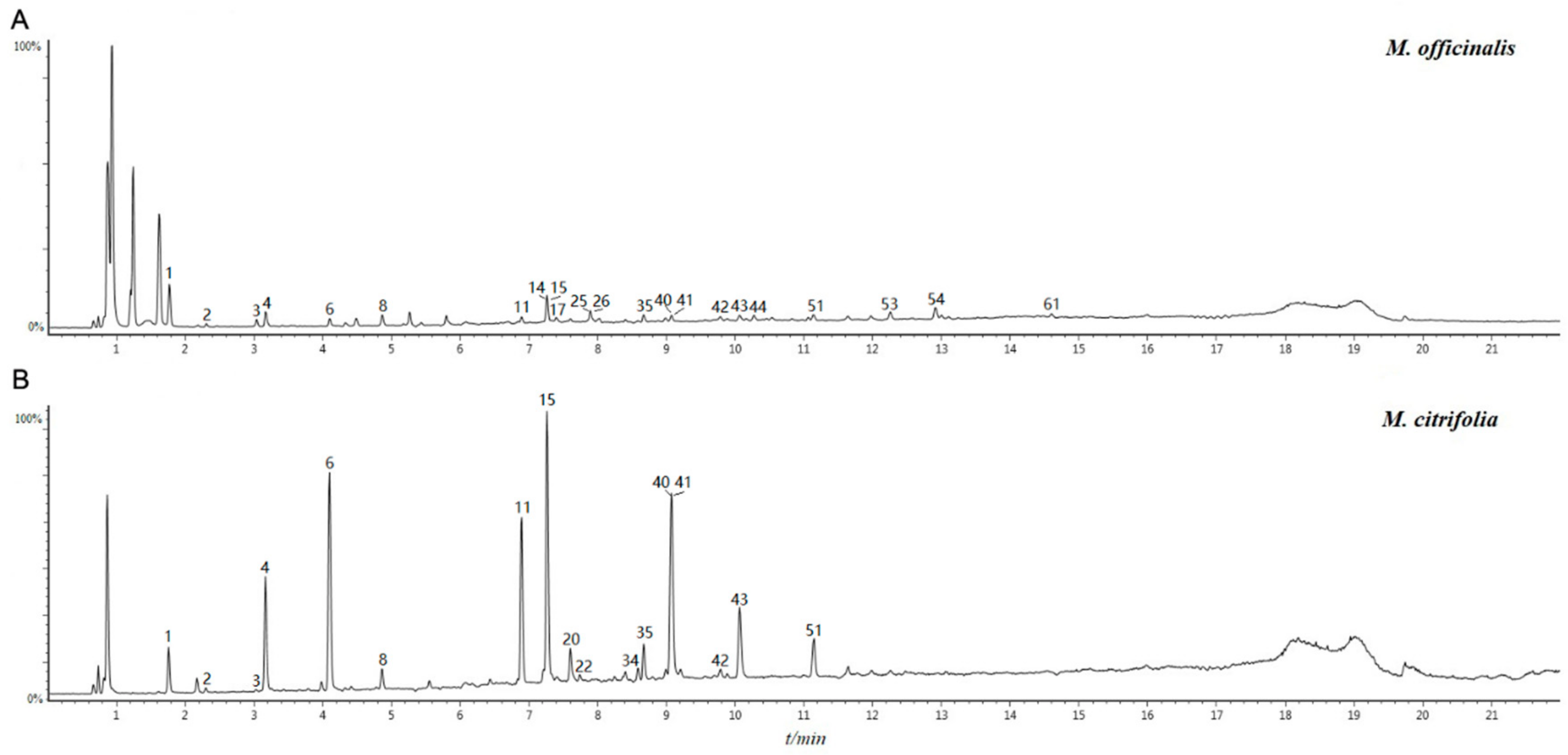
| Peak No. | Rte (min) | Formula | Quasi-Molecular Ion | MS2 Ions | Molecular Weight | Mass Error (ppm) | Identification | Type | Source | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Observed | Theoretical | M. O | M. C | ||||||||
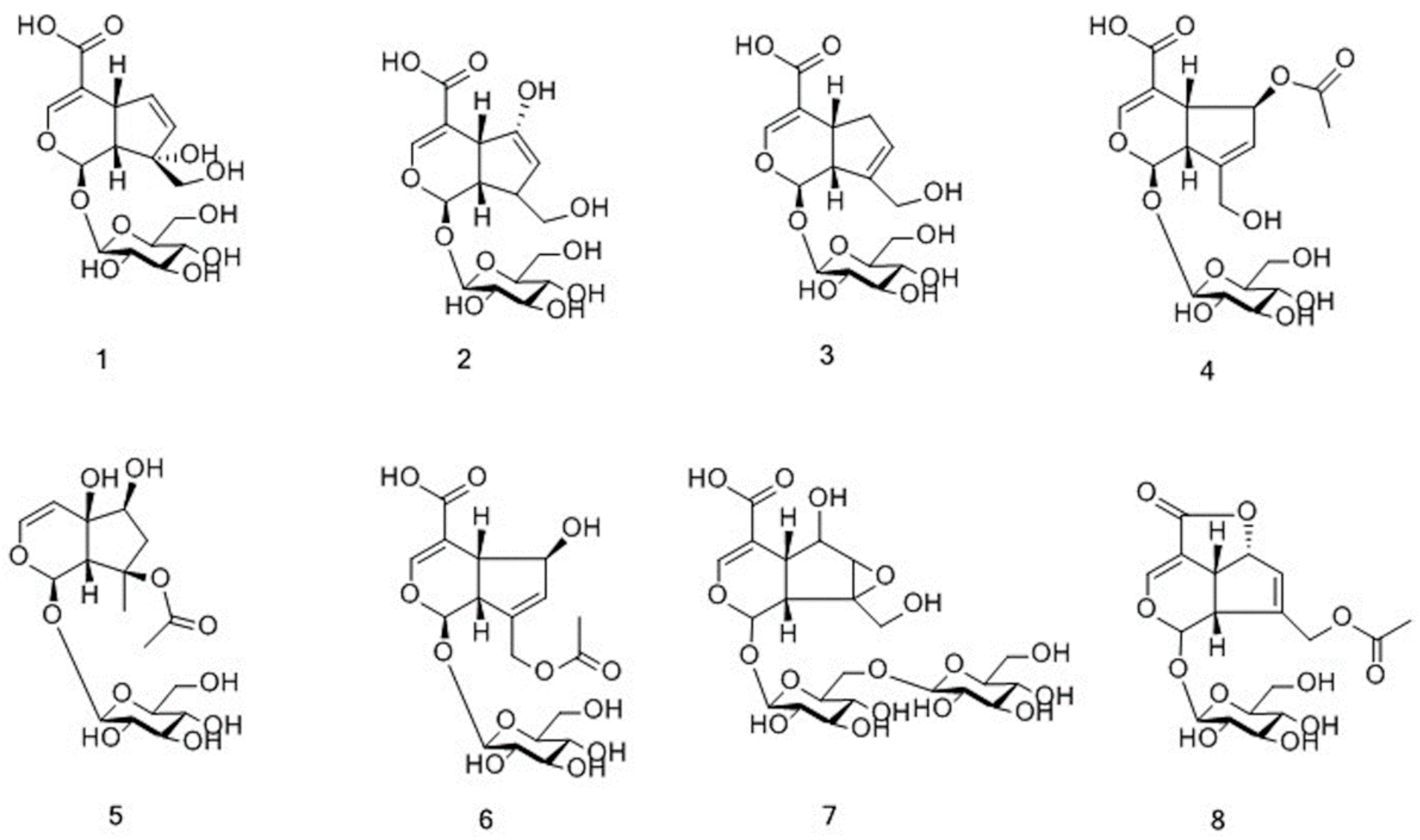
| 1 | 1.76 | C16H22O11 | 389.1087 [M − H]− | 209.0457 137.0610 | 390.1159 | 390.1162 | −0.7 | monotropein | I | √ | √ |
| 2 | 2.30 | C16H22O11 | 389.1089 [M − H]− | - | 390.1162 | 390.1162 | −0.1 | diacetylasperulosidic acid | I | √ | √ |
| 3 | 3.03 | C16H22O10 | 419.1191 [M + HCOO]− | 211.0613 123.0454 | 374.1209 | 374.1213 | −1.0 | geniposidic acid | I | √ | √ |
| 4 | 3.17 | C18H24O12 | 477.1248 [M + HCOO]− | 417.1039 371.0983 209.0453 191.0350 165.0559 161.0248 147.0454 | 432.1266 | 432.1268 | −0.4 | isoasperulosidic acid | I | √ | √ |
| 5 | 3.67 | C17H26O11 | 451.1454 [M+HCOO]− | 243.0875 | 406.1472 | 406.1475 | −0.8 | harpagide acetate | I | √ | √ |
| 6 | 4.10 | C18H24O12 | 431.1196 [M − H]− | 371.0974 251.0563 165.0560 | 432.1269 | 432.1268 | 0.3 | asperulosidic acid | I | √ | √ |
| 7 | 4.78 | C21H32O15 | 523.1666 [M − H]− | 477.1613 293.0876 233.0669 | 524.1739 | 524.1741 | −0.4 | rehmannioside A | I | √ | √ |
| 8 | 4.86 | C18H22O11 | 459.1143 [M + HCOO]− | 147.0454 | 414.1161 | 414.1162 | −0.3 | asperuloside | I | √ | √ |
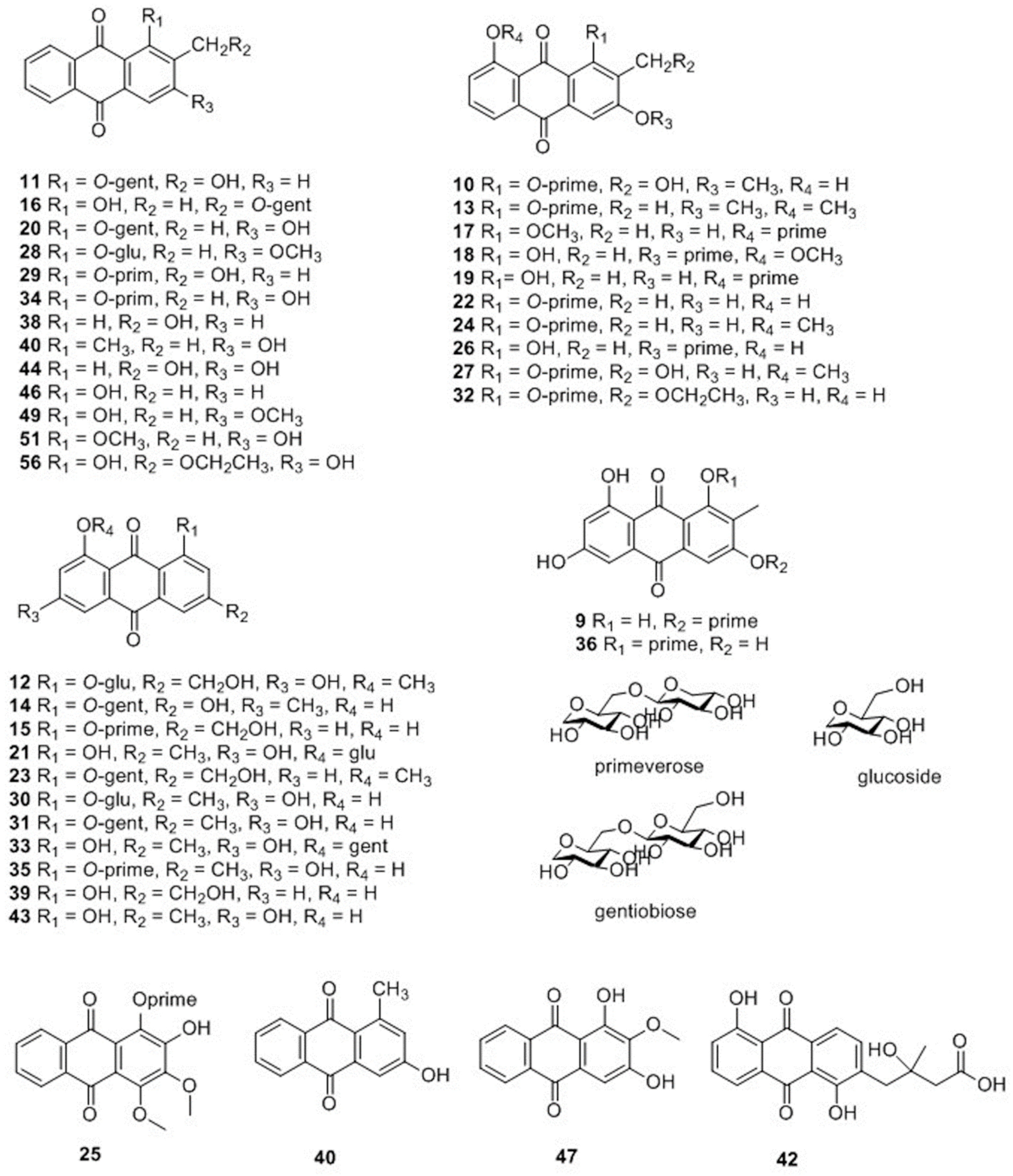
| 9 | 6.44 | C26H28O15 | 579.1342 [M − H]− | 413.1078 285.0398 146.9650 | 580.1415 | 580.1428 | −2.4 | 3-O-primeverose-1,6,8-trihydroxy-2-methyl-anthraquinone | A | √ | |
| 10 | 6.84 | C27H30O15 | 593.1507 [M − H]− | 253.0506 | 594.1580 | 594.1585 | −0.8 | 1-O-primeverose-3-methoxy-8-hydroxy-2-methylol-anthraquinone | A | √ | √ |
| 11 | 6.89 | C27H30O14 | 623.1616 [M + HCOO]− | 283.0612 253.0506 146.9654 | 578.1634 | 578.1636 | −0.2 | 1-O-gentiobiose-2-methylol-anthraquinone | A | √ | √ |
| 12 | 7.21 | C22H22O10 | 491.1195 [M + HCOO]− | 283.0613 | 446.1213 | 446.1213 | −0.1 | 1-O-β-d-glycopyranosyl-8-methoxy-emodin | A | √ | |
| 13 | 7.23 | C28H32O14 | 637.1771 [M + HCOO]− | 547.1449 307.0609 297.0763 283.0613 | 592.1789 | 592.1792 | −0.4 | 1-O-primeverose-3,8-dimethoxy-2-methyl-anthraquinone | A | √ | |
| 14 | 7.25 | C27H30O15 | 593.1510 [M − H]− | 269.0456 251.0350 146.9655 | 594.1583 | 594.1585 | −0.3 | 1-O-gentiobiose-emodin | A | √ | |
| 15 | 7.27 | C26H28O14 | 563.1405 [M − H]− | 269.0457 251.0352 237.0557 | 564.1478 | 564.1479 | −0.2 | 1-O-primeverose-aloeemodin | A | √ | √ |
| 16 | 7.34 | C27H30O14 | 577.1561 [M − H]− | 253.0504 | 578.1634 | 578.1636 | −0.3 | 3-O-gentiobiose -1-hydroxy-2-methyl-anthraquinone | A | √ | √ |
| 17 | 7.40 | C27H30O14 | 623.1617[M + HCOO]− | 269.0455 | 578.1635 | 578.1636 | −0.1 | 8−O-primeverose-1-methoxy-3-hydroxy-2- methyl -anthraquinone | A | √ | |
| 18 | 7.53 | C27H30O14 | 577.1559 [M − H]− | 283.0619 | 578.1632 | 578.1636 | −0.7 | 3-O-primeverose-8-methoxy-1-hydroxy- 2- methyl -anthraquinone | A | √ | |
| 19 | 7.57 | C26H28O14 | 609.1461 [M + HCOO]− | 415.1024 269.0457 | 564.1479 | 564.1479 | 0.0 | 8-O-primeverose-1,3-dihydroxy-2-methyl -anthraquinone | A | √ | |
| 20 | 7.61 | C27H30O13 | 607.1663 [M + HCOO]− | 253.0505 | 562.1681 | 562.1686 | −0.8 | 1-O-gentiobiose -3-hydroxy- 2-methyl -anthraquinone | A | √ | √ |
| 21 | 7.65 | C21H20O10 | 431.0977 [M − H]− | 269.0455 251.0347 | 432.1049 | 432.1057 | −1.7 | emodin 1-O-β-d-glycopyranosyl | A | √ | |
| 22 | 7.74 | C26H28O14 | 563.1404 [M − H]− | 269.0456 251.0348 223.0407 | 564.1477 | 564.1479 | −0.4 | 1-O-primeverose-3,8-dihydroxy-2-methyl -anthraquinone | A | √ | √ |
| 23 | 7.75 | C28H32O15 | 607.1665 [M − H]− | 563.1403 283.0617 267.0661 251.0348 | 608.1738 | 608.1741 | −0.6 | 1-O-gentiobiose-8-methoxy-aloeemodin | A | √ | |
| 24 | 7.80 | C27H30O14 | 577.1556 [M − H]− | 283.0618 | 578.1629 | 578.1636 | −1.2 | 1-O-primeverose-8-methoxy-3-hydroxy-2-methyl-anthraquinone | A | √ | |
| 25 | 7.87 | C27H30O15 | 639.1558 [M + HCOO]− | 299.0562 | 594.1576 | 594.1585 | −1.3 | 1-O-primeverose-3,4-dimethoxy-2-hydroxy-anthraquinone | A | √ | |
| 26 | 7.89 | C26H28O14 | 563.1405 [M − H]− | 269.0457 | 564.1478 | 564.1479 | −0.2 | 3-O-primeverose-1,8-dihydroxy-2-methyl -anthraquinone | A | √ | |
| 27 | 7.93 | C27H30O15 | 593.1502 [M − H]− | 547.1452 283.0608 285.0405 251.0347 | 594.1574 | 594.1585 | −1.7 | 1-O-primeverose-2- methylol-3-hydroxy-8-methoxy-anthraquinone | A | √ | |
| 28 | 8.08 | C22H22O9 | 475.1243 [M + HCOO]− | 267.0658 253.0506 | 430.1261 | 430.1264 | −0.5 | 1-O-β-d-glycopyranosylrubiadin3-methyl ether | A | √ | |
| 29 | 8.10 | C26H28O13 | 547.1451 [M − H]− | 253.0506 | 548.1524 | 548.1530 | −1.1 | 1-O-primeverose-2-methylol-anthraquinone | A | √ | |
| 30 | 8.15 | C21H20O10 | 431.0977 [M−H]− | 269.0456 | 432.1050 | 432.1057 | −1.5 | 1-O-β-d-glycopyranosyl-emodin | A | √ | |
| 31 | 8.25 | C27H30O15 | 593.1514 [M − H]− | 269.0457 265.0501 | 594.1587 | 594.1585 | 0.4 | 1-O-gentiobiose-emodin | A | √ | √ |
| 32 | 8.37 | C28H32O15 | 653.1720 [M + HCOO]− | 313.0717 298.0478 | 608.1738 | 608.1741 | −0.6 | 1-O-primeverose-8-hydroxy-ibericin | A | √ | |
| 33 | 8.40 | C27H30O15 | 593.1512 [M − H]− | 547.1455 431.0981 269.0455 | 594.1585 | 594.1585 | 0.1 | 8-O-gentiobiose-emodin | A | √ | √ |
| 34 | 8.59 | C26H28O13 | 593.1513 [M + HCOO]− | 253.0508 146.9655 | 548.1531 | 548.1530 | 0.2 | 1-O-primeverose-rubiadin | A | √ | √ |
| 35 | 8.67 | C26H28O14 | 563.1407 [M − H]− | - | 564.1479 | 564.1479 | 0.0 | 1-O-primeverose-emodin | A | √ | √ |
| 36 | 8.81 | C26H28O15 | 579.1353 [M − H]− | 285.0403 | 580.1426 | 580.1428 | −0.4 | 1-O-primeverose-2-methyl-3,6,8-trihydroxy-anthraquinone | A | √ | |
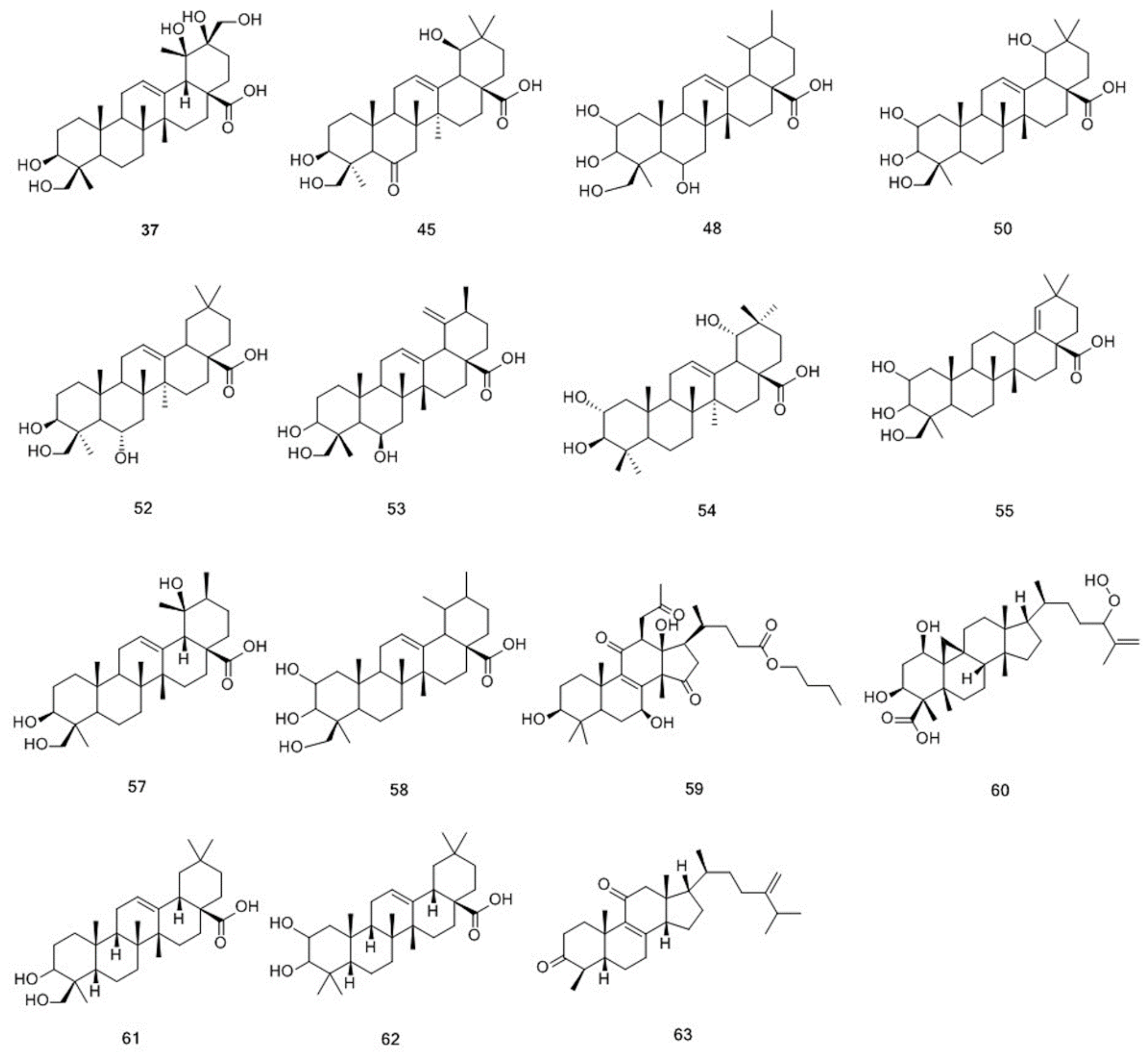
| 37 | 8.84 | C30H48O7 | 519.3323 [M − H]− | 489.3215 | 520.3396 | 520.3400 | −0.8 | (3α)-3,19,20,24,30-pentahydroxyurs-12-en-28-oic acid | T | √ | |
| 38 | 8.98 | C15H10O4 | 253.0509 [M − H]− | 223.0402 | 254.0582 | 254.0579 | 1.1 | 1-hydroxy-2-methylol-anthraquinone | A | √ | √ |
| 39 | 9.00 | C15H10O5 | 269.0457 [M − H]− | - | 270.0530 | 270.0528 | 0.5 | aloe-emodin | A | √ | |
| 40 | 9.07 | C15H10O3 | 283.0614 [M + HCOO]− | - | 238.0632 | 238.0630 | 0.9 | 1-methyl-3-hydroxy-anthraquinone | A | √ | √ |
| 41 | 9.08 | C15H10O4 | 253.0510 [M − H]− | - | 254.0582 | 254.0579 | 1.3 | rubiadin | A | √ | √ |
| 42 | 9.79 | C19H16O7 | 401.0877 [M + HCOO]- | 283.0607 269.0452 | 356.0895 | 356.0896 | −0.3 | fridamycin E | A | √ | √ |
| 43 | 10.07 | C15H10O5 | 269.0458 [M − H]− | 251.0353 | 270.0531 | 270.0528 | 0.9 | emodin | A | √ | √ |
| 44 | 10.27 | C15H10O4 | 253.0511 [M − H]− | 238.0275 | 254.0584 | 254.0579 | 1.8 | 3-hydroxy-2-methylol-anthraquinone | A | √ | |
| 45 | 10.46 | C30H46O6 | 501.3222 [M − H]− | 483.3111 457.3323 | 502.3295 | 502.3294 | 0.1 | (3α,19β)-3,19,23-trihydroxy-6-oxoolean-12-en-28-oic acid | T | √ | |
| 46 | 10.53 | C15H10O3 | 283.0614 [M + HCOO]− | - | 238.0632 | 238.0630 | 0.7 | 1-hydroxy-2-methyl-anthraquinone | A | √ | |
| 47 | 10.82 | C15H10O5 | 269.0457 [M − H]− | - | 270.0530 | 270.0528 | 0.6 | 1,3-dihydroxy-2-methoxy-anthraquinone | A | √ | |
| 48 | 10.97 | C30H48O6 | 549.3426 [M + HCOO]- | 485.3259 | 504.3444 | 504.3451 | −1.2 | 2,3,6,23-tetrahydroxyurs-12-en-28-oic acid | T | √ | |
| 49 | 11.00 | C16H12O4 | 267.0658 [M − H]- | 252.0424 224.0470 | 268.0731 | 268.0736 | −1.7 | rubiadin 3-methyl ether | A | √ | |
| 50 | 11.06 | C30H48O6 | 503.3377 [M − H]- | 485.3268 | 504.3449 | 504.3451 | −0.3 | 2,3,19,23-tetrahydroxyolean-12-en-28-oic acid | T | √ | |
| 51 | 11.14 | C16H12O4 | 267.0665 [M − H]- | 224.0476 | 268.0738 | 268.0736 | 0.7 | rubiadin 1-methyl ether | A | √ | √ |
| 52 | 11.97 | C30H48O5 | 487.3424 [M − H]- | 469.3322 437.3048 | 488.3497 | 488.3502 | −1.0 | (3β,6α)-3,6,24-trihydroxyolean-12-en-28-oic acid | T | √ | |
| 53 | 12.26 | C30H48O5 | 485.3273 [M − H]- | 467.3164 441.3372 423.3266 | 486.3346 | 486.3345 | 0.2 | (3β,6α)-3,6,23-trihydroxyursa-12,19(29)-dien-28-oic acid | T | √ | |
| 54 | 12.91 | C30H48O5 | 487.3433 [M − H]- | 469.3324 443.3528 425.3422 | 488.3506 | 488.3502 | 0.9 | (2α,3β,19α)-2,3,19-trihydroxyolean-12-en-28-oic acid | T | √ | |
| 55 | 13.01 | C30H48O5 | 487.3429 [M − H]- | 441.3368 425.3424 409.3109 | 488.3501 | 488.3502 | −0.1 | (2α,3α)-2,3,24-trihydroxyolean-18-en-28-oic acid | T | √ | |
| 56 | 13.06 | C17H14O5 | 297.0766 [M − H]- | 251.0348 | 298.0839 | 298.08412 | −0.8 | ibericin | A | √ | |
| 57 | 13.11 | C30H48O5 | 487.3425 [M − H]- | 441.3368 425.3424 409.3109 | 488.3498 | 488.3502 | −0.8 | (3α)-3,19,23-trihydroxyurs-12-en-28-oic acid | T | √ | |
| 58 | 13.26 | C30H48O5 | 533.3475 [M+HCOO]- | 441.3364 | 488.3493 | 488.3502 | −1.7 | 2,3,23-trihydroxyurs-12-en-28-oic acid | T | √ | |
| 59 | 13.53 | C33H50O8 | 573.3427 [M − H]- | - | 574.3499 | 574.3506 | −1.1 | butyl (3β,7β,12β)-12-acetoxy-3,7-dihydroxy-4,4,14-trimethyl-11,15-dioxochol-8-en-24-oate | T | √ | |
| 60 | 14.30 | C31H50O6 | 517.3527 [M − H]- | 471.3470 | 518.3600 | 518.3607 | −1.4 | (1α,3β,9β)-24-hydroperoxy-1,3-dihydroxy-5-methyl-9,19-cyclolanost-25-en-28-oic acid | T | √ | |
| 61 | 14.61 | C30H48O4 | 471.3474 [M − H]- | 453.3369 427.3583 | 472.3547 | 472.3553 | −1.2 | hederagenin | T | √ | |
| 62 | 15.95 | C30H48O4 | 517.3528 [M + HCOO]- | 423.3266 | 472.3546 | 472.3553 | −1.3 | maslinic acid | T | √ | |
| 63 | 19.52 | C29H44O2 | 423.3264 [M − H]- | 325.0718 | 424.3337 | 424.3341 | −1.1 | camphoratin H | T | √ | √ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Wang, Q.; Yang, Q.; Yan, X.; Feng, S.; Wang, Z. Comparison of Anthraquinones, Iridoid Glycosides and Triterpenoids in Morinda officinalis and Morinda citrifolia Using UPLC/Q-TOF-MS and Multivariate Statistical Analysis. Molecules 2020, 25, 160. https://doi.org/10.3390/molecules25010160
Wang M, Wang Q, Yang Q, Yan X, Feng S, Wang Z. Comparison of Anthraquinones, Iridoid Glycosides and Triterpenoids in Morinda officinalis and Morinda citrifolia Using UPLC/Q-TOF-MS and Multivariate Statistical Analysis. Molecules. 2020; 25(1):160. https://doi.org/10.3390/molecules25010160
Chicago/Turabian StyleWang, Maoyuan, Qinglong Wang, Qing Yang, Xiaoxia Yan, Shixiu Feng, and Zhunian Wang. 2020. "Comparison of Anthraquinones, Iridoid Glycosides and Triterpenoids in Morinda officinalis and Morinda citrifolia Using UPLC/Q-TOF-MS and Multivariate Statistical Analysis" Molecules 25, no. 1: 160. https://doi.org/10.3390/molecules25010160
APA StyleWang, M., Wang, Q., Yang, Q., Yan, X., Feng, S., & Wang, Z. (2020). Comparison of Anthraquinones, Iridoid Glycosides and Triterpenoids in Morinda officinalis and Morinda citrifolia Using UPLC/Q-TOF-MS and Multivariate Statistical Analysis. Molecules, 25(1), 160. https://doi.org/10.3390/molecules25010160




