1. Introduction
-Aescin or
-escin belongs to the vast family of natural surfactants, the so-called saponins [
1]. All saponins follow very similar structural motifs. They consist of an aglycone (hydrophobic part of the molecule) which is linked to one or several oligosaccharide chains. Saponins are found in hundreds of plants and a lot of them have interesting pharmacological activities which have already been used in ancient times for the treatment of various diseases. The very characteristic amphiphilic nature of the saponins leads to diverse applications in, among others, industry and pharmacy [
2,
3,
4,
5,
6,
7,
8].
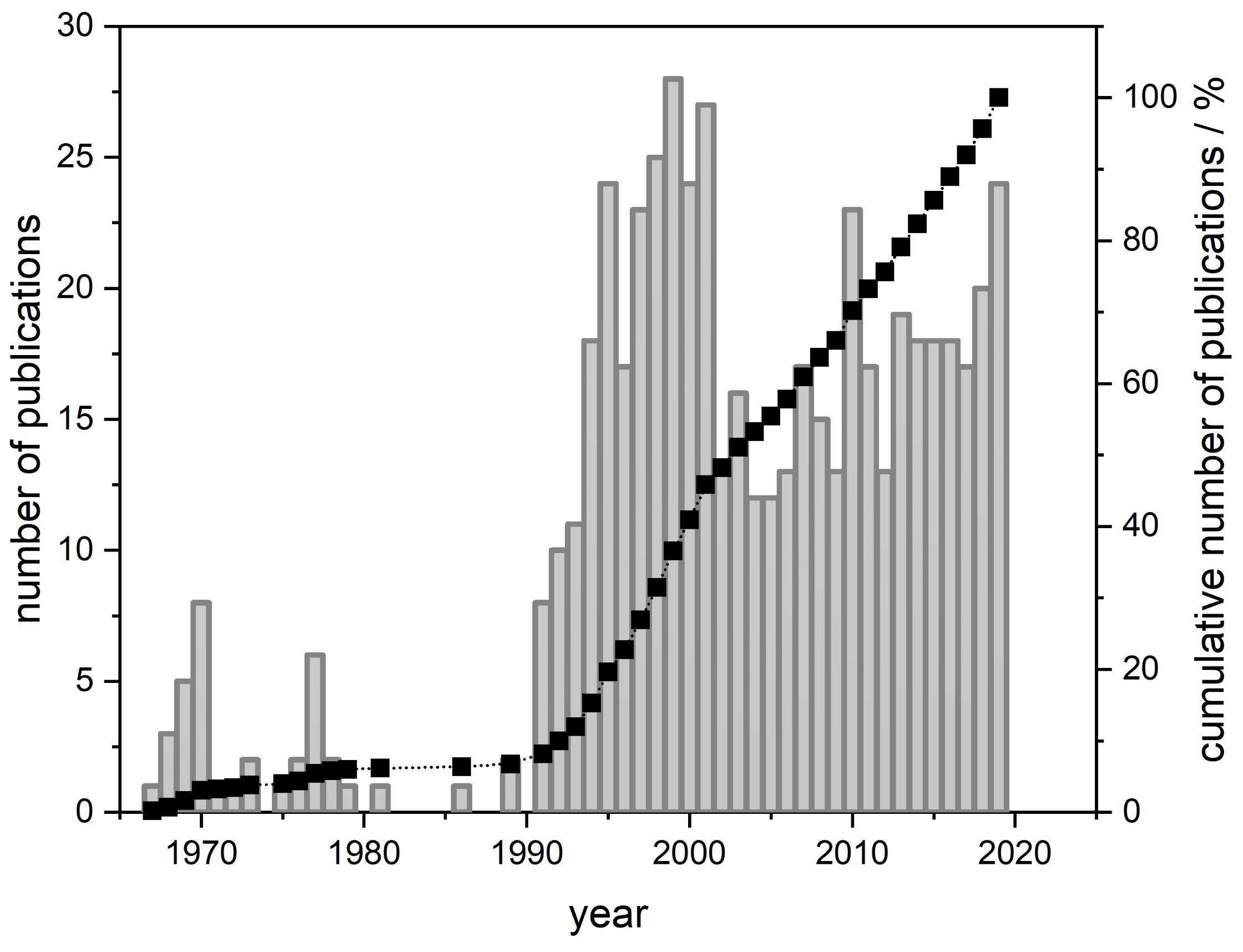
A simple search in the ”The Web of Science Core Collection” database yields 550 hits for
-aescin at the moment (November 2019, compare
Figure 1). More than one third of these works was published in the last 10 years. This shows the continuous interest in this natural surfactant molecule produced by the horse chestnut tree
Aesculus hippocastanum. However, most of the published works are pharmacological studies which have been recently reviewed by Xinxin Zhang et al. [
9].
-Aescin has been proven to be safe and very tolerable [
10,
11]. It is, e.g., successfully used in the treatment of the post thrombotic syndrome [
12,
13], chronic venous insufficiency [
10,
14,
15], shows promising effects in the treatment of arthritis [
16,
17], and seems to have potential as an anti-cancer drug [
18,
19]. Especially remarkable is
-aescins action against glioblastoma-initiating cells (GIC) which have the ability to initiate glioblastoma growth [
20]. Glioblastoma multiform is the most abundant primary tumor of the human nervous system.
-Aescin was more efficient in diminishing GIC growth compared to present clinically used drugs. Moreover, as other saponins, it shows a hemolytic effect and seems to interact with non-steroidal anti-inflammatory drugs inside lipid membranes [
21]. Vinarov et al. have shown that
-aescin might also help to solubilize hydrophobic drugs [
22]. Also most of the available reviews mainly treat the biological activity of the
-aescin molecule. The most recent one was published by Luca Galleli in September 2019 [
23].
The physical properties of -aescin are less investigated and its interaction with biological membranes on a molecular level, which is the basis of several of its effects, is only sparsely studied. Hence, in the present focus review, we will try to summarize recent progress in this area.
The review is organized as follows.
Section 2 will introduce the chemical structure of
-aescin. In
Section 3 the micelle formation properties of
-aescin will be investigated. Afterwards, in
Section 4, the behavior of
-aescin at interfaces is focused upon. In
Section 5, we will review and discuss the interaction of
-aescin with bare lipid membranes, followed by
Section 6 which describes synergistic effects between other membrane active compounds like e.g., cholesterol or drugs. In
Section 7, the interaction with Langmuir monolayers is summarized. The focus review will end with a conclusion (
Section 8) also comprising remarks about future perspectives in
-aescin research.
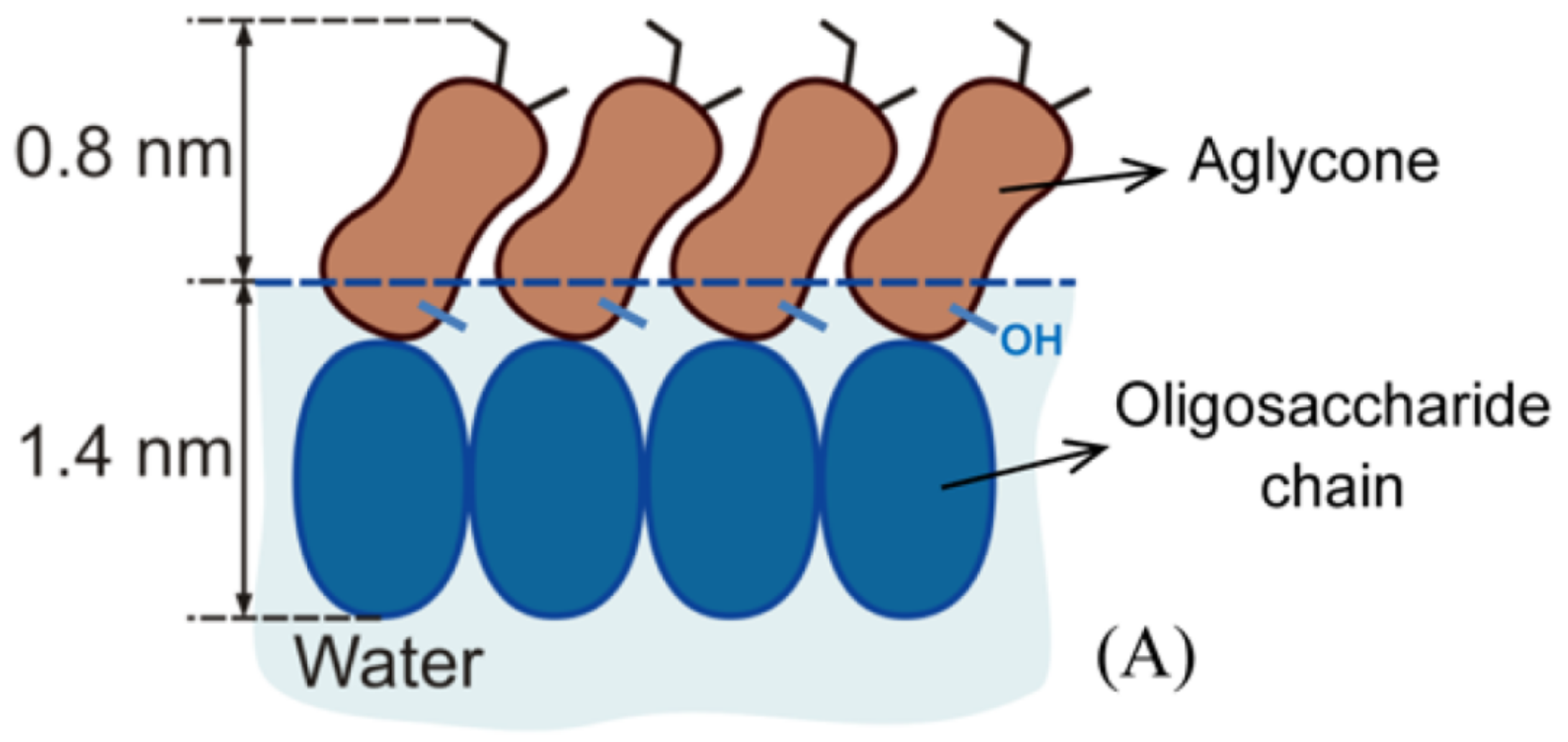
2. The Saponin -Aescin: Chemical Structure and Specifications
Saponins generally share a similar structure, which is built by an either triterpenic or steroidic hydrophobic backbone, to which a different number of sugar chains is attached [
1,
2,
3,
4,
5,
6,
7,
8]. Aescin itself obtained from the seeds of the horse chestnut tree is not a pure compound but rather a complex mixture of about 30 different molecules [
7,
10,
24,
25]. This mixture is based on two different hydrophobic, triterpenic backbones, namely, protoaescigenin and barringtogenol C [
2]. Both backbones differ only at the C-4 position which carries a methyl group in the case of barringtogenol C and a methoxy group in case of protoaescigenin [
7]. In all cases, a trisaccharide is attached to the C-3 position of the backbone. Thereby, a glucuronic acid acts as linker for glucose, xylose or galactose molecules [
7]. Hydrolysis experiments showed that the main fraction consists of only glucose and glucuronic acid [
24].
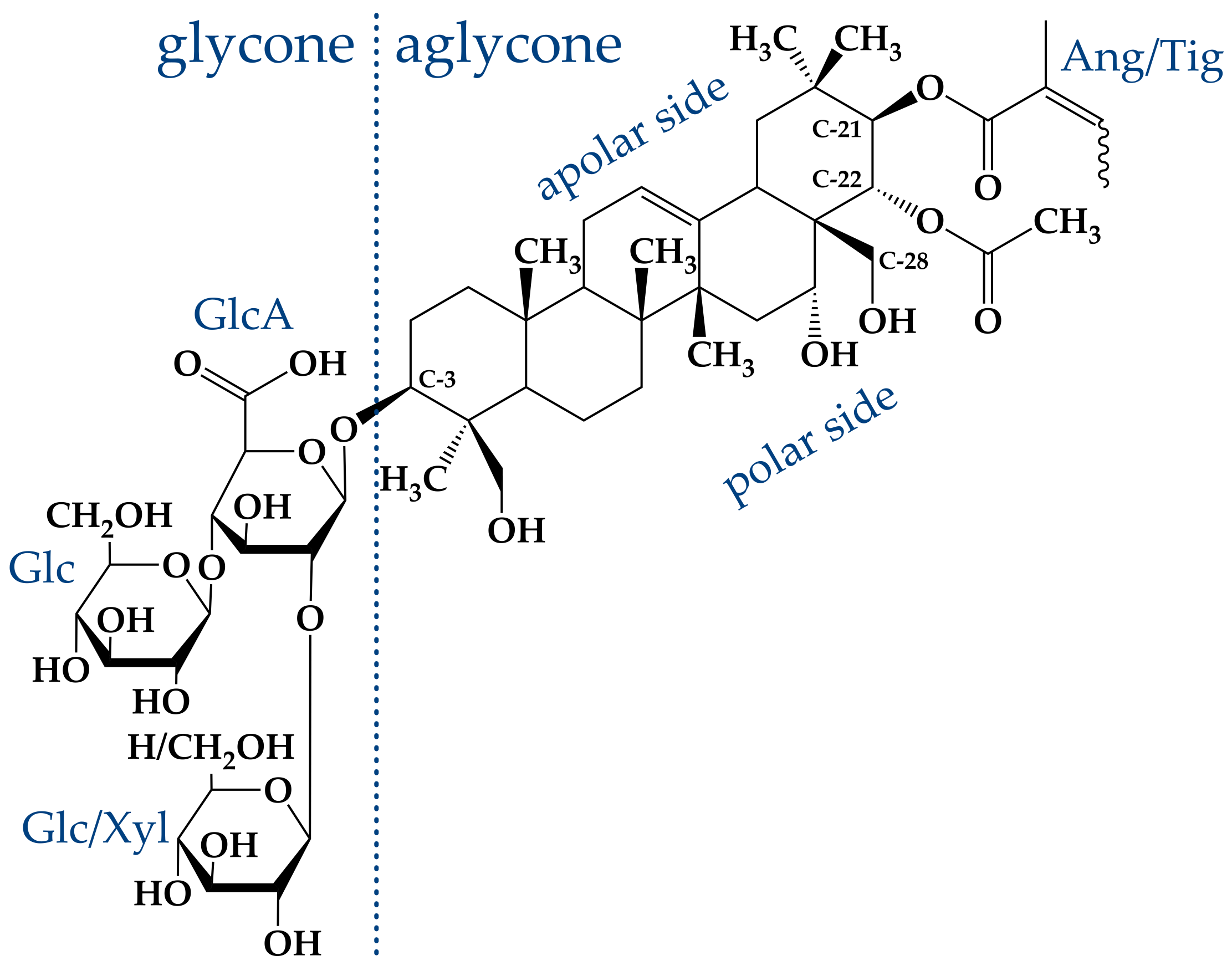
The main compound in this “aescin mixture”, which is present in a share of ≈60%, consists of a protoaescigenin backbone which is esterified at the C-22 position with acetic acid and with angelic/tiglic acid at C-21 [
6,
7,
10,
26]. This fraction is called
-aescin and the most abundant structures of this mixture are shown in
Figure 2. One of the two glucose molecules attached to the glucuronic acid can be substituted by xylose [
6,
24,
27]. Besides
-aescin, two other fractions are identified in the aescin mixture. These are called
- and crypto-aescin, whereby
-aescin is a mixture of crypto- and
-aescin [
7,
10,
28].
- and crypto-aescin differ in the position of the acetyl group in the backbone [
15,
28]. Whereas for crypto-aescin this group is located at C-28, it can be found at C-22 for
-aescin. Both forms can be distinguished by the solubility in water, the melting point and the hemolytic index [
15]. In comparison to
-aescin,
-aescin shows a much higher pharmacological activity and
-aescin is the main active component in the pharmaceutically used horse chestnut tree extract [
10,
25].
According to the literature, the amount of the general saponin mixture in the horse chestnut tree seeds is ≈3–10%, whereby the amount of the aescin mixture is ≈2–3% [
2,
7,
10,
28,
30]. In conventional extraction, saponins are dissolved in a solvent [
4]. In the case of aescin, the horse chestnut seeds are first dried and ground up to a powder. This powder is afterwards treated with ≈60%
ethanol (or methanol) to dissolve the aescin fraction [
31,
32]. By e.g., addition of acid and therewith lowering of the pH value, the less soluble protonated form of
-aescin sediments and can be separated afterwards from the
-aescin fraction and other compounds [
32]. In 1991, E. Horvath proposed a different approach, which does not require alcohol for extraction and which results in better yields of
-aescin.
-Aescin was extracted from fresh, frozen and ground seeds by the usage of only water as solvent. The pure substance
-aescin is obtained by acidification and recrystallization. A separation of both forms is not necessary, because an isomerization of the
-form to
-aescin induced by drying the seeds is avoided [
32].
-Aescin is commercially available (CAS: 6805-41-0) and can e.g., be purchased from Merck/Sigma-Aldrich Company in purity of >95 %. The structures present in this mixture were determined by combination of analytical reversed-phase high performance liquid chromatography (RP-HPLC) and the analysis techniques mass spectrometry and
1H-NMR spectroscopy [
29]. Two separable fractions with an exact mass of 1130.5 g/mol were obtained.
1H-NMR spectroscopy identified both fractions as cis-trans-isomers with either tiglic or angelic acid attached to the C-21 carbon atom of the triterpenic backbone [
29,
33,
34]. The presence of xylose, which would lead to a different molar mass, was not proven. Hence, these structures correspond to
-aescin. The solubility of the compound in an aqueous solution is limited, because especially the acidic form of the molecule shows a bad solubility [
32]. The solubility of
-aescin can be significantly increased by increase of pH value (and the usage of buffer). The carboxylic group located in the glycone of
-aescin (
Figure 2) induces intrinsic pH-dependence of the
-aescin form. Dargel et al. [
29] recently determined the
value of
-aescin in water to be 4.7 ± 0.2. Therefore, it is assumed that
-aescin exists in its neutral form below and in a deprotonated ionized form above a pH value of 4.7. Deprotonation leads to strongly enhanced solubility of
-aescin in water. Moreover,
-aescin is soluble in alcohols such as ethanol and methanol, which are also used in the extraction procedure [
31,
32].
3. Micelle Formation
The amphiphilic nature of
-aescin causes formation of micelles in aqueous solution at a critical micelle concentration (
). Values for the
of
-aescin have been determined by different groups under different conditions such as varying buffer and pH value. Determination was thereby mainly performed by tensiometry (T) or measurement of the autofluorescence (AF) of
-aescin. The reported values are summarized in
Table 1, indicating the pH, solvent, temperature and method used to determine the
.
The
-values are mostly consistent but, however, show a slight dependence on pH value. Slightly smaller values were obtained at a lower pH value and this observation might be explained by the increased charge of the
-aescin due to deprotonation at higher pH values.
-Aescin molecules were found to exhibit peculiar viscoelastic properties at e.g., the
-aescin–water interface [
38,
40,
41]. These works demonstrated that
and pH value are important parameters leading to modified structural and elastic properties of e.g., an adsorbed
-aescin monolayer at the water–air interface. This will be discussed in more detail in
Section 4. Also, the state of protonation was found to modify the inter-molecular interactions and thus the attractive and repulsive forces between
-aescin molecules [
42,
43].
Besides the numerous reports on the
-values of
-aescin, there are only two works elucidating the actual structure of
-aescin micelles in solution [
29,
39]. De Groot et al. [
39] report on rather flat and spherically shaped
-aescin micelles in pure water and ambient temperature (Figure 13A) at unspecified pH. In this study, the micelle shape was visualized by transmission electron microscopy (TEM) and an average length/diameter of the
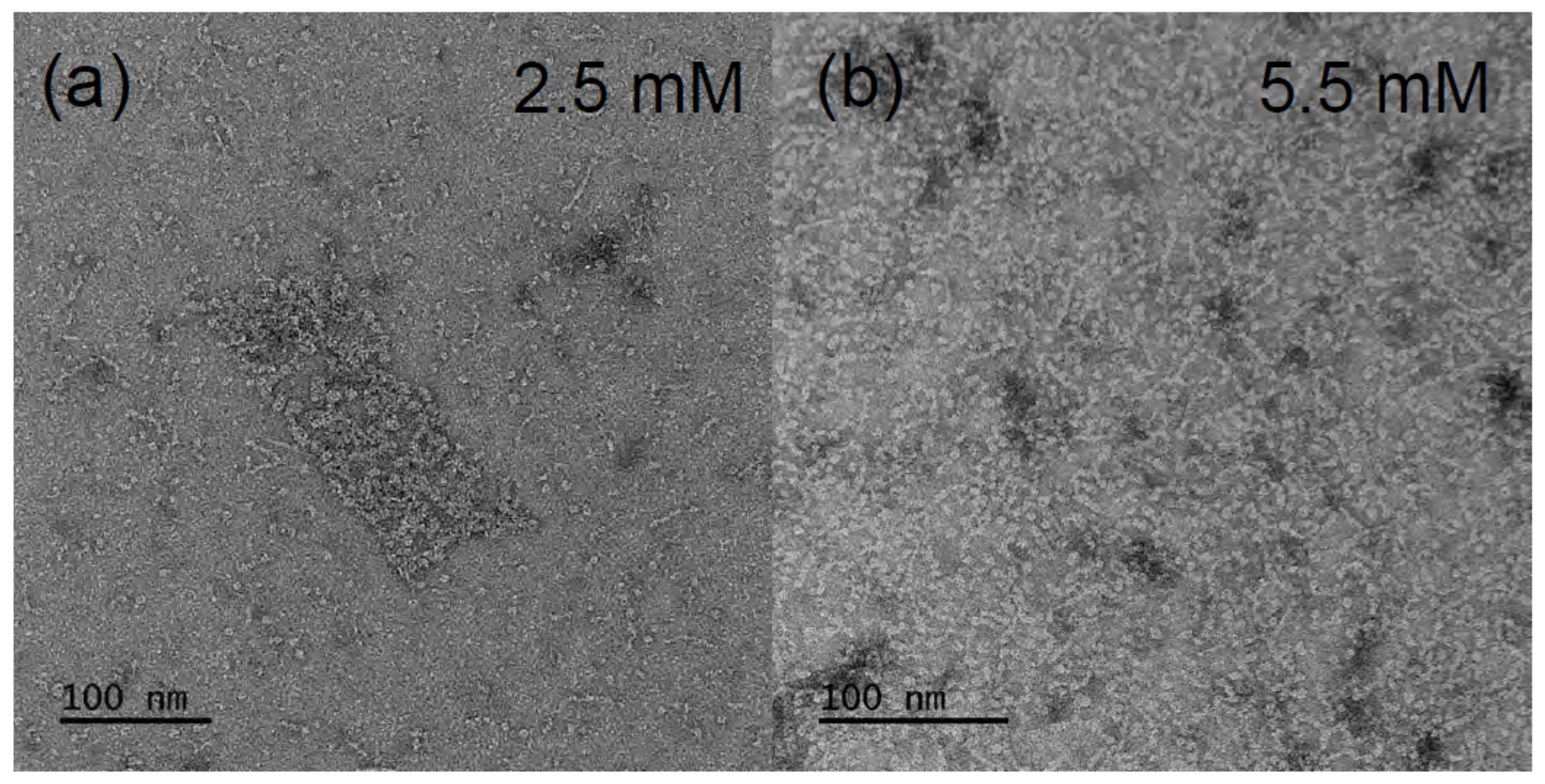
-aescin micelles of 200 Å was obtained. Another work by Dargel et al. [
29] characterizes the
-aescin structures in dependence of
-aescin concentration ranging from 1.7–9.5 mM at temperatures of 10
C and 40
C. The analysis was conducted by TEM and small-angle X-ray scattering (SAXS) experiments and clearly shows that micellar shape varies with temperature whereas the concentration only plays a marginal role above the
.
Figure 3 shows TEM images at different concentrations and low temperature (6
C). The contour of the micelles suggests that they exhibit a rather cylindrical shape with a cross-sectional diameter of 25–30 Å and a length of 70–150 Å. From a closer look, it becomes visible that the rods contain smaller segments which could be stacked smaller discoidal micelles. However, by the SAXS results, a temperature-dependent behavior was evidenced. Whereas, the cylindrical shape could be confirmed at low temperature of 10
C, ellipsoidal structures form at 40
C. The rods show a cross-sectional diameter ranging from 16.8 Å to 18.5 Å and a length in the range between 74 Å and 90 Å, which slightly decreased with increasing
-aescin concentration. The equatorial radius of the ellipsoids was around 31 Å and the polar radius between 13.2 and 16.8 Å. The modified geometry of
-aescin micelles with temperature may be the result of changed attractive or repulsive interactions, e.g., modified hydrogen bonds, with temperature. However, the clear cause for this behavior could not be identified within this work.
5. -Aescin Interaction with Bare Lipid Model Membranes
The interaction of
-aescin with bare lipid model membranes from the physicochemical perspective is subject of works published over the last 2 years by Sreij et al. [
46,
47,
48] and Geisler et al. [
49]. The work is done exemplarily on phospholipid bilayers composed of 1,2-dimyristoyl-
sn-glycero-3-phosphocholine (DMPC) at constant environmental conditions, i.e., in 50 mM phosphate buffer at physiological p(H)≈ p(D) of about 7.4 and a DMPC mass concentration of
w(DMPC) = 15 mg/mL. All samples were prepared by the lipid film hydration method; i.e., the dry thin lipid film is hydrated by a
-aescin containing buffer solution and subsequently exposed to five freeze-thaw cycles. The
-aescin concentrations investigated range from 0 (pure lipid bilayers) to 30 mol%
-aescin molecules with respect to the number of DMPC molecules. Here,
-aescin concentrations below and above
(
-aescin) are covered. The
is crossed at about 3–4 mol% of
-aescin with respect to the lipid. The interaction of the lower contents was generally studied on bilayers in the form of extruded small unilamellar vesicles (SUVs). Polycarbonate membranes for extrusion had a pore size of 500 Å.
The successful incorporation of
-aescin into the bilayer of the samples prepared as mentioned above was shown by a changing cross-sectional electron density profile of samples with varying
-aescin concentration obtained from SAXS measurements [
47].
In these works, these authors elucidated a concentration- and lipid phase state-dependent structure formation caused by the interaction of
-aescin with the DMPC bilayer. In general, they divided the studied range into three parts (III and IV belong to the same concentration range), similar to the three-stage model of membrane solubilization by classical detergents and bile salts [
50,
51,
52], whereby each of them represents a concentration regime where similar interactions and structures are obtained. An overall summary of the concentration-dependent interactions and structures formed is illustrated schematically in
Figure 8.
At low
-aescin contents (region I) which ranges up to ≈0.8 mol%,
-aescin can be incorporated into stable SUVs and the structural parameters of these SUVs were studied by small-angle scattering with X-rays (SAXS) and neutrons (SANS) [
47]. It was found that
-aescin incorporation leads to higher radii of gyration (
) with rising
-aescin content while the vesicles temperature-driven phase transition due to the conformational transition of DMPC’s alkyl chains remains conserved. The membrane thickness is only slightly affected by
-aescin incorporation.
Membrane dynamics affected by the incorporation of
-aescin were addressed by Sreij et al. [
47] by means of neutron spin-echo spectroscopy (NSE) measurements for DMPC SUVs in the mentioned
-aescin content regime. For
-aescin, the bilayer bending modulus
depends on
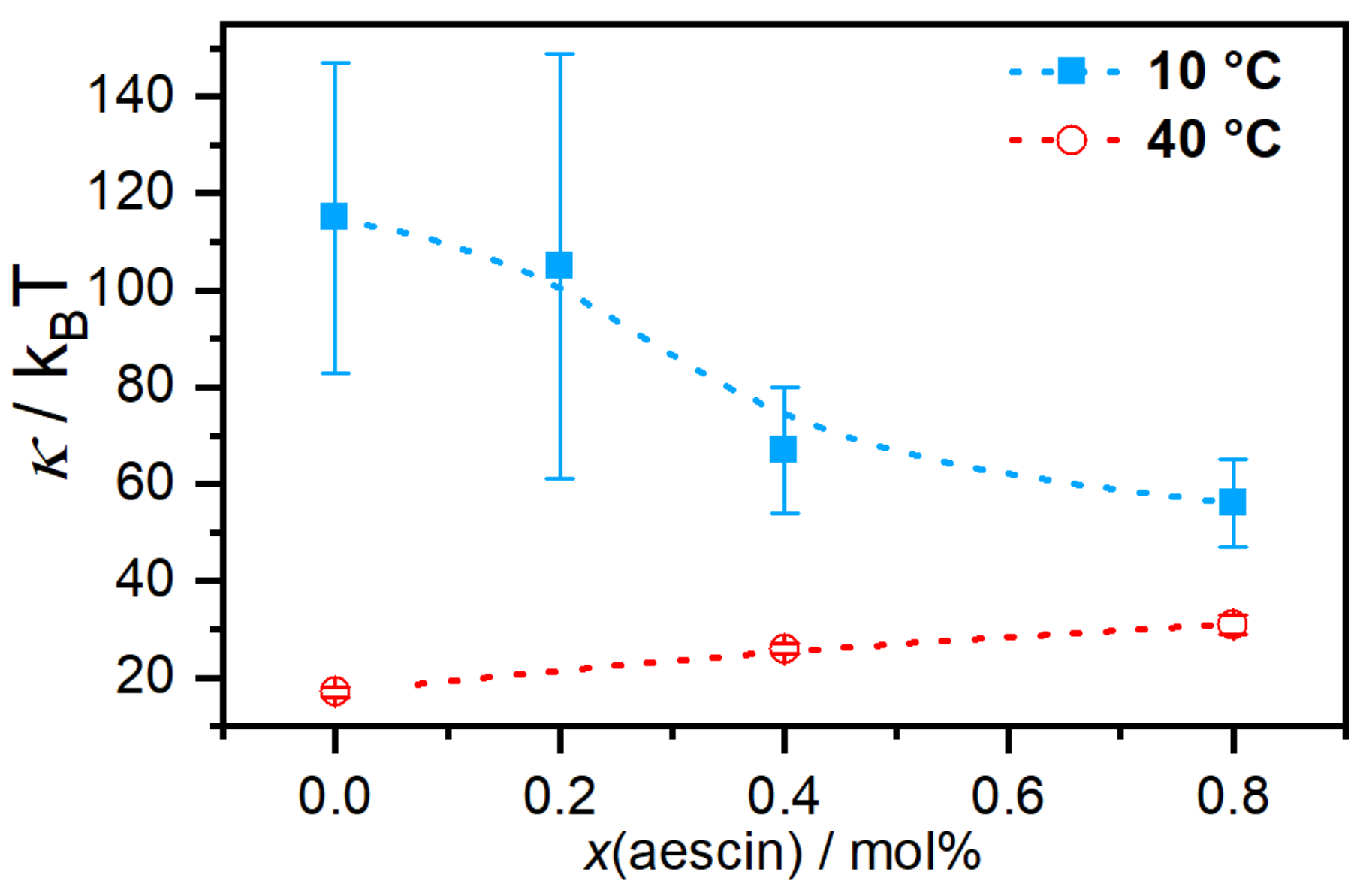
-aescin concentration and lipid phase state. For the phospholipid DMPC, the main phase transition occurs at a temperature of
23.6
C [
46]. In the gel phase of DMPC, i.e., at 10
C, the interactions of
-aescin with the bilayer lower the bending modulus and the membrane softens in a concentration-dependent manner (
Figure 9).
Above the melting temperature of DMPC, the bilayer rigidifies in presence of
-aescin and the value of
increases. To explain these effects, the authors used analogies to similar molecules since there are no comparable experiments on saponin molecules. The softening most likely results from headgroup-headgroup interactions between the saponins sugar moieties and DMPCs phosphocholine groups, similar to the effect of nonsteroidal anti-inflammatory drugs (NSAIDs) known to interact with the headgroups of phospholipid bilayers [
53]. This becomes especially convincing when taking into account that both, the NSAID molecules and also the
-aescin used in this work, are deprotonated and carry a single charge. The stiffening effect at the phospholipids fluid phase was explained by the insertion of the stiff triterpene backbone of
-aescin into the flexible bilayer, similar to the known effect of cholesterol incorporation.
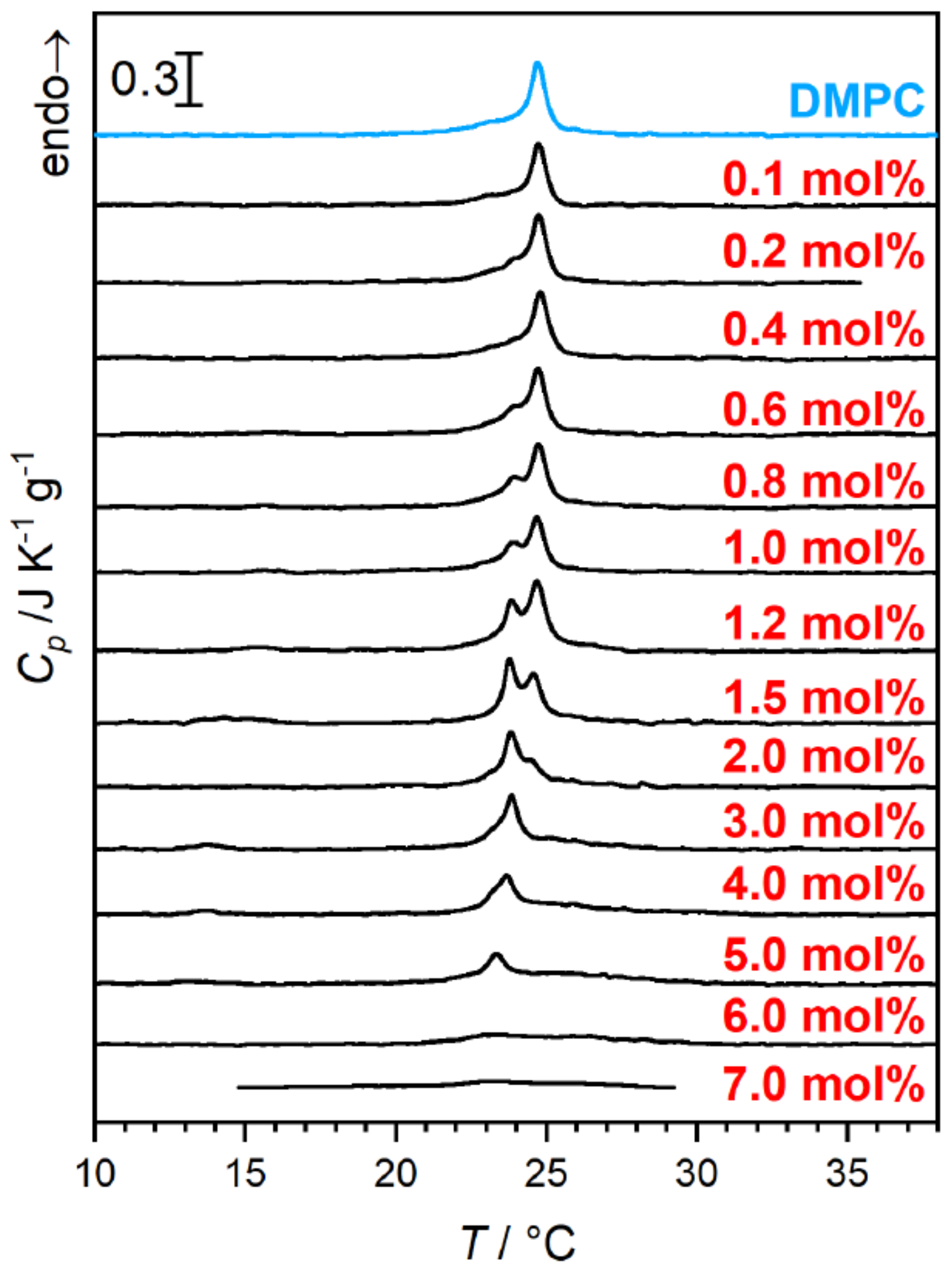
Upon rising the
-aescin content to values higher than ≈1 mol%,
-aescin incorporation increases the effect of phase separation inside the lipid bilayer. This shows clearly in the endotherms measured by differential scanning calorimetry (DSC) (
Figure 10) and was moreover confirmed by wide-angle X-ray scattering (WAXS) experiments [
46]. The formation of saponin-poor and saponin-rich domains becomes obvious in the presented data and is expressed by the rising intensity of the peak at lower temperature. Similar effects resulting from headgroup-drug interactions were observed for non-steroidal anti-inflammatory drugs (NSAIDs) known to interact with phospholipids headgroups from the inside of the bilayer [
54]. The presence of the stiff saponin backbone in the bilayer distorts the temperature-driven cooperative phase transition of DMPC molecules and the bilayer becomes fluidized at lower temperatures [
21]. At around 6 mol% of the saponin the signal broadens significantly and is only weakly visible indicating different structures in the samples.
A study devoted to the “mid” concentration regime between 1 and 7 mol% [
48] (region II) showed that an increasing
-aescin concentration leads to aggregated SUVs which start to deform and solubilize at concentrations around
(
-aescin). The diverse structures simultaneously present in this regime are visualized by cryogenic transmission electron microscopy (cryo-TEM) for a sample with 4 mol%
-aescin (
Figure 11). The figure shows the manifold of coexisting structures in the “mid” concentration regime, as this concentration is at the onset of complete decomposition into free-standing discoidal micelles, also called “bicelles” or “nanodisks”. At this
-aescin concentration, the presence of
-aescin leads to deformation (arrow 1), elongation (arrow 2) of and the presence of peculiar features like edges (arrow 3) in gel phase DMPC SUVs. Additionally to this, also a certain fraction of already solubilized small membrane fragments/bicelles is visible (arrow 4).
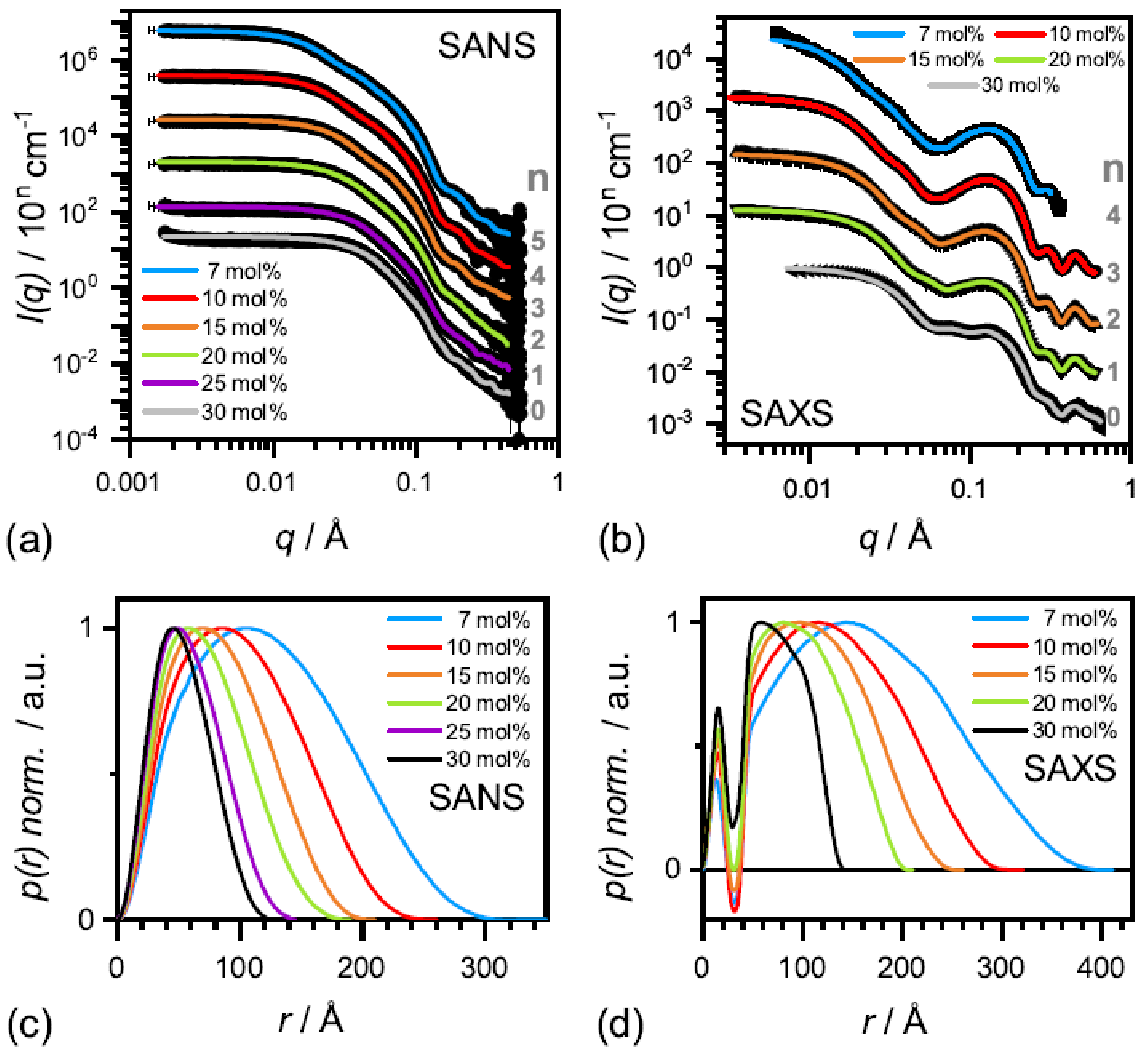
The effect of complete solubilization of a DMPC membrane at
-aescin contents >7 mol% (region III), was subject of a recent work published by Geisler et al. [
49]. This work has shown that well-defined bicelles form at
-aescin concentrations far above its
and at a temperature below the lipids
. Thereby, the disk radius is determined by the number of
-aescin molecules and decreases with increasing
-aescin content from around 350 Å at 7 mol% to 120 Å at 30 mol%
-aescin (
Figure 12). Disk radii were obtained from SAXS and SANS. The bicelles result to be tunable in size by composition. Moreover, the authors identified the onset of a fourth (IV) regime at around 25 mol%, where the radius of the bicelles becomes smaller than the thickness of the bilayer. Above this
-aescin content, a slightly different interaction with the bilayers is expected.
It was found that the
-aescin disks self-assemble at low temperature, i.e., at temperatures where the phospholipid is in its rigid gel state. At this lipid phase state and temperature (10
C), the phospholipid chains of DMPC are tightly packed and the formation of intermolecular hydrogen bonds (involving lipid and
-aescin molecules) is especially favorable. Here, the model assumes that the bicelles have circular discoidal shape with a surrounding rim composed of
-aescin molecules. The size of the discoidal structures is determined by the amount of
-aescin molecules. At a lower
-aescin-to-DMPC ratio, larger structures are stabilized and vice versa. Moreover, there are indications that the bicelles undergo a temperature-dependent phase transition into large vesicles and stacked bilayers [
48,
49], similar to phospholipid-bile salt systems [
56]. In addition to the DMPC-
-aescin system, solubilization of a 1,2-dipalmitoyl-
sn-glycero-3-phosphocholine (DPPC) membrane by
-aescin in the lipids gel-phase was reported in a study of de Groot et al. [
39]. By TEM, small and oval shaped structures, partially stacked in a worm-like arrangement were found for a DPPC-
-aescin mixture in a ratio of 1:3. These structures are depicted in
Figure 13D,G and also correspond to the formation of bicelles.
6. Synergies of -Aescin, Cholesterol and Drug Molecules
The effect of cholesterol addition on the structural parameters of
-aescin-containing phospholipid bilayers composed of DMPC was investigated by Sreij et al. [
55]. In this work, the cholesterol contents studied were 1, 5, and 10 mol% and the
-aescin contents ranged from 0 to 6 mol% with respect to the amount of DMPC in solution. Thereby, the formation of
-aescin-cholesterol complexes in DMPC bilayers is confirmed which was already shown for other saponins. The complex formation was investigated by various methods, including SAXS & SANS, wide-angle X-ray scattering (WAXS) and differential scanning calorimetry (DSC). DSC confirmed complexation of cholesterol with
-aescin. This was revealed by a modification of the endothermic signal whereas the effect increases with rising steroid content. Although the lowest amount (1 mol% of cholesterol) seems to reduce the
-aescin-driven phase segregation, 5 and 10 mol% cholesterol lead to strong broadening of the endothermic peaks, resulting from the distortion of the cooperative phase transition of hydrocarbon chains through insertion of the stiff steroid molecules. WAXS data show that already 0.5 to 1 mol% of
-aescin are able to reverse the distorting effect of 10 mol% cholesterol on the WAXS pattern. Moreover, it could be shown that
is generally lowered by about 1
C and the area per headgroup (
) increased by around 0.3 Å
upon cholesterol (10 mol%) addition. SAXS and SANS demonstrate how
-aescin–cholesterol complexes modify structural parameters of SUVs investigated. It becomes visible that the structural parameters (e.g., inner core radius and bilayer thickness) are dominated by the effects resulting from
-aescin. Nevertheless, the presence of cholesterol enhances inter-vesicular effects such as aggregation of SUVs.
De Groot et al. [
39] investigated the interaction of
-aescin with DPPC and cholesterol at relatively high lipid-to-cholesterol-to-saponin ratios inspired by the quantities reported for the formation of immune stimulating complex matrices (ISCOMs). In their work, the authors report on the formation of circularly and ovally shaped structures in either water or Tris buffer solution at pH 7.4. TEM images illustrating these structures (
Figure 13) reveal that the final form of aggregates obtained strongly depends on composition.
-Aescin micelles (in water) exhibit circular shape (A) and the binary DMPC/
-aescin mixtures (panels D & G) form discoidal structures which assemble into worm-like aggregates. This finding is in agreement with the results on bicelle formation in DMPC-
-aescin mixtures reported by Geisler et al. [
49]. The ternary system (panels B & C), on the other hand, leads to the formation of slightly different structures. These are significantly larger and posses partially oval shape (arrow in panel B). The authors suggest that these structures are involved in the ISCOM formation process. However, another interesting observation is that similar but almost spherical aggregates are obtained in the binary
-aescin-cholesterol mixtures (panels E & H) in the absence of DPPC, indicating that cholesterol is completely solubilized by
-aescin. The comparison of the TEM images also suggests that DPPC has a major influence on the alignment of the flat disks in solution.
The reverse effect to the one of cholesterol was found for the non-steroidal anti-inflammatory drug (NSAID) ibuprofen [
21] at similar experimental conditions. In this work, which was conducted at pH 7.4 in a 50 mM phosphate buffer solution, both, the
-aescin and ibuprofen molecules are deprotonated. The
-aescin-contents range from 0 to 6 mol% and ibuprofen was set constant at either 5 or 10 mol%, all with respect to the amount of DMPC. Contrarily to the effect of cholesterol, NSAIDs in general and therefore also ibuprofen, are known to interact with the phospholipid headgroups from the inside of the bilayer. The study was again conducted by means of SAXS, WAXS, and DSC. In contrast to the behavior with cholesterol, strong complexation of
-aescin and the ibuprofen molecules was not found. Nevertheless, synergistic effects of both types of molecules were observed on the structural parameters of DMPC bilayer. DSC showed that the concentration-dependent phase segregation of
-aescin-molecules in the bilayer is affected by the presence of ibuprofen. A general shift of the entire endothermic signal to generally lower temperatures is observed. This effect enhances with rising ibuprofen content. In addition to this, the peak at lower temperatures caused by
-aescin (
Figure 10) forms first at even lower
-aescin contents compared to the NSAID-free system and is still observed for the highest saponin-contents studied, compared to the NSAID-free system. WAXS, which investigates the acyl chain correlations and gives therefore information about the effect on the lateral arrangement of DMPC molecules, shows that the effect of ibuprofen (10 mol%) is different from that of cholesterol. Here, contrarily to the steroid effect, no prominent impact on area per lipid molecule
could be shown.
is obtained from WAXS as described by Equation (3) in Reference [
21]. Therefore, no direct complexation of these two molecules is seen. However, it could be shown that the presence of ibuprofen decreases
especially at lower
-aescin (<1 mol%) contents. This effect becomes negligible at 5 mol% of the saponin. SAXS experiments revealed that the
-aescin concentration dominates the vesicle size parameters obtained. In addition, the most prominent effect is observed in a temperature-dependent study conducted from 10 to 50
C at 5 mol%
-aescin and 10 mol% of the NSAID. Here, and contrarily to the aggregation effect of cholesterol, it could be shown that ibuprofen reduces the amount of aggregation between SUVs and bilayers in general. More precisely, high amounts of ibuprofen could reduce the temperature-driven membrane stacking as observed for the binary
-aescin-DMPC mixtures, especially for the higher temperatures. Already 10 mol% ibuprofen leads to the formation of free-standing and extended membrane sheets whose size exceeded instrument resolution.
7. Interaction of -Aescin with Langmuir Monolayers from Langmuir Tensiometry (LT) Experiments
Up to now, there are only a few reports on the effect of
-aescin on a DMPC monolayer [
39,
46]. Sreij et al. [
46] studied the penetration effect of
-aescin into a DMPC monolayer by subphase exchange experiments. Thereby, the DMPC monolayer was adsorbed at the buffer–air interface and was studied in either the liquid condensed (LC) or liquid expanded (LE) phase at 4
C or 38
C, respectively. In these subphase-exchange experiments
-aescin gently incorporates into the adsorption layer from the bulk solution.
From surface pressure-time (
) adsorption kinetics faster adsorption at higher temperature, which is due to faster diffusional adsorption of
-aescin from the solution, was found (
Figure 14). In these
-kinetics a special feature (an intermediate cusp) was found after initial rapid pressure increase and before reaching the equilibrium surface pressure
. Based on this finding, the authors assume that an adsorption barrier arises leading to a transient meta-stable state before reaching the final penetration/reorganization of
-aescin inside the DMPC monolayer, or transient domain-formation within the monolayer, or both with time. This effect was found to take longer time at low than at high temperature (
Figure 14). Moreover, it was found that penetration of
-aescin into a disordered LE phase (e.g., at weak initial compression of the lipid film) is facilitated and independent of temperature (
Figure 15). This shows in the pressure difference
which is calculated as a free energy difference between the initial pure lipid state (
) and the final equilibrium plateau (
) corresponding to the mixed
-aescin-lipid monolayer after
-aescin has penetrated the DMPC monolayer from the solution. From the maximum insertion pressure (MIP) it becomes evident, that the strength of penetration depends on the phospholipid phase state. The LC phase is much more penetrable (MIP
62 ± 2 mNm
) by
-aescin than the LE phase (MIP
38 ± 3 mNm
). From these results the authors conclude that the higher MIP value may be the result of a more pronounced impact of
-aescin molecules in the gel-phase bilayer-like LC phase than in the LE phase. However, an alternative explanation which was suggested by one of the referees of the present review differs from this explanation. He pointed out that a different explanation might be concluded from the higher slope of the surface pressure isotherm in the LC state than in the LE state. Due to the steepness of the curve even small increases of surface concentration (decrease of area per molecule) could result in a huge jump of surface pressure.
De Groot et al. [
39] studied the interaction of
-aescin with DPPC and cholesterol monolayers and combinations of both also by Langmuir tensiometry (LT). In their experiment they compare the adsorption of
-aescin, which is injected in the subphase, with either a DPPC or a cholesterol monolayer or a mixture of both. The work was carried out at 20
C and the subphase-exchange experiments were recorded for a duration of 1000 s, i.e., 16.6 min. They found that the most prominent effect of
-aescin in the subphase occurs on cholesterol-containing monolayers confirming the strong affinity of
-aescin and cholesterol molecules. These results are in concordance with the effects of
-aescin on DMPC bilayers in the presence of cholesterol as discussed previously [
55] (
Section 6).
8. Conclusions
The results summarized in this review related to the self-assembly of -aescin in solution as well as at interfaces and the interaction with lipid-model membranes show special interactions caused by the specific molecular structure of the saponin -aescin. In particular, several studies show the influence of the pH and thus the protonation state of -aescin on the self-assembly of -aescin. A deprotonation of the acidic residue in the glyconic molecular part drastically modifies the properties of -aescin monolayers at interfaces and in foams. This knowledge plays an important role in the various applications of -aescin, e.g., in the cosmetics sector.
The interaction of -aescin with phospholipid model membranes further demonstrated decomposition and solubilisation into small lipid membrane fragments. The formation of bicelles at high -aescin concentration may have potential in the use of bicelles for the structural determination of membrane proteins by nuclear magnetic resonance (NMR). Furthermore, results from studies with membrane additives such as cholesterol and ibuprofen can provide important, fundamental insights that help to better understand the impact of -aescin on the human body. This is particularly important as -aescin is present in a variety of freely available and advertised products, but the background is unknown for many of the observed beneficial effects. In this context, the results presented in this review can serve as a basis for model systems closer to the human organism. This includes, for example, the elucidation of the interactions of -aescin with animal tissues such as skin.
Moreover, the reviewed works reveal that -aescin shows strong synergistic effects with a drug like ibuprofen. This effect should be further elucidated and it would be of great interest to relate this found molecular interaction to changes in efficiency of the drug. Also, other NSAIDs should be evaluated in this context. Exploiting such synergies could lead to reduced doses of drugs with e.g., less burden for the liver.