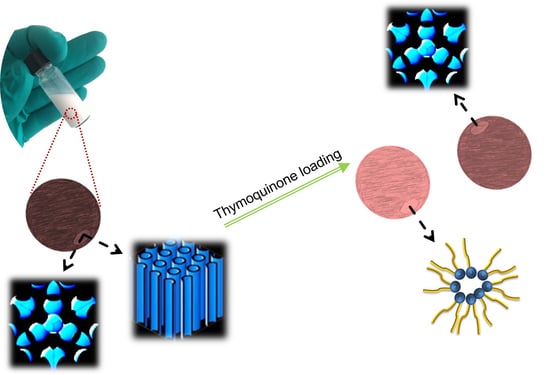
Non-Lamellar Liquid Crystalline Nanocarriers for Thymoquinone Encapsulation
Abstract
1. Introduction
2. Results and Discussion
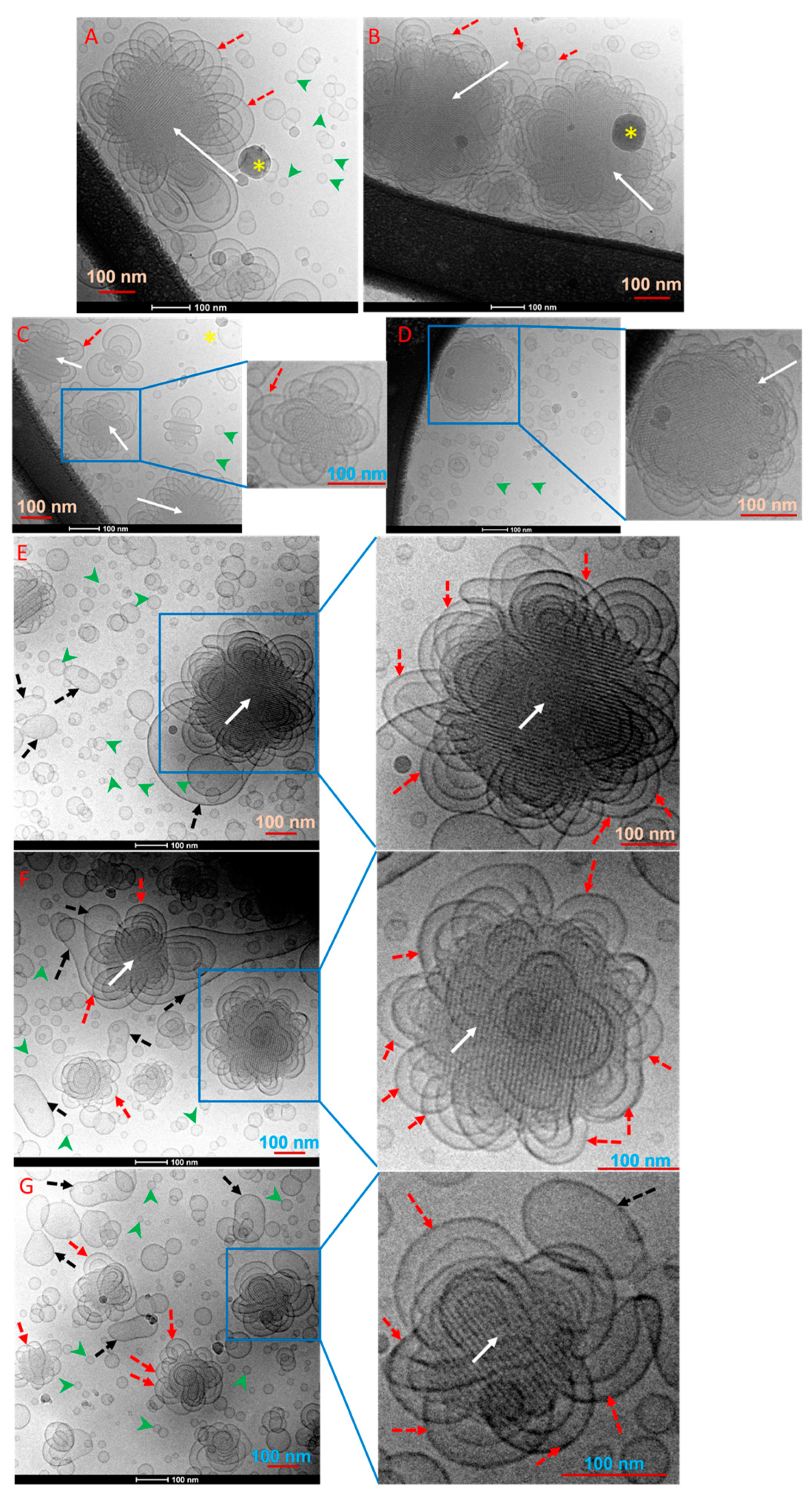
2.1. Biophysical Characteristics of Drug-Free GMO/Vit E Nano-Self-Assemblies
2.2. Effect of TQ Loading on Biophysical Characteristics of GMO/Vit E Nano-Self-Assemblies
2.3. Encapsulation Efficiency of TQ-Loaded GMO/Vit E Nano-Self-Assemblies
3. Materials and Methods
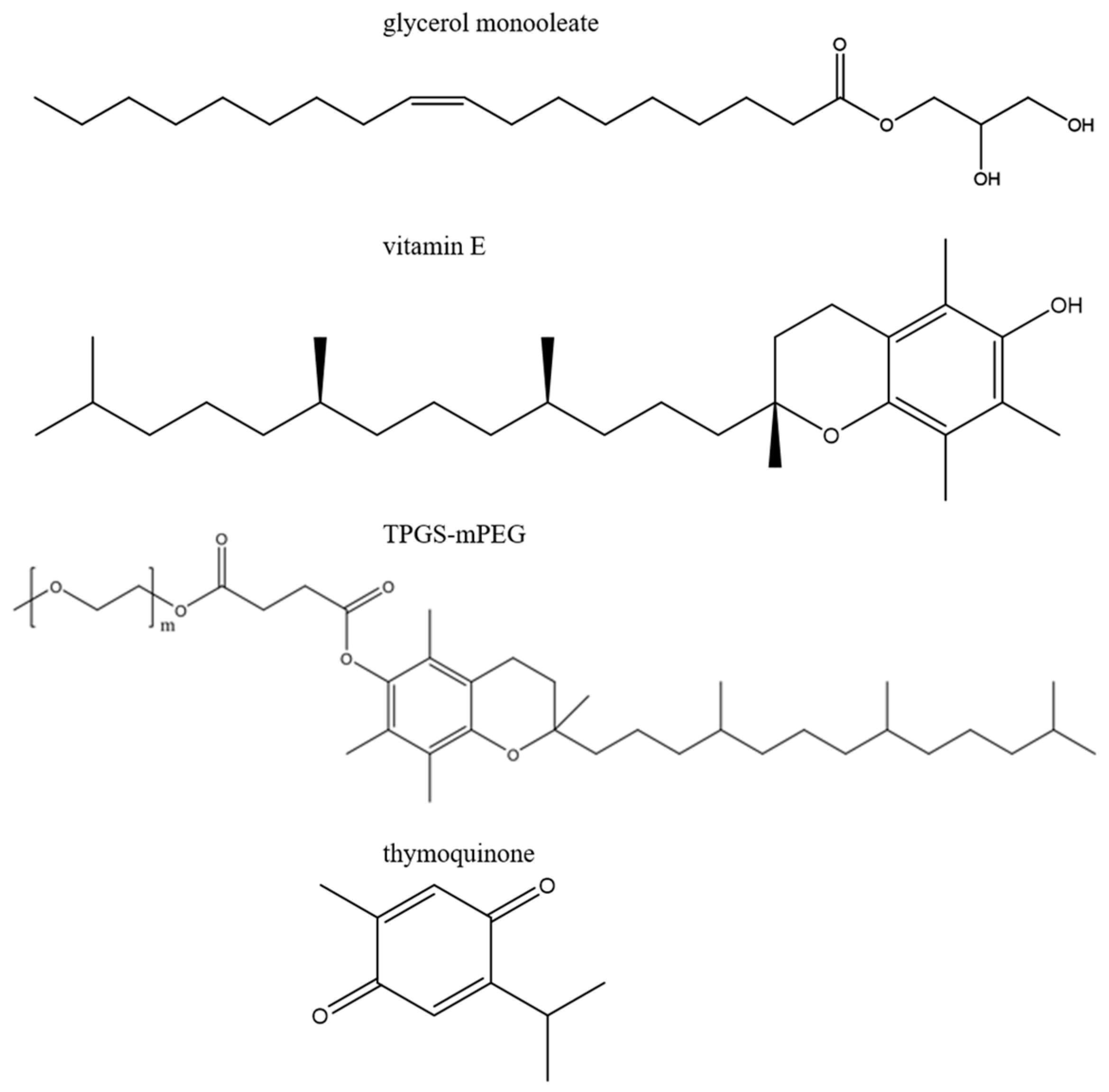
3.1. Materials
3.2. Preparation of Thymoquinone-Free and Thymoquinone-Loaded GMO/Vit E Nanodispersions
3.3. Size Characterization of GMO/Vit E Nanodispersions
3.4. Cryo-Transmission Electron Microscopy (Cryo-TEM)
3.5. Synchrotron Small-Angle X-ray Scattering (SAXS)
3.6. Encapsulation Efficiency
4. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Amin, B.; Hosseinzadeh, H. Black Cumin (Nigella sativa) and Its Active Constituent, Thymoquinone: An Overview on the Analgesic and Anti-inflammatory Effects. Planta Med. 2016, 82, 8–16. [Google Scholar] [CrossRef]
- Salem, M.L. Immunomodulatory and therapeutic properties of the Nigella sativa L. seed. Int. Immunopharmacol. 2005, 5, 1749–1770. [Google Scholar] [CrossRef]
- Sheikh, B.Y.; Sarker, M.M.R.; Kamarudin, M.N.A.; Ismail, A. Prophetic medicine as potential functional food elements in the intervention of cancer: A review. Biomed. Pharmacother. 2017, 95, 614–648. [Google Scholar] [CrossRef] [PubMed]
- Asaduzzaman Khan, M.; Tania, M.; Fu, S.; Fu, J. Thymoquinone, as an anticancer molecule: From basic research to clinical investigation. Oncotarget 2017, 8, 51907–51919. [Google Scholar] [CrossRef] [PubMed]
- Darakhshan, S.; Bidmeshki Pour, A.; Hosseinzadeh Colagar, A.; Sisakhtnezhad, S. Thymoquinone and its therapeutic potentials. Pharmacol. Res. 2015, 95–96, 138–158. [Google Scholar] [CrossRef] [PubMed]
- Burits, M.; Bucar, F. Antioxidant activity of Nigella sativa essential oil. Phytother. Res. 2000, 14, 323–328. [Google Scholar] [CrossRef]
- Mazaheri, Y.; Torbati, M.; Azadmard-Damirchi, S.; Savage, G.P. A comprehensive review of the physicochemical, quality and nutritional properties of Nigella sativa oil. Food Rev. Int. 2019, 35, 342–362. [Google Scholar] [CrossRef]
- Ong, Y.S.; Saiful Yazan, L.; Ng, W.K.; Noordin, M.M.; Sapuan, S.; Foo, J.B.; Tor, Y.S. Acute and subacute toxicity profiles of thymoquinone-loaded nanostructured lipid carrier in BALB/c mice. Int. J. Nanomed. 2016, 11, 5905–5915. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, M.K.; Arfuso, F.; Kumar, A.P.; Wang, L.Z.; Goh, B.C.; Ahn, K.S.; Bishayee, A.; Sethi, G. Modulation of diverse oncogenic transcription factors by thymoquinone, an essential oil compound isolated from the seeds of Nigella sativa Linn. Pharmacol. Res. 2018, 129, 357–364. [Google Scholar] [CrossRef]
- Schneider-Stock, R.; Fakhoury, I.H.; Zaki, A.M.; El-Baba, C.O.; Gali-Muhtasib, H.U. Thymoquinone: Fifty years of success in the battle against cancer models. Drug Discov. Today 2014, 19, 18–30. [Google Scholar] [CrossRef]
- Ng, W.K.; Saiful Yazan, L.; Yap, L.H.; Wan Nor Hafiza, W.A.; How, C.W.; Abdullah, R. Thymoquinone-loaded nanostructured lipid carrier exhibited cytotoxicity towards breast cancer cell lines (MDA-MB-231 and MCF-7) and cervical cancer cell lines (HeLa and SiHa). BioMed Res. Int. 2015, 2015, 263131. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Rauf, A.; Khan, I.A.; Shahbaz, M.; Qaisrani, T.B.; Fatmawati, S.; Abu-Izneid, T.; Imran, A.; Rahman, K.U.; Gondal, T.A. Thymoquinone: A novel strategy to combat cancer: A review. Biomed. Pharmacother. 2018, 106, 390–402. [Google Scholar] [CrossRef] [PubMed]
- Mostofa, A.G.M.; Hossain, M.K.; Basak, D.; Bin Sayeed, M.S. Thymoquinone as a Potential Adjuvant Therapy for Cancer Treatment: Evidence from Preclinical Studies. Front. Pharmacol. 2017, 8, 295. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, M.K.; Ahn, K.S.; Hsu, A.; Woo, C.C.; Yuan, Y.; Tan, K.H.B.; Chinnathambi, A.; Alahmadi, T.A.; Alharbi, S.A.; Koh, A.P.F.; et al. Thymoquinone Inhibits Bone Metastasis of Breast Cancer Cells Through Abrogation of the CXCR4 Signaling Axis. Front. Pharmacol. 2018, 9, 1294. [Google Scholar] [CrossRef] [PubMed]
- Bonifacio, B.V.; Silva, P.B.; Ramos, M.A.; Negri, K.M.; Bauab, T.M.; Chorilli, M. Nanotechnology-based drug delivery systems and herbal medicines: A review. Int. J. Nanomed. 2014, 9, 1–15. [Google Scholar] [CrossRef]
- Jakaria, M.; Cho, D.Y.; Haque, M.E.; Karthivashan, G.; Kim, I.S.; Ganesan, P.; Choi, D.K. Neuropharmacological Potential and Delivery Prospects of Thymoquinone for Neurological Disorders. Oxid. Med. Cell Longev. 2018, 2018. [Google Scholar] [CrossRef]
- Odeh, F.; Ismail, S.I.; Abu-Dahab, R.; Mahmoud, I.S.; Al Bawab, A. Thymoquinone in liposomes: A study of loading efficiency and biological activity towards breast cancer. Drug Deliv. 2012, 19, 371–377. [Google Scholar] [CrossRef]
- Singh, A.; Ahmad, I.; Akhter, S.; Jain, G.K.; Iqbal, Z.; Talegaonkar, S.; Ahmad, F.J. Nanocarrier based formulation of Thymoquinone improves oral delivery: Stability assessment, in vitro and in vivo studies. Colloid Surf. B 2013, 102, 822–832. [Google Scholar] [CrossRef]
- Jain, A.; Pooladanda, V.; Bulbake, U.; Doppalapudi, S.; Rafeeqi, T.A.; Godugu, C.; Khan, W. Liposphere mediated topical delivery of thymoquinone in the treatment of psoriasis. Nanomed. Nanotechnol. 2017, 13, 2251–2262. [Google Scholar] [CrossRef]
- Fakhoury, I.; Saad, W.; Bouhadir, K.; Nygren, P.; Schneider-Stock, R.; Gali-Muhtasib, H. Uptake, delivery, and anticancer activity of thymoquinone nanoparticles in breast cancer cells. J. Nanoparticle Res. 2016, 18, 1–16. [Google Scholar] [CrossRef]
- Assadpour, E.; Jafari, S.M. A systematic review on nanoencapsulation of food bioactive ingredients and nutraceuticals by various nanocarriers. Crit. Rev. Food Sci. 2018, 1–47. [Google Scholar] [CrossRef] [PubMed]
- Yaghmur, A.; Glatter, O. Characterization and potential applications of nanostructured aqueous dispersions. Adv. Colloid Interface Sci. 2009, 147–148, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Rakotoarisoa, M.; Angelov, B.; Garamus, V.M.; Angelova, A. Curcumin-and Fish Oil-Loaded Spongosome and Cubosome Nanoparticles with Neuroprotective Potential against H2O2-Induced Oxidative Stress in Differentiated Human SH-SY5Y Cells. ACS Omega 2019, 4, 3061–3073. [Google Scholar] [CrossRef]
- Azmi, I.D.; Moghimi, S.M.; Yaghmur, A. Cubosomes and hexosomes as versatile platforms for drug delivery. Ther. Deliv. 2015, 6, 1347–1364. [Google Scholar] [CrossRef]
- Angelova, A.; Drechsler, M.; Garamus, V.M.; Angelov, B. Liquid Crystalline Nanostructures as PEGylated Reservoirs of Omega-3 Polyunsaturated Fatty Acids: Structural Insights toward Delivery Formulations against Neurodegenerative Disorders. ACS Omega 2018, 3, 3235–3247. [Google Scholar] [CrossRef]
- Bor, G.; Mat Azmi, I.D.; Yaghmur, A. Nanomedicines for cancer therapy: Current status, challenges and future prospects. Ther. Deliv. 2019, 10, 113–132. [Google Scholar] [CrossRef]
- Cortesi, R.; Cappellozza, E.; Drechsler, M.; Contado, C.; Baldisserotto, A.; Mariani, P.; Carducci, F.; Pecorelli, A.; Esposito, E.; Valacchi, G. Monoolein aqueous dispersions as a delivery system for quercetin. Biomed. Microdevices 2017, 19, 41. [Google Scholar] [CrossRef]
- Yaghmur, A. Nanoencapsulation of food ingredients by cubosomes and hexosomes. In Lipid-based Nanostructures for Food Encapsulation Purposes; Jafari, S.M., Ed.; Elsevier: Edinburgh, UK, 2019; Volume 2, pp. 483–522. [Google Scholar]
- Prajapati, R.; Larsen, S.W.; Yaghmur, A. Citrem-phosphatidylcholine nano-self-assemblies: Solubilization of bupivacaine and its role in triggering colloidal transition from vesicles to cubosomes and hexosomes. Phys. Chem. Chem. Phys. 2019, 21, 15142–15150. [Google Scholar] [CrossRef]
- Angelova, A.; Garamus, V.M.; Angelov, B.; Tian, Z.; Li, Y.; Zou, A. Advances in structural design of lipid-based nanoparticle carriers for delivery of macromolecular drugs, phytochemicals and anti-tumor agents. Adv. Colloid Interface Sci. 2017, 249, 331–345. [Google Scholar] [CrossRef]
- Prajapati, R.; Gontsarik, M.; Yaghmur, A.; Salentinig, S. pH-Responsive Nano-Self-Assemblies of the Anticancer Drug 2-Hydroxyoleic Acid. Langmuir 2019, 35, 7954–7961. [Google Scholar] [CrossRef]
- Azmi, I.D.; Wibroe, P.P.; Wu, L.P.; Kazem, A.I.; Amenitsch, H.; Moghimi, S.M.; Yaghmur, A. A structurally diverse library of safe-by-design citrem-phospholipid lamellar and non-lamellar liquid crystalline nano-assemblies. J. Control. Release 2016, 239, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ou, N.; Sun, Y.; Zhou, S.; Gu, P.; Liu, Z.; Bo, R.; Hu, Y.; Liu, J.; Wang, D. Evaluation of optimum conditions for Achyranthes bidentata polysaccharides encapsulated in cubosomes and immunological activity in vitro. Int. J. Biol. Macromol. 2018, 109, 748–760. [Google Scholar] [CrossRef] [PubMed]
- Yaghmur, A.; Al-Hosayni, S.; Amenitsch, H.; Salentinig, S. Structural investigation of bulk and dispersed inverse lyotropic hexagonal liquid crystalline phases of eicosapentaenoic acid monoglyceride. Langmuir 2017, 33, 14045–14057. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Bor, G.; Al-Hosayni, S.; Salentinig, S.; Yaghmur, A. Structural characterization of self-assemblies of new omega-3 lipids: Docosahexaenoic acid and docosapentaenoic acid monoglycerides. Phys. Chem. Chem. Phys. 2018, 20, 23928–23941. [Google Scholar] [CrossRef]
- Azmi, I.D.M.; Ostergaard, J.; Sturup, S.; Gammelgaard, B.; Urtti, A.; Moghimi, S.M.; Yaghmur, A. Cisplatin encapsulation generates morphologically different multicompartments in the internal nanostructures of nonlamellar liquid-crystalline self-assemblies. Langmuir 2018, 34, 6570–6581. [Google Scholar] [CrossRef]
- Gontsarik, M.; Yaghmur, A.; Ren, Q.; Maniura-Weber, K.; Salentinig, S. From Structure to Function: pH-Switchable Antimicrobial Nano-Self-Assemblies. ACS Appl. Mater. Interfaces 2019, 11, 2821–2829. [Google Scholar] [CrossRef]
- Yaghmur, A.; Rappolt, M. The Micellar Cubic Fd3m Phase: Recent Advances in Structural Characterization and Potential Applications. In Advances in Planar Lipid Bilayers and Liposomes; Igles, A., Ed.; Elsevier Book Series; Elsevier: Edinburgh, UK, 2013; Volume 18, pp. 111–145. [Google Scholar]
- Madheswaran, T.; Kandasamy, M.; Bose, R.J.C.; Karuppagounder, V. Current potential and challenges in the advances of liquid crystalline nanoparticles as drug delivery systems. Drug Discov. Today 2019, 24, 1405–1412. [Google Scholar] [CrossRef]
- Zabara, M.; Senturk, B.; Gontsarik, M.; Ren, Q.; Rottmar, M.; Maniura-Weber, K.; Mezzenga, R.; Bolisetty, S.; Salentinig, S. Multifunctional Nano-Biointerfaces: Cytocompatible Antimicrobial Nanocarriers from Stabilizer-Free Cubosomes. Adv. Funct. Mater. 2019, 29, 1904007. [Google Scholar] [CrossRef]
- Faria, A.R.; Silvestre, O.F.; Maibohm, C.; Adao, R.M.R.; Silva, B.F.B.; Nieder, J.B. Cubosome nanoparticles for enhanced delivery of mitochondria anticancer drug elesclomol and therapeutic monitoring via sub-cellular NAD(P)H multi-photon fluorescence lifetime imaging. Nano Res. 2019, 12, 991–998. [Google Scholar] [CrossRef]
- Rappolt, M.; Cacho-Nerin, F.; Morello, C.; Yaghmur, A. How the chain configuration governs the packing of inverted micelles in the cubic Fd3m-phase. Soft Matter 2013, 9, 6291–6300. [Google Scholar] [CrossRef]
- Nakano, M.; Teshigawara, T.; Sugita, A.; Leesajakul, W.; Taniguchi, A.; Kamo, T.; Matsuoka, H.; Handa, T. Dispersions of liquid crystalline phases of the monoolein/oleic acid/Pluronic F127 system. Langmuir 2002, 18, 9283–9288. [Google Scholar] [CrossRef]
- Yaghmur, A.; Sartori, B.; Rappolt, M. Self-assembled nanostructures of fully hydrated monoelaidin-elaidic acid and monoelaidin-oleic acid systems. Langmuir 2012, 28, 10105–10119. [Google Scholar] [CrossRef] [PubMed]
- Yaghmur, A.; De Campo, L.; Salentinig, S.; Sagalowicz, L.; Leser, M.E.; Glatter, O. Oil-loaded monolinolein-based particles with confined inverse discontinuous cubic structure (Fd3m). Langmuir 2006, 22, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Yaghmur, A.; Kriechbaum, M.; Amenitsch, H.; Steinhart, M.; Laggner, P.; Rappolt, M. Effects of pressure and temperature on the self-assembled fully hydrated nanostructures of monoolein-oil systems. Langmuir 2010, 26, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Gontsarik, M.; Mohammadtaheri, M.; Yaghmur, A.; Salentinig, S. pH-Triggered nanostructural transformations in antimicrobial peptide/oleic acid self-assemblies. Biomater. Sci.-UK 2018, 6, 803–812. [Google Scholar] [CrossRef]
- Neophytou, C.; Constantinou, A.I. Drug Delivery Innovations for Enhancing the Anticancer Potential of Vitamin E Isoforms and Their Derivatives. BioMed Res. Int. 2015. [Google Scholar] [CrossRef]
- Hao, T.; Chen, D.; Liu, K.; Qi, Y.; Tian, Y.; Sun, P.; Liu, Y.; Li, Z. Micelles of d-α-tocopheryl polyethylene glycol 2000 succinate (TPGS 2K) for doxorubicin delivery with reversal of multidrug resistance. ACS Appl. Mater. Interface 2015, 7, 18064–18075. [Google Scholar] [CrossRef]
- Zhai, J.; Hinton, T.M.; Waddington, L.J.; Fong, C.; Tran, N.; Mulet, X.; Drummond, C.J.; Muir, B.W. Lipid-PEG conjugates sterically stabilize and reduce the toxicity of phytantriol-based lyotropic liquid crystalline nanoparticles. Langmuir 2015, 31, 10871–10880. [Google Scholar] [CrossRef]
- Nilsson, C.; Ostergaard, J.; Larsen, S.W.; Larsen, C.; Urtti, A.; Yaghmur, A. PEGylation of phytantriol-based lyotropic liquid crystalline particles--the effect of lipid composition, PEG chain length, and temperature on the internal nanostructure. Langmuir 2014, 30, 6398–6407. [Google Scholar] [CrossRef]
- Helvig, S.; Azmi, I.D.M.; Moghimi, S.M.; Yaghmur, A. Recent advances in cryo-TEM imaging of soft lipid nanoparticles. Aims Biophys. 2015, 2, 116–130. [Google Scholar] [CrossRef]
- Gustafsson, J.; Ljusberg-Wahren, H.; Almgren, M.; Larsson, K. Submicron particles of reversed lipid phases in water stabilized by a nonionic amphiphilic polymer. Langmuir 1997, 13, 6964–6971. [Google Scholar] [CrossRef]
- Yaghmur, A.; de Campo, L.; Sagalowicz, L.; Leser, M.E.; Glatter, O. Emulsified microemulsions and oil-containing liquid crystalline phases. Langmuir 2005, 21, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Sagalowicz, L.; Michel, M.; Adrian, M.; Frossard, P.; Rouvet, M.; Watzke, H.J.; Yaghmur, A.; de Campo, L.; Glatter, O.; Leser, M.E. Crystallography of dispersed liquid crystalline phases studied by cryo-transmission electron microscopy. J. Microsc-Oxf. 2006, 221, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Wibroe, P.P.; Mat Azmi, I.D.; Nilsson, C.; Yaghmur, A.; Moghimi, S.M. Citrem modulates internal nanostructure of glyceryl monooleate dispersions and bypasses complement activation: Towards development of safe tunable intravenous lipid nanocarriers. Nanomed. Nanotechnol. 2015, 11, 1909–1914. [Google Scholar] [CrossRef] [PubMed]
- Almgren, M.; Edwards, K.; Karlsson, G. Cryo transmission electron microscopy of liposomes and related structures. Colloid Surface A 2000, 174, 3–21. [Google Scholar] [CrossRef]
- Yaghmur, A.; Laggner, P.; Almgren, M.; Rappolt, M. Self-assembly in monoelaidin aqueous dispersions: Direct vesicles to cubosomes transition. PLoS ONE 2008, 3, e3747. [Google Scholar] [CrossRef]
- Barauskas, J.; Johnsson, M.; Tiberg, F. Self-assembled lipid superstructures: Beyond vesicles and liposomes. Nano Lett. 2005, 5, 1615–1619. [Google Scholar] [CrossRef]
- de Campo, L.; Yaghmur, A.; Sagalowicz, L.; Leser, M.E.; Watzke, H.; Glatter, O. Reversible phase transitions in emulsified nanostructured lipid systems. Langmuir 2004, 20, 5254–5261. [Google Scholar] [CrossRef]
- Yaghmur, A.; de Campo, L.; Sagalowicz, L.; Leser, M.E.; Glatter, O. Control of the internal structure of MLO-based isasomes by the addition of diglycerol monooleate and soybean phosphatidylcholine. Langmuir 2006, 22, 9919–9927. [Google Scholar] [CrossRef]
- Sagalowicz, L.; Guillot, S.; Acquistapace, S.; Schmitt, B.; Maurer, M.; Yaghmur, A.; de Campo, L.; Rouvet, M.; Leser, M.; Glatter, O. Influence of vitamin E acetate and other lipids on the phase behavior of mesophases based on unsaturated monoglycerides. Langmuir 2013, 29, 8222–8232. [Google Scholar] [CrossRef]
- Yaghmur, A.; Rappolt, M.; Ostergaard, J.; Larsen, C.; Larsen, S.W. Characterization of bupivacaine-loaded formulations based on liquid crystalline phases and microemulsions: The effect of lipid composition. Langmuir 2012, 28, 2881–2889. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.D.; Larson, I.; Hanley, T.; Boyd, B.J. Bulk and dispersed aqueous phase behavior of phytantriol: Effect of vitamin E acetate and F127 polymer on liquid crystal nanostructure. Langmuir 2006, 22, 9512–9518. [Google Scholar] [CrossRef] [PubMed]
- Yaghmur, A.; Laggner, P.; Zhang, S.; Rappolt, M. Tuning curvature and stability of monoolein bilayers by designer lipid-like peptide surfactants. PLoS ONE 2007, 2, e479. [Google Scholar] [CrossRef] [PubMed]
- Wadsater, M.; Barauskas, J.; Nylander, T.; Tiberg, F. Formation of highly structured cubic micellar lipid nanoparticles of soy phosphatidylcholine and glycerol dioleate and their degradation by triacylglycerol lipase. ACS Appl. Mater. Interface 2014, 6, 7063–7069. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.Y.; Zhu, Y.X.; Bu, J.Y.; Li, G.W.; Zhou, J.H.; Zhou, S.P. Evaluation of Neuroprotective Effect of Thymoquinone Nanoformulation in the Rodent Cerebral Ischemia-Reperfusion Model. BioMed Res. Int. 2016. [Google Scholar] [CrossRef]
- Nii, T.; Ishii, F. Encapsulation efficiency of water-soluble and insoluble drugs in liposomes prepared by the microencapsulation vesicle method. Int. J. Pharm. 2005, 298, 198–205. [Google Scholar] [CrossRef]
- Salmani, J.M.M.; Asghar, S.; Lv, H.X.; Zhou, J.P. Aqueous Solubility and Degradation Kinetics of the Phytochemical Anticancer Thymoquinone; Probing the Effects of Solvents, pH and Light. Molecules 2014, 19, 5925–5939. [Google Scholar] [CrossRef]
- Moghimi, S.M.; Simberg, D.; Skotland, T.; Yaghmur, A.; Hunter, A.C. The Interplay Between Blood Proteins, Complement, and Macrophages on Nanomedicine Performance and Responses. J. Pharmacol. Exp. Ther. 2019, 370, 581–592. [Google Scholar] [CrossRef]
- Wibroe, P.P.; Ahmadvand, D.; Oghabian, M.A.; Yaghmur, A.; Moghimi, S.M. An integrated assessment of morphology, size, and complement activation of the PEGylated liposomal doxorubicin products Doxil(R), Caelyx(R), DOXOrubicin, and SinaDoxosome. J. Control. Release 2016, 221, 1–8. [Google Scholar] [CrossRef]
- Wibroe, P.P.; Anselmo, A.C.; Nilsson, P.H.; Sarode, A.; Gupta, V.; Urbanics, R.; Szebeni, J.; Hunter, A.C.; Mitragotri, S.; Mollnes, T.E.; et al. Bypassing adverse injection reactions to nanoparticles through shape modification and attachment to erythrocytes. Nat. Nanotechnol. 2017, 12, 589–594. [Google Scholar] [CrossRef]
- Leong, H.S.; Butler, K.S.; Brinker, C.J.; Azzawi, M.; Conlan, S.; Dufes, C.; Owen, A.; Rannard, S.; Scott, C.; Chen, C.Y.; et al. On the issue of transparency and reproducibility in nanomedicine. Nat. Nanotechnol. 2019, 14, 629–635. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |
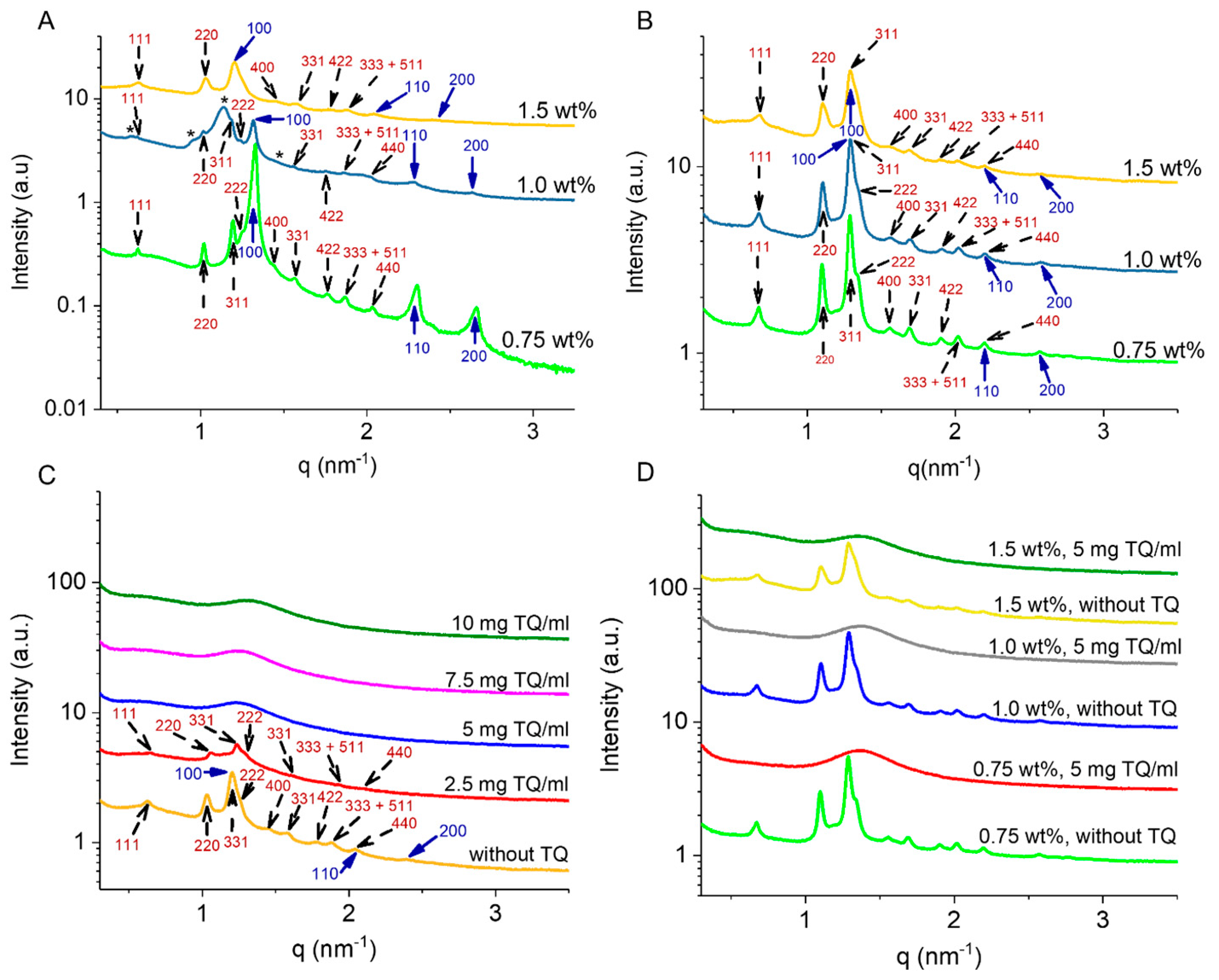

| Sample | Lipids | Stabilizer (wt%) | TQ (mg/mL) | Space Group | Lattice Parameter (nm) |
|---|---|---|---|---|---|
| (GMO/Vit E Ratio) | |||||
| 1 | 70:30 | 0.75 | 0 | Fd3m H2 | 17.45 5.45 |
| 2 | 70:30 | 1.0 | 0 | Fd3m Fd3ma H2 | 17.45 18.60 5.50 |
| 3 | 70:30 | 1.5 | 0 | Fd3m H2 | 17.34 6.02 |
| 4 | 60:40 | 0.75 | - | Fd3m H2 | 16.20 5.64 |
| 5 | 60:40 | 1.0 | - | Fd3m H2 | 16.20 5.64 |
| 6 | 60:40 | 1.5 | - | Fd3m H2 | 16.20 5.64 |
| 7 | 70:30 | 1.5 | 2.5 | Fd3m | 16.81 |
| 8 | 70:30 | 1.5 | 5 | L2b | 4.95 |
| 9 | 70:30 | 1.5 | 7.5 | L2b | 4.95 |
| 10 | 70:30 | 1.5 | 10 | L2b | 4.69 |
| 11 | 60:40 | 0.75 | 5 | L2b | 4.55 |
| 12 | 60:40 | 1.0 | 5 | L2b | 4.53 |
| 13 | 60:40 | 1.5 | 5 | L2b | 4.53 |
| Sample | Lipids | Stabilizer (wt%) | TQ (mg/mL) | Mean Particle Size ± SD (nm) | Mode ± SD (nm) | EE ± SD (%) | EE ± SD (%) |
|---|---|---|---|---|---|---|---|
| (GMO/Vit E Ratio) | Preparation Day | 5 Days | |||||
| 1 | 70:30 | 1.5 | 0 | 145 ± 49 | 126 ± 11 | ||
| 4 | 60:40 | 0.75 | 0 | 119 ± 49 | 95 ± 3 | ||
| 6 | 60:40 | 1.5 | 0 | 141 ± 53 | 131 ± 3 | ||
| 8 | 70:30 | 1.5 | 5 | 160 ± 65 | 134 ± 7 | - | - |
| 11 | 60:40 | 0.75 | 5 | 127 ± 43 | 118 ± 3 | 98.8 ± 0.09 | 97.6 ± 0.11 |
| 13 | 60:40 | 1.5 | 5 | 168 ± 67 | 136 ± 1 | 98.9 ± 0.10 | 97.9 ± 0.09 |
| 14 | 70:30 | 1.5 | 7.5 | - | - | 99.4 ± 0.05 | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yaghmur, A.; Tran, B.V.; Moghimi, S.M. Non-Lamellar Liquid Crystalline Nanocarriers for Thymoquinone Encapsulation. Molecules 2020, 25, 16. https://doi.org/10.3390/molecules25010016
Yaghmur A, Tran BV, Moghimi SM. Non-Lamellar Liquid Crystalline Nanocarriers for Thymoquinone Encapsulation. Molecules. 2020; 25(1):16. https://doi.org/10.3390/molecules25010016
Chicago/Turabian StyleYaghmur, Anan, Boi Vi Tran, and Seyed Moein Moghimi. 2020. "Non-Lamellar Liquid Crystalline Nanocarriers for Thymoquinone Encapsulation" Molecules 25, no. 1: 16. https://doi.org/10.3390/molecules25010016
APA StyleYaghmur, A., Tran, B. V., & Moghimi, S. M. (2020). Non-Lamellar Liquid Crystalline Nanocarriers for Thymoquinone Encapsulation. Molecules, 25(1), 16. https://doi.org/10.3390/molecules25010016