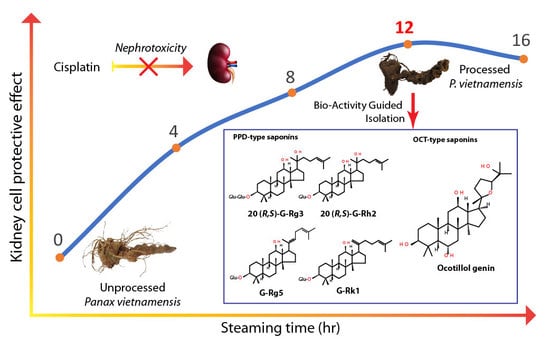
Increase in Protective Effect of Panax vietnamensis by Heat Processing on Cisplatin-Induced Kidney Cell Toxicity
Abstract
1. Introduction
2. Results
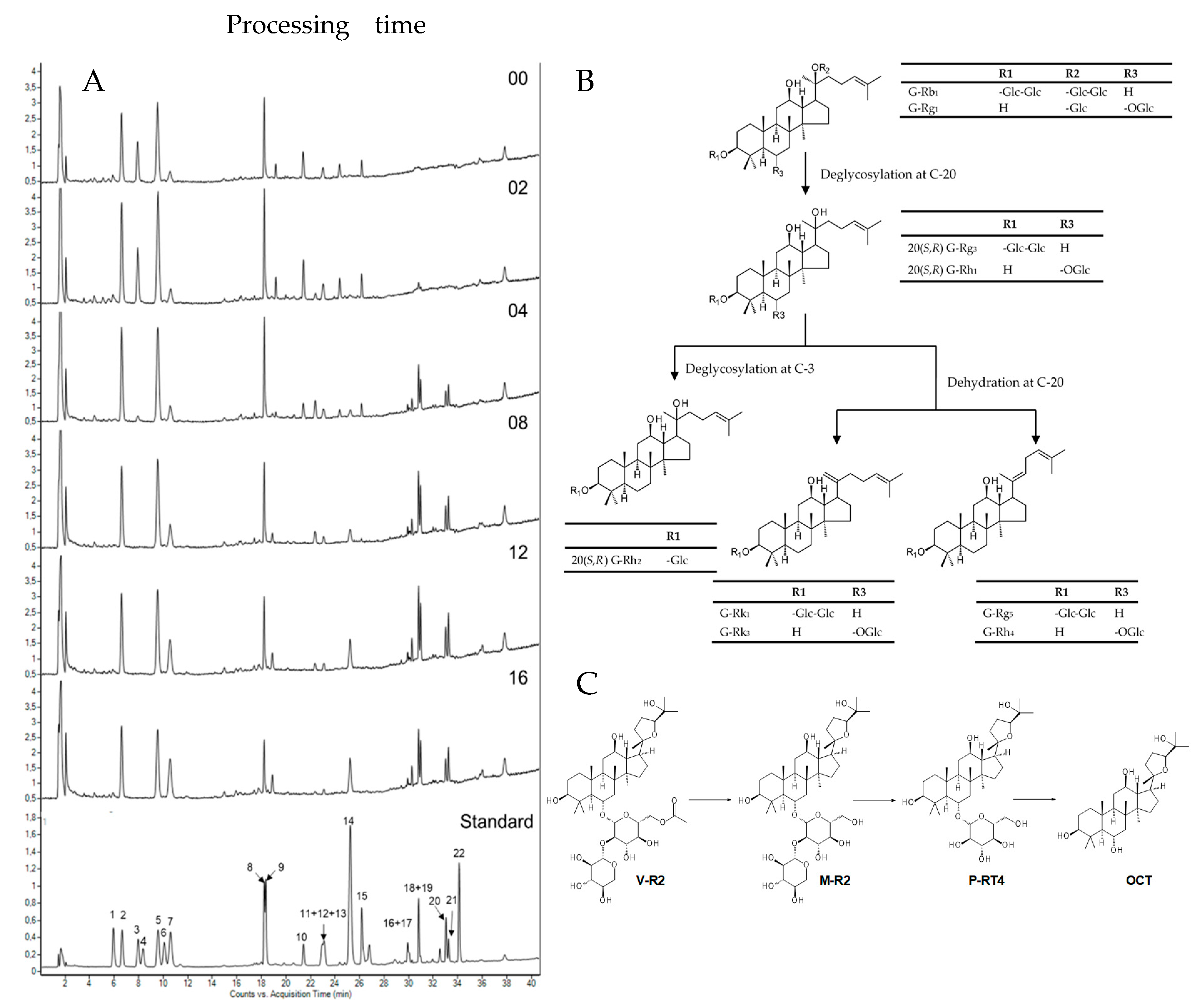
2.1. Chemical Changes in Panax Vietnamensis by Steaming
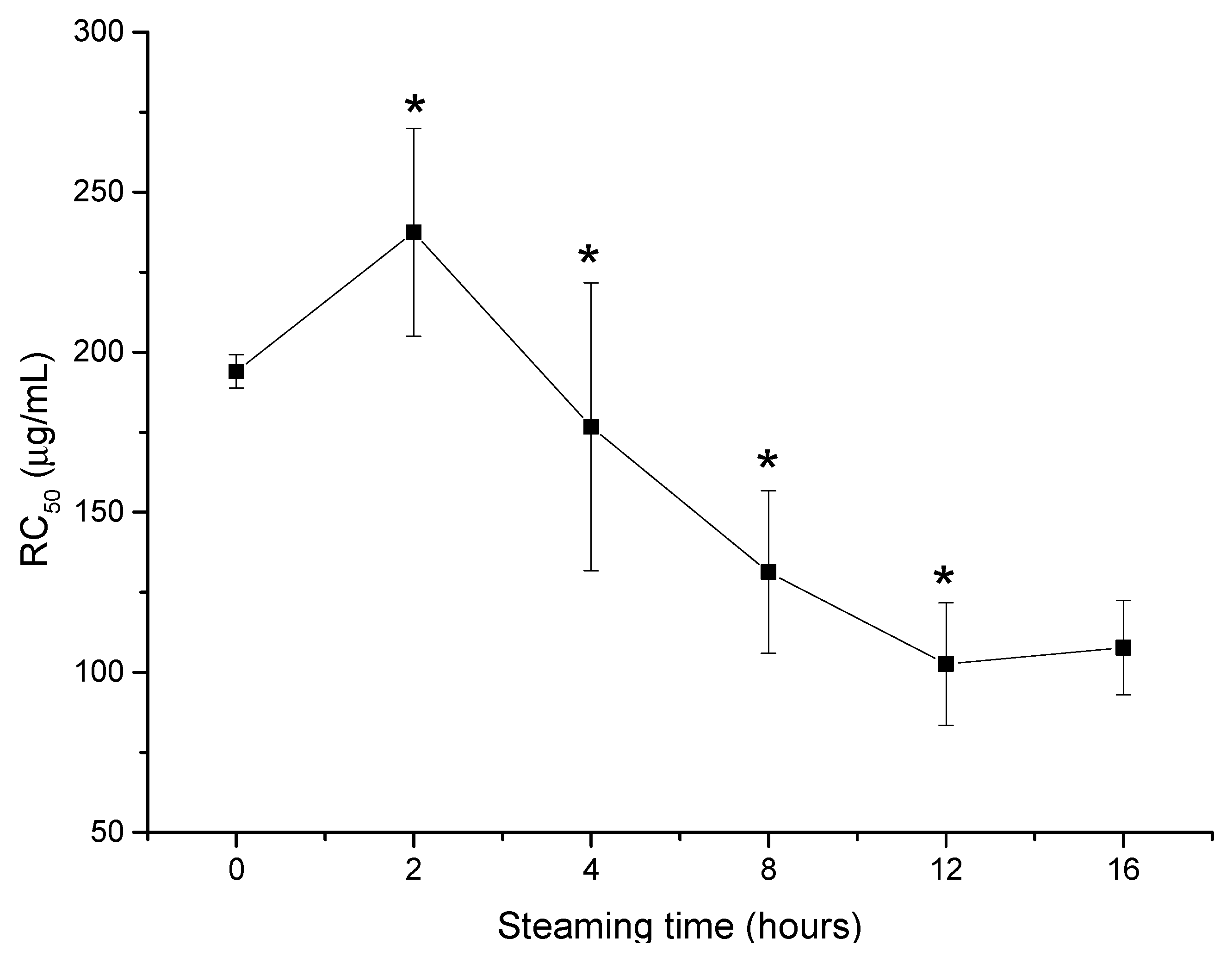
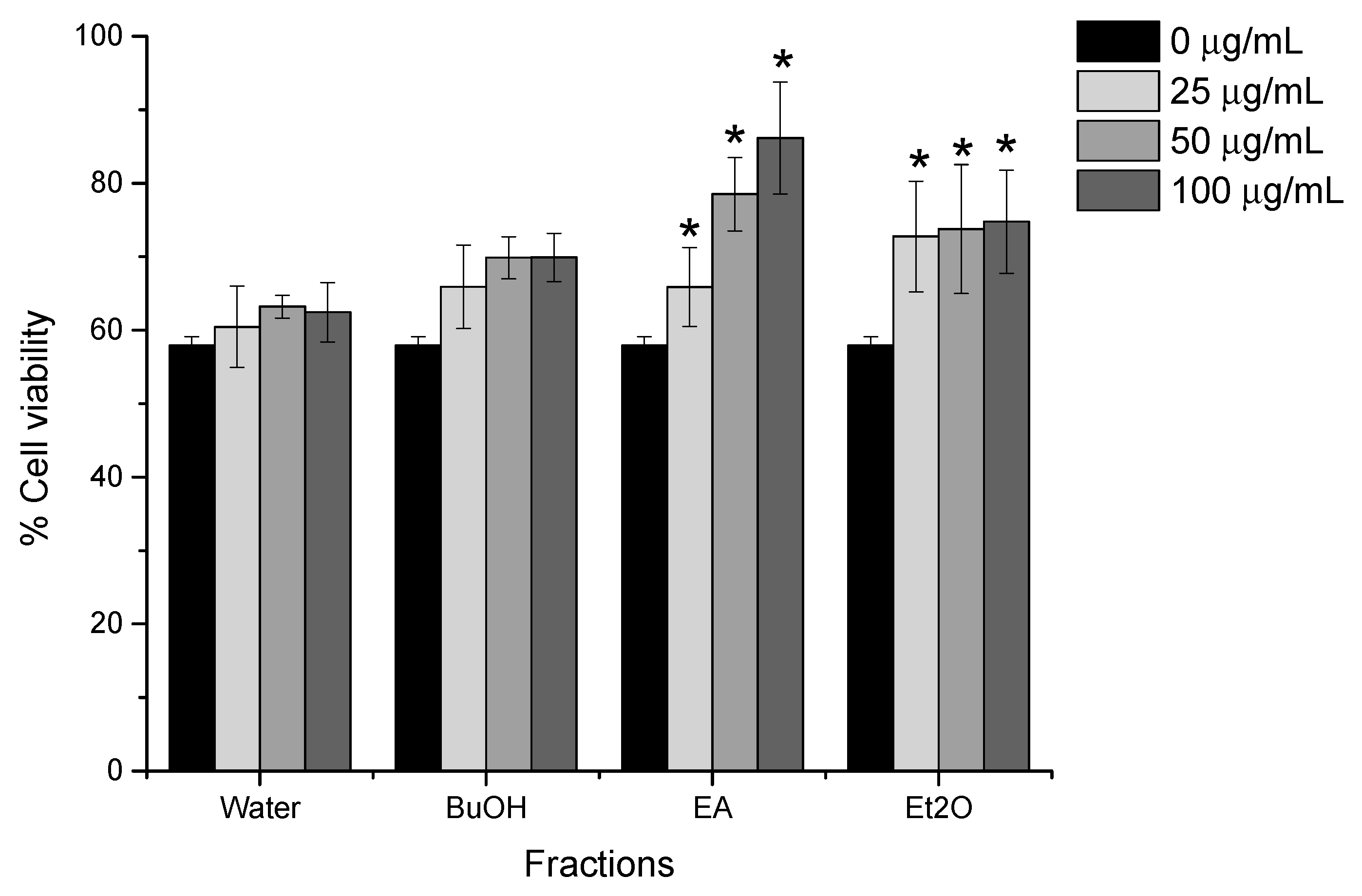
2.2. Heating Increases the Kidney Cell Protective Effect of VG
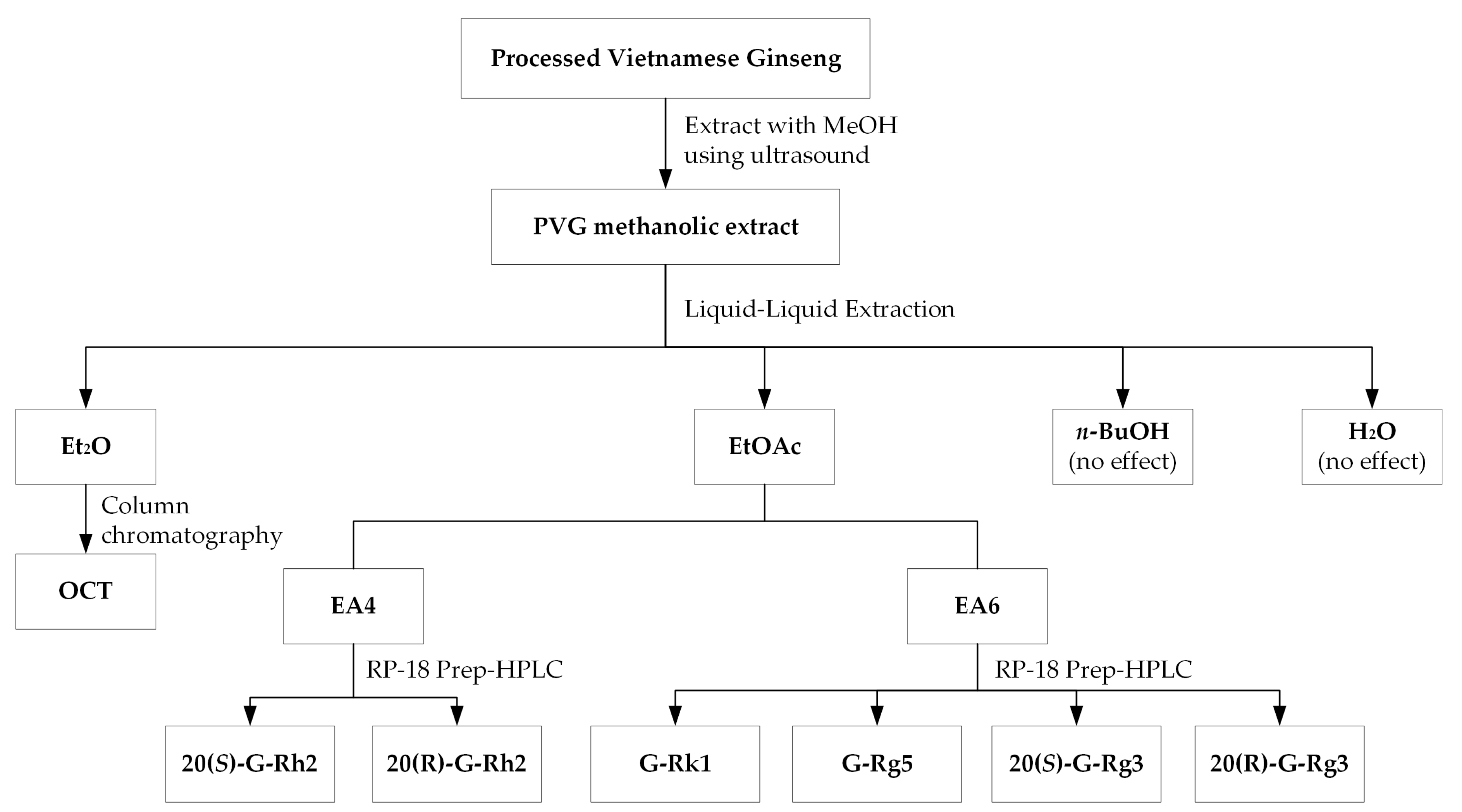
2.3. Bioactivity-Guided Extraction
2.4. Comparison of Protection Effects of Isolated Compounds
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Preparation of Processed Vietnamese Ginseng at Different Times
4.2.2. Processed Vietnamese Ginseng Extraction and Isolation
4.2.3. Liquid Chromatography–QToF Mass Spectrometry Analysis
4.2.4. Nuclear Magnetic Resonance (NMR) Spectroscopy
4.2.5. Cell Culture and Cells Viability Assay
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rosenberg, B.; Van Camp, L.; Krigas, T. Inhibition of cell division in Escherichia coli by electrolysis products from a platinum electrode. Nature 1965, 205, 698–699. [Google Scholar] [CrossRef] [PubMed]
- FDA PLATINOL. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/018057s080lbl.pdf (accessed on 21 November 2019).
- Planting, A.S.; Catimel, G.; de Mulder, P.H.; de Graeff, A.; Höppener, F.; Verweij, J.; Oster, W.; Vermorken, J.B. Randomized study of a short course of weekly cisplatin with or without amifostine in advanced head and neck cancer. EORTC Head and Neck Cooperative Group. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 1999, 10, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Gatzemeier, U.; von Pawel, J.; Gottfried, M.; ten Velde, G.P.; Mattson, K.; de Marinis, F.; Harper, P.; Salvati, F.; Robinet, G.; Lucenti, A.; et al. Phase III comparative study of high-dose cisplatin versus a combination of paclitaxel and cisplatin in patients with advanced non-small-cell lung cancer. J. Clin. Oncol. 2000, 18, 3390–3399. [Google Scholar] [CrossRef] [PubMed]
- Noda, K.; Nishiwaki, Y.; Kawahara, M.; Negoro, S.; Sugiura, T.; Yokoyama, A.; Fukuoka, M.; Mori, K.; Watanabe, K.; Tamura, T.; et al. Irinotecan plus Cisplatin Compared with Etoposide plus Cisplatin for Extensive Small-Cell Lung Cancer. N. Engl. J. Med. 2002, 346, 85–91. [Google Scholar] [CrossRef]
- Rose, P.G.; Bundy, B.N.; Watkins, E.B.; Thigpen, J.T.; Deppe, G.; Maiman, M.A.; Clarke-Pearson, D.L.; Insalaco, S. Concurrent Cisplatin-Based Radiotherapy and Chemotherapy for Locally Advanced Cervical Cancer. N. Engl. J. Med. 1999, 340, 1144–1153. [Google Scholar] [CrossRef]
- Hartmann, J.T.; Lipp, H.-P. Toxicity of platinum compounds. Expert Opin. Pharmacother. 2003, 4, 889–901. [Google Scholar] [CrossRef]
- Kociba, R.J.; Sleight, S.D. Acute toxicologic and pathologic effects of cis-diamminedichloroplatinum (NSC-119875) in the male rat. Cancer Chemother. Rep. 1971, 55, 1–8. [Google Scholar]
- Pabla, N.; Murphy, R.F.; Liu, K.; Dong, Z. The copper transporter Ctr1 contributes to cisplatin uptake by renal tubular cells during cisplatin nephrotoxicity. Am. J. Physiol. Physiol. 2009, 296, F505–F511. [Google Scholar] [CrossRef]
- Ishida, S.; Lee, J.; Thiele, D.J.; Herskowitz, I. Uptake of the anticancer drug cisplatin mediated by the copper transporter Ctr1 in yeast and mammals. Proc. Natl. Acad. Sci. USA 2002, 99, 14298–14302. [Google Scholar] [CrossRef]
- Filipski, K.K.; Loos, W.J.; Verweij, J.; Sparreboom, A. Interaction of Cisplatin with the Human Organic Cation Transporter 2. Clin. Cancer Res. 2008, 14, 3875–3880. [Google Scholar] [CrossRef]
- Ciarimboli, G.; Ludwig, T.; Lang, D.; Pavenstädt, H.; Koepsell, H.; Piechota, H.-J.; Haier, J.; Jaehde, U.; Zisowsky, J.; Schlatter, E. Cisplatin Nephrotoxicity Is Critically Mediated via the Human Organic Cation Transporter 2. Am. J. Pathol. 2005, 167, 1477–1484. [Google Scholar] [CrossRef]
- Ramesh, G.; Reeves, W.B. TNFR2-mediated apoptosis and necrosis in cisplatin-induced acute renal failure. Am. J. Physiol. Physiol. 2003, 285, F610–F618. [Google Scholar] [CrossRef] [PubMed]
- Pabla, N.; Dong, Z. Cisplatin nephrotoxicity: Mechanisms and renoprotective strategies. Kidney Int. 2008, 73, 994–1007. [Google Scholar] [CrossRef] [PubMed]
- Tarladacalisir, Y.T.; Kanter, M.; Uygun, M. Protective Effects of Vitamin C on Cisplatin-Induced Renal Damage: A Light and Electron Microscopic Study. Ren. Fail. 2008, 30, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Nazıroǧlu, M.; Karaoğlu, A.; Aksoy, A.O. Selenium and high dose vitamin E administration protects cisplatin-induced oxidative damage to renal, liver and lens tissues in rats. Toxicology 2004, 195, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Nisar, S.; Feinfeld, D.A. N-acetylcysteine as salvage therapy in cisplatin nephrotoxicity. Ren. Fail. 2002, 24, 529–533. [Google Scholar] [CrossRef]
- Ridzuan, N.R.A.; Rashid, N.A.; Othman, F.; Budin, S.B.; Hussan, F.; Teoh, S.L. Protective role of natural products in cisplatin-induced nephrotoxicity. Mini Rev. Med. Chem. 2019, 19, 1134–1143. [Google Scholar] [CrossRef]
- Yokozawa, T.; Cho, E.J.; Hara, Y.; Kitani, K. Antioxidative Activity of Green Tea Treated with Radical Initiator 2,2‘-Azobis(2-amidinopropane) Dihydrochloride. J Agric Food Chem 2000, 48, 5068–5073. [Google Scholar] [CrossRef]
- It, I.C.; Kuhlmann, M.K.; Horsch, E.; Burkhardt, G.; Wagner, M.; Ko, H. Reduction of cisplatin toxicity in cultured renal tubular cells by the bio-avonoid quercetin. Arch. Toxicol. 1998, 72, 536–540. [Google Scholar]
- Al-Majed, A.A.; Abd-Allah, A.R.A.; Al-Rikabi, A.C.; Al-Shabanah, O.A.; Mostafa, A.M. Effect of oral administration of arabic gum on cisplatin-induced nephrotoxicity in rats. J. Biochem. Mol. Toxicol. 2003, 17, 146–153. [Google Scholar] [CrossRef]
- Park, J.Y.; Choi, P.; Kim, T.; Ko, H.; Kim, H.K.; Kang, K.S.; Ham, J. Protective Effects of Processed Ginseng and Its Active Ginsenosides on Cisplatin-Induced Nephrotoxicity: In Vitro and in Vivo Studies. J. Agric. Food Chem. 2015, 63, 5964–5969. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhang, W.; Yan, X.; Hou, J.; Wang, Z.; Ding, C.; Liu, W.; Zheng, Y.; Chen, C.; Li, Y.; et al. Arginyl-fructosyl-glucose (AFG), A Major Maillard Reaction Product of Red Ginseng, Attenuates Cisplatin-Induced Acute Kidney Injury by Regulating NF-$κ$B and PI3K/Akt Signaling Pathways. J. Agric. Food Chem. 2019. [Google Scholar]
- Nguyen, M.D.; Nguyen, T.N.; Kasai, R.; Ito, A.; Yamasaki, K.; Tanaka, O. Saponins from Vietnamese ginseng, Panax vietnamensis Ha et Grushv. Collected in central Vietnam. I. Chem Pharm Bull 1993, 41, 2010–2014. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.D.; Kasai, R.; Ohtani, K.; Ito, A.; Nguyen, T.N.; Yamasaki, K.; Tanaka, O. Saponins from Vietnamese Ginseng, Panax vietnamensis Ha et Grushv. Collected in central Vietnam. II. Chem Pharm Bull 1994, 42, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.D.; Nguyen, T.N.; Kasai, R.; Ito, A.; Yamasaki, K.; Tanaka, O. Saponins from Vietnamese ginseng, Panax vietnamensis Ha et Grushv. collected in central Vietnam. III. Chem. Pharm. Bull. 1994, 42, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Duc, N.M.; Kasai, R.; Yamasaki, K.; Nham, N.T.; Tanaka, O. New dammarane saponins from Vietnamese ginseng. In Studies in Plant Science; Chong-Ren, Y., Osamu, T., Eds.; Elsevier: Kunming, China, 1999; Volume 6, pp. 77–82. ISBN 0928-3420. [Google Scholar]
- Konoshima, T.; Takasaki, M.; Tokuda, H.; Nishino, H.; Duc, N.M.; Kasai, R.; Yamasaki, K. Anti-tumor-promoting activity of majonoside-R2 from Vietnamese ginseng, Panax vietnamensis Ha et Grushv. (I). Biol Pharm Bull 1998, 21, 834–838. [Google Scholar] [CrossRef][Green Version]
- Tran, Q.L.; Adnyana, I.K.; Tezuka, Y.; Nagaoka, T.; Tran, Q.K.; Kadota, S. Triterpene saponins from Vietnamese ginseng (Panax vietnamensis) and their hepatocytoprotective activity. J. Nat. Prod. 2001, 64, 456–461. [Google Scholar] [CrossRef]
- Wang, H.; Kong, L.; Zhang, J.; Yu, G.; Lv, G.; Zhang, F. The pseudoginsenoside F11 ameliorates cisplatin-induced nephrotoxicity without compromising its anti-tumor activity in vivo. Sci. Rep. 2014, 4, 4986. [Google Scholar] [CrossRef]
- Baek, S.H.; Piao, X.L.; Lee, U.J.; Kim, H.Y.; Park, J.H. Reduction of Cisplatin-induced nephrotoxicity by ginsenosides isolated from processed ginseng in cultured renal tubular cells. Biol. Pharm. Bull. 2006, 29, 2051–2055. [Google Scholar] [CrossRef][Green Version]
- Yang, H.; Kim, J.Y.; Kim, S.O.; Yoo, Y.H.; Sung, S.H. Complete 1H-NMR and13C-NMR spectral analysis of the pairs of 20(S) and 20(R) ginsenosides. J. Ginseng Res. 2014, 38, 194–202. [Google Scholar] [CrossRef]
- Hirakura, K.; Morita, M.; Nakajima, K.; Ikeya, Y.; Mitsuhashi, H. Polyacetylenes from the roots of Panax ginseng. Phytochemistry 1991, 30, 3327–3333. [Google Scholar] [CrossRef]
- Nguyen, M.D.; Kasai, R.; Ohtani, K.; Ito, A.; Yamasaki, K.; Nguyen, T.N.; Tanaka, O. New saponins from Vietnamese ginseng: highlights on biogenesis of dammarane triterpenoids. In Advances in Experimental Medicine and Biology; Springer Nature: Kunming, China, 1996; Volume 404, pp. 129–149. [Google Scholar]
- Han, M.S.; Han, I.H.; Lee, D.; An, J.M.; Kim, S.N.; Shin, M.S.; Yamabe, N.; Hwang, G.S.; Yoo, H.H.; Choi, S.J.; et al. Beneficial effects of fermented black ginseng and its ginsenoside 20(S)-Rg3 against cisplatin-induced nephrotoxicity in LLC-PK1 cells. J. Ginseng Res. 2015, 40, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.L.; Kang, K.S. Protective effect of ginsenoside Rh3 against anticancer drug-induced apoptosis in LLC-PK1 kidney cells. J. Ginseng Res. 2017, 41, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Li, W.; Tan, J.; Wang, C.; Lin, H.; Zhou, B.; Liu, J.; Li, P. Effect of ginsenoside Rh2 on renal apoptosis in cisplatin-induced nephrotoxicity in vivo. Phytomedicine 2019, 61, 152862. [Google Scholar] [CrossRef] [PubMed]
- Le, T.H.; Lee, S.Y.; Kim, T.R.; Kim, J.Y.; Kwon, S.W.; Nguyen, N.K.; Park, J.H.; Nguyen, M.D. Processed Vietnamese ginseng: Preliminary results in chemistry and biological activity. J. Ginseng Res. 2014, 38, 154–159. [Google Scholar] [CrossRef]
- Jeong, J.-J.; Van Le, T.H.; Lee, S.-Y.; Eun, S.-H.; Nguyen, M.D.; Park, J.H.; Kim, D.-H. Anti-inflammatory effects of vina-ginsenoside R2 and majonoside R2 isolated from Panax vietnamensis and their metabolites in lipopolysaccharide-stimulated macrophages. Int. Immunopharmacol. 2015, 28, 700–706. [Google Scholar] [CrossRef]
- Wang, H.; Yu, P.; Gou, H.; Zhang, J.; Zhu, M.; Wang, Z.; Tian, J.; Jiang, Y.; Fu, F. Cardioprotective Effects of 20(S)-Ginsenoside Rh2 against Doxorubicin-Induced Cardiotoxicity In Vitro and In Vivo. Evidence-Based Complement. Altern. Med. 2012, 2012, 1–8. [Google Scholar]
- Wang, Z.; Zheng, Q.; Liu, K.; Li, G.; Zheng, R. Ginsenoside Rh2 Enhances Antitumour Activity and Decreases Genotoxic Effect of Cyclophosphamide. Basic Clin. Pharmacol. Toxicol. 2006, 98, 411–415. [Google Scholar] [CrossRef]
- Tang, C.; Wang, Y.; Long, Y.; An, X.; Shen, J.; Ni, Y. Anchoring 20(R)-Ginsenoside Rg3 onto Cellulose Nanocrystals To Increase the Hydroxyl Radical Scavenging Activity. ACS Sustain. Chem. Eng. 2017, 5, 7507–7513. [Google Scholar] [CrossRef]
- Yue, P.Y.K.; Wong, D.Y.L.; Wu, P.K.; Leung, P.Y.; Mak, N.K.; Yeung, H.W.; Liu, L.; Cai, Z.; Jiang, Z.-H.; Fan, T.P.D.; et al. The angiosuppressive effects of 20(R)- ginsenoside Rg3. Biochem. Pharmacol. 2006, 72, 437–445. [Google Scholar] [CrossRef]
- Wei, X.; Su, F.; Su, X.; Hu, T.; Hu, S. Stereospecific antioxidant effects of ginsenoside Rg3 on oxidative stress induced by cyclophosphamide in mice. Fitoterapia 2012, 83, 636–642. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 20(S)-ginsenoside-Rh2, 20(R)-Ginsenoside-Rh2, 20(S)-Ginsenoside-Rg3, 20(R)-Ginsenoside-Rg3, Ginsenoside Rk1, Ginsenoside Rg5, Ocotillol genin are available from the authors. |
| No. | Compounds | RC50 (µM) ± SD | RCmax (µM)/Recovery Rate (%) |
|---|---|---|---|
| 1. | 20(R)-Ginsenoside-Rh2 | 6.67 ± 0.42 | 10/73.9 |
| 2. | 20(R)-Ginsenoside-Rg3 | 8.39 ± 0.3 | 25/69.9 |
| 3. | 20(S)-Ginsenoside-Rh2 | 46.15 ± 9.66 | 50/75.7 |
| 4. | Ginsenoside Rk1 | 62.69 ± 17.3 | 50/40.6 |
| 5. | 20(S)-Ginsenoside-Rg3 | 88.4 ± 54.62 | 200/59.3 |
| 6. | Ginsenoside Rg5 | 180.83 ± 33.27 | 200/43.5 |
| 7. | Ocotillol genin | 226.19 ± 66.16 | 200/43.9 |
| 8. | N-acetyl cysteine | 1543.6 ± 74.07 | 4000/67.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vu-Huynh, K.L.; Le, T.H.V.; Nguyen, H.T.; Kim, H.M.; Kang, K.S.; Park, J.H.; Nguyen, M.D. Increase in Protective Effect of Panax vietnamensis by Heat Processing on Cisplatin-Induced Kidney Cell Toxicity. Molecules 2019, 24, 4627. https://doi.org/10.3390/molecules24244627
Vu-Huynh KL, Le THV, Nguyen HT, Kim HM, Kang KS, Park JH, Nguyen MD. Increase in Protective Effect of Panax vietnamensis by Heat Processing on Cisplatin-Induced Kidney Cell Toxicity. Molecules. 2019; 24(24):4627. https://doi.org/10.3390/molecules24244627
Chicago/Turabian StyleVu-Huynh, Kim Long, Thi Hong Van Le, Huy Truong Nguyen, Hyung Min Kim, Ki Sung Kang, Jeong Hill Park, and Minh Duc Nguyen. 2019. "Increase in Protective Effect of Panax vietnamensis by Heat Processing on Cisplatin-Induced Kidney Cell Toxicity" Molecules 24, no. 24: 4627. https://doi.org/10.3390/molecules24244627
APA StyleVu-Huynh, K. L., Le, T. H. V., Nguyen, H. T., Kim, H. M., Kang, K. S., Park, J. H., & Nguyen, M. D. (2019). Increase in Protective Effect of Panax vietnamensis by Heat Processing on Cisplatin-Induced Kidney Cell Toxicity. Molecules, 24(24), 4627. https://doi.org/10.3390/molecules24244627